An Updated Review on Electrochemical Nanobiosensors for Neurotransmitter Detection
Abstract
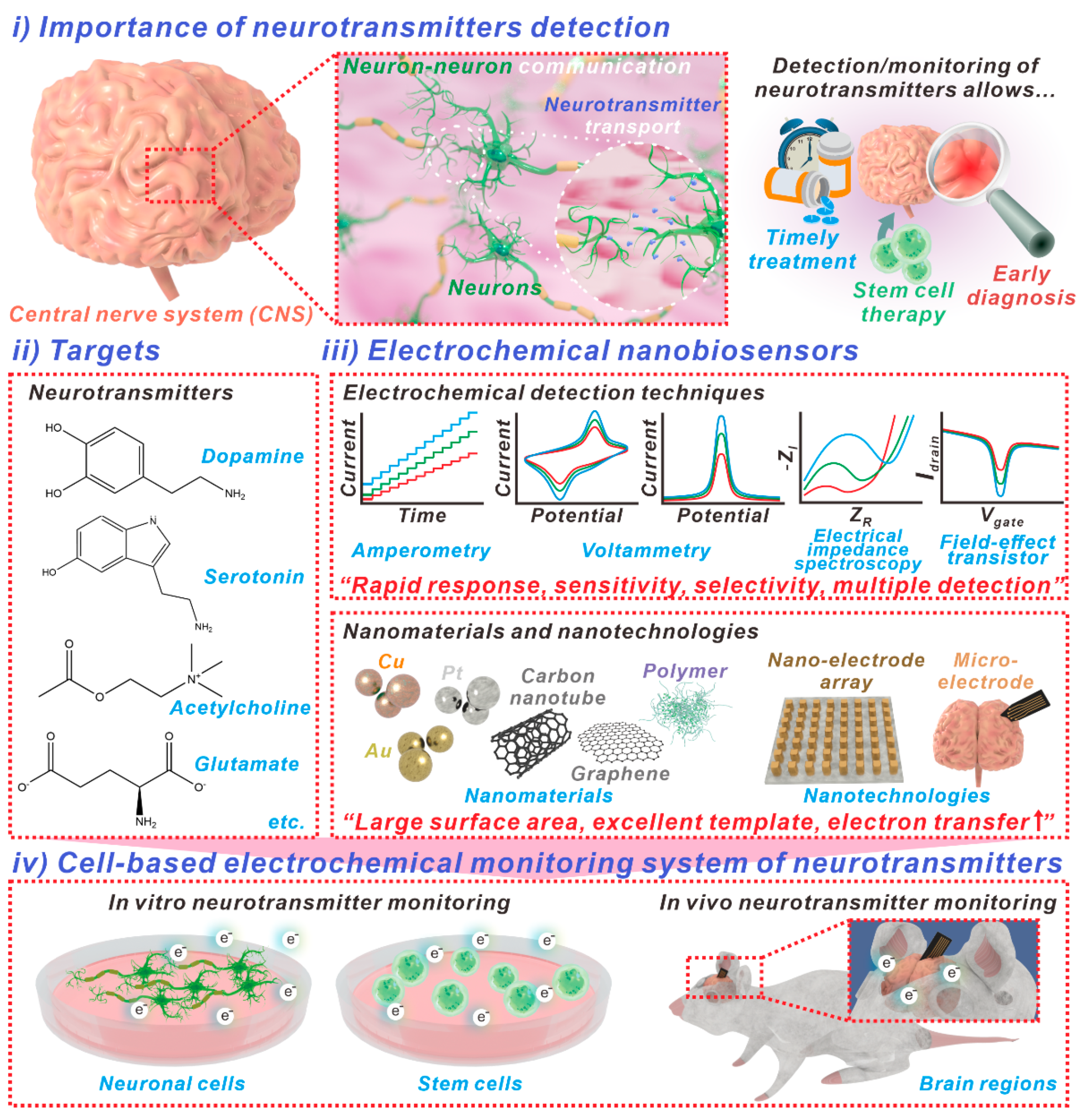
:1. Introduction
2. Neurotransmitters for Early Diagnosis and Pathophysiological Monitoring

3. Recent Advances in Electrochemical Nanobiosensors for Neurotransmitter Detection
3.1. Amperometric Biosensors
3.2. Voltammetric Biosensors
3.2.1. Cyclic Voltammetry
3.2.2. Differential Pulse Voltammetry
3.3. Electrochemical Impedance Spectroscopy Biosensors
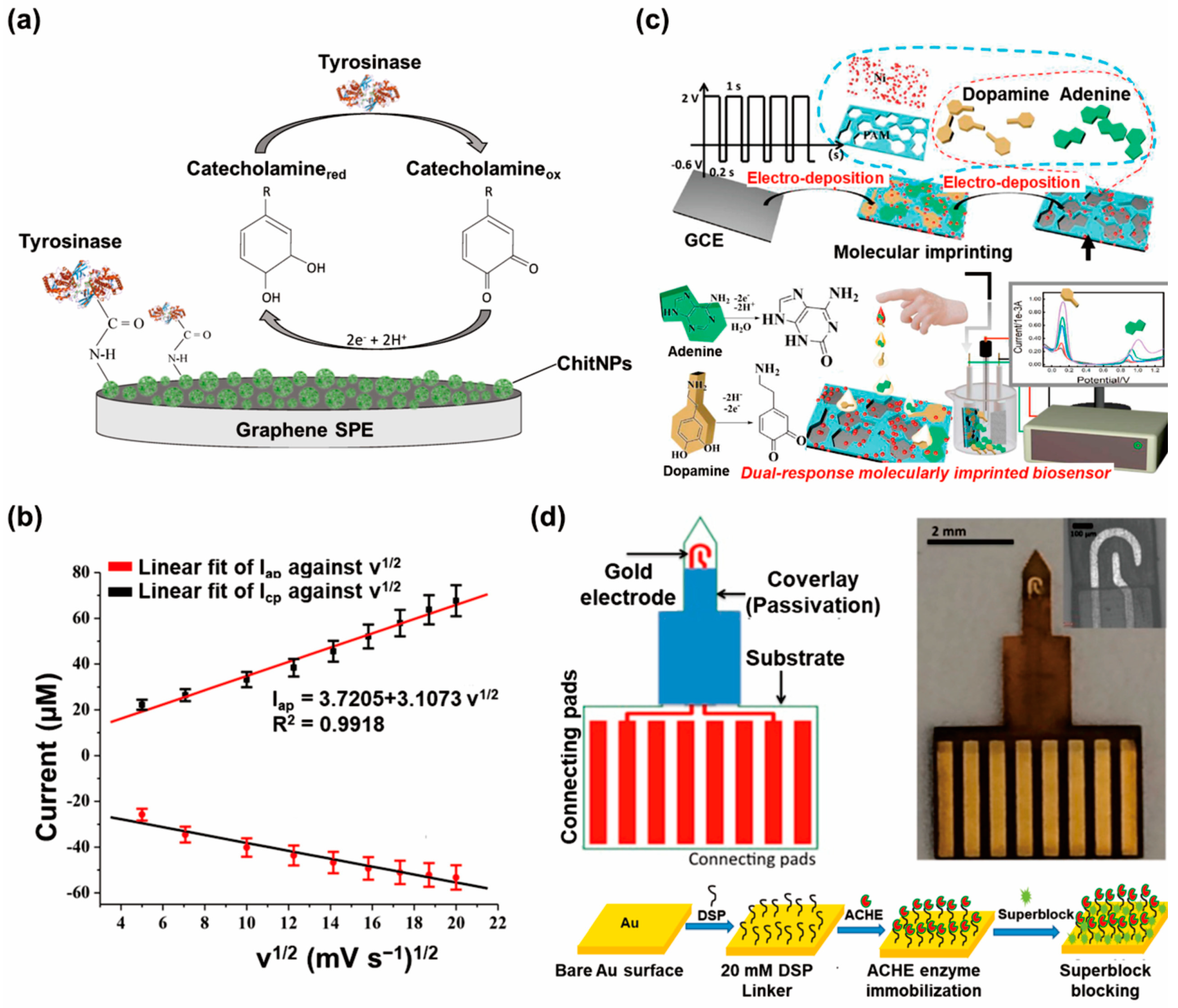
3.4. Field Effect Transistor Biosensors
4. Cell-Based Electrochemical Monitoring Systems for Neurotransmitters
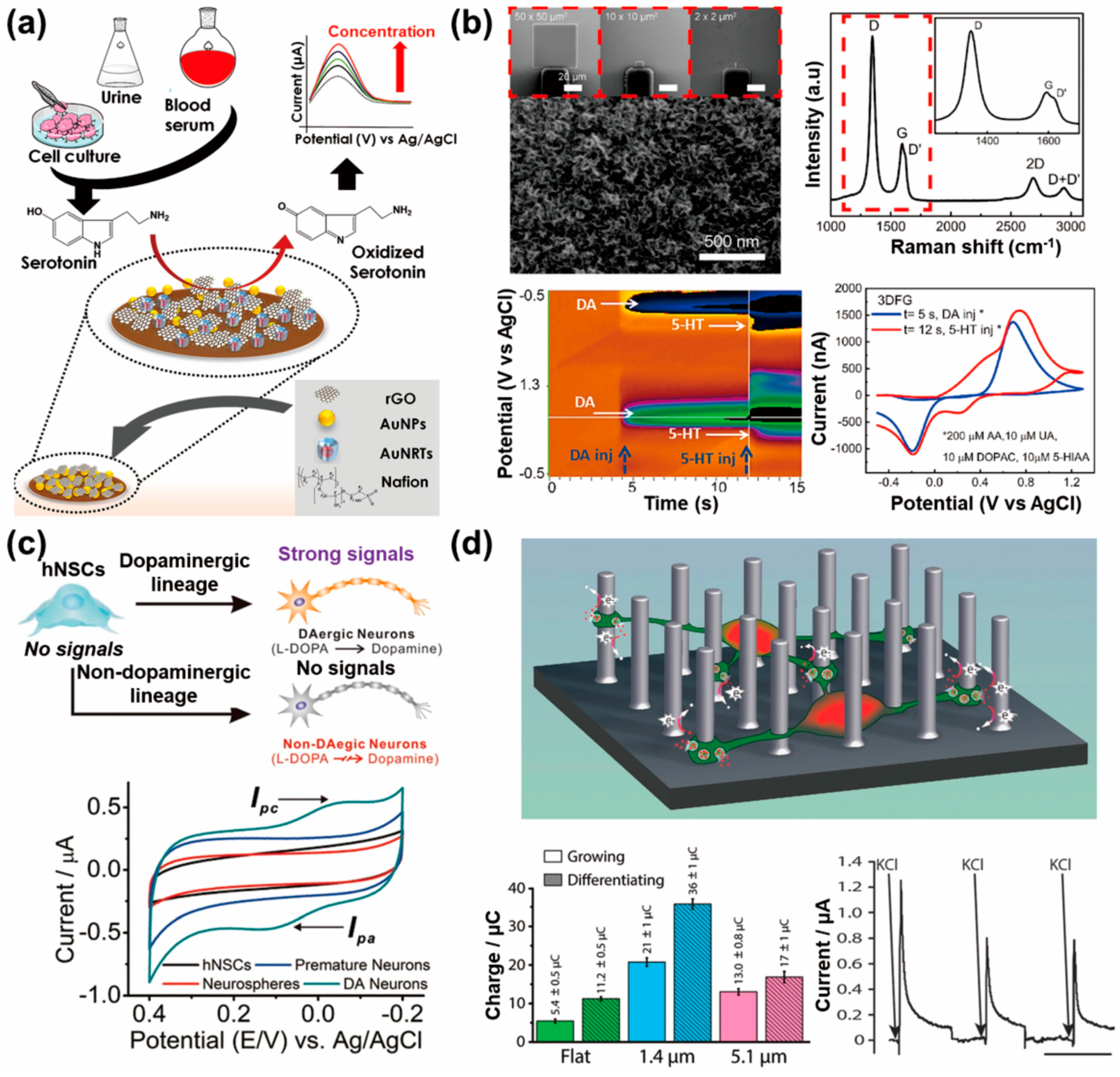
4.1. In Vitro Neurotransmitter Detection System from Neuronal Cells Using Electrochemical Sensors
4.2. Stem-Cell-Released Neurotransmitter Detection Systems Using Electrochemical Sensors
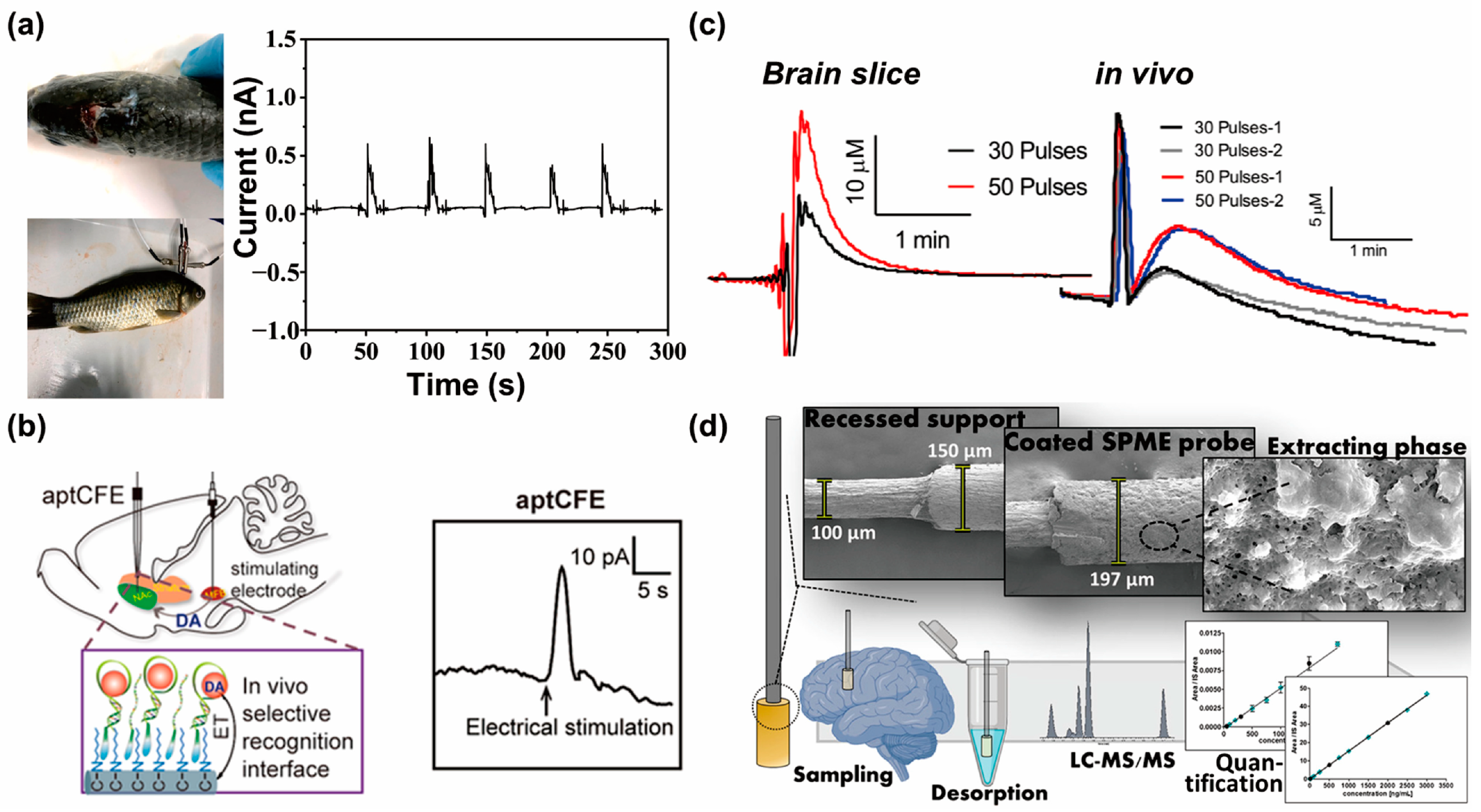
4.3. In Vivo Neurotransmitter Monitoring Systems Using Electrochemical Sensors
5. Conclusions and Future Perspectives
| Sensing Techniques | Targets | Nanomaterials/Nanotechniques | Sample Type | Limit of Detection | Reference |
|---|---|---|---|---|---|
| Amperometric | Dopamine | CDs-CPTMS | Cell-free | 1.0 nM | [53] |
| Catecholamine | Chitosan nanoparticle | Cell-free | 0.17 µM | [54] | |
| Acetylcholine | Poly(3,4-ethylenedioxythiophene) (PEDOT)/carbon electrodes | Cell-free | Not applicable (N/A) | [55] | |
| Acetylcholine | Platinum nanoparticles/porous graphene oxide nano sheets | Cell-free | 1 nM | [56] | |
| Serotonin | Pt NPs on the carbon nanotubes (CNTs)-Cu2O-CuO | HaCaT cell 4t1 cell P815 cell | 3 nM | [92] | |
| Glutamateγ-aminobutyric acid | Microwire | Rat hippocampal slices | N/A | [97] | |
| Glutamate | Platinum microelectrode array | Astrocyte | 6.3 µM | [99] | |
| Dopamine | Three-dimensional carbon scaffolds (p3D-carbon) | Human neural stem cells | N/A | [102] | |
| Glutamate | Borosilicate glass capillaries | Human cerebral organoids | 5.6 µM | [103] | |
| Dopamine | Interdigitated gold microsensor | Human midbrain organoids | 476 nM | [104] | |
| Dopamine | Carbon fiber electrode (CFE) | Rat nucleus accumbens | N/A | [109] | |
| Glutamate | Poly o-phenylenediamine (PPD) membrane/glutamate oxidase/ascorbate oxidase | Rat brain slices and in vivo | 0.044 µM | [110] | |
| Voltametric | Dopamine | Polypyrrole-derived carbon nanotube | Cell-free | 0.2 µM | [63] |
| Epinephrine | Carbon quantum dot/copper oxide nanocomplex | Cell-free | 15.99 µM | [64] | |
| Dopamine | Reduced graphene oxide sheets | Cell-free | 0.11 μM | [72] | |
| Dopamine, Adenine | Ni-polyacrylamide-molecular imprinted polymer (PAM-MIP) matrix | Cell-free | 0.12 μM 0.15 μM | [73] | |
| Serotonin | Gold (Au)-nanorattles (AuNRTs)/reduced graphene oxide (rGO)/Au nanoparticles (AuNPs) | Neuronal cell | 0.39 µM | [90] | |
| Dopamine | Graphene quantum dots (GQDs)/multiwalled carbon nanotubes (MWCNTs) | PC12 cell | 0.87 nM | [94] | |
| Dopamine | Cavity carbon nanopipette electrodes (CNPEs) | Mouse-brain slices | 56 nM | [95] | |
| Dopamine | Overoxidized polypyrrole/sodium dodecyl sulfate (OPPy/SDS)-CNT electrode | PC12 cell | 136 pM | [96] | |
| Dopamine | Homogeneous nanocup electrode arrays | Human neural stem cells | 100 nM | [17] | |
| Dopamine | Cylindrical gold nanoelectrode | Human neural stem cells | 5.83 µM | [101] | |
| Dopamine | PEDOT/CNT-functionalized CFEs | Rat dorsal striatum | 2.03 nM | [106] | |
| Dopamine | PEDOT: PSS-coated diamond films | Ventral tegmental area of the mouse | 0.5 μM | [107] | |
| Melatonin | CFEs | Mice brain | 20.02 nM | [111] | |
| Dopamine, Serotonin | Graphene-based biosensing neural interface | Mouse brain/colon | 5.6 nM 3.5 nM | [115] | |
| EIS | Acetylcholine | Gold microelectrodes | Cell-free | N/A | [78] |
| Anandamide | Nickel nanoparticles/Imprinted polymer | Cell-free | 0.01 nM | [79] | |
| Dopamine | Zinc oxide-embedded polyvinyl alcohol nanoplatelets | Cell-free | 5.0 nM | [80] | |
| Dopamine | Fullerene-pyrrole-pyrrole-3-carboxylic acid nanocomposite | Cell-free | 8.77 ng/mL | [81] | |
| Serotonin | Quartz nanopipettes | Neurobasal medium | N/A | [91] | |
| FET | Dopamine, Epinephrine | Single-walled carbon nanotubes/microfluidics | Cell-free | N/A | [87] |
| Dopamine, Epinephrine, Norepinephrine | Conductive metal–organic framework (c-MOF)-gated field-effect transistor (FET) arrays: Cu3HHTP2 (HTP) (i) and Cu2TCPP (TCP) types (ii) | Cell-free | 1.74 nM (i) 1.95 nM (ii) 1.66 nM (i) 1.88 nM (ii) 1.52 nM (i) 1.55 nM (ii) | [88] | |
| Acetylcholine | Polyaniline/reduced graphene oxide | Cell-free | 72.3 nM | [89] | |
| Glutamate | Graphene | Primary embryonic rat hippocampal neurons | 1 fM | [98] | |
| Dopamine | Gold-coated magnetic nanoparticles | Fish brain | 3.3 nM | [105] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, R.H. The neurotransmitter cycle and quantal size. Neuron 2007, 55, 835–858. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, T.S.; Edwards, R.H. Neurotransmitter corelease: Mechanism and physiological role. Annu. Rev. Physiol. 2012, 74, 225–243. [Google Scholar] [CrossRef]
- Spitzer, N.C. Neurotransmitter Switching in the Developing and Adult Brain. Annu. Rev. Neurosci. 2017, 40, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hornykiewicz, O. Brain neurotransmitter changes in Parkinson’s disease. In Movement Disorders; Marsden, C.D., Fahn, S., Eds.; Butterworth-Heinemann: Oxford, UK, 1981; pp. 41–58. [Google Scholar]
- Reinikainen, K.J.; Soininen, H.; Riekkinen, P.J. Neurotransmitter changes in Alzheimer’s disease: Implications to diagnostics and therapy. J. Neurosci. Res. 1990, 27, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Kumar, P. Insight Into the Emerging Role of Striatal Neurotransmitters in the Pathophysiology of Parkinson’s Disease and Huntington’s Disease: A Review. Curr. Neuropharmacol. 2019, 17, 165–175. [Google Scholar] [CrossRef]
- Hansen, J.Y.; Shafiei, G.; Markello, R.D.; Smart, K.; Cox, S.M.L.; Norgaard, M.; Beliveau, V.; Wu, Y.; Gallezot, J.D.; Aumont, E.; et al. Mapping neurotransmitter systems to the structural and functional organization of the human neocortex. Nat. Neurosci. 2022, 25, 1569–1581. [Google Scholar] [CrossRef]
- Jiang, S.H.; Hu, L.P.; Wang, X.; Li, J.; Zhang, Z.G. Neurotransmitters: Emerging targets in cancer. Oncogene 2020, 39, 503–515. [Google Scholar] [CrossRef]
- Lyu, Z.; Park, J.; Kim, K.M.; Jin, H.J.; Wu, H.; Rajadas, J.; Kim, D.H.; Steinberg, G.K.; Lee, W. A neurovascular-unit-on-a-chip for the evaluation of the restorative potential of stem cell therapies for ischaemic stroke. Nat. Biomed. Eng. 2021, 5, 847–863. [Google Scholar] [CrossRef]
- Ojeda, J.; Avila, A. Early Actions of Neurotransmitters During Cortex Development and Maturation of Reprogrammed Neurons. Front. Synaptic. Neurosci. 2019, 11, 33. [Google Scholar] [CrossRef]
- Leopold, A.V.; Shcherbakova, D.M.; Verkhusha, V.V. Fluorescent Biosensors for Neurotransmission and Neuromodulation: Engineering and Applications. Front. Cell. Neurosci. 2019, 13, 474. [Google Scholar] [CrossRef]
- Hussein, M.A.; El-Said, W.A.; Abu-Zied, B.M.; Choi, J.W. Nanosheet composed of gold nanoparticle/graphene/epoxy resin based on ultrasonic fabrication for flexible dopamine biosensor using surface-enhanced Raman spectroscopy. Nano Converg. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Eom, Y.-S.; Kim, T.-H. Recent Advances in Aptamer-Based Sensors for Sensitive Detection of Neurotransmitters. Biosensors 2023, 13, 413. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, C.C. Recent Advances on Electrochemical Biosensing Strategies toward Universal Point-of-Care Systems. Angew. Chem. 2019, 58, 12355–12368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sen, P.; Adhikari, B.R.; Li, Y.; Soleymani, L. Development of Nucleic-Acid-Based Electrochemical Biosensors for Clinical Applications. Angew. Chem. Int. Ed. 2022, 61, e202212496. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme immobilized nanomaterials as electrochemical biosensors for detection of biomolecules. Enzyme Microb. Technol. 2022, 156, 110006. [Google Scholar] [CrossRef]
- Kim, T.H.; Yea, C.H.; Chueng, S.T.; Yin, P.T.; Conley, B.; Dardir, K.; Pak, Y.; Jung, G.Y.; Choi, J.W.; Lee, K.B. Large-Scale Nanoelectrode Arrays to Monitor the Dopaminergic Differentiation of Human Neural Stem Cells. Adv. Mater. 2015, 27, 6356–6362. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, T.H.; El-Said, W.A.; Lee, J.H.; Yang, L.; Conley, B.; Choi, J.W.; Lee, K.B. In Situ Detection of Neurotransmitters from Stem Cell-Derived Neural Interface at the Single-Cell Level via Graphene-Hybrid SERS Nanobiosensing. Nano Lett. 2020, 20, 7670–7679. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.O.; Akanji, S.P.; Orimolade, B.O.; Olorundare, F.O.; Azizi, S.; Mamba, B.; Maaza, M. Using Nanomaterials as Excellent Immobilisation Layer for Biosensor Design. Biosensors 2023, 13, 192. [Google Scholar] [CrossRef]
- Li, L.; He, R.D.; Yan, H.L.; Leng, Z.W.; Zhu, S.; Gu, Z.J. Nanotechnology for the diagnosis and treatment of Alzheimer’s disease: A bibliometric analysis. Nano Today 2022, 47, 101654. [Google Scholar] [CrossRef]
- Hyman, S.E. Neurotransmitters. Curr. Biol. 2005, 15, R154–R158. [Google Scholar] [CrossRef]
- Entschladen, F.; Drell, T.L.t.; Lang, K.; Joseph, J.; Zaenker, K.S. Tumour-cell migration, invasion, and metastasis: Navigation by neurotransmitters. Lancet Oncol. 2004, 5, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Dasgupta, P.S. Dopamine, a neurotransmitter, influences the immune system. J. Neuroimmunol. 2000, 102, 113–124. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Tao, M.; Zhang, C.; He, Y.; Xu, W.; Liu, Y.; Zhu, W. Microelectrode-Based Electrochemical Sensing Technology for in Vivo Detection of Dopamine: Recent Developments and Future Prospects. Crit. Rev. Anal. Chem. 2022, 52, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Chagraoui, A.; Boulain, M.; Juvin, L.; Anouar, Y.; Barrière, G.; Deurwaerdère, P.D. L-DOPA in Parkinson’s Disease: Looking at the “False” Neurotransmitters and Their Meaning. Int. J. Mol. Sci. 2020, 21, 294. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.-A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Reyes-Resina, I.; Navarro, G. Dopamine in Health and Disease: Much More Than a Neurotransmitter. Biomedicines 2021, 9, 109. [Google Scholar] [CrossRef]
- Mossner, R.; Lesch, K.P. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav. Immun. 1998, 12, 249–271. [Google Scholar] [CrossRef]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Cox, M.A.; Bassi, C.; Saunders, M.E.; Nechanitzky, R.; Morgado-Palacin, I.; Zheng, C.; Mak, T.W. Beyond neurotransmission: Acetylcholine in immunity and inflammation. J. Intern. Med. 2020, 287, 120–133. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Venkataraman, S.; Rajendran, D.S.; Vinoth Kumar, V.; Kumar, V.V.; Rangasamy, G. Acetylcholinesterase biosensors for electrochemical detection of neurotoxic pesticides and acetylcholine neurotransmitter: A literature review. Environ. Res. 2023, 227, 115724. [Google Scholar] [CrossRef]
- Soldatkin, O.O.; Soldatkina, O.V.; Piliponskiy, I.I.; Rieznichenko, L.S.; Gruzina, T.G.; Dybkova, S.M.; Dzyadevych, S.V.; Soldatkin, A.P. Application of gold nanoparticles for improvement of analytical characteristics of conductometric enzyme biosensors. Appl. Nanosci. 2021, 12, 995–1003. [Google Scholar] [CrossRef]
- Hassani-Marand, M.; Fahimi-Kashani, N.; Hormozi-Nezhad, M.R. Machine-learning assisted multiplex detection of catecholamine neurotransmitters with a colorimetric sensor array. Anal. Methods 2023, 15, 1123–1134. [Google Scholar] [CrossRef]
- Renjini, S.; Abraham, P.; Anitha Kumary, V.; Chithra, P.G.; Sreevalsan, K. Review—Progress on Carbon-Based Electrochemical Sensors for Epinephrine and Norepinephrine. J. Electrochem. Soc. 2022, 169, 046519. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Hughes, G.; Pemberton, R.M.; Fielden, P.R.; Hart, J.P. The design, development and application of electrochemical glutamate biosensors. TrAC Trends Anal. Chem. 2016, 79, 106–113. [Google Scholar] [CrossRef]
- Tang, L.; Zhu, Y.; Xu, L.; Yang, X.; Li, C. Amperometric glutamate biosensor based on self-assembling glutamate dehydrogenase and dendrimer-encapsulated platinum nanoparticles onto carbon nanotubes. Talanta 2007, 73, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, M.S.; Troncone, L.R. Glycine as a neurotransmitter in the forebrain: A short review. J. Neural Transm. 2009, 116, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Schousboe, A.; Verkhratsky, A. Astrocyte energy and neurotransmitter metabolism in Alzheimer’s disease: Integration of the glutamate/GABA-glutamine cycle. Prog. Neurobiol. 2022, 217, 102331. [Google Scholar] [CrossRef]
- Reeves, K.C.; Shah, N.; Munoz, B.; Atwood, B.K. Opioid Receptor-Mediated Regulation of Neurotransmission in the Brain. Front. Mol. Neurosci. 2022, 15, 919773. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Chakravarthy, H.; Sharma, S.; Shree, S.; Bhat, A.R.; Pradeepkiran, J.A.; Devanathan, V. Neurotransmitter systems in the etiology of major neurological disorders: Emerging insights and therapeutic implications. Ageing Res. Rev. 2023, 89, 101994. [Google Scholar] [CrossRef]
- Mobed, A.; Hasanzadeh, M.; Ahmadalipour, A.; Fakhari, A. Recent advances in the biosensing of neurotransmitters: Material and method overviews towards the biomedical analysis of psychiatric disorders. Anal. Methods 2020, 12, 557–575. [Google Scholar] [CrossRef]
- He, J.; Spanolios, E.; Froehlich, C.E.; Wouters, C.L.; Haynes, C.L. Recent Advances in the Development and Characterization of Electrochemical and Electrical Biosensors for Small Molecule Neurotransmitters. ACS Sens. 2023, 8, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Tedjo, W.; Chen, T. An Integrated Biosensor System With a High-Density Microelectrode Array for Real-Time Electrochemical Imaging. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 20–35. [Google Scholar] [CrossRef]
- Hatamie, A.; He, X.; Zhang, X.W.; Oomen, P.E.; Ewing, A.G. Advances in nano/microscale electrochemical sensors and biosensors for analysis of single vesicles, a key nanoscale organelle in cellular communication. Biosens. Bioelectron. 2023, 220, 114899. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Karnam, J.B.; Brabazon, D.; Takai, M.; Ahad, I.U.; Balaguru Rayappan, J.B.; Krishnan, U.M. “Nano”: An Emerging Avenue in Electrochemical Detection of Neurotransmitters. ACS Chem. Neurosci. 2020, 11, 4024–4047. [Google Scholar] [CrossRef] [PubMed]
- Da, Y.; Luo, S.; Tian, Y. Real-Time Monitoring of Neurotransmitters in the Brain of Living Animals. ACS Appl. Mater. Interfaces 2023, 15, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Tvorynska, S.; Barek, J.; Josypcuk, B. High-performance amperometric biosensor for flow injection analysis consisting of a replaceable lactate oxidase-based mini-reactor and a silver amalgam screen-printed electrode. Electrochim. Acta 2023, 445, 142033. [Google Scholar] [CrossRef]
- Deepa; Nohwal, B.; Chaudhary, R.; Pundir, C.S. Amperometric detection of tumor suppressor protein p53 via pencil graphite electrode for fast cancer diagnosis. Anal. Biochem. 2022, 639, 114528. [Google Scholar] [CrossRef]
- Fredj, Z.; Singh, B.; Bahri, M.; Qin, P.; Sawan, M. Enzymatic Electrochemical Biosensors for Neurotransmitters Detection: Recent Achievements and Trends. Chemosensors 2023, 11, 388. [Google Scholar] [CrossRef]
- Sciurti, E.; Biscaglia, F.; Prontera, C.T.; Giampetruzzi, L.; Blasi, L.; Francioso, L. Nanoelectrodes for intracellular and intercellular electrochemical detection: Working principles, fabrication techniques and applications. J. Electroanal. Chem. 2023, 929, 117125. [Google Scholar] [CrossRef]
- Hu, K.; Jia, R.; Hatamie, A.; Le Vo, K.L.; Mirkin, M.V.; Ewing, A.G. Correlating Molecule Count and Release Kinetics with Vesicular Size Using Open Carbon Nanopipettes. J. Am. Chem. Soc. 2020, 142, 16910–16914. [Google Scholar] [CrossRef] [PubMed]
- Akbıyık, M.A.; Bodur, O.C.; Keskin, M.; Kara, M.; Dinç, S.; Arslan, H.; Özmen, M.; Arslan, F. A Sensitive Amperometric Biosensor Based on Carbon Dot 3-Chloropropyl-trimethoxysilane Modified Electrode for Detection of Neurotransmitter Dopamine. J. Electrochem. Soc. 2023, 170, 037517. [Google Scholar] [CrossRef]
- Gigli, V.; Tortolini, C.; Capecchi, E.; Angeloni, A.; Lenzi, A.; Antiochia, R. Novel Amperometric Biosensor Based on Tyrosinase/Chitosan Nanoparticles for Sensitive and Interference-Free Detection of Total Catecholamine. Biosensors 2022, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Muhamad Rapidi, H.I.; Ahmed, S.; Abel, D.K.; Garcia, K.J.; Chen, R.; Iwai, N.T.; Shen, M. PEDOT/PVC-modified amperometric carbon electrodes for acetylcholine detection. Chem. Commun. 2022, 58, 13218–13221. [Google Scholar] [CrossRef]
- Ahlawat, J.; Sharma, M.; Pundir, C.S. An Amperometric Acetylcholine Biosensor Based on Co-Immobilization of Enzyme Nanoparticles onto Nanocomposite. Biosensors 2023, 13, 386. [Google Scholar] [CrossRef]
- McCarty, G.S.; Dunaway, L.E.; Denison, J.D.; Sombers, L.A. Neurotransmitter Readily Escapes Detection at the Opposing Microelectrode Surface in Typical Amperometric Measurements of Exocytosis at Single Cells. Anal. Chem. 2022, 94, 9548–9556. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Du, J.; Zhou, J.; Cao, L.; Li, X. Cisplatin Inhibits Neurotransmitter Release during Exocytosis from Single Chromaffin Cells Monitored with Single Cell Amperometry. Electroanalysis 2021, 34, 981–986. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Wang, M.; Zhang, J.; Liu, C.; Li, X. Recent Progress in Quantitatively Monitoring Vesicular Neurotransmitter Release and Storage With Micro/Nanoelectrodes. Front. Chem. 2020, 8, 591311. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 396. [Google Scholar] [CrossRef]
- Kaur, H.; Siwal, S.S.; Saini, R.V.; Singh, N.; Thakur, V.K. Significance of an Electrochemical Sensor and Nanocomposites: Toward the Electrocatalytic Detection of Neurotransmitters and Their Importance within the Physiological System. ACS Nanosci. Au 2023, 3, 1–27. [Google Scholar] [CrossRef]
- Banerjee, S.; McCracken, S.; Hossain, M.F.; Slaughter, G. Electrochemical Detection of Neurotransmitters. Biosensors 2020, 10, 101. [Google Scholar] [CrossRef]
- Wang, Y.Y.C.; Yao, W.Z.; Huang, H.B.; Huang, J.; Li, L.; Yu, X.H. Polypyrrole-derived carbon nanotubes for potential application in electrochemical detection of dopamine. Solid State Sci. 2022, 134, 107038. [Google Scholar] [CrossRef]
- Elugoke, S.E.; Fayemi, O.E.; Adekunle, A.S.; Ganesh, P.S.; Kim, S.Y.; Ebenso, E.E. Sensitive and selective neurotransmitter epinephrine detection at a carbon quantum dots/copper oxide nanocomposite. J. Electroanal. Chem. 2023, 929, 117120. [Google Scholar] [CrossRef]
- Venton, B.J.; Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 2020, 145, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Grinevich, V.P.; Zakirov, A.N.; Berseneva, U.V.; Gerasimova, E.V.; Gainetdinov, R.R.; Budygin, E.A. Applying a Fast-Scan Cyclic Voltammetry to Explore Dopamine Dynamics in Animal Models of Neuropsychiatric Disorders. Cells 2022, 11, 1533. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, P.; Johnson, M.A. Characterization of D3 Autoreceptor Function in Whole Zebrafish Brain with Fast-Scan Cyclic Voltammetry. ACS Chem. Neurosci. 2022, 13, 2863–2873. [Google Scholar] [CrossRef]
- Schapira, I.; O’Neill, M.R.; Russo-Savage, L.; Narla, T.; Laprade, K.A.; Stafford, J.M.; Ou, Y. Measuring tryptophan dynamics using fast scan cyclic voltammetry at carbon fiber microelectrodes with improved sensitivity and selectivity. RSC Adv. 2023, 13, 26203–26212. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, H.Q.; Hao, T.T.; Hu, K.Y.; Qin, L.X.; Ren, X.X.; Guo, Z.Y.; Wang, S.; Hu, Y.F. A fully integrated fast scan cyclic voltammetry electrochemical method: Improvements in reaction kinetics and signal stability for specific Ag(I) and Hg(II) analysis. J. Electroanal. Chem. 2022, 910, 116208. [Google Scholar] [CrossRef]
- Bhimani, R.V.; Yates, R.; Bass, C.E.; Park, J. Distinct limbic dopamine regulation across olfactory-tubercle subregions through integration of in vivo fast-scan cyclic voltammetry and optogenetics. J. Neurochem. 2022, 161, 53–68. [Google Scholar] [CrossRef]
- Yang, D.; Liu, G.; Li, H.; Liu, A.; Guo, J.; Shan, Y.; Wang, Z.; He, J. The fabrication of a gold nanoelectrode-nanopore nanopipette for dopamine enrichment and multimode detection. Analyst 2020, 145, 1047–1055. [Google Scholar] [CrossRef]
- Gaidukevic, J.; Aukstakojyte, R.; Barkauskas, J.; Niaura, G.; Murauskas, T.; Pauliukaite, R. A novel electrochemical sensor based on thermally reduced graphene oxide for the sensitive determination of dopamine. Appl. Surf. Sci. 2022, 592, 153257. [Google Scholar] [CrossRef]
- Zhang, T.; Xuan, X.; Li, M.; Li, C.; Li, P.; Li, H. Molecularly imprinted Ni-polyacrylamide-based electrochemical sensor for the simultaneous detection of dopamine and adenine. Anal. Chim. Acta 2022, 1202, 339689. [Google Scholar] [CrossRef]
- Kaushik, A.; Jayant, R.D.; Tiwari, S.; Vashist, A.; Nair, M. Nano-biosensors to detect beta-amyloid for Alzheimer’s disease management. Biosens. Bioelectron. 2016, 80, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.H.; Muthuswamy, J. Acoustic biosensor for monitoring antibody immobilization and neurotransmitter GABA in real-time. Sens. Actuators B Chem. 2004, 101, 8–19. [Google Scholar] [CrossRef]
- Lopez, L.; Hernandez, N.; Reyes Morales, J.; Cruz, J.; Flores, K.; Gonzalez-Amoretti, J.; Rivera, V.; Cunci, L. Measurement of Neuropeptide Y Using Aptamer-Modified Microelectrodes by Electrochemical Impedance Spectroscopy. Anal. Chem. 2021, 93, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Brazaca, L.C.; Sampaio, I.; Zucolotto, V.; Janegitz, B.C. Applications of biosensors in Alzheimer’s disease diagnosis. Talanta 2020, 210, 120644. [Google Scholar] [CrossRef]
- Chen, J.; Lin, K.C.; Prasad, S.; Schmidtke, D.W. Label free impedance based acetylcholinesterase enzymatic biosensors for the detection of acetylcholine. Biosens. Bioelectron. 2023, 235, 115340. [Google Scholar] [CrossRef] [PubMed]
- Jain, U.; Soni, S.; Balhara, Y.P.S.; Khanuja, M.; Chauhan, N. Dual-Layered Nanomaterial-Based Molecular Pattering on Polymer Surface Biomimetic Impedimetric Sensing of a Bliss Molecule, Anandamide Neurotransmitter. ACS Omega 2020, 5, 10750–10758. [Google Scholar] [CrossRef] [PubMed]
- Emadoddin, M.; Mozaffari, S.A.; Ebrahimi, F. An antifouling impedimetric sensor based on zinc oxide embedded polyvinyl alcohol nanoplatelets for wide range dopamine determination in the presence of high concentration ascorbic acid. J. Pharm. Biomed. Anal. 2021, 205, 114278. [Google Scholar] [CrossRef]
- Ertuğrul Uygun, H.D.; Demir, M.N. A Novel Fullerene-Pyrrole-Pyrrole-3-Carboxylic Acid Nanocomposite Modified Molecularly Imprinted Impedimetric Sensor for Dopamine Determination in Urine. Electroanalysis 2020, 32, 1971–1976. [Google Scholar] [CrossRef]
- Hu, Z.H.; Li, Y.Q.; Figueroa-Miranda, G.; Musall, S.; Li, H.Y.; Martinez-Roque, M.A.; Hu, Q.Y.; Feng, L.Y.; Mayer, D.; Offenhausser, A. Aptamer based biosensor platforms for neurotransmitters analysis. TrAC Trends Anal. Chem. 2023, 162, 117021. [Google Scholar] [CrossRef]
- Hao, R.; Liu, L.; Yuan, J.; Wu, L.; Lei, S. Recent Advances in Field Effect Transistor Biosensors: Designing Strategies and Applications for Sensitive Assay. Biosensors 2023, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Gwyther, R.E.A.; Freeley, M.; Jones, D.; Palma, M. Fabrication and Functionalisation of Nanocarbon-Based Field-Effect Transistor Biosensors. Chembiochem 2022, 23, e202200282. [Google Scholar] [CrossRef] [PubMed]
- Chadha, U.; Bhardwaj, P.; Agarwal, R.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; et al. Recent progress and growth in biosensors technology: A critical review. J. Ind. Eng. Chem. 2022, 109, 21–51. [Google Scholar] [CrossRef]
- Zhao, C.; Cheung, K.M.; Huang, I.W.; Yang, H.; Nakatsuka, N.; Liu, W.; Cao, Y.; Man, T.; Weiss, P.S.; Monbouquette, H.G.; et al. Implantable aptamer-field-effect transistor neuroprobes for in vivo neurotransmitter monitoring. Sci. Adv. 2021, 7, eabj7422. [Google Scholar] [CrossRef]
- Clement, P.; Ackermann, J.; Sahin-Solmaz, N.; Herbertz, S.; Boero, G.; Kruss, S.; Brugger, J. Comparison of electrical and optical transduction modes of DNA-wrapped SWCNT nanosensors for the reversible detection of neurotransmitters. Biosens. Bioelectron. 2022, 216, 114642. [Google Scholar] [CrossRef]
- Keum, C.; Park, S.; Kim, H.; Kim, H.; Lee, K.H.; Jeong, Y. Modular conductive MOF-gated field-effect biosensor for sensitive discrimination on the small molecular scale. Chem. Eng. J. 2023, 456, 141079. [Google Scholar] [CrossRef]
- Park, D.; Lee, D.; Kim, H.J.; Yoon, D.S.; Hwang, K.S. Scalable Functionalization of Polyaniline-Grafted rGO Field-Effect Transistors for a Highly Sensitive Enzymatic Acetylcholine Biosensor. Biosensors 2022, 12, 279. [Google Scholar] [CrossRef]
- Mahato, K.; Purohit, B.; Bhardwaj, K.; Jaiswal, A.; Chandra, P. Novel electrochemical biosensor for serotonin detection based on gold nanorattles decorated reduced graphene oxide in biological fluids and in vitro model. Biosens. Bioelectron. 2019, 142, 111502. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Failletaz, A.; Eggemann, D.; Forro, C.; Voros, J.; Momotenko, D. Aptamer Conformational Change Enables Serotonin Biosensing with Nanopipettes. Anal. Chem. 2021, 93, 4033–4041. [Google Scholar] [CrossRef]
- Ashraf, G.; Asif, M.; Aziz, A.; Iftikhar, T.; Liu, H. Rice-Spikelet-like Copper Oxide Decorated with Platinum Stranded in the CNT Network for Electrochemical In Vitro Detection of Serotonin. ACS Appl. Mater. Interfaces 2021, 13, 6023–6033. [Google Scholar] [CrossRef]
- Castagnola, E.; Garg, R.; Rastogi, S.K.; Cohen-Karni, T.; Cui, X.T. 3D fuzzy graphene microelectrode array for dopamine sensing at sub-cellular spatial resolution. Biosens. Bioelectron. 2021, 191, 113440. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.T.; Lin, X.F.; Tong, L.L.; Tong, Q.X. Graphene Quantum Dots/Multiwalled Carbon Nanotubes Composite-Based Electrochemical Sensor for Detecting Dopamine Release from Living Cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650. [Google Scholar] [CrossRef]
- Yang, C.; Hu, K.; Wang, D.; Zubi, Y.; Lee, S.T.; Puthongkham, P.; Mirkin, M.V.; Venton, B.J. Cavity Carbon-Nanopipette Electrodes for Dopamine Detection. Anal. Chem. 2019, 91, 4618–4624. [Google Scholar] [CrossRef]
- Eom, G.; Oh, C.; Moon, J.; Kim, H.; Kim, M.K.; Kim, K.; Seo, J.W.; Kang, T.; Lee, H.J. Highly sensitive and selective detection of dopamine using overoxidized polypyrrole/sodium dodecyl sulfate-modified carbon nanotube electrodes. J. Electroanal. Chem. 2019, 848, 113295. [Google Scholar] [CrossRef]
- Doughty, P.T.; Hossain, I.; Gong, C.; Ponder, K.A.; Pati, S.; Arumugam, P.U.; Murray, T.A. Novel microwire-based biosensor probe for simultaneous real-time measurement of glutamate and GABA dynamics in vitro and in vivo. Sci. Rep. 2020, 10, 12777. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Jin, X.; Tang, L.; Lv, W.L.; Xiao, M.M.; Zhang, Z.Y.; Gao, C.; Zhang, G.J. Receptor-Mediated Field Effect Transistor Biosensor for Real-Time Monitoring of Glutamate Release from Primary Hippocampal Neurons. Anal. Chem. 2019, 91, 8229–8236. [Google Scholar] [CrossRef] [PubMed]
- Scoggin, J.L.; Tan, C.; Nguyen, N.H.; Kansakar, U.; Madadi, M.; Siddiqui, S.; Arumugam, P.U.; DeCoster, M.A.; Murray, T.A. An enzyme-based electrochemical biosensor probe with sensitivity to detect astrocytic versus glioma uptake of glutamate in real time in vitro. Biosens. Bioelectron. 2019, 126, 751–757. [Google Scholar] [CrossRef]
- Kim, T.H.; El-Said, W.A.; An, J.H.; Choi, J.W. ITO/gold nanoparticle/RGD peptide composites to enhance electrochemical signals and proliferation of human neural stem cells. Nanomedicine 2013, 9, 336–344. [Google Scholar] [CrossRef]
- Kim, D.S.; Kang, E.S.; Baek, S.; Choo, S.S.; Chung, Y.H.; Lee, D.; Min, J.; Kim, T.H. Electrochemical detection of dopamine using periodic cylindrical gold nanoelectrode arrays. Sci. Rep. 2018, 8, 14049. [Google Scholar] [CrossRef]
- Amato, L.; Heiskanen, A.; Caviglia, C.; Shah, F.; Zor, K.; Skolimowski, M.; Madou, M.; Gammelgaard, L.; Hansen, R.; Seiz, E.G.; et al. Pyrolysed 3D-Carbon Scaffolds Induce Spontaneous Differentiation of Human Neural Stem Cells and Facilitate Real-Time Dopamine Detection. Adv. Funct. Mater. 2014, 24, 7042–7052. [Google Scholar] [CrossRef]
- Nasr, B.; Chatterton, R.; Yong, J.H.; Jamshidi, P.; D’Abaco, G.M.; Bjorksten, A.R.; Kavehei, O.; Chana, G.; Dottori, M.; Skafidas, E. Self-Organized Nanostructure Modified Microelectrode for Sensitive Electrochemical Glutamate Detection in Stem Cells-Derived Brain Organoids. Biosensors 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, C.; Spitz, S.; Berger, E.; Bolognin, S.; Smits, L.M.; Crepaz, P.; Rothbauer, M.; Rosser, J.M.; Marchetti-Deschmann, M.; Schwamborn, J.C.; et al. Monitoring the neurotransmitter release of human midbrain organoids using a redox cycling microsensor as a novel tool for personalized Parkinson’s disease modelling and drug screening. Analyst 2021, 146, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xiang, X.; Fu, L.; Cao, Q.; Huang, R.; Liu, H.; Han, G.; Wu, L. Regenerative field effect transistor biosensor for in vivo monitoring of dopamine in fish brains. Biosens. Bioelectron. 2021, 188, 113340. [Google Scholar] [CrossRef]
- Taylor, I.M.; Patel, N.A.; Freedman, N.C.; Castagnola, E.; Cui, X.T. Direct in Vivo Electrochemical Detection of Resting Dopamine Using Poly(3,4-ethylenedioxythiophene)/Carbon Nanotube Functionalized Microelectrodes. Anal. Chem. 2019, 91, 12917–12927. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Cai, X.; Xie, Y.; Wang, T.; Cheng, D.; Li, L.; Li, R.; Deng, Y.; Ding, H.; et al. A wireless, implantable optoelectrochemical probe for optogenetic stimulation and dopamine detection. Microsyst. Nanoeng. 2020, 6, 64. [Google Scholar] [CrossRef]
- Raju, D.; Mendoza, A.; Wonnenberg, P.; Mohanaraj, S.; Sarbanes, M.; Truong, C.; Zestos, A.G. Polymer Modified Carbon Fiber-Microelectrodes and Waveform Modifications Enhance Neurotransmitter Metabolite Detection. Anal. Methods 2019, 11, 1620–1630. [Google Scholar] [CrossRef]
- Hou, H.; Jin, Y.; Wei, H.; Ji, W.; Xue, Y.; Hu, J.; Zhang, M.; Jiang, Y.; Mao, L. A Generalizable and Noncovalent Strategy for Interfacing Aptamers with a Microelectrode for the Selective Sensing of Neurotransmitters In Vivo. Angew. Chem. Int. Ed. 2020, 59, 18996–19000. [Google Scholar] [CrossRef]
- Ganesana, M.; Trikantzopoulos, E.; Maniar, Y.; Lee, S.T.; Venton, B.J. Development of a novel micro biosensor for in vivo monitoring of glutamate release in the brain. Biosens. Bioelectron. 2019, 130, 103–109. [Google Scholar] [CrossRef]
- Castagnola, E.; Robbins, E.M.; Woeppel, K.M.; McGuier, M.; Golabchi, A.; Taylor, I.M.; Michael, A.C.; Cui, X.T. Real-Time Fast Scan Cyclic Voltammetry Detection and Quantification of Exogenously Administered Melatonin in Mice Brain. Front. Bioeng. Biotechnol. 2020, 8, 602216. [Google Scholar] [CrossRef]
- Stuart, T.; Jeang, W.J.; Slivicki, R.A.; Brown, B.J.; Burton, A.; Brings, V.E.; Alarcon-Segovia, L.C.; Agyare, P.; Ruiz, S.; Tyree, A.; et al. Wireless, Battery-Free Implants for Electrochemical Catecholamine Sensing and Optogenetic Stimulation. ACS Nano 2023, 17, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, E.; Robbins, E.M.; Wu, B.; Pwint, M.Y.; Garg, R.; Cohen-Karni, T.; Cui, X.T. Flexible Glassy Carbon Multielectrode Array for In Vivo Multisite Detection of Tonic and Phasic Dopamine Concentrations. Biosensors 2022, 12, 540. [Google Scholar] [CrossRef] [PubMed]
- Lendor, S.; Hassani, S.A.; Boyaci, E.; Singh, V.; Womelsdorf, T.; Pawliszyn, J. Solid Phase Microextraction-Based Miniaturized Probe and Protocol for Extraction of Neurotransmitters from Brains In Vivo. Anal. Chem. 2019, 91, 4896–4905. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Yuan, L.; Zhang, B.; Bishop, E.S.; Wang, K.; Tang, J.; Zheng, Y.Q.; Xu, W.; Niu, S.; et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature 2022, 606, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, N.; Lin, X.; Wang, Z.; Zhao, X.; Fang, P.; Yue, H.; Kim, J.; Luo, J.; Cui, S.; et al. Organic electrochemical transistor arrays for real-time mapping of evoked neurotransmitter release in vivo. eLife 2020, 9, e50345. [Google Scholar] [CrossRef]
- Zhang, X.W.; Hatamie, A.; Ewing, A.G. Nanoelectrochemical analysis inside a single living cell. Curr. Opin. Electrochem. 2020, 22, 94–101. [Google Scholar] [CrossRef]
- Li, X.; Dunevall, J.; Ewing, A.G. Quantitative Chemical Measurements of Vesicular Transmitters with Electrochemical Cytometry. Acc. Chem. Res. 2016, 49, 2347–2354. [Google Scholar] [CrossRef]
- Li, X.; Majdi, S.; Dunevall, J.; Fathali, H.; Ewing, A.G. Quantitative measurement of transmitters in individual vesicles in the cytoplasm of single cells with nanotip electrodes. Angew. Chem. 2015, 54, 11978–11982. [Google Scholar] [CrossRef]
- Jeong, E.; Choi, S.; Cho, S.W. Recent Advances in Brain Organoid Technology for Human Brain Research. ACS Appl. Mater. Interfaces 2023, 15, 200–219. [Google Scholar] [CrossRef]
- Li, R.Q.; Zhao, X.H.; Zhu, Q.; Liu, T.; Hondermarck, H.; Thorne, R.F.; Zhang, X.D.; Gao, J.N. Exploring neurotransmitters and their receptors for breast cancer prevention and treatment. Theranostics 2023, 13, 1109–1129. [Google Scholar] [CrossRef]
- Murphy, S.E.; Sweedler, J.V. Metabolomics-based mass spectrometry methods to analyze the chemical content of 3D organoid models. Analyst 2022, 147, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pardo, J.; Novio, F.; Nador, F.; Cavaliere, I.; Suarez-Garcia, S.; Lope-Piedrafita, S.; Candiota, A.P.; Romero-Gimenez, J.; Rodriguez-Galvan, B.; Bove, J.; et al. Bioinspired Theranostic Coordination Polymer Nanoparticles for Intranasal Dopamine Replacement in Parkinson’s Disease. ACS Nano 2021, 15, 8592–8609. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Takano, T.; Hansen, A.J. Beyond the role of glutamate as a neurotransmitter. Nat. Rev. Neurosci. 2002, 3, 748–755. [Google Scholar] [CrossRef]
- Costa, N.G.; Antunes, J.C.; Paleo, A.J.; Rocha, A.M. A Review on Flexible Electrochemical Biosensors to Monitor Alcohol in Sweat. Biosensors 2022, 12, 252. [Google Scholar] [CrossRef]
- Yu, Y.; Nyein, H.Y.Y.; Gao, W.; Javey, A. Flexible Electrochemical Bioelectronics: The Rise of In Situ Bioanalysis. Adv. Mater. 2020, 32, 1902083. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Liu, Y.; Wang, W.; Ren, R.; Li, M.; Liu, D.; Liu, Y.; Liu, Y.; Pang, G. Electrochemical Signal Amplification Strategies and Their Use in Olfactory and Taste Evaluation. Biosensors 2022, 12, 566. [Google Scholar] [CrossRef]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing Biosensors with Machine Learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef]
| Neurotransmitter | Function | Location |
|---|---|---|
| Acetylcholine | Muscle control Memory | Central nervous system Peripheral nervous system |
| Serotonin | Intestinal movement Mode regulation Sleep | Central nervous system Gut |
| Dopamine | Voluntary muscle movement Cognition Reward pathways | Hypothalamus |
| Norepinephrine | Fight/flight response | Adrenal medulla |
| GABA | Inhibits central nerve system | Brain |
| Glutamate | Excitatory neurotransmitter Memory | Central nervous system Peripheral nervous system |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.K.; Choi, J.-H.; Yoon, J. An Updated Review on Electrochemical Nanobiosensors for Neurotransmitter Detection. Biosensors 2023, 13, 892. https://doi.org/10.3390/bios13090892
Choi HK, Choi J-H, Yoon J. An Updated Review on Electrochemical Nanobiosensors for Neurotransmitter Detection. Biosensors. 2023; 13(9):892. https://doi.org/10.3390/bios13090892
Chicago/Turabian StyleChoi, Hye Kyu, Jin-Ha Choi, and Jinho Yoon. 2023. "An Updated Review on Electrochemical Nanobiosensors for Neurotransmitter Detection" Biosensors 13, no. 9: 892. https://doi.org/10.3390/bios13090892
APA StyleChoi, H. K., Choi, J.-H., & Yoon, J. (2023). An Updated Review on Electrochemical Nanobiosensors for Neurotransmitter Detection. Biosensors, 13(9), 892. https://doi.org/10.3390/bios13090892





