Microlens-Assisted Light-Scattering Imaging of Plasmonic Nanoparticles at the Single Particle Level
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
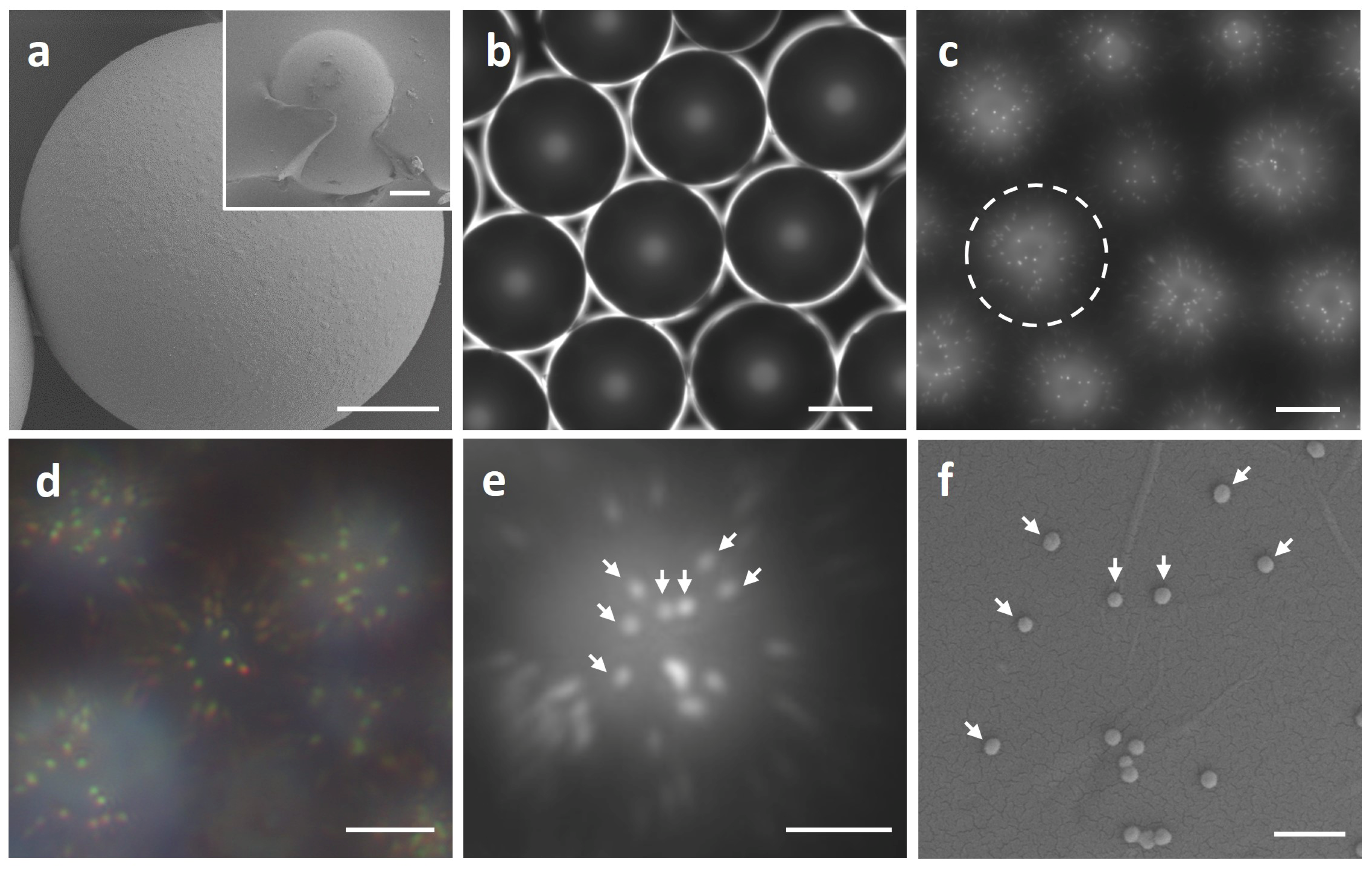
2.2.1. Preparation of the Microlenses
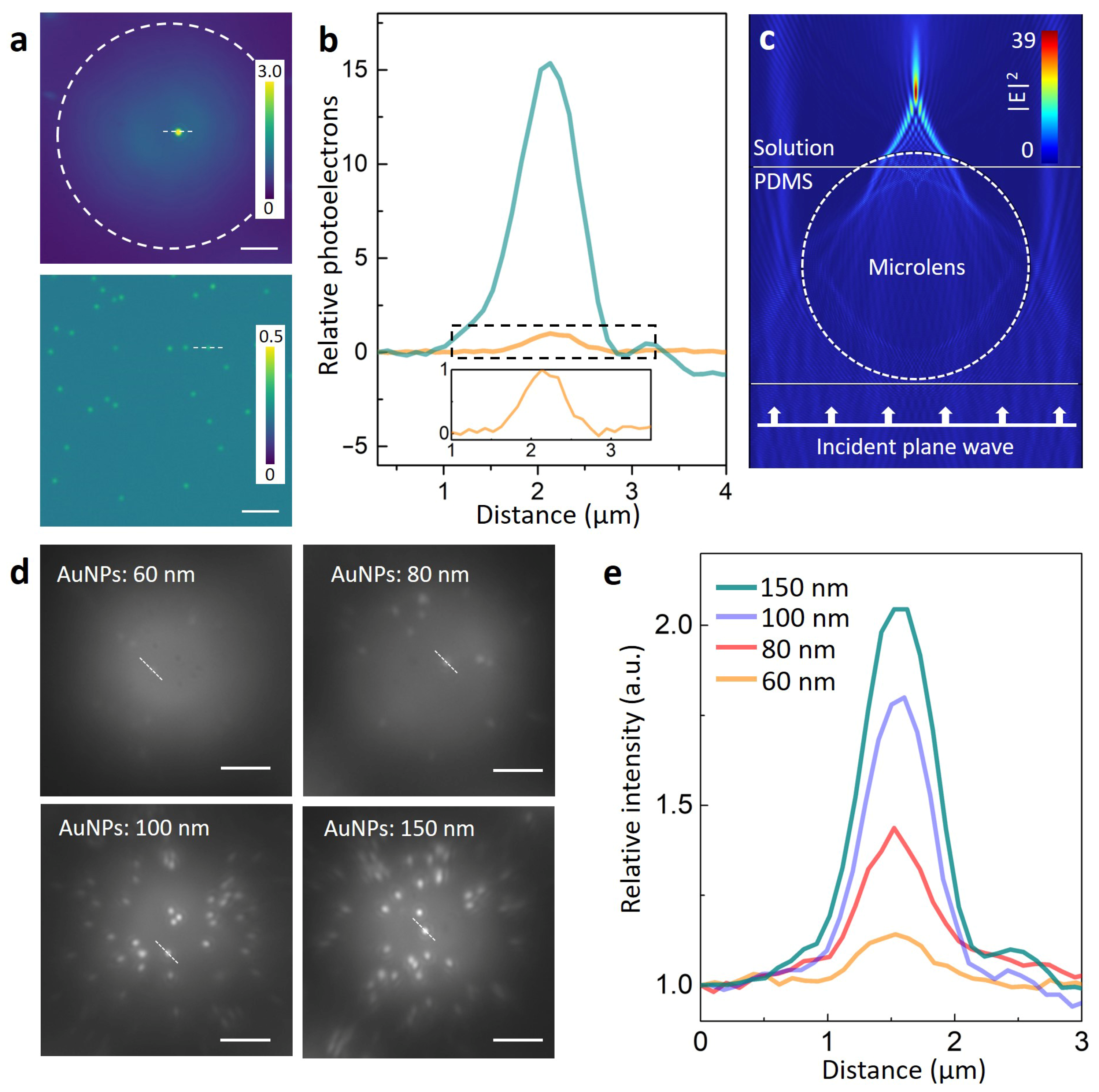
2.2.2. Microlens Assisted Imaging of the Plasmonic Nanoparticles
2.2.3. Simulation of Photonic Hotspot Generated by the Microlens
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.F.; Zhang, Y.J.; Ding, S.Y.; Panneerselvam, R.; Tian, Z.Q. Core-Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
- Kurt, H.; Pishva, P.; Pehlivan, Z.S.; Arsoy, E.G.; Saleem, Q.; Bayazit, M.K.; Yuce, M. Nanoplasmonic biosensors: Theory, structure, design, and review of recent applications. Anal. Chim. Acta 2021, 1185, 338842. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.S.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. Phys. Soc. 2013, 76, 046401. [Google Scholar] [CrossRef]
- Taylor, A.B.; Zijlstra, P. Single-Molecule Plasmon Sensing: Current Status and Future Prospects. ACS Sens. 2017, 2, 1103–1122. [Google Scholar] [CrossRef]
- Xie, T.; Jing, C.; Long, Y.T. Single plasmonic nanoparticles as ultrasensitive sensors. Analyst 2017, 142, 409–420. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.Y.; Choi, H.K.; Lee, J.Y.; Choi, J.W. Application of Gold Nanoparticle to Plasmonic Biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef]
- Ringe, E.; Sharma, B.; Henry, A.I.; Marks, L.D.; Van Duyne, R.P. Single nanoparticle plasmonics. Phys. Chem. Chem. Phys. PCCP 2013, 15, 4110–4129. [Google Scholar] [CrossRef]
- Olson, J.; Dominguez-Medina, S.; Hoggard, A.; Wang, L.Y.; Chang, W.S.; Link, S. Optical characterization of single plasmonic nanoparticles. Chem. Soc. Rev. 2015, 44, 40–57. [Google Scholar] [CrossRef]
- Al-Zubeidi, A.; McCarthy, L.A.; Rafiei-Miandashti, A.; Heiderscheit, T.S.; Link, S. Single-particle scattering spectroscopy: Fundamentals and applications. Nanophotonics 2021, 10, 1621–1655. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, X.; Xiao, L. Single-Particle Tracking with Scattering-Based Optical Microscopy. Anal. Chem. 2019, 91, 15327–15334. [Google Scholar] [CrossRef]
- Priest, L.; Peters, J.S.; Kukura, P. Scattering-based Light Microscopy: From Metal Nanoparticles to Single Proteins. Chem. Rev. 2021, 121, 11937–11970. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Liu, H.; Wei, L.; Xiao, L. Recent advances in optical microscopic methods for single-particle tracking in biological samples. Anal. Bioanal. Chem. 2019, 411, 4445–4463. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Kappes, M.; Cicha, I.; Tietze, R.; Braun, C.; Schneider-Stock, R.; Nagy, R.; Alexiou, C.; Janko, C. Optical Microscopy Systems for the Detection of Unlabeled Nanoparticles. Int. J. Nanomed. 2022, 17, 2139–2163. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, W.; Li, L.; Luk’yanchuk, B.; Khan, A.; Liu, Z.; Chen, Z.; Hong, M. Optical virtual imaging at 50 nm lateral resolution with a white-light nanoscope. Nat. Commun. 2011, 2, 218. [Google Scholar] [CrossRef]
- Yang, H.; Moullan, N.; Auwerx, J.; Gijs, M.A.M. Super-Resolution Biological Microscopy Using Virtual Imaging by a Microsphere Nanoscope. Small 2014, 10, 1712–1718. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Microsphere-aided optical microscopy and its applications for super-resolution imaging. Opt. Commun. 2017, 404, 32–41. [Google Scholar] [CrossRef]
- Huszka, G.; Yang, H.; Gijs, M.A.M. Microsphere-based super-resolution scanning optical microscope. Opt. Express 2017, 25, 15079–15092. [Google Scholar] [CrossRef]
- Huszka, G.; Gijs, M.A.M. Super-resolution optical imaging: A comparison. Micro Nano Eng. 2019, 2, 7–28. [Google Scholar] [CrossRef]
- Chen, Z.; Taflove, A.; Backman, V. Photonic nanojet enhancement of backscattering of light by nanoparticles: A potential novel visible-light ultramicroscopy technique. Opt. Express 2004, 12, 1214–1220. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Taflove, A.; Backman, V. Optical analysis of nanoparticles via enhanced backscattering facilitated by 3-D photonic nanojets. Opt. Express 2005, 13, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.L.; Teh, D.B.L.; Dinh, N.D.; Chen, W.D.; Chen, Q.S.; Wu, Y.M.; Chowdhury, S.; Yamanaka, A.; Sum, T.C.; Chen, C.H.; et al. Upconversion amplification through dielectric superlensing modulation. Nat. Commun. 2019, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cornaglia, M.; Gijs, M.A. Photonic nanojet array for fast detection of single nanoparticles in a flow. Nano Lett. 2015, 15, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xin, H.; Liu, X.; Zhang, Y.; Lei, H.; Li, B. Trapping and Detection of Nanoparticles and Cells Using a Parallel Photonic Nanojet Array. ACS Nano 2016, 10, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yan, B.; Gu, G.; Yu, Z.; Chen, X.; Wang, Z.; Yang, H. Localized photonic nanojet based sensing platform for highly efficient signal amplification and quantitative biosensing. Sens. Actuators B Chem. 2022, 357, 131401. [Google Scholar] [CrossRef]
- Lu, D.; Pedroni, M.; Labrador-Paez, L.; Marques, M.I.; Jaque, D.; Haro-Gonzalez, P. Nanojet Trapping of a Single Sub-10 nm Upconverting Nanoparticle in the Full Liquid Water Temperature Range. Small 2021, 17, e2006764. [Google Scholar] [CrossRef]
- Kovrov, A.; Novitsky, A.; Karabchevsky, A.; Shalin, A.S. A Photonic Nanojet as Tunable and Polarization-Sensitive Optical Tweezers. Ann. Der Phys. 2018, 530, 1800129. [Google Scholar] [CrossRef]
- Lu, D.; Retama, J.R.; Marin, R.; Marques, M.I.; Calderon, O.G.; Melle, S.; Haro-Gonzalez, P.; Jaque, D. Thermoresponsive Polymeric Nanolenses Magnify the Thermal Sensitivity of Single Upconverting Nanoparticles. Small 2022, 18, e2202452. [Google Scholar] [CrossRef]
- Darafsheh, A.; Walsh, G.F.; Dal Negro, L.; Astratov, V.N. Optical super-resolution by high-index liquid-immersed microspheres. Appl. Phys. Lett. 2012, 101, 141128. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zhan, T.; Xue, S.; Yang, H. Microlens-Assisted Light-Scattering Imaging of Plasmonic Nanoparticles at the Single Particle Level. Biosensors 2023, 13, 871. https://doi.org/10.3390/bios13090871
Zhang P, Zhan T, Xue S, Yang H. Microlens-Assisted Light-Scattering Imaging of Plasmonic Nanoparticles at the Single Particle Level. Biosensors. 2023; 13(9):871. https://doi.org/10.3390/bios13090871
Chicago/Turabian StyleZhang, Pengcheng, Tingting Zhan, Sha Xue, and Hui Yang. 2023. "Microlens-Assisted Light-Scattering Imaging of Plasmonic Nanoparticles at the Single Particle Level" Biosensors 13, no. 9: 871. https://doi.org/10.3390/bios13090871
APA StyleZhang, P., Zhan, T., Xue, S., & Yang, H. (2023). Microlens-Assisted Light-Scattering Imaging of Plasmonic Nanoparticles at the Single Particle Level. Biosensors, 13(9), 871. https://doi.org/10.3390/bios13090871





