Bright NIR-Emitting Styryl Pyridinium Dyes with Large Stokes’ Shift for Sensing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedure for Synthesis
2.2. Fluorescence Quantum Yields Calculation and Photophysical Property Evaluation
2.3. Fluorescence Microscopy Imaging
3. Results
3.1. Optical Properties
3.2. Sensing Human Serum Albumin (HSA) by Fluorometry
3.3. Low-Temperature and Fluorescence Lifetime Studies
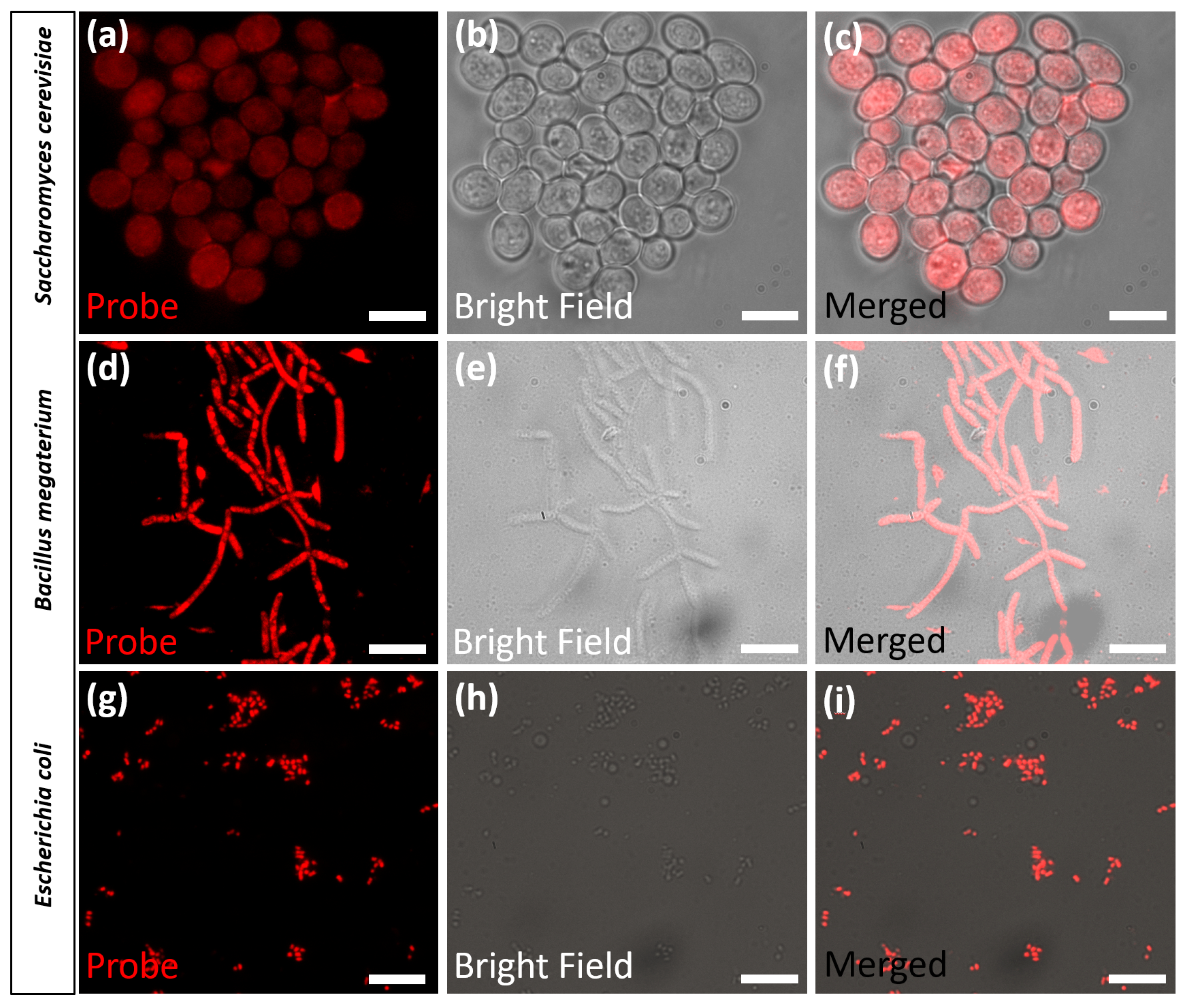
3.4. Visualizing Microorganisms via Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deligeorgiev, T.; Vasilev, A.; Kaloyanova, S.; Vaquero, J.J. Styryl Dyes–Synthesis and Applications during the Last 15 Years. Color. Technol. 2010, 126, 55–80. [Google Scholar] [CrossRef]
- Zhao, C.F.; Gvishi, R.; Narang, U.; Ruland, G.; Prasad, P.N. Structures, Spectra, and Lasing Properties of New (Aminostyryl) Pyridinium Laser Dyes. J. Phys. Chem. 1996, 100, 4526–4532. [Google Scholar] [CrossRef]
- Li, T.-Y.; Su, C.; Akula, S.B.; Sun, W.-G.; Chien, H.-M.; Li, W.-R. New Pyridinium Ylide Dyes for Dye Sensitized Solar Cell Applications. Org. Lett. 2016, 18, 3386–3389. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.; Spence, M.T.Z. The Handbook: A Guide to Fluorescent Probes and Labeling Technologies; Molecular Probes Inc.: Eugene, OR, USA, 2010. [Google Scholar]
- Shindy, H.A. Fundamentals in the Chemistry of Cyanine Dyes: A Review. Dyes Pigment. 2017, 145, 505–513. [Google Scholar] [CrossRef]
- Bohländer, P.R.; Wagenknecht, H.-A. Synthesis and Evaluation of Cyanine–Styryl Dyes with Enhanced Photostability for Fluorescent DNA Staining. Org. Biomol. Chem. 2013, 11, 7458–7462. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Wijesinghe, K.J.; Plescia, C.B.; Fisher, L.S.; Goodson, T., III; Stahelin, R.V.; Pang, Y. A Pyrene-Based Two-Photon Excitable Fluorescent Probe to Visualize Nuclei in Live Cells. Photochem. Photobiol. Sci. 2020, 19, 1152–1159. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Pang, Y. Synthesis of a Bis [2-(2′-Hydroxyphenyl) Benzoxazole] Pyridinium Derivative: The Fluoride-Induced Large Spectral Shift for Ratiometric Response. New J. Chem. 2021, 45, 9102–9108. [Google Scholar] [CrossRef]
- Abeywickrama, C.S. Large Stokes Shift Benzothiazolium Cyanine Dyes with Improved Intramolecular Charge Transfer (ICT) for Cell Imaging Applications. Chem. Commun. 2022, 58, 9855–9869. [Google Scholar] [CrossRef]
- Ayadi, A.; Szukalski, A.; El-Ghayoury, A.; Haupa, K.; Zouari, N.; Myśliwiec, J.; Kajzar, F.; Kulyk, B.; Sahraoui, B. TTF Based Donor-Pi-Acceptor Dyads Synthesized for NLO Applications. Dyes Pigment. 2017, 138, 255–266. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Wijesinghe, K.J.; Stahelin, R.V.; Pang, Y. Bright Red-Emitting Highly Reliable Styryl Probe with Large Stokes Shift for Visualizing Mitochondria in Live Cells under Wash-Free Conditions. Sens. Actuators B Chem. 2019, 285, 76–83. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Bertman, K.A.; Pang, Y. From Nucleus to Mitochondria to Lysosome Selectivity Switching in a Cyanine Probe: The Phenolic to Methoxy Substituent Conversion Affects Probe’s Selectivity. Bioorg. Chem. 2020, 99, 103848. [Google Scholar] [CrossRef]
- Kurtaliev, E.N. Spectroscopic Study of Interaction of Styrylcyanine Dye Sbt and Its Derivatives with Bovine Serum Albumin. J. Lumin. 2012, 132, 2281–2287. [Google Scholar] [CrossRef]
- Dahal, D.; Ojha, K.R.; Alexander, N.; Konopka, M.; Pang, Y. An NIR-Emitting ESIPT Dye with Large Stokes Shift for Plasma Membrane of Prokaryotic (E. coli) Cells. Sens. Actuators B Chem. 2018, 259, 44–49. [Google Scholar] [CrossRef]
- Yashchuk, V.M.; Gusak, V.V.; Dmytruk, I.M.; Prokopets, V.M.; Kudrya, V.Y.; Losytskyy, M.Y.; Tokar, V.P.; Gumenyuk, Y.O.; Yarmoluk, S.M.; Kovalska, V.B. Two-Photon Excited Luminescent Styryl Dyes as Probes for the DNA Detection and Imaging. Photostability and Phototoxic Influence on DNA. Mol. Cryst. Liq. Cryst. 2007, 467, 325–338. [Google Scholar] [CrossRef]
- Bhadani, A.; Singh, S. Novel Gemini Pyridinium Surfactants: Synthesis and Study of Their Surface Activity, DNA Binding, and Cytotoxicity. Langmuir 2009, 25, 11703–11712. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kitchen, J.A.; Gunnlaugsson, T.; Kelly, J.M. The Effect of the 4-Amino Functionality on the Photophysical and DNA Binding Properties of Alkyl-Pyridinium Derived 1, 8-Naphthalimides. Org. Biomol. Chem. 2013, 11, 5642–5655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Rao, Q.; Bu, Y.; Xu, T.; Zhu, X.; Zhang, J.; Tian, Y.; Zhou, H. Tuning the Hydrophobicity of Pyridinium-Based Probes to Realize the Mitochondria-Targeted Photodynamic Therapy and Mitophagy Tracking. Sens. Actuators B Chem. 2020, 321, 128460. [Google Scholar] [CrossRef]
- Reedy, J.L.; Hedlund, D.K.; Gabr, M.T.; Henning, G.M.; Pigge, F.C.; Schultz, M.K. Synthesis and Evaluation of Tetraarylethylene-Based Mono-, Bis-, and Tris (Pyridinium) Derivatives for Image-Guided Mitochondria-Specific Targeting and Cytotoxicity of Metastatic Melanoma Cells. Bioconjug. Chem. 2016, 27, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Loew, L.M.; Cohen, L.B.; Dix, J.; Fluhler, E.N.; Montana, V.; Salama, G.; Jian-young, W. A Naphthyl Analog of the Aminostyryl Pyridinium Class of Potentiometric Membrane Dyes Shows Consistent Sensitivity in a Variety of Tissue, Cell, and Model Membrane Preparations. J. Membr. Biol. 1992, 130, 1–10. [Google Scholar] [CrossRef]
- Ermakova, Y.G.; Sen, T.; Bogdanova, Y.A.; Smirnov, A.Y.; Baleeva, N.S.; Krylov, A.I.; Baranov, M.S. Pyridinium Analogues of Green Fluorescent Protein Chromophore: Fluorogenic Dyes with Large Solvent-Dependent Stokes Shift. J. Phys. Chem. Lett. 2018, 9, 1958–1963. [Google Scholar] [CrossRef]
- Li, Y.; Dahal, D.; Abeywickrama, C.S.; Pang, Y. Progress in Tuning Emission of the Excited-State Intramolecular Proton Transfer (ESIPT)-Based Fluorescent Probes. ACS Omega 2021, 6, 6547–6553. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Qian, X. Novel Cyanine Dyes as Fluorescent PH Sensors: PET, ICT Mechanism or Resonance Effect? J. Photochem. Photobiol. A Chem. 2007, 190, 1–8. [Google Scholar] [CrossRef]
- Coto, P.B.; Serrano-Andrés, L.; Gustavsson, T.; Fujiwara, T.; Lim, E.C. Intramolecular Charge Transfer and Dual Fluorescence of 4-(Dimethylamino)Benzonitrile: Ultrafast Branching Followed by a Two-Fold Decay Mechanism. Phys. Chem. Chem. Phys. 2011, 13, 15182. [Google Scholar] [CrossRef]
- Song, S.; Ju, D.; Li, J.; Li, D.; Wei, Y.; Dong, C.; Lin, P.; Shuang, S. Synthesis and Spectral Characteristics of Two Novel Intramolecular Charge Transfer Fluorescent Dyes. Talanta 2009, 77, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Drummen, G.P.C.; Konishi, G. Recent Advances in Twisted Intramolecular Charge Transfer (TICT) Fluorescence and Related Phenomena in Materials Chemistry. J. Mater. Chem. C 2016, 4, 2731–2743. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yokoyama, K. Design and Synthesis of Intramolecular Charge Transfer-Based Fluorescent Reagents for the Highly-Sensitive Detection of Proteins. J. Am. Chem. Soc. 2005, 127, 17799–17802. [Google Scholar] [CrossRef]
- Keij, J.F.; Bell-Prince, C.; Steinkamp, J.A. Staining of Mitochondrial Membranes with 10-nonyl Acridine Orange MitoFluor Green, and MitoTracker Green Is Affected by Mitochondrial Membrane Potential Altering Drugs. Cytom. J. Int. Soc. Anal. Cytol. 2000, 39, 203–210. [Google Scholar] [CrossRef]
- Rytting, E.; Bryan, J.; Southard, M.; Audus, K.L. Low-Affinity Uptake of the Fluorescent Organic Cation 4-(4-(Dimethylamino) Styryl)-N-Methylpyridinium Iodide (4-Di-1-ASP) in BeWo Cells. Biochem. Pharmacol. 2007, 73, 891–900. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A Review of NIR Dyes in Cancer Targeting and Imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Escobedo, J.O.; Rusin, O.; Lim, S.; Strongin, R.M. NIR Dyes for Bioimaging Applications. Curr. Opin. Chem. Biol. 2010, 14, 64–70. [Google Scholar] [CrossRef]
- Daehne, S.; Resch-Genger, U.; Wolfbeis, O.S. Near-Infrared Dyes for High Technology Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 52, ISBN 9401151024. [Google Scholar]
- Samanta, A.; Vendrell, M.; Das, R.; Chang, Y.-T. Development of Photostable Near-Infrared Cyanine Dyes. Chem. Commun. 2010, 46, 7406–7408. [Google Scholar] [CrossRef]
- Swamy, P.C.A.; Sivaraman, G.; Priyanka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) Absorbing Dyes as Promising Photosensitizer for Photo Dynamic Therapy. Coord. Chem. Rev. 2020, 411, 213233. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Baumann, H.J.; Alexander, N.; Shriver, L.P.; Konopka, M.; Pang, Y. NIR-Emitting Benzothiazolium Cyanines with an Enhanced Stokes Shift for Mitochondria Imaging in Live Cells. Org. Biomol. Chem. 2018, 16, 3382–3388. [Google Scholar] [CrossRef]
- Carlotti, B.; Benassi, E.; Barone, V.; Consiglio, G.; Elisei, F.; Mazzoli, A.; Spalletti, A. Effect of the π Bridge and Acceptor on Intramolecular Charge Transfer in Push–Pull Cationic Chromophores: An Ultrafast Spectroscopic and TD-DFT Computational Study. ChemPhysChem 2015, 16, 1440–1450. [Google Scholar] [CrossRef]
- Agnihotri, H.; Vasu, A.K.; Palakollu, V.; Kanvah, S. Neutral and Cationic Pyridylbutadienes: Solvatochromism and Fluorescence Response with Sodium Cholate. Photochem. Photobiol. Sci. 2015, 14, 2159–2167. [Google Scholar] [CrossRef]
- Li, C.; Plamont, M.-A.; Aujard, I.; Le Saux, T.; Jullien, L.; Gautier, A. Design and Characterization of Red Fluorogenic Push–Pull Chromophores Holding Great Potential for Bioimaging and Biosensing. Org. Biomol. Chem. 2016, 14, 9253–9261. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Li, Y.; Ramanah, A.; Owitipana, D.N.; Wijesinghe, K.J.; Pang, Y. Albumin-Induced Large Fluorescence Turn ON in 4-(Diphenylamino)Benzothiazolium Dyes for Clinical Applications in Protein Detection. Sens. Actuators B Chem. 2022, 368, 132199. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Mishra, A.K. Inner Filter Effect in Fluorescence Spectroscopy: As a Problem and as a Solution. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100318. [Google Scholar]
- Chen, S.; Yu, Y.-L.; Wang, J.-H. Inner Filter Effect-Based Fluorescent Sensing Systems: A Review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Wang, T.; Zeng, L.-H.; Li, D.-L. A Review on the Methods for Correcting the Fluorescence Inner-Filter Effect of Fluorescence Spectrum. Appl. Spectrosc. Rev. 2017, 52, 883–908. [Google Scholar] [CrossRef]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative Fluorescence Quantum Yields Using a Computer-Controlled Luminescence Spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Langhals, H.; Karolin, J.; Johansson, L.B.-Å. Spectroscopic Properties of New and Convenient Standards for Measuring Fluorescence Quantum Yields. J. Chem. Soc. Faraday Trans. 1998, 94, 2919–2922. [Google Scholar]
- Bertman, K.A.; Abeywickrama, C.S.; Pang, Y. A NIR Emitting Cyanine with Large Stokes’ Shift for Mitochondria and Identification of Their Membrane Potential Disruption. ChemBioChem 2022, 23, e202100516. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Matsubara, Y.; Ochi, T.; Wakamiya, T.; Yoshida, Z. How the π Conjugation Length Affects the Fluorescence Emission Efficiency. J. Am. Chem. Soc. 2008, 130, 13867–13869. [Google Scholar] [CrossRef]
- Duncan, T.V.; Susumu, K.; Sinks, L.E.; Therien, M.J. Exceptional Near-Infrared Fluorescence Quantum Yields and Excited-State Absorptivity of Highly Conjugated Porphyrin Arrays. J. Am. Chem. Soc. 2006, 128, 9000–9001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, G.; Wang, J.; Sun, S.; Zhang, Z. The Mechanisms of Large Stokes Shift and Fluorescence Quantum Yields in Anilino Substituted Rhodamine Analogue: TICT and PICT. Comput. Theor. Chem. 2016, 1095, 44–53. [Google Scholar]
- Lu, H.; Rutan, S.C. Solvatochromic Studies on Reversed-Phase Liquid Chromatographic Phases. 2. Characterization of Stationary and Mobile Phases. Anal. Chem. 1996, 68, 1387–1393. [Google Scholar] [CrossRef]
- Cha, S.; Choi, M.G.; Jeon, H.R.; Chang, S.-K. Negative Solvatochromism of Merocyanine Dyes: Application as Water Content Probes for Organic Solvents. Sens. Actuators B Chem. 2011, 157, 14–18. [Google Scholar]
- Muraoka, H.; Obara, T.; Ogawa, S. Systematic Synthesis, Comparative Studies of the Optical Properties, and the ICT-Based Sensor Properties of a Series of 2, 4, 6-Tri (5-Aryl-2-Thienyl) Pyrimidines with the D–π–A System. Tetrahedron Lett. 2016, 57, 3011–3015. [Google Scholar] [CrossRef]
- Abeywickrama, C.S.; Wijesinghe, K.J.; Stahelin, R.V.; Pang, Y. Lysosome Imaging in Cancer Cells by Pyrene-Benzothiazolium Dyes: An Alternative Imaging Approach for LAMP-1 Expression Based Visualization Methods to Avoid Background Interference. Bioorg. Chem. 2019, 91, 103144. [Google Scholar]
- Maillard, J.; Klehs, K.; Rumble, C.; Vauthey, E.; Heilemann, M.; Fürstenberg, A. Universal Quenching of Common Fluorescent Probes by Water and Alcohols. Chem. Sci. 2021, 12, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Vérolet, Q.; Fürstenberg, A. Improved Super-Resolution Microscopy with Oxazine Fluorophores in Heavy Water. Angew. Chem. Int. Ed. 2013, 52, 8948–8951. [Google Scholar] [CrossRef]
- Cao, J.; Wu, T.; Hu, C.; Liu, T.; Sun, W.; Fan, J.; Peng, X. The Nature of the Different Environmental Sensitivity of Symmetrical and Unsymmetrical Cyanine Dyes: An Experimental and Theoretical Study. Phys. Chem. Chem. Phys. 2012, 14, 13702–13708. [Google Scholar]
- Er, J.C.; Tang, M.K.; Chia, C.G.; Liew, H.; Vendrell, M.; Chang, Y.-T. MegaStokes BODIPY-Triazoles as Environmentally Sensitive Turn-on Fluorescent Dyes. Chem. Sci. 2013, 4, 2168–2176. [Google Scholar] [CrossRef]
- Wagner, B.D. The Use of Coumarins as Environmentally-Sensitive Fluorescent Probes of Heterogeneous Inclusion Systems. Molecules 2009, 14, 210–237. [Google Scholar] [PubMed]
- Wang, C.; Sato, Y.; Kudo, M.; Nishizawa, S.; Teramae, N. Ratiometric Fluorescent Signaling of Small Molecule, Environmentally Sensitive Dye Conjugates for Detecting Single-Base Mutations in DNA. Chem. Eur. J. 2012, 18, 9481–9484. [Google Scholar] [PubMed]
- Kim, Y.; Shin, E.; Jung, W.; Kim, M.K.; Chong, Y. A Near-Infrared Turn-on Fluorescent Sensor for Sensitive and Specific Detection of Albumin from Urine Samples. Sensors 2020, 20, 1232. [Google Scholar] [CrossRef]
- van der Vusse, G.J. Albumin as Fatty Acid Transporter. Drug Metab. Pharmacokinet. 2009, 24, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Ràfols, C.; Amézqueta, S.; Fuguet, E.; Bosch, E. Molecular Interactions between Warfarin and Human (HSA) or Bovine (BSA) Serum Albumin Evaluated by Isothermal Titration Calorimetry (ITC), Fluorescence Spectrometry (FS) and Frontal Analysis Capillary Electrophoresis (FA/CE). J. Pharm. Biomed. Anal. 2018, 150, 452–459. [Google Scholar]
- Mallick, A.; Haldar, B.; Chattopadhyay, N. Spectroscopic Investigation on the Interaction of ICT Probe 3-Acetyl-4-Oxo-6,7-Dihydro-12H Indolo-[2,3-a] Quinolizine with Serum Albumins. J. Phys. Chem. B 2005, 109, 14683–14690. [Google Scholar] [CrossRef]
- Li, Y.; He, W.; Dong, Y.; Sheng, F.; Hu, Z. Human Serum Albumin Interaction with Formononetin Studied Using Fluorescence Anisotropy, FT-IR Spectroscopy, and Molecular Modeling Methods. Bioorg. Med. Chem. 2006, 14, 1431–1436. [Google Scholar]
- Chatterjee, S.; Mukherjee, T.K. Effect of Self-Association of Bovine Serum Albumin on the Stability of Surfactant-Induced Aggregates of Allylamine-Capped Silicon Quantum Dots. J. Phys. Chem. B 2013, 117, 16110–16116. [Google Scholar]
- Yang, F.; Zhang, Y.; Liang, H. Interactive Association of Drugs Binding to Human Serum Albumin. Int. J. Mol. Sci. 2014, 15, 3580–3595. [Google Scholar] [CrossRef] [PubMed]
- Epps, D.E.; Raub, T.J.; Caiolfa, V.; Chiari, A.; Zamai, M. Determination of the Affinity of Drugs toward Serum Albumin by Measurement of the Quenching of the Intrinsic Tryptophan Fluorescence of the Protein. J. Pharm. Pharmacol. 1999, 51, 41–48. [Google Scholar] [CrossRef]
- Tayyab, S.; Izzudin, M.M.; Kabir, M.Z.; Feroz, S.R.; Tee, W.-V.; Mohamad, S.B.; Alias, Z. Binding of an Anticancer Drug, Axitinib to Human Serum Albumin: Fluorescence Quenching and Molecular Docking Study. J. Photochem. Photobiol. B Biol. 2016, 162, 386–394. [Google Scholar]
- Ramadass, R.; Bereiter-Hahn, J. Photophysical Properties of DASPMI as Revealed by Spectrally Resolved Fluorescence Decays. J. Phys. Chem. B 2007, 111, 7681–7690. [Google Scholar] [PubMed]
- Xiong, W.; Wang, L.; Chen, X.; Tang, H.; Cao, D.; Zhang, G.; Chen, W. Pyridinium-Substituted Tetraphenylethylene Salt-Based Photosensitizers by Varying Counter Anions: A Highly Efficient Photodynamic Therapy for Cancer Cell Ablation and Bacterial Inactivation. J. Mater. Chem. B 2020, 8, 5234–5244. [Google Scholar] [PubMed]
- Yuan, H.; Liu, Z.; Liu, L.; Lv, F.; Wang, Y.; Wang, S. Cationic Conjugated Polymers for Discrimination of Microbial Pathogens. Adv. Mater. 2014, 26, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, W.; Jia, S.; Yuan, H.; Gao, L.-H. Design and Application of Conjugated Polymer Nanomaterials for Detection and Inactivation of Pathogenic Microbes. ACS Appl. Bio Mater. 2020, 4, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Gujrati, V.; Werner, J.P.F.; Mishra, K.; Anzenhofer, P.; Stiel, A.C.; Mettenleiter, G.; Feuchtinger, A.; Walch, A.; Ntziachristos, V. Bacterial Outer Membrane Vesicles as Cationic Dye Carriers for Optoacoustics-Guided Phototherapy of Cancer. Cancer Nanotechnol. 2023, 14, 36. [Google Scholar] [CrossRef]
- Budin, G.; Chung, H.J.; Lee, H.; Weissleder, R. A ‘Magnetic’Gram Stain for Bacterial Detection. Angew. Chem. Int. Ed. Engl. 2012, 51, 7752. [Google Scholar] [CrossRef] [PubMed]

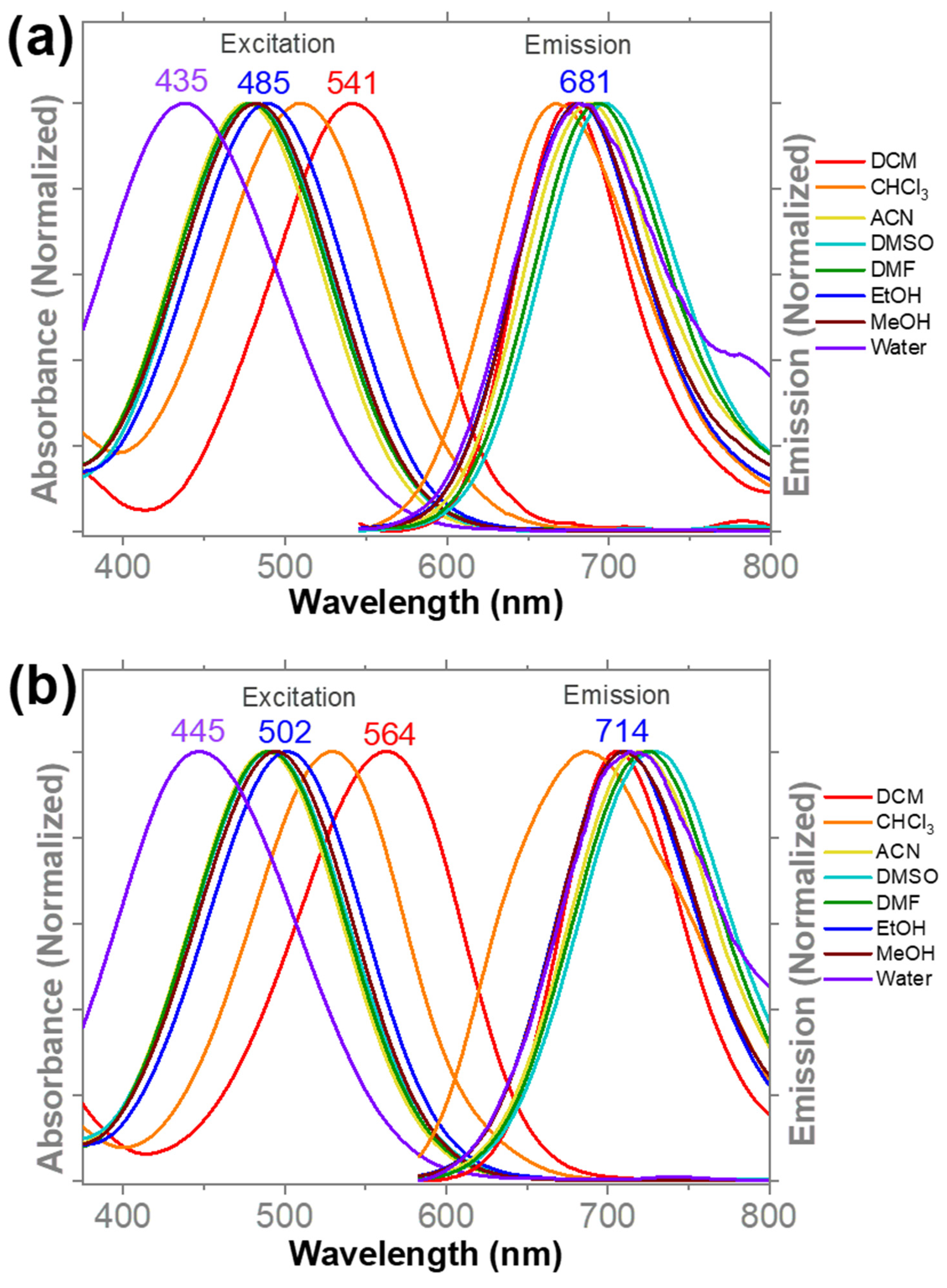



| Solvent | DCM | CHCl3 | ACN | DMSO | DMF | EtOH | MeOH | Water |
|---|---|---|---|---|---|---|---|---|
| Probe 1a | ||||||||
| λabs (nm) | 541 | 512 | 478 | 477 | 476 | 488 | 482 | 438 |
| λem (nm) | 678 | 667 | 687 | 698 | 694 | 684 | 688 | 687 |
| ∆λ (nm) | 137 | 155 | 209 | 221 | 218 | 196 | 206 | 249 |
| ϕfl | 0.61 | 0.29 | 0.33 | 0.68 | 0.40 | 0.24 | 0.38 | 0.01 |
| ε (M−1·cm−1) | 47,023 | 42,456 | 41,482 | 41,470 | 40,700 | 42,428 | 39,647 | 33,162 |
| Probe 1b | ||||||||
| λabs (nm) | 561 | 530 | 487 | 489 | 490 | 501 | 492 | 446 |
| λem (nm) | 706 | 688 | 718 | 731 | 724 | 714 | 712 | 718 |
| ∆λ (nm) | 145 | 158 | 231 | 242 | 234 | 213 | 220 | 272 |
| ϕfl | 0.72 | 0.47 | 0.24 | 0.61 | 0.48 | 0.38 | 0.31 | 0.005 |
| ε (M−1·cm−1) | 48,120 | 43,442 | 43,100 | 49,406 | 42,100 | 42,159 | 40,477 | 25,670 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickramasinghe, N.I.; Corbin, B.; Kanakarathna, D.Y.; Pang, Y.; Abeywickrama, C.S.; Wijesinghe, K.J. Bright NIR-Emitting Styryl Pyridinium Dyes with Large Stokes’ Shift for Sensing Applications. Biosensors 2023, 13, 799. https://doi.org/10.3390/bios13080799
Wickramasinghe NI, Corbin B, Kanakarathna DY, Pang Y, Abeywickrama CS, Wijesinghe KJ. Bright NIR-Emitting Styryl Pyridinium Dyes with Large Stokes’ Shift for Sensing Applications. Biosensors. 2023; 13(8):799. https://doi.org/10.3390/bios13080799
Chicago/Turabian StyleWickramasinghe, Nirasha I., Brian Corbin, Devni Y. Kanakarathna, Yi Pang, Chathura S. Abeywickrama, and Kaveesha J. Wijesinghe. 2023. "Bright NIR-Emitting Styryl Pyridinium Dyes with Large Stokes’ Shift for Sensing Applications" Biosensors 13, no. 8: 799. https://doi.org/10.3390/bios13080799
APA StyleWickramasinghe, N. I., Corbin, B., Kanakarathna, D. Y., Pang, Y., Abeywickrama, C. S., & Wijesinghe, K. J. (2023). Bright NIR-Emitting Styryl Pyridinium Dyes with Large Stokes’ Shift for Sensing Applications. Biosensors, 13(8), 799. https://doi.org/10.3390/bios13080799






