Liquid Metal-Based Flexible Bioelectrodes for Management of In-Stent-Restenosis: Potential Application
Abstract
1. Introduction
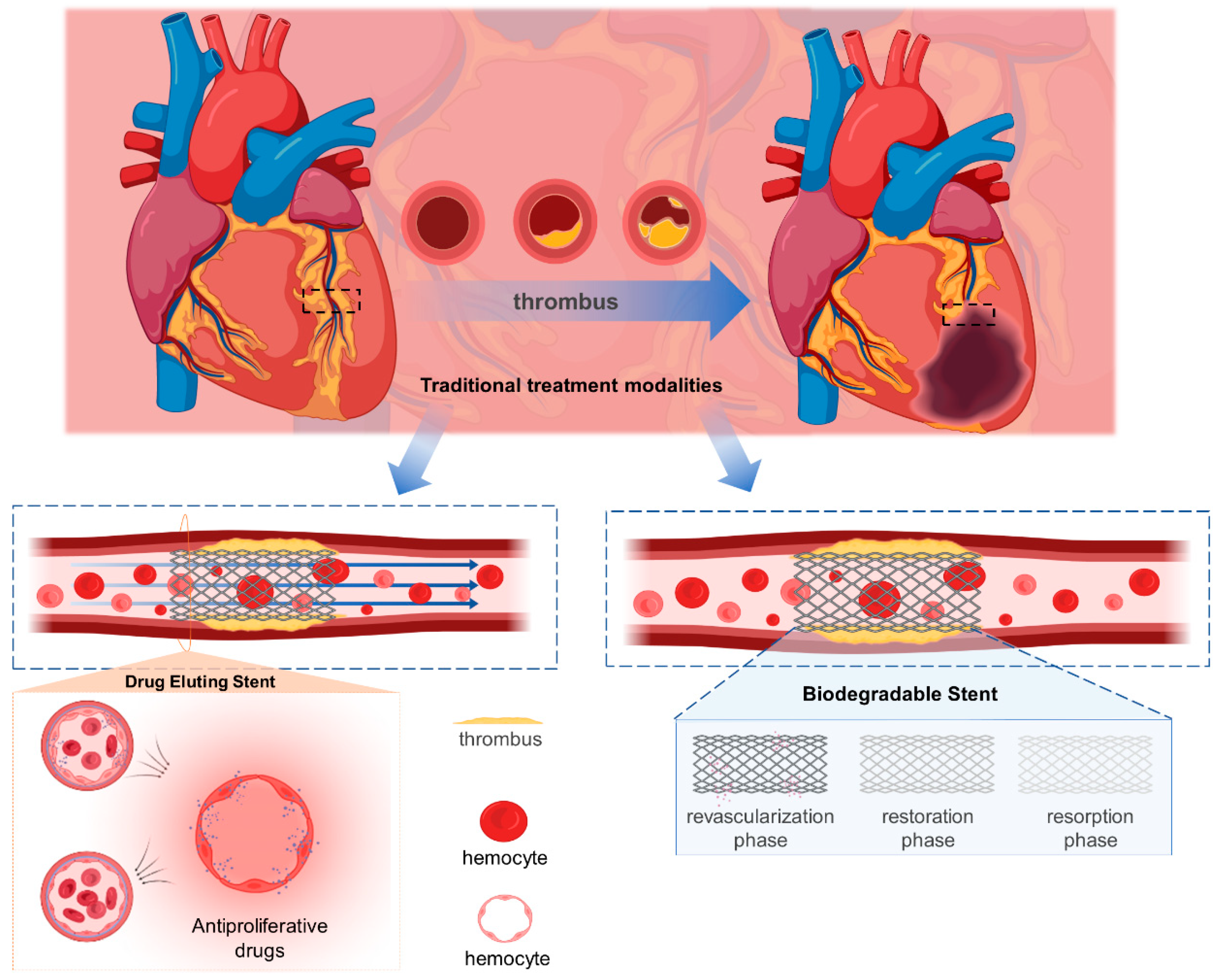
2. Techniques for the Treatment of In-Stent Restenosis
2.1. Drug-Eluting Stent
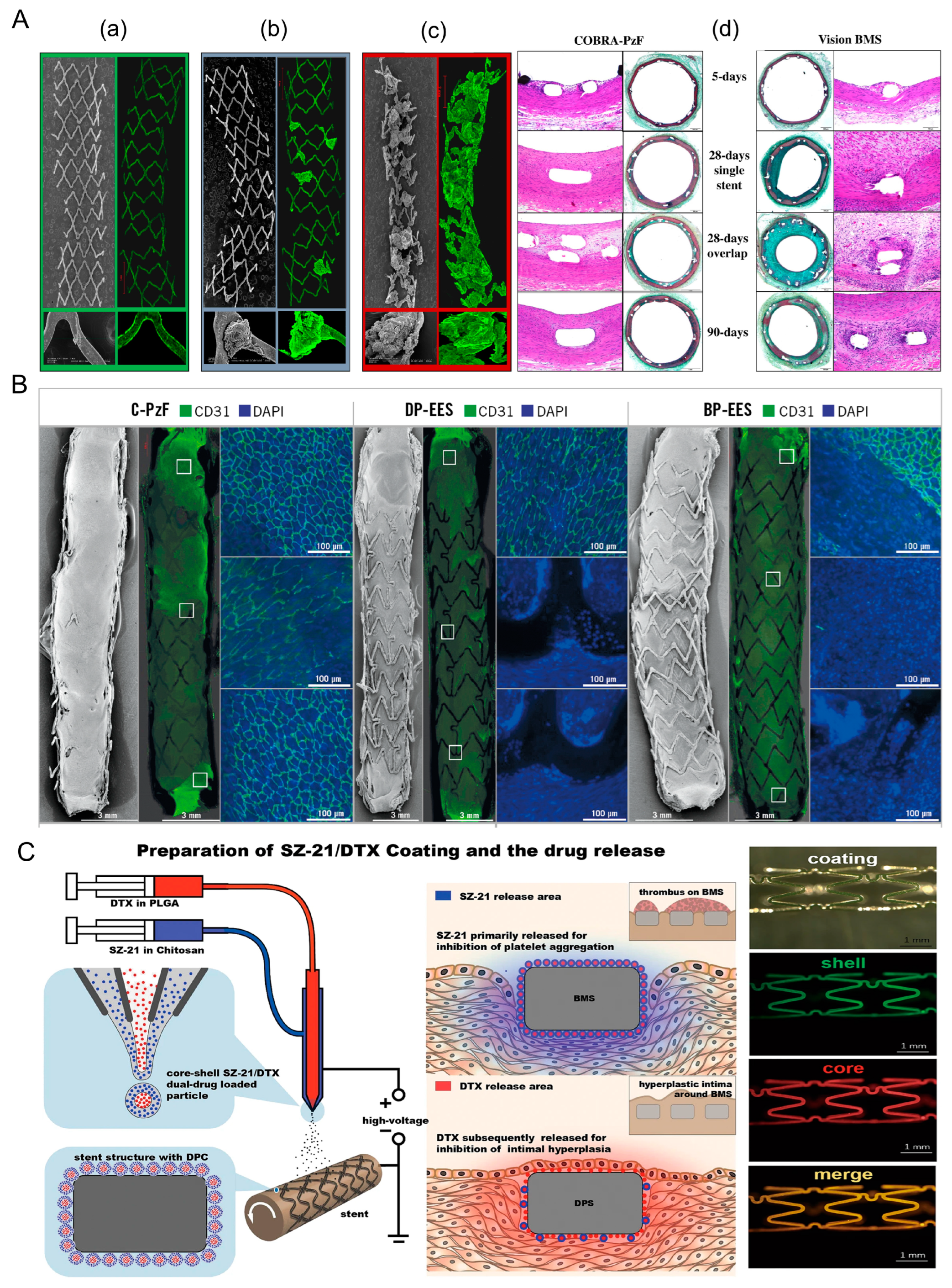
2.1.1. First Generation
2.1.2. Second Generation
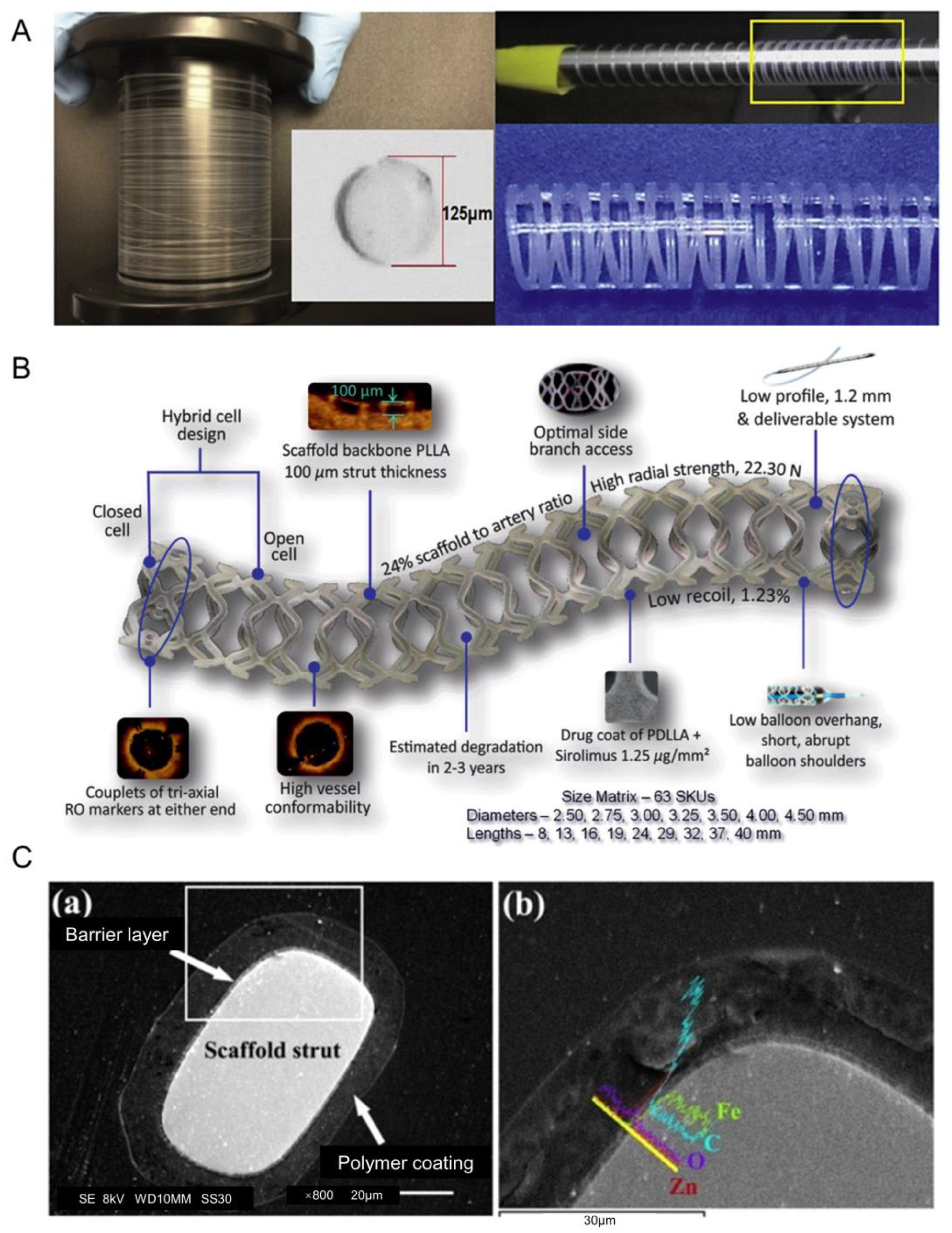
2.1.3. Optimization of DES
2.2. Biodegradable Stent
2.2.1. Metal Degradable Stent
2.2.2. Polymer-Based Degradable Stent
2.2.3. Optimization of BDS
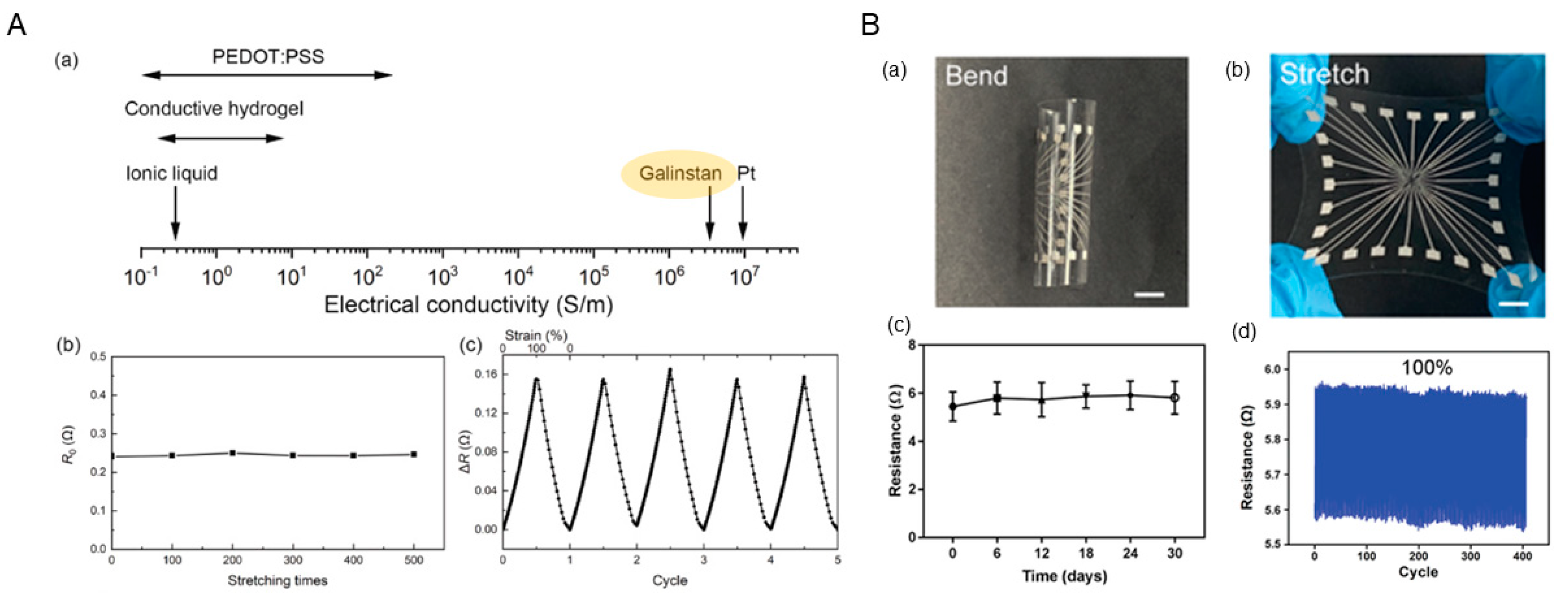
3. The Potential of Liquid-Metal-Based Electrodes Combined with Vascular Stents
3.1. Advantages of Liquid-Metal-Based Electrodes
3.2. Challenges of Liquid-Metal-Based Electrodes for Vascular Stent
4. Perspective
4.1. Structural Design of Integrated Vascular Stent Based on Liquid Metal
4.2. Future Prospect of Integrated Vascular Stents Based on Liquid Metal
4.2.1. Detection of Neointimal Hyperplasia Thickness
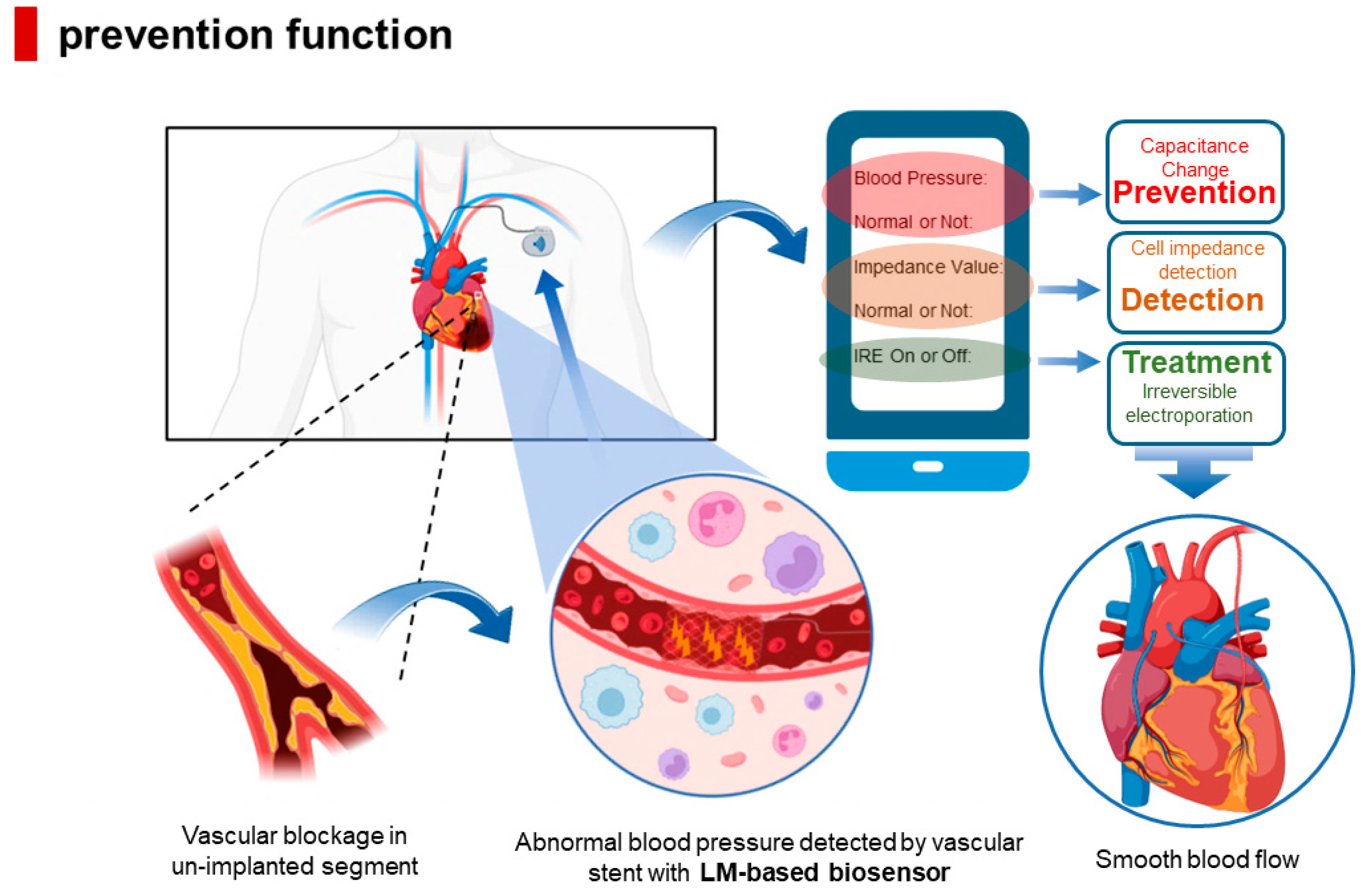
4.2.2. Permanent Prevention of ISR
4.2.3. Blood Pressure Monitoring
4.2.4. Reduce the Accumulation of Blood Cells
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roadmap for the Implementation of the Global Action Plan for the Prevention and Control of Non-communicable Diseases 2013–2030. Available online: https://www.who.int/publications-detail-redirect/9789241506236 (accessed on 25 August 2021).
- Buccheri, D.; Piraino, D.; Andolina, G.; Cortese, B. Understanding and managing in-stent restenosis: A review of clinical data; from pathogenesis to treatment. J. Thorac. Dis. 2016, 8, E1150–E1162. [Google Scholar] [CrossRef] [PubMed]
- Camenzind, E.; Steg, P.; Wijns, W. Stent thrombosis late after implantation of first-generation drug-eluting stents: A cause for concern. Circulation 2007, 115, 1440–1455. [Google Scholar] [CrossRef]
- Scafa Udriște, A.; Niculescu, A.G.; Grumezescu, A.M.; Bădilă, E. Cardiovascular Stents: A Review of Past, Current, and Emerging Devices. Materials 2021, 14, 2498. [Google Scholar] [CrossRef] [PubMed]
- Bønaa, K.H.; Mannsverk, J.; Wiseth, R.; Aaberge, L.; Myreng, Y.; Nygard, O.; Nilsen, D.W.; Kløw, N.E.; Uchto, M.; Trovik, T. Drug-eluting or bare-metal stents for coronary artery disease. N. Engl. J. Med. 2016, 375, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Wykrzykowska, J.J.; Kraak, R.P.; Hofma, S.H.; van der Schaaf, R.J.; Arkenbout, E.K.; IJsselmuiden, A.J.; Elias, J.; van Dongen, I.M.; Tijssen, R.Y.; Koch, K.T. Bioresorbable scaffolds versus metallic stents in routine PCI. N. Engl. J. Med. 2017, 376, 2319–2328. [Google Scholar] [CrossRef]
- Oliver, A.A.; Sikora-Jasinska, M.; Demir, A.G.; Guillory, R.J., II. Recent advances and directions in the development of bioresorbable metallic cardiovascular stents: Insights from recent human and in vivo studies. Acta Biomater. 2021, 127, 1–23. [Google Scholar] [CrossRef]
- Cockerill, I.; See, C.W.; Young, M.L.; Wang, Y.; Zhu, D. Designing better cardiovascular stent materials: A learning curve. Adv. Funct. Mater. 2021, 31, 2005361. [Google Scholar] [CrossRef]
- Bussooa, A.; Hoare, D.; Kirimi, M.T.; Mitra, S.; Mirzai, N.; Neale, S.L.; Mercer, J.R. Impedimetric detection and electromediated apoptosis of vascular smooth muscle using microfabricated biosensors for diagnosis and therapeutic intervention in cardiovascular diseases. Adv. Sci. 2020, 7, 1902999. [Google Scholar] [CrossRef]
- Napotnik, T.B.; Polajžer, T.; Miklavčič, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Song, J.M.; Lui, T.S.; Chang, Y.L.; Chen, L.H. Compositional effects on the microstructure and vibration fracture properties of Sn–Zn–Bi alloys. J. Alloys Compd. 2005, 403, 191–196. [Google Scholar] [CrossRef]
- David, R.; Miki, N. Liquid metal electrodes micro-array for electrical stimulation via direct contact. In Proceedings of the 2018 IEEE Micro Electro Mechanical Systems (MEMS), Belfast, UK, 1 January 2018. [Google Scholar]
- Li, Y.; Nayak, S.; Luo, Y.; Liu, Y.; Salila Vijayalal Mohan, H.K.; Pan, J.; Liu, Z.; Heng, C.H.; Thean, A.V.Y. A soft polydimethylsiloxane liquid metal interdigitated capacitor sensor and its integration in a flexible hybrid system for on-body respiratory sensing. Materials 2019, 12, 1458. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Wang, L.; Hang, C.; Chen, Z.; Liu, X.; Zhong, L.; Qi, J.; Huang, Y.; Liu, S.; Wang, L. Printed stretchable liquid metal electrode arrays for in vivo neural recording. Small 2021, 17, 2006612. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M. Principles of regenerative biology. In Principles of Regenerative Biology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 325–369. [Google Scholar]
- Oberhoff, M.; Herdeg, C.; Baumbach, A.; Karsch, K.R. Stent-based antirestenotic coatings (sirolimus/paclitaxel). Catheter. Cardiovasc. Interv. 2002, 55, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Waugh, J.; Wagstaff, A.J. The paclitaxel (TAXUS™)-eluting stent: A review of its use in the management of de novo coronary artery lesions. Am. J. Cardiovasc. Drugs 2004, 4, 257–268. [Google Scholar] [CrossRef]
- Blagosklonny, M.V.; Darzynkiewicz, Z.; Halicka, H.D.; Pozarowski, P.; Demidenko, Z.N.; Barry, J.J.; Kamath, K.R.; Herrmann, R.A. Paclitaxel induces primary and postmitotic G1 arrest in human arterial smooth muscle cells. Cell Cycle 2004, 3, 1048–1054. [Google Scholar] [CrossRef]
- Wessely, R.; Schömig, A.; Kastrati, A. Sirolimus and paclitaxel on polymer-based drug-eluting stents: Similar but different. J. Am. Coll. Cardiol. 2006, 47, 708–714. [Google Scholar] [CrossRef]
- Stone, G.W.; Moses, J.W.; Ellis, S.G.; Schofer, J.; Dawkins, K.D.; Morice, M.C.; Colombo, A.; Schampaert, E.; Grube, E.; Kirtane, A.J. Safety and efficacy of sirolimus-and paclitaxel-eluting coronary stents. N. Engl. J. Med. 2007, 356, 998–1008. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takano, M.; Murakami, D.; Okamatsu, K.; Seino, Y.; Mizuno, K. Comparative angioscopic evaluation of neointimal coverage and thrombus between TAXUS-Express and TAXUS-Liberté stents: Is the stent platform type associated with the vascular response? Int. J. Cardiol. 2010, 145, 587–589. [Google Scholar] [CrossRef]
- Miyazawa, A.; Ako, J.; Hongo, Y.; Hur, S.H.; Tsujino, I.; Courtney, B.K.; Hassan, A.H.; Kandzari, D.E.; Honda, Y.; Fitzgerald, P.J. Comparison of vascular response to zotarolimus-eluting stent versus sirolimus-eluting stent: Intravascular ultrasound results from ENDEAVOR III. Am. Heart J. 2008, 155, 108–113. [Google Scholar] [CrossRef]
- Raber, L.; Windecker, S. Current Status of Drug-Eluting Stents. Cardiovasc. Ther. 2011, 29, 176–189. [Google Scholar] [CrossRef]
- Wessely, R. New drug-eluting stent concepts. Nat. Rev. Cardiol. 2010, 7, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Jinnouchi, H.; Diljon, C.; Torii, S.; Sakamoto, A.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. A new category stent with novel polyphosphazene surface modification. Future Cardiol. 2018, 14, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Koppara, T.; Sakakura, K.; Pacheco, E.; Cheng, Q.; Zhao, X.Q.; Acampado, E.; Finn, A.V.; Barakat, M.; Maillard, L.; Ren, J. Preclinical evaluation of a novel polyphosphazene surface modified stent. Int. J. Cardiol. 2016, 222, 217–225. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Mori, H.; Cheng, Q.; Kutyna, M.; Torii, S.; Sakamoto, A.; Guo, L.; Acampado, E.; Gupta, A.; Kolodgie, F.D. Thromboresistance and functional healing in the COBRA PzF stent versus competitor DES: Implications for dual antiplatelet therapy. EuroIntervention 2019, 15, e342–e353. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Wang, Y.; Huang, Y.; Zhao, Y.; Zhang, D.; Du, D.; Zhang, Y.; Li, Z.; McGinty, S.; Pontrelli, G. Design and testing of hydrophobic core/hydrophilic shell nano/micro particles for drug-eluting stent coating. NPG Asia Mater. 2018, 10, 642–658. [Google Scholar] [CrossRef]
- Li, S.; Wei, Y.; Wei, Z.; Wenjie, T.; Jingliang, T.; Yizhe, W. e0473 Rapid re-endothelialization and anti-intimal hyperplasia coronary stent system with a novel biomacromolecular prohealing coating. Heart 2010, 96, A148. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable; implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Scarcello, E.; Herpain, A.; Tomatis, M.; Turci, F.; Jacques, P.; Lison, D. Hydroxyl radicals and oxidative stress: The dark side of Fe corrosion. Colloids Surf. B Biointerfaces 2020, 185, 110542. [Google Scholar] [CrossRef]
- Scarcello, E.; Lobysheva, I.; Bouzin, C.; Jacques, P.; Lison, D.; Dessy, C. Ndothelial dysfunction induced by hydroxyl radicals–the hidden face of biodegradable Fe-based materials for coronary stents. Mater. Sci. Eng. C 2020, 112, 110938. [Google Scholar] [CrossRef]
- Lin, W.; Qin, L.; Qi, H.; Zhang, D.; Zhang, G.; Gao, R.; Qiu, H.; Xia, Y.; Cao, P.; Wang, X. Long-term in vivo corrosion behavior; biocompatibility and bioresorption mechanism of a bioresorbable nitrided iron scaffold. Acta Biomater. 2017, 54, 454–468. [Google Scholar] [CrossRef]
- Peuster, M.; Wohlsein, P.; Brügmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal—Results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Schinhammer, M.; Gerber, I.; Hänzi, A.C.; Uggowitzer, P.J. On the cytocompatibility of biodegradable Fe-based alloys. Mater. Sci. Eng. C 2013, 33, 782–789. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Luo, L.; He, Y.; Li, B.; Cai, Y.; Hu, S.; Xiao, X. Hierarchical porous silicon structures with extraordinary mechanical strength as high-performance lithium-ion battery anodes. Nat. Commun. 2020, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, B.H.; Cai, Z.X. Recent Progress on Mg- and Zn-Based Alloys for Biodegradable Vascular Stent Applications. J. Nanomater. 2019, 2019, 1310792. [Google Scholar] [CrossRef]
- Nishio, S.; Kosuga, K.; Igaki, K.; Okada, M.; Kyo, E.; Tsuji, T.; Takeuchi, E.; Inuzuka, Y.; Takeda, S.; Hata, T. Long-term (>10 years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents: Igaki-Tamai stents. Circulation 2012, 125, 2343–2353. [Google Scholar] [CrossRef]
- Ali, Z.A.; Serruys, P.W.; Kimura, T.; Gao, R.; Ellis, S.G.; Kereiakes, D.J.; Onuma, Y.; Simonton, C.; Zhang, Z.; Stone, G.W. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: A systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet 2017, 390, 760–772. [Google Scholar] [CrossRef]
- Stone, G.W.; Abizaid, A.; Onuma, Y.; Seth, A.; Gao, R.; Ormiston, J.; Kimura, T.; Chevalier, B.; Ben-Yehuda, O.; Dressler, O. Effect of technique on outcomes following bioresorbable vascular scaffold implantation: Analysis from the ABSORB trials. J. Am. Coll. Cardiol. 2017, 70, 2863–2874. [Google Scholar] [CrossRef]
- Regazzoli, D.; Leone, P.P.; Colombo, A.; Latib, A. New generation bioresorbable scaffold technologies: An update on novel devices and clinical results. J. Thorac. Dis. 2017, 9, S979. [Google Scholar] [CrossRef]
- Tenekecioglu, E.; Serruys, P.W.; Onuma, Y.; Costa, R.; Chamié, D.; Sotomi, Y.; Yu, T.B.; Abizaid, A.; Liew, H.B.; Santoso, T. Randomized comparison of absorb bioresorbable vascular scaffold and mirage microfiber sirolimus-eluting scaffold using multimodality imaging. JACC Cardiovasc. Interv. 2017, 10, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Onuma, Y.; Costa, R.; Chandra, P.; Bahl, V.K.; C Manjunath, N.; Mahajan, A.U.; Kumar, V.; Goel, P.K.; Wander, G.S. First-in-human evaluation of a novel poly-L-lactide based sirolimus-eluting bioresorbable vascular scaffold for the treatment of de novo native coronary artery lesions: MeRes-1 trial. EuroIntervention 2017, 13, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Wu, Y.Z.; Shen, L.; Zhang, F.; Yao, Z.F.; Yin, J.S.; Ji, M.; Wang, Q.B.; Ge, L.; Qian, J.Y. First-in-man implantation of the XINSORB bioresorbable sirolimus-eluting scaffold in China. Chin. Med. J. 2015, 128, 1275–1276. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, S.; Luo, X.; Liu, Z. Theoretical and numerical analysis of mechanical behaviors of a metamaterial-based shape memory polymer stent. Polymers 2020, 12, 1784. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Cui, L.Y.; Zeng, R.C.; Li, S.Q.; Chen, X.B.; Zheng, Y.; Kannan, M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Li, M.; Jiang, M.W.; Gao, Y.F.; Zheng, Y.; Liu, Z.; Zhou, C.; Huang, T.; Gu, X.N.; Li, A.; Fang, J.C.; et al. Current status and outlook of biodegradable metals in neuroscience and their potential applications as cerebral vascular stent materials. Bioact. Mater. 2022, 11, 140–153. [Google Scholar] [CrossRef]
- Amani, S.; Faraji, G. Processing and properties of biodegradable magnesium microtubes for using as vascular stents: A brief review. Met. Mater. Int. 2019, 25, 1341–1359. [Google Scholar] [CrossRef]
- Lin, W.J.; Zhang, D.Y.; Zhang, G.; Sun, H.T.; Qi, H.P.; Chen, L.P.; Liu, Z.Q.; Gao, R.L.; Zheng, W. Design and characterization of a novel biocorrodible iron-based drug-eluting coronary scaffold. Mater. Des. 2016, 91, 72–79. [Google Scholar] [CrossRef]
- Hoare, D.; Tsiamis, A.; Marland, J.R.; Czyzewski, J.; Kirimi, M.T.; Holsgrove, M.; Russell, E.; Neale, S.L.; Mirzai, N.; Mitra, S. Predicting Cardiovascular Stent Complications Using Self-Reporting Biosensors for Noninvasive Detection of Disease. Adv. Sci. 2022, 9, 2105285. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, B.X.; Gao, J.R.; Lang, Y.; Lv, X.; Deng, Z.S.; Gui, L.; Liu, J.; Tang, R.Y.; Li, L. Liquid Metal-Based Electrode Array for Neural Signal Recording. Bioengineering 2023, 10, 578. [Google Scholar] [CrossRef]
- Tang, R.Y.; Zhang, C.L.; Liu, B.X.; Jiang, C.; Wang, L.; Zhang, X.; Huang, Q.; Liu, J.; Li, L. Towards an artificial peripheral nerve: Liquid metal-based fluidic cuff electrodes for long-term nerve stimulation and recording. Biosens. Bioelectron. 2022, 216, 114600. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.H.; Liu, X.Y.; Cheng, S.Y.; Tang, L.X.; Chen, M.; Zhong, L.N.; Chen, Z.; Liu, X.Q.; Jiang, X.Y. Highly stretchable metal–polymer conductor electrode array for Electrophysiology. Adv. Healthc. Mater. 2021, 10, 2000641. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, R.; Li, L.; Zhang, L.; Liu, B.; Deng, Z.; Wang, L.; Gui, L. A liquid metal based capacitive soft pressure microsensor. Lab Chip 2019, 19, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Yao, K.M.; Wu, M.G.; Lei, Z.G.; Chen, F.; Li, J.Y.; Mei, Q.J.; Zhou, Y.Y.; Huang, Q.Y.; Zhao, X.; et al. Wafer-patterned, permeable, and stretchable liquid metal microelectrodes for implantable bioelectronics with chronic biocompatibility. Sci. Adv. 2023, 9, eadg8602. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jung, J.; Lee, Y.; Lee, D.; Vlassak, J.J.; Park, Y.L. Liquid-metal micro-networks with strain-induced conductivity for soft electronics and robotic skin. npj Flex. Electron. 2022, 6, 81. [Google Scholar] [CrossRef]
- Foremny, K.; Nagels, S.; Kreienmeyer, M.; Doll, T.; Deferme, W. Biocompatibility Testing of Liquid Metal as an Interconnection Material for Flexible Implant Technology. Nanomaterials 2021, 11, 3251. [Google Scholar] [CrossRef]
- Zhu, B.; Cai, Y.; Huang, H.; Liang, X.; Li, X.; Yang, H. Red blood cell stretching manipulation using the microfluidics chip integrated with liquid metal electrode. In Proceedings of the 2019 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (AIM), Hong Kong, China, 8–12 July 2019. [Google Scholar]
- Cheng, S.; Hang, C.; Ding, L.; Jia, L.; Tang, L.; Mou, L.; Qi, J.; Dong, R.; Zheng, W.; Zhang, Y. Electronic blood vessel. Matter 2020, 3, 1664–1684. [Google Scholar] [CrossRef]

| Name | Affiliated Company |
|---|---|
| Cypher stent | Cordis Corp–Johnson and Johnson company, New Brunswick, NJ, USA |
| Taxus stent | Boston Scientific, Natick, MA, USA |
| Taxus Liberte stent | Boston Scientific, Natick, MA, USA |
| Endeavor stent | Medtronic CardioVascular, Minneapolis, MN, USA |
| Xience V stent | Abbott Vascular, Markham, Ontario, Canada |
| COBRA PzF stent | Alta Biomaterials, Carlsbad, CA, USA |
| AMSorb stent | Beijing Advanced Medical Technologies, Ltd., Inc. Beijing, China |
| IBS stent | Lifetech Scientific, Shenzhen, GuangDong, China |
| Mirage BRMS stent | Manli Cardiology, Singapore |
| Sirolimus Stent Set | BDS Set L | Paclitaxel Stent Set | BDS Set Z | |
|---|---|---|---|---|
| 4-year thrombosis rate | 1.2% | 0.6% | 1.3% | 0.9% |
| Number of thrombosis after 1 year | 5 | 0 | 9 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, L.; Deng, Z. Liquid Metal-Based Flexible Bioelectrodes for Management of In-Stent-Restenosis: Potential Application. Biosensors 2023, 13, 795. https://doi.org/10.3390/bios13080795
Zhang X, Li L, Deng Z. Liquid Metal-Based Flexible Bioelectrodes for Management of In-Stent-Restenosis: Potential Application. Biosensors. 2023; 13(8):795. https://doi.org/10.3390/bios13080795
Chicago/Turabian StyleZhang, Xilong, Lei Li, and Zhongshan Deng. 2023. "Liquid Metal-Based Flexible Bioelectrodes for Management of In-Stent-Restenosis: Potential Application" Biosensors 13, no. 8: 795. https://doi.org/10.3390/bios13080795
APA StyleZhang, X., Li, L., & Deng, Z. (2023). Liquid Metal-Based Flexible Bioelectrodes for Management of In-Stent-Restenosis: Potential Application. Biosensors, 13(8), 795. https://doi.org/10.3390/bios13080795





