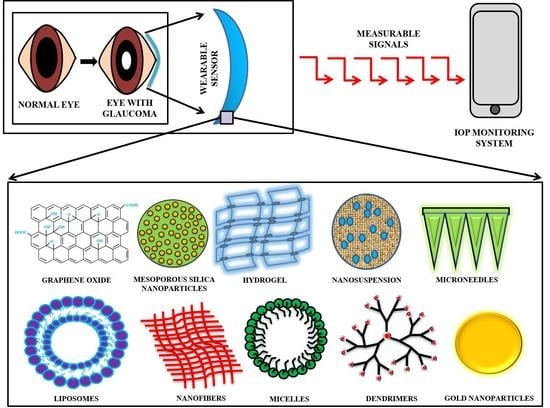
Current Innovations in Intraocular Pressure Monitoring Biosensors for Diagnosis and Treatment of Glaucoma—Novel Strategies and Future Perspectives
Abstract
1. Introduction
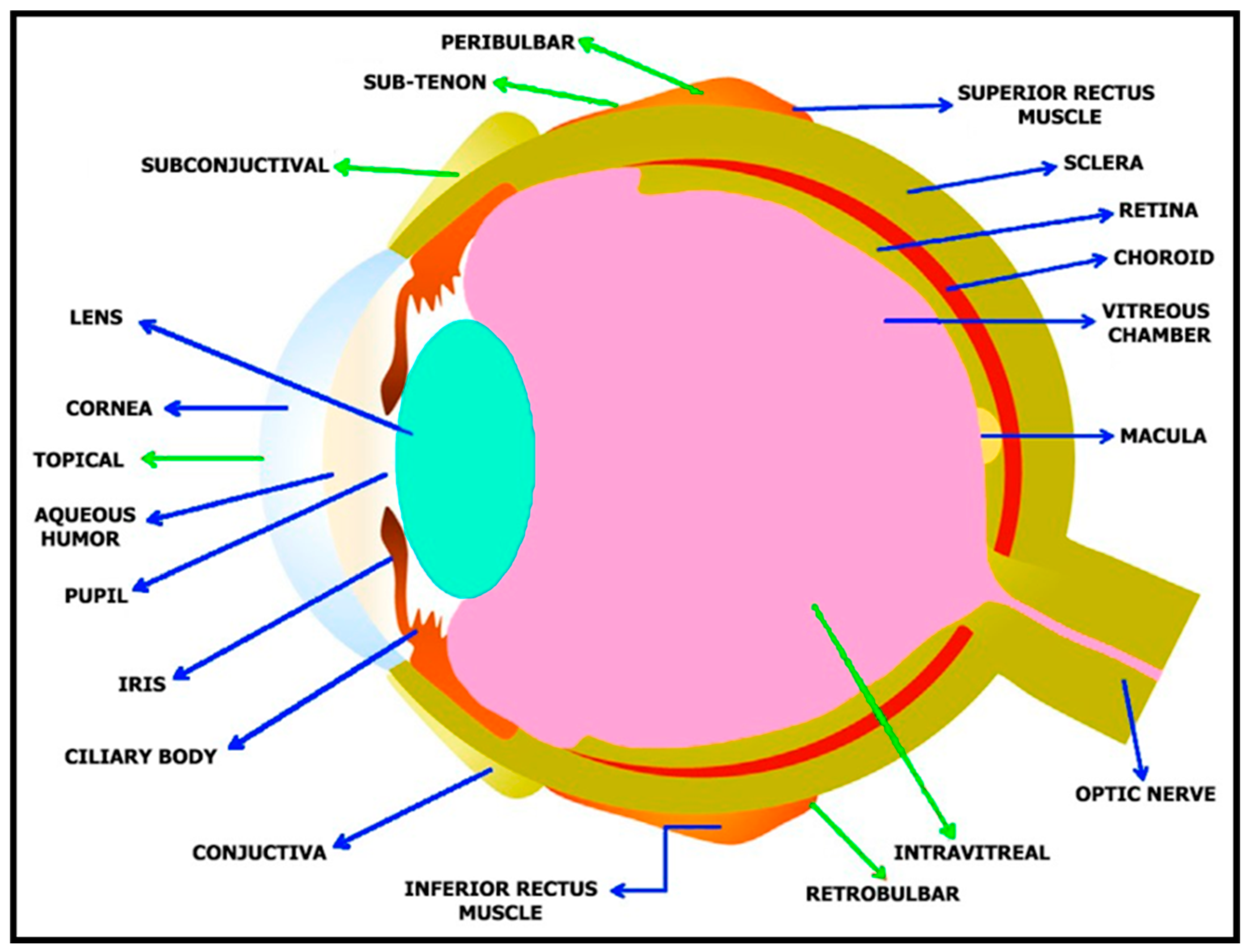
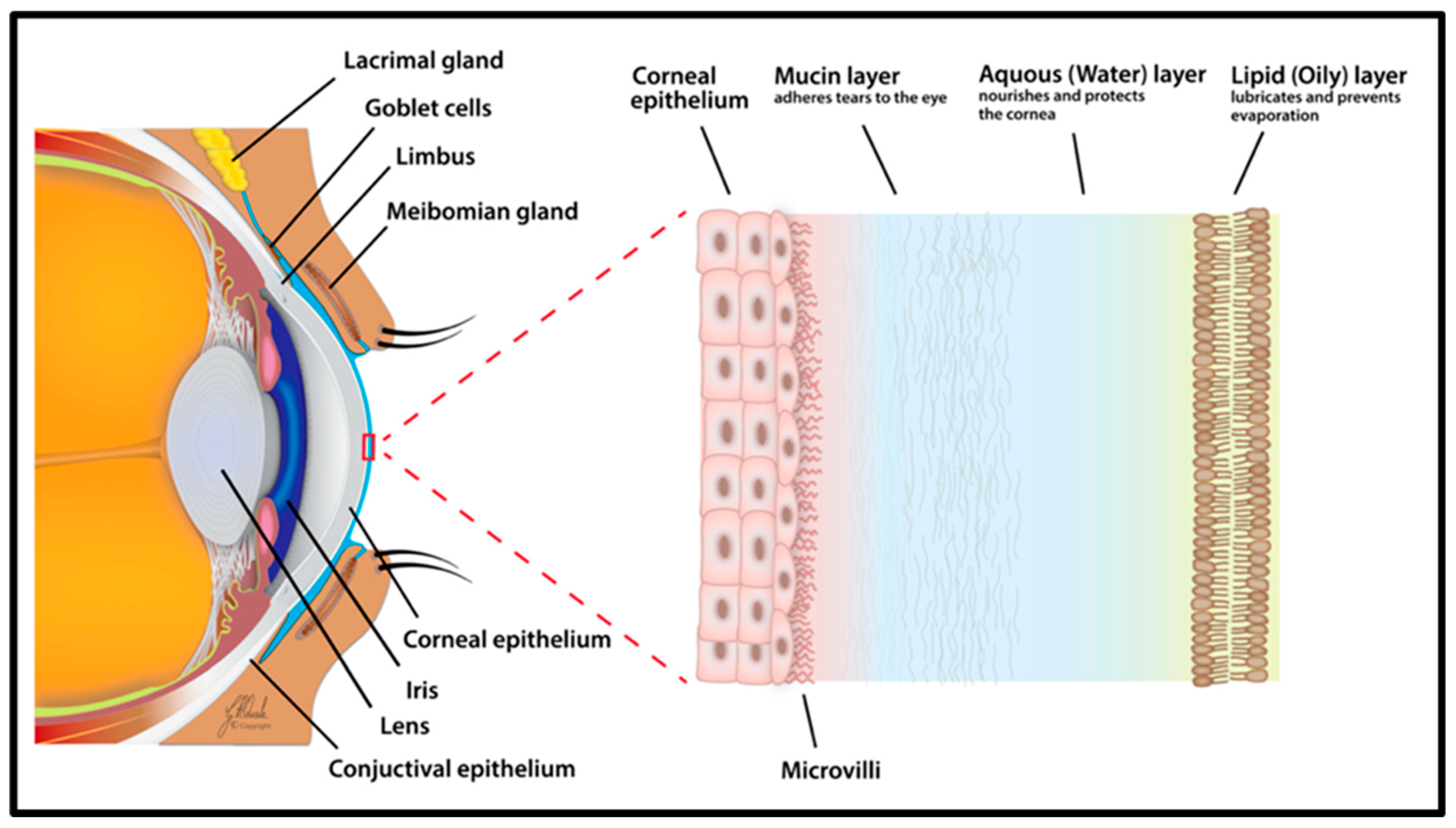
2. Anatomy of the Human Eye
3. Ocular Diseases
3.1. Age-Related Macular Degeneration (AMD)
3.2. Cataracts
3.3. Diabetic Retinopathy
3.4. Dry Eye
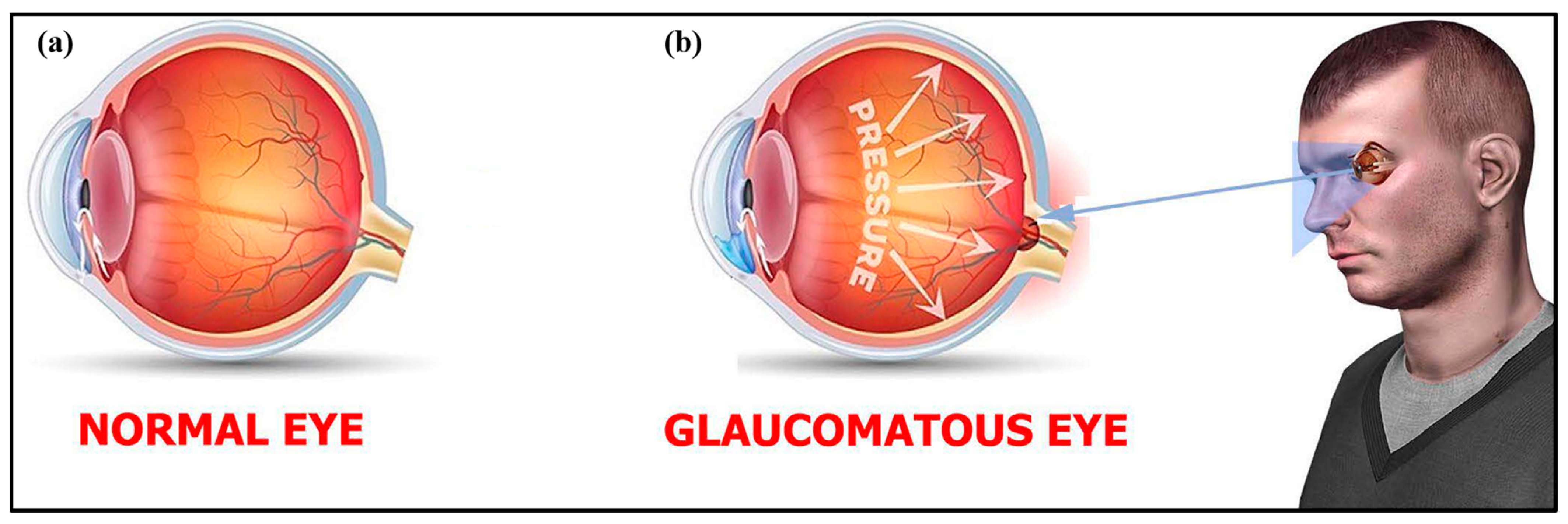
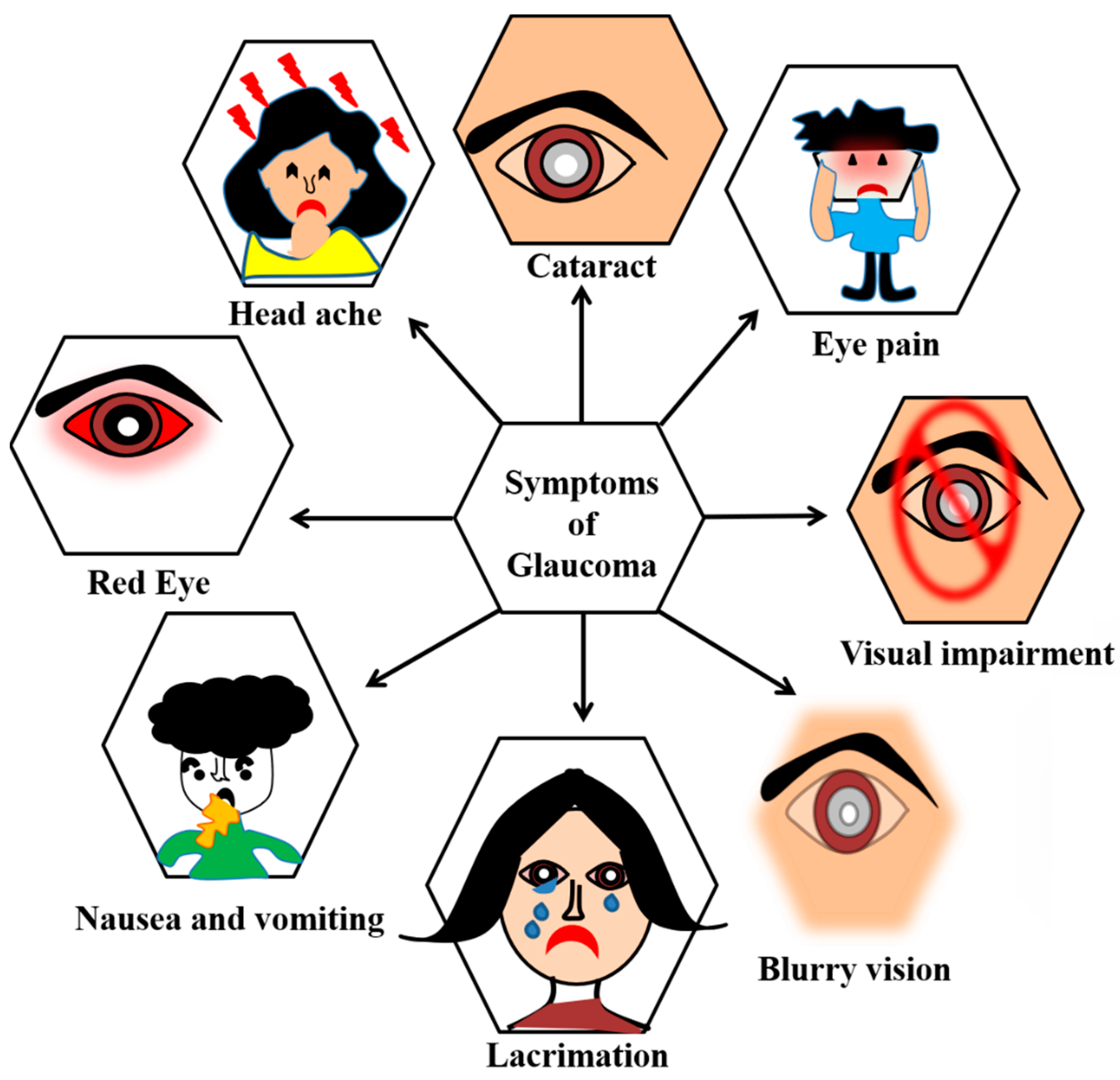
3.5. Glaucoma
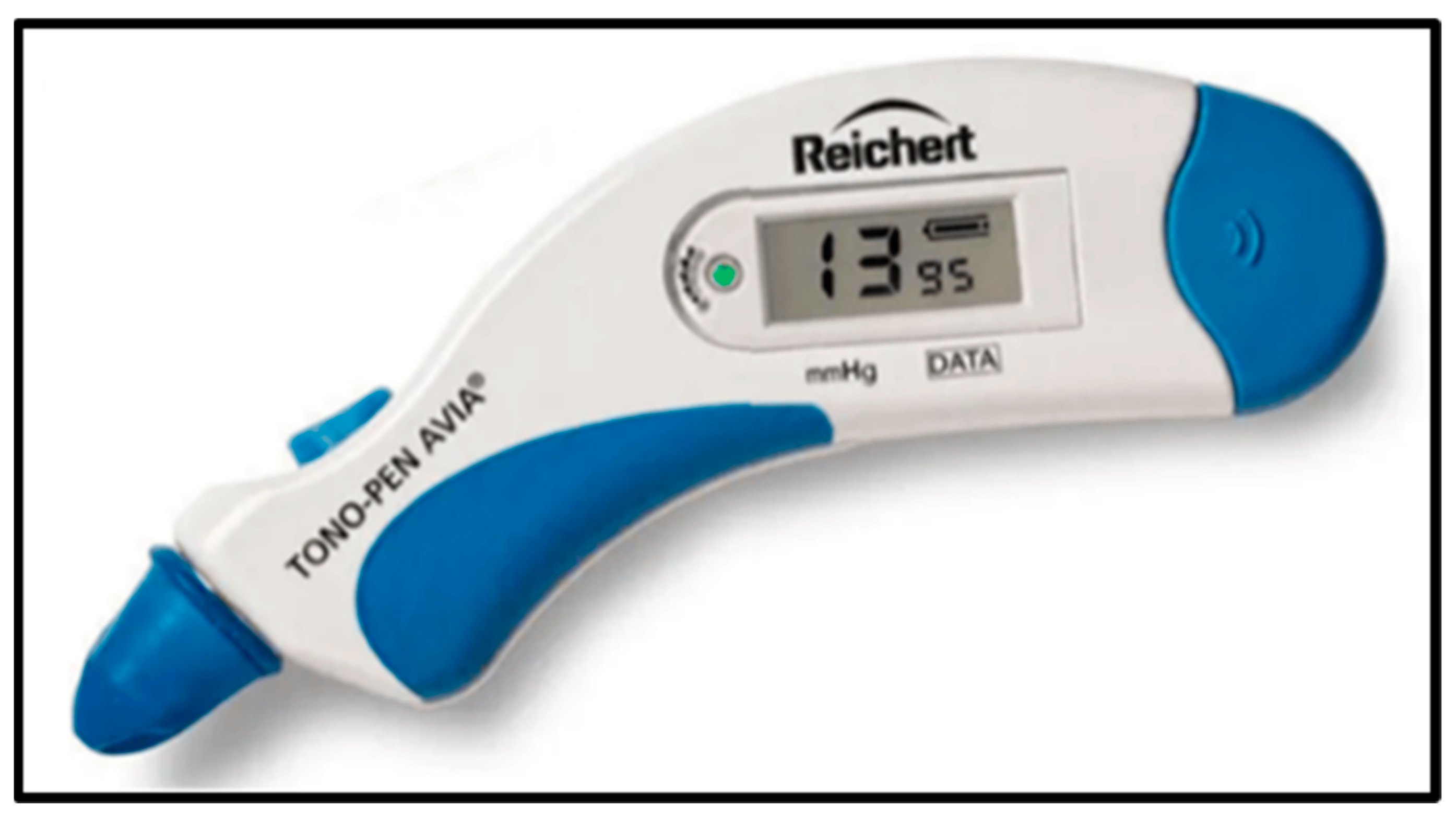
4. Intraocular Pressure (IOP)
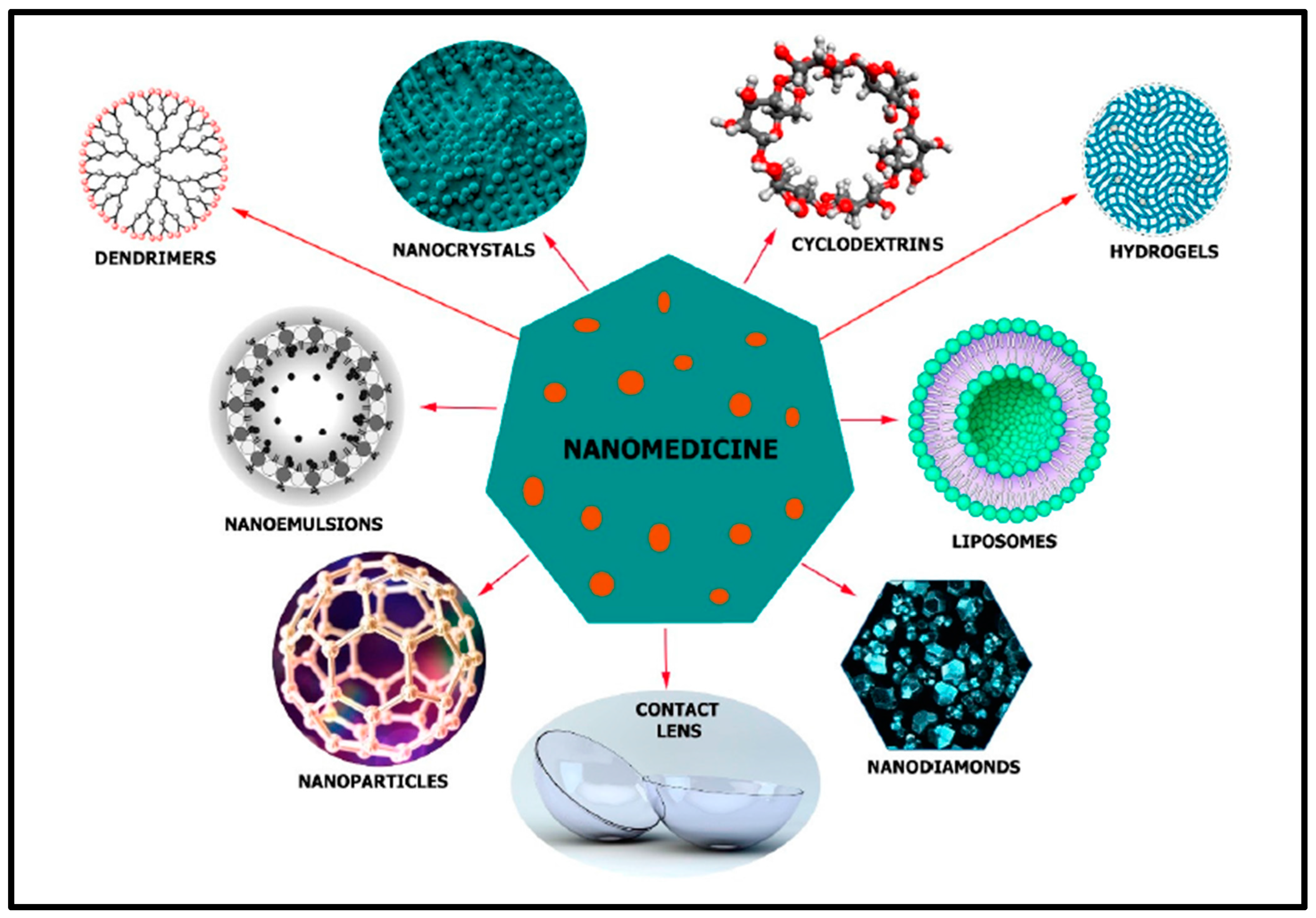
5. Nanotechnology
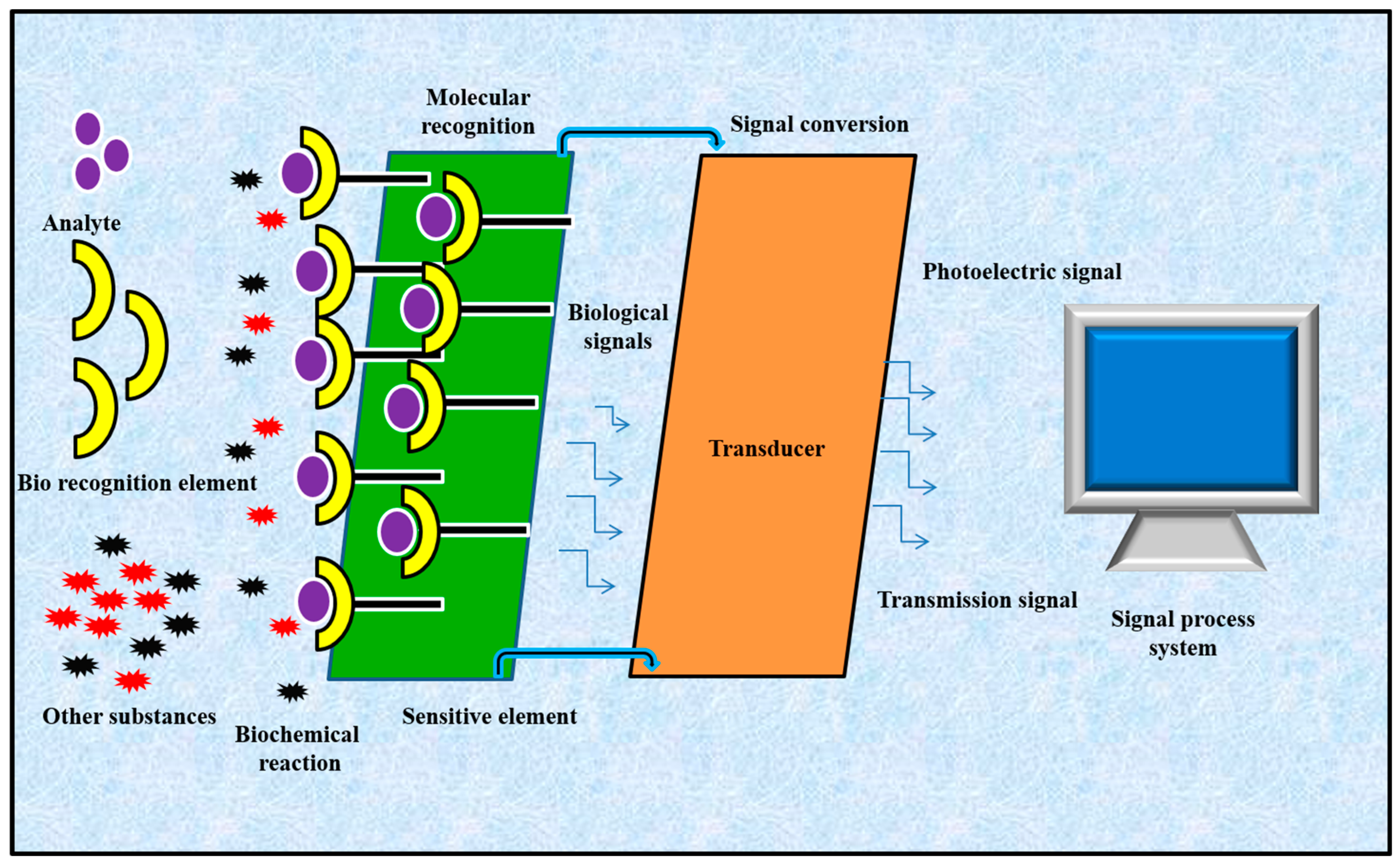
6. IOP Monitoring with Nano-Based Sensors
| Key Materials | Fabrication | Model Used for the Study | Ref. |
|---|---|---|---|
| Polydimethylsiloxane (PDMS) and polyurethane-based elastomers | Soft lithography | Enucleated porcine eye | [23] |
| Polydimethylsiloxane (PDMS), parylene C | Soft lithography | Porcine eye | [24] |
| Silver nanowires | Spin coating, photolithography, wet etching | In vitro: polydimethylsiloxane model eye; in vivo: rabbit eye | [55] |
| Graphene, polydimethylsiloxane (PDMS), parylene C | Chemical vapor deposition (CVD) | Silicone eyeball | [56] |
| Graphene nanowalls, polydimethylsiloxane (PDMS), silver wires | Plasma-assisted chemical vapor deposition (PACVD), spin coating | Porcine eye | [81] |
| Si-nanomembrane, Au-loaded coil, Cu-inductive coil | 3D printer, spinning, Photolithography | Rat eye | [82] |
| Monodisperse silica nanoparticles, gold nanobowl (AuNB) substrate | Hydrofluoric acid (HF) etching technique | Porcine eye | [83] |
| Platinum-stain gauge, polyimide, polydimethylsiloxane (PDMS) | MEMS (microelectromechanical system) process, spin coating | Silicone eyeball | [84] |
| Gold hollow nanowires | Spin coating | Rabbit eye | [85] |
| Polydimethylsiloxane (PDMS) | Casting process with the molds manufactured with a high-speed micromilling machine | Enucleated porcine eye | [86] |
| Polydimethylsiloxane (PDMS), polystyrene | Spin coating | In vitro: artificial silicone eye model; ex vivo: porcine eyeball | [87] |
| Polydimethylsiloxane (PDMS), polyethylene terephthalate (PET) | Chemical-assisted bonding and thermoforming technologies | Porcine eye | [88] |
| Silver conductive paint | Painting | Finite-element based model | [89] |
| Polydimethylsiloxane (PDMS) | Casting method | Enucleated porcine eyes | [90] |
| Nanostructured Si3N4-membrane, poly(methyl methacrylate) (PMMA) and polystyrene | Bottom-up fabrication process based on polymer phase separation, spin coating, E-Beam evaporation, Reactive Ion etching | Rabbit eye | [91] |
| Silicon dioxide (SiO2), silicon nitride (SiN), Al2O3 layer | Low-pressure chemical vapor-deposition (LPCVD), reactive ion etching, photolithography, electron beam lithography | Rabbit eye | [92] |
| Graphene | Chemical vapor deposition (CVD) | Porcine eye | [93] |
| Graphene–silver nanowires (AgNW), polyethylene terephthalate and polydimethylsiloxane (PDMS), PMMA | Spin coating, CVD method, etching, photolithography | Bovine eye | [94] |
| Silicon wafer, ferrite, PDMS | Etching, coating | Rabbit eye | [95] |
| Poly-2-hydroxyethyl methacrylate (poly HEMA), parylene C | Cast-molding method | Artificial anterior chamber | [96] |
| SU-8 photoresist, AZ9260 resin, Copper | Spin coating, sputtering, Lithography, etching | Silicone Rubber model eye | [97] |
| Liquid silicone elastomer, copper foil | Molding, etching | Porcine eye | [98] |
| PDMS, copper, gold, titanium, parylene C | Chemical vapor deposition (CVD), plasma etching, UV lithography | Canine eye | [99] |
| SiN, Titanium, polyimide, Titanium/Copper | Low-pressure chemical vapor deposition (LPCVD), sputtering, spin coating, photolithography | Rabbit eye | [100] |
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karayilan, M.; Clamen, L.; Becker, M.L. Polymeric Materials for Eye Surface and Intraocular Applications. Biomacromolecules 2021, 22, 223–261. [Google Scholar] [CrossRef]
- Rodrigues, F.S.C.; Campos, A.; Martins, J.; Ambrósio, A.F.; Campos, E.J. Emerging Trends in Nanomedicine for Improving Ocular Drug Delivery: Light-Responsive Nanoparticles, Mesoporous Silica Nanoparticles, and Contact Lenses. ACS Biomater. Sci. Eng. 2020, 6, 6587–6597. [Google Scholar] [CrossRef]
- World Report on Vision. Available online: https://www.WHO.Int/Publications/i/Item/9789241516570 (accessed on 8 October 2019).
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for Ocular Drug Delivery: Current Status and Translational Opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-Based Strategies for Treatment of Ocular Disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, N.; Bikkannavar, P.; Cordeiro, M.F.; Yetisen, A.K. Ophthalmic Sensing Technologies for Ocular Disease Diagnostics. Analyst 2021, 146, 6416–6444. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpa, R.Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625. [Google Scholar] [CrossRef]
- Abellan-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallon, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885. [Google Scholar] [CrossRef] [PubMed]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.Z. Corrigendum to “A wearable multisensing patch for continuous sweat monitoring”. Biosens. Bioelectron. 2017, 94, 730. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.; Kim, J.; Won, J.E.; Kang, W.; Park, Y.G.; Park, J.; Lee, J.-H.; Cheon, J.; Lee, H.H.; Park, J.-U. Smart, soft contact lens for wireless immunosensing of cortisol. Sci. Adv. 2020, 6, eabb2891. [Google Scholar] [CrossRef]
- Chen, L.Y.; Tee, B.C.K.; Chortos, A.L.; Schwartz, G.; Tse, V.; Lipomi, D.J.; Wong, H.S.P.; McConnell, M.V.; Bao, Z. Continuous wireless pressure monitoring and mapping with ultra-small passive sensors for health monitoring and critical care. Nat. Commun. 2014, 5, 5028. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47. [Google Scholar] [CrossRef]
- Zhao, Y.; You, S.S.; Zhang, A.; Lee, J.-H.; Huang, J.; Lieber, C.M. Scalable ultrasmall three-dimensional nanowire transistor probes for intracellular recording. Nat. Nanotechnol. 2019, 14, 783. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, S.; Rastogi, M. A review on biosensors and recent development. ACADEMICIA An. Int. Multidiscip. Res. J. 2021, 11, 613–620. [Google Scholar]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, X.; Li, X.; Yang, C.; Zhang, T.; Wu, Q.; Liu, D.; Lin, H.; Chen, W.; Hu, N.; et al. Wearable and Implantable Intraocular Pressure Biosensors: Recent Progress and Future Prospects. Adv. Sci. 2021, 8, 2002971. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Liu, Z.; Bai, W.; Liu, Y.; Yan, Y.; Xue, Y.; Kandela, I.; Pezhouh, M.; MacEwan, M.R.; Huang, Y.; et al. Bioresorbable Optical Sensor Systems for Monitoring of Intracranial Pressure and Temperature. Sci. Adv. 2019, 5, eaaw1899. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-Invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Shetti, N.P.; Mishra, A.; Basu, S.; Mascarenhas, R.J.; Kakarla, R.R.; Aminabhavi, T.M. Skin-patchable electrodes for biosensor applications: A review. ACS Biomater. Sci. Eng. 2020, 6, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G. Design challenges of implantable pressure monitoring system. Front. Neurosci. 2010, 4, 2. [Google Scholar] [CrossRef]
- Mukherjee, S.; Suleman, S.; Pilloton, R.; Narang, J.; Rani, K. State of the Art in Smart Portable, Wearable, Ingestible and Implantable Devices for Health Status Monitoring and Disease Management. Sensors 2022, 22, 4228. [Google Scholar] [CrossRef]
- Agaoglu, S.; Diep, P.; Martini, M.; Samudhyatha, K.T.; Baday, M.; Araci, I.E. Ultra-sensitive microfluidic wearable strain sensor for intraocular pressure monitoring. Lab. Chip 2018, 18, 3471. [Google Scholar] [CrossRef]
- Araci, I.E.; Su, B.; Quake, S.R.; Mandel, Y. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat. Med. 2014, 20, 1074. [Google Scholar] [CrossRef]
- Kim, Y.C.; Chiang, B.; Wu, X.; Prausnitz, M.R. Ocular Delivery of Macromolecules. J. Control. Release 2014, 190, 172–181. [Google Scholar] [CrossRef]
- Imanishi, S.; Tomita, Y.; Negishi, K.; Tsubota, K.; Kurihara, T. Molecular and Cellular Regulations in the Development of the Choroidal Circulation System. Int. J. Mol. Sci. 2023, 24, 5371. [Google Scholar] [CrossRef]
- Kronfeld, P.C. The gross anatomy and embryology of the eye. In Vegetative Physiology and Biochemistry; Academic Press: Cambridge, MA, USA, 1962; pp. 1–62. [Google Scholar]
- Yao, Q.; Hu, Y.; Yu, F.; Zhang, W.; Fu, Y. A Novel Application of Electrospun Silk Fibroin/Poly(l-lactic acid-co-ε-caprolactone) Scaffolds for Conjunctiva Reconstruction. RSC Adv. 2018, 8, 18372–18380. [Google Scholar] [CrossRef] [PubMed]
- Juliana, F.R.; Kesse, S.; Boakye-Yiadom, K.O.; Veroniaina, H.; Wang, H.; Sun, M. Promising approach in the treatment of glaucoma using nanotechnology and nanomedicine-based systems. Molecules 2019, 24, 3805. [Google Scholar] [CrossRef] [PubMed]
- Litzinger, T.C.; Rio-Tsonis, K. Del Eye Anatomy. In eLS; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Godfrey, K.B.; Eglen, S.J. Theoretical Models of Spontaneous Activity Generation and Propagation in the Developing Retina. Mol. Biosyst. 2009, 5, 1527. [Google Scholar] [CrossRef]
- Dartt, D.A.; Willcox, M.D. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp. Eye Res. 2013, 117, 1. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Seo, H.; Chung, W.G.; Joo, B.J.; Jang, J.; Park, J.U. Recent progress on wearable point-of-care devices for ocular systems. Lab. Chip 2021, 21, 1269–1286. [Google Scholar] [CrossRef]
- Gugleva, V.; Andonova, V. Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Ocular Drug Delivery Platforms. Pharmaceuticals 2023, 16, 474. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Elgstøen, K.B.; Rootwelt, H.; Shahdadfar, A.; Utheim, Ø.A.; Utheim, T.P. Tear metabolomics in dry eye disease: A review. Int. J. Mol. Sci. 2019, 20, 3755. [Google Scholar] [CrossRef]
- Cheng, K.-J.; Hsieh, C.-M.; Nepali, K.; Liou, J.-P. Ocular Disease Therapeutics: Design and Delivery of Drugs for Diseases of the Eye. J. Med. Chem. 2020, 63, 10533–10593. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Lai, J.-Y. Advancing the Stimuli Response of Polymer-Based Drug Delivery Systems for Ocular Disease Treatment. Polym. Chem. 2020, 11, 6988–7008. [Google Scholar] [CrossRef]
- Chatterjee, S.; Upadhyay, P.; Mishra, M.; Srividya, M.; Akshara, M.R.; Kamali, N.; Zaidi, Z.S.; Iqbal, S.F.; Misra, S.K. Advances in Chemistry and Composition of Soft Materials for drug Releasing Contact Lenses. RSC Adv. 2020, 10, 36751–36777. [Google Scholar] [CrossRef]
- Kim, T.W.; Kang, J.W.; Ahn, J.; Lee, E.K.; Cho, K.-C.; Han, B.N.R.; Hong, N.Y.; Park, J.; Kim, K.P. Proteomic Analysis of the Aqueous Humor in Age-Related Macular Degeneration (AMD) Patients. J. Proteome Res. 2012, 11, 4034–4043. [Google Scholar] [CrossRef]
- Kang, G.-Y.; Bang, J.Y.; Choi, A.J.; Yoon, J.; Lee, W.-C.; Choi, S.; Yoon, S.; Kim, H.C.; Baek, J.-H.; Park, H.S.; et al. Exosomal Proteins in the Aqueous Humor as Novel Biomarkers in Patients with Neovascular Age-Related Macular Degeneration. J. Proteome Res. 2014, 13, 581–595. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Bari, K.J.; Sharma, S. A Perspective on Biophysical Studies of Crystallin Aggregation and Implications for Cataract Formation. J. Phys. Chem. B 2020, 124, 11041–11054. [Google Scholar] [CrossRef]
- Loukovaara, S.; Nurkkala, H.; Tamene, F.; Gucciardo, E.; Liu, X.; Repo, P.; Lehti, K.; Varjosalo, M. Quantitative Proteomics Analysis of Vitreous Humor from Diabetic Retinopathy Patients. J. Proteome Res. 2015, 14, 5131–5143. [Google Scholar] [CrossRef]
- Kim, D.; Choi, S.W.; Cho, J.; Been, J.-H.; Choi, K.; Jiang, W.; Han, J.; Oh, J.; Park, C.; Choi, S.; et al. Discovery of Novel Small-Molecule Antiangiogenesis Agents to Treat Diabetic Retinopathy. J. Med. Chem. 2021, 64, 5535–5550. [Google Scholar] [CrossRef] [PubMed]
- Hancox, Z.; Keshel, S.H.; Yousaf, S.; Saeinasab, M.; Shahbazi, M.-A.; Sefat, F. The Progress in Corneal Translational Medicine. Biomater. Sci. 2020, 8, 6469–6504. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Maria, D.N.; Mishra, S.R.; Guragain, D.; Wang, X.; Jablonski, M.M. Once daily pregabalin eye drops for management of glaucoma. ACS Nano. 2019, 13, 13728–13744. [Google Scholar] [CrossRef] [PubMed]
- Schnichels, S.; Hurst, J.; de Vries, J.W.; Ullah, S.; Frößl, K.; Gruszka, A.; Löscher, M.; Bartz-Schmidt, K.U.; Spitzer, M.S.; Herrmann, A. Improved treatment options for glaucoma with brimonidine-loaded lipid DNA nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 9445–9456. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M. Particulate systems in the enhancement of the antiglaucomatous drug pharmacodynamics: A meta-analysis study. ACS Omega 2019, 4, 21909–21913. [Google Scholar] [CrossRef]
- Killer, H.E.; Pircher, A. Normal Tension Glaucoma: Review of Current Understanding and Mechanisms of the Pathogenesis. Eye 2018, 32, 924–930. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Garg, A.; Ahmad, M.Z.; Alqahtani, A.A.; Walbi, I.A.; Ahmad, J. Recent Progress in Chitosan-Based Nanomedicine for Its Ocular Application in Glaucoma. Pharmaceutics 2023, 15, 681. [Google Scholar] [CrossRef]
- Kim, K.E.; Oh, S.; Baek, S.U.; Ahn, S.J.; Park, K.H.; Jeoung, J.W. Ocular Perfusion Pressure and the Risk of Open-Angle Glaucoma: Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 10056. [Google Scholar] [CrossRef]
- Brusini, P.; Salvetat, M.L.; Zeppieri, M. How to measure intraocular pressure: An updated review of various tonometers. J. Clin. Med. 2021, 10, 3860. [Google Scholar] [CrossRef]
- Xu, J.; Li, R.; Xu, H.; Yang, Y.; Zhang, S.; Ren, T.L. Recent progress of continuous intraocular pressure monitoring. Nano Select. 2022, 3, 1–9. [Google Scholar] [CrossRef]
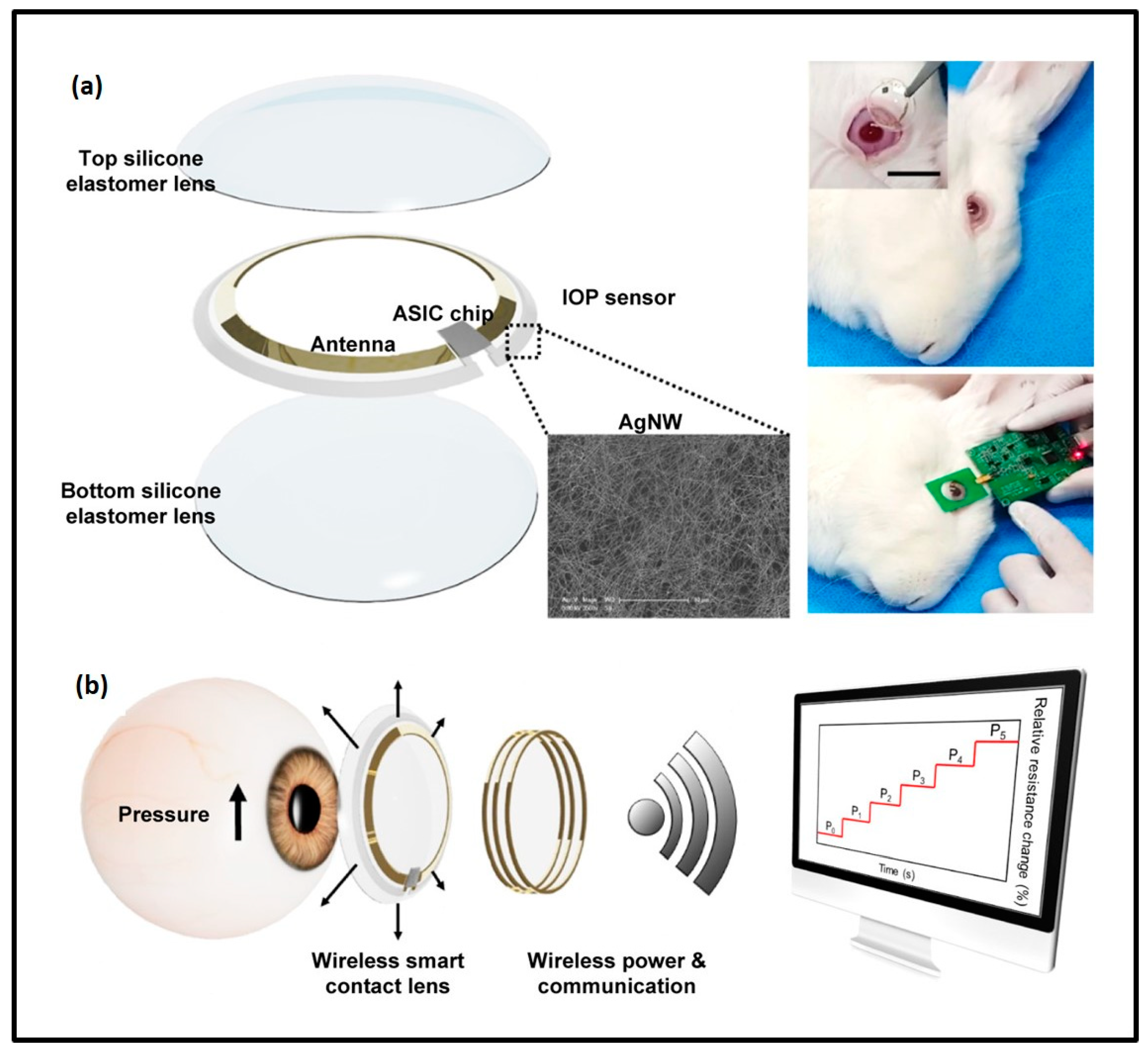
- Kim, J.; Park, J.; Park, Y.G.; Cha, E.; Ku, M.; An, H.S.; Lee, K.P.; Huh, M.I.; Kim, J.; Kim, T.S.; et al. A soft and transparent contact lens for the wireless quantitative monitoring of intraocular pressure. Nat. Biomed. Eng. 2021, 5, 772–782. [Google Scholar] [CrossRef]
- Kim, T.Y.; Shin, S.; Choi, H.; Jeong, S.H.; Myung, D.; Hahn, S.K. Smart Contact Lenses with a Transparent Silver Nanowire Strain Sensor for Continuous Intraocular Pressure Monitoring. ACS Appl. Bio Mater. 2021, 4, 4532–4541. [Google Scholar] [CrossRef]
- Xu, J.; Cui, T.; Hirtz, T.; Qiao, Y.; Li, X.; Zhong, F.; Han, X.; Yang, Y.; Zhang, S.; Ren, T.L. Highly transparent and sensitive graphene sensors for continuous and non-invasive intraocular pressure monitoring. ACS Appl. Mater. Interfaces 2020, 12, 18375–18384. [Google Scholar] [CrossRef] [PubMed]
- Sakthi Devi, R.; Girigoswami, A.; Siddharth, M.; Girigoswami, K. Applications of gold and silver nanoparticles in theranostics. Appl. Biochem. Biotechnol. 2022, 194, 4187–4219. [Google Scholar] [CrossRef] [PubMed]
- Al-Halafi, A.M. Nanocarriers of Nanotechnology in Retinal Diseases. Saudi J. Ophthalmol. 2014, 28, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Andreescu, S. Nanotechnology-Based Approaches for Food Sensing and Packaging Applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Ong, Y.; Schirhagl, R.; Tahir, M.A.; Khan, W.S.; Bajwa, S.Z. Nanosensors for Diagnosis with Optical, Electric and Mechanical Transducers. RSC Adv. 2019, 9, 6793–6803. [Google Scholar] [CrossRef]
- Deepachitra, R.; Chamundeeswari, M.; Krithiga, G.; Prabu, P.; Devi, M.P.; Sastry, T.P. Osteo mineralization of fibrin-decorated graphene oxide. Carbon 2013, 56, 64–76. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Lee, C.-H.; Chen, S.-T.; Lai, J.-Y.; Wu, K.C.-W. Gelatin-Functionalized Mesoporous Silica Nanoparticles with Sustained Release Properties for Intracameral Pharmacotherapy of Glaucoma. J. Mater. Chem. B 2017, 5, 7008–7013. [Google Scholar] [CrossRef]
- Vedakumari, S.W.; Jancy, S.J.V.; Prabakaran, L.; Raja Pravin, Y.; Senthil, R. A Review on Background, Process and Application of Electrospun Nanofibers for Tissue Regeneration. Proc. Inst. Mech. Eng. H 2023, 237, 529–541. [Google Scholar] [CrossRef]
- Hu, X.; Tan, H.; Chen, P.; Wang, X.; Pang, J. Polymer Micelles Laden Hydrogel Contact Lenses for Ophthalmic Drug Delivery. J. Nanosci. Nanotechnol. 2016, 16, 5480–5488. [Google Scholar] [CrossRef]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.-M.; Majoral, J.-P. Dendrimers in Combination with Natural Products and Analogues as Anti-Cancer Agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef]
- Soliman, G.M.; Sharma, A.; Maysinger, D.; Kakkar, A. Dendrimers and Miktoarm Polymers Based Multivalent Nanocarriers for Efficient and Targeted Drug Delivery. Chem. Commun. 2011, 47, 9572. [Google Scholar] [CrossRef]
- Satija, J.; Sai, V.V.R.; Mukherji, S. Dendrimers in Biosensors: Concept and Applications. J. Mater. Chem. 2011, 21, 14367. [Google Scholar] [CrossRef]
- Liu, Z.; Kompella, U.B.; Chauhan, A. Gold Nanoparticle Synthesis in Contact Lenses for Drug-Less Ocular Cystinosis Treatment. Eur. J. Pharm. Biopharm. 2021, 165, 271–278. [Google Scholar] [CrossRef]
- Occhiutto, M.L.; Maranhão, R.C.; Costa, V.P.; Konstas, A.G. Nanotechnology for Medical and Surgical Glaucoma Therapy—A Review. Adv. Ther. 2020, 37, 155–199. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Alqattan, B.; Jackson, T.; Pikramenou, Z.; Sun, X.W.; Wang, K.; Butt, H. Cost-Efficient Printing of Graphene Nanostructures on Smart Contact Lenses. ACS Appl. Mater. Interfaces 2020, 12, 10820–10828. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-N.; Ko, S.A.; Park, C.G.; Lee, S.H.; Huh, B.K.; Park, Y.H.; Kim, Y.K.; Ha, A.; Park, K.H.; Choy, Y. Bin Amino-Functionalized Mesoporous Silica Particles for Ocular Delivery of Brimonidine. Mol. Pharm. 2018, 15, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, A.; Makhmalzadeh, B.S.; Feghhi, M.; SoleimaniBiatiani, E. Cationic Liposomes as Promising Vehicles for Timolol/Brimonidine Combination Ocular Delivery in Glaucoma: Formulation Development and in Vitro/in Vivo Evaluation. Drug. Deliv. Transl. Res. 2023, 13, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Gangadeep; Garg, T.; Malik, B.; Rath, G.; Goyal, A.K. Development and Characterization of Nano-Fiber Patch for the Treatment of Glaucoma. Eur. J. Pharm. Sci. 2014, 53, 10–16. [Google Scholar]
- Xu, J.; Ge, Y.; Bu, R.; Zhang, A.; Feng, S.; Wang, J.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; et al. Co-Delivery of Latanoprost and Timolol from Micelles-Laden Contact Lenses for the Treatment of Glaucoma. J. Control. Release 2019, 305, 18–28. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Huang, D.; Norat, P.; Grannonico, M.; Cooper, R.C.; Gui, Q.; Nam Chow, W.; Liu, X.; Yang, H. Nano-in-Nano Dendrimer Gel Particles for Efficient Topical Delivery of Antiglaucoma Drugs into the Eye. Chem. Eng. J. 2021, 425, 130498. [Google Scholar] [CrossRef]
- Sonntag, T.; Froemel, F.; Stamer, W.D.; Ohlmann, A.; Fuchshofer, R.; Breunig, M. Distribution of gold nanoparticles in the anterior chamber of the eye after intracameral injection for glaucoma therapy. Pharmaceutics 2021, 13, 901. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Brobeck, L. Poly(Amidoamine) Dendrimers as Ophthalmic Vehicles for Ocular Delivery of Pilocarpine Nitrate and Tropicamide. J. Control. Release 2005, 102, 23–38. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Patil, R.J.; Desai, A.R.; Shukla, M.R.; Vaidya, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Shah, D.O. Effect of Gold Nanoparticles on Timolol Uptake and Its Release Kinetics from Contact Lenses: In Vitro and in Vivo Evaluation. Acta Biomater. 2019, 86, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, J.; Wu, L.; Wang, B.; Wang, Z.; Xu, Q.; Xin, H. Ophthalmic Delivery of Brinzolamide by Liquid Crystalline Nanoparticles: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2013, 14, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
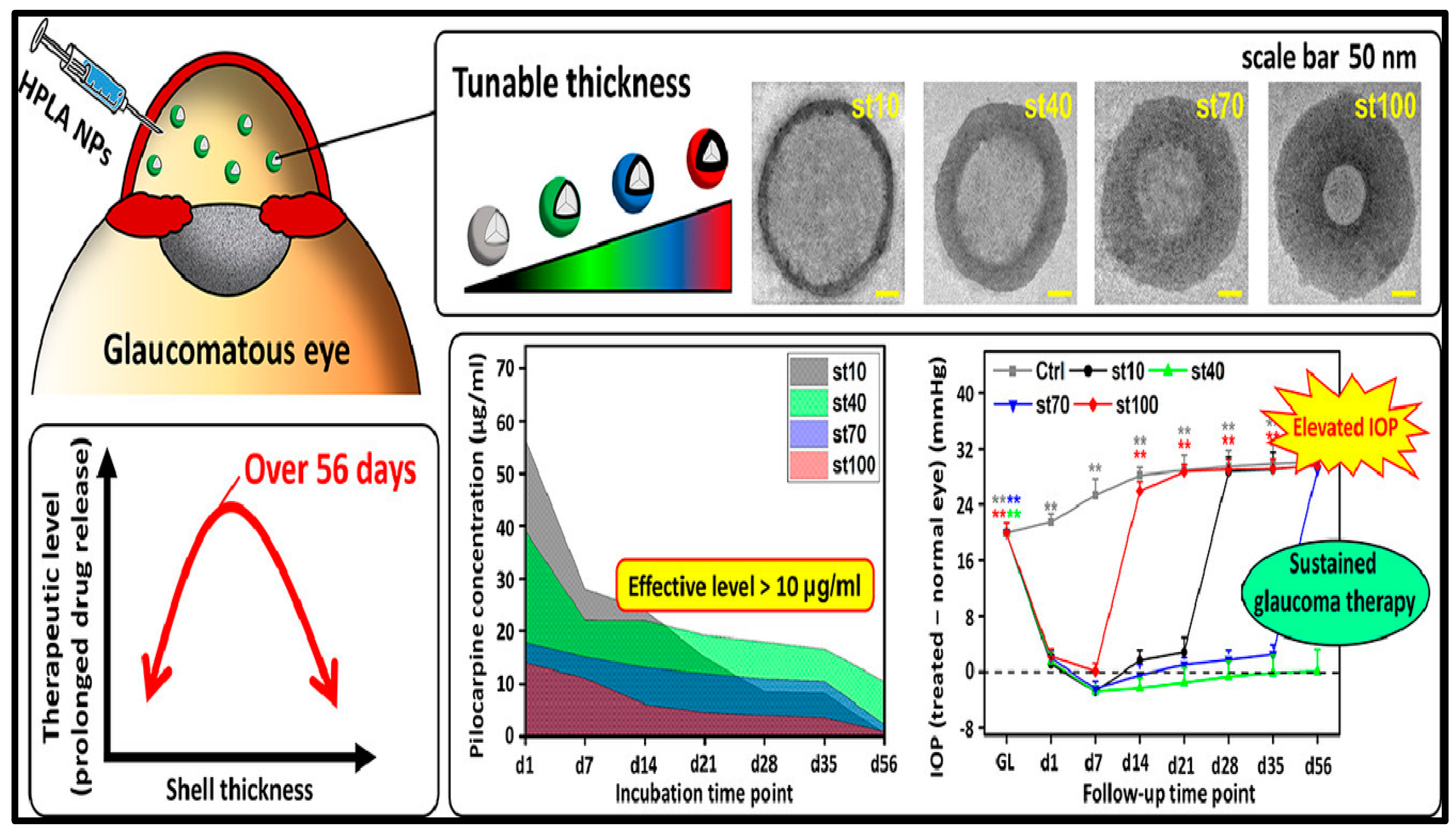
- Nguyen, D.D.; Luo, L.-J.; Lai, J.-Y. Effects of Shell Thickness of Hollow Poly(Lactic Acid) Nanoparticles on Sustained Drug Delivery for Pharmacological Treatment of Glaucoma. Acta Biomater. 2020, 111, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, G.; Pei, W.; Wei, C.; Wu, X.; Dou, Z.; Li, Y.; Wang, Y.; Chen, H. Application of graphene nanowalls in an intraocular pressure sensor. J. Mater. Chem. B 2020, 8, 8794–8802. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Ku, M.; Cha, E.; Ju, S.; Park, W.Y.; Kim, K.H.; Kim, D.W.; Berggren, P.O.; Park, J.U. Intraocular pressure monitoring following islet transplantation to the anterior chamber of the eye. Nano Lett. 2019, 20, 1517–1525. [Google Scholar] [CrossRef]
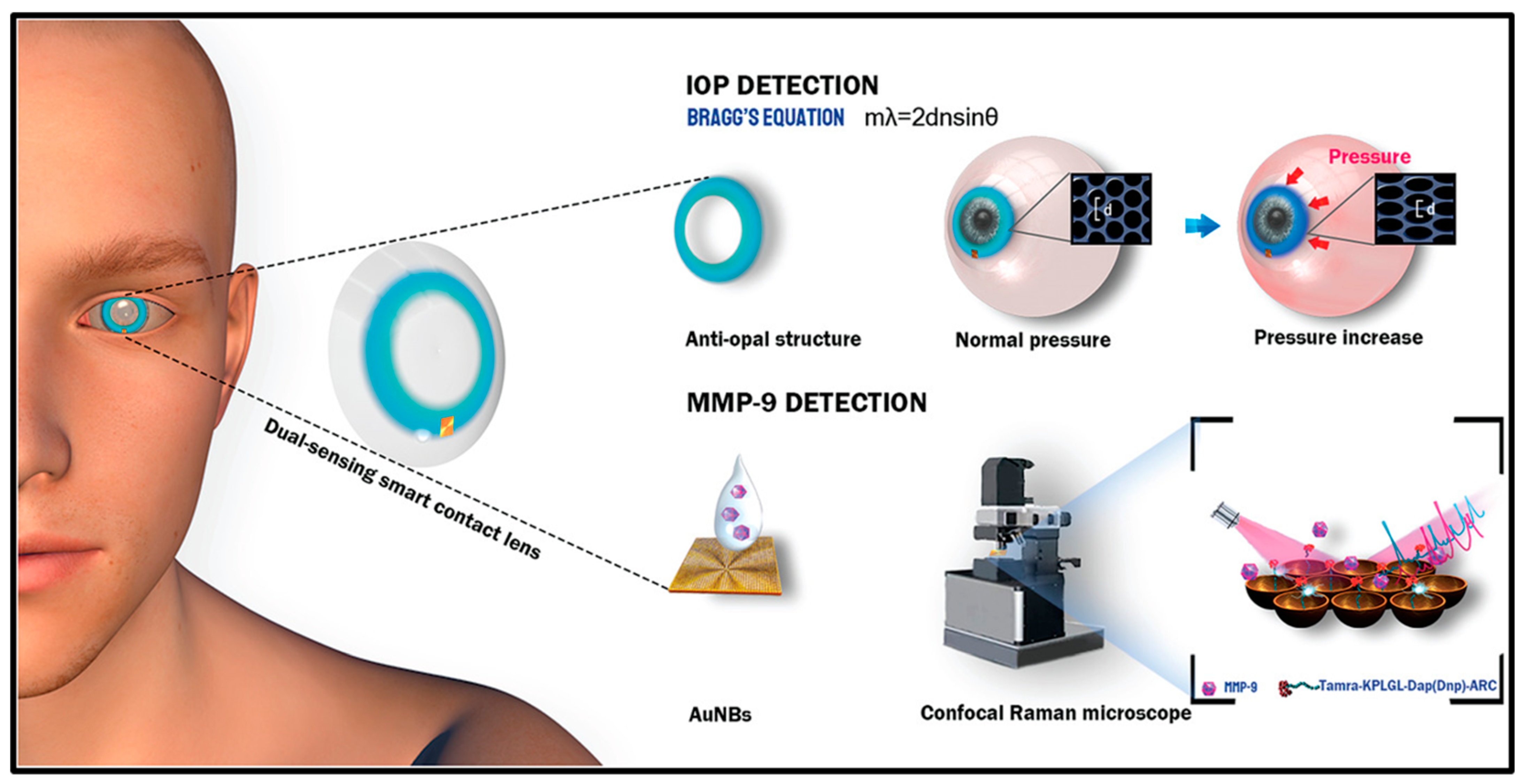
- Ye, Y.; Ge, Y.; Zhang, Q.; Yuan, M.; Cai, Y.; Li, K.; Li, Y.; Xie, R.; Xu, C.; Jiang, D.; et al. Smart contact lens with dual-sensing platform for monitoring intraocular pressure and matrix metalloproteinase-9. Adv. Sci. 2022, 9, 2104738. [Google Scholar] [CrossRef]
- Dou, Z.; Tang, J.; Liu, Z.; Su, Q.; Wang, Y.; Li, Y.; Yuan, M.; Wu, H.; Wang, Y.; Pei, W.; et al. Wearable contact lens sensor for non-invasive continuous monitoring of intraocular pressure. Micromachines 2021, 12, 108. [Google Scholar] [CrossRef]
- Kim, T.Y.; Mok, J.W.; Hong, S.H.; Jeong, S.H.; Choi, H.; Shin, S.; Joo, C.K.; Hahn, S.K. Wireless theranostic smart contact lens for monitoring and control of intraocular pressure in glaucoma. Nat. Commun. 2022, 13, 6801. [Google Scholar] [CrossRef]
- Campigotto, A.; Lai, Y. A novel non-invasive wearable sensor for intraocular pressure measurement. Med. Devices Sens. 2020, 3, e10086. [Google Scholar] [CrossRef]
- Maeng, B.; Chang, H.K.; Park, J. Photonic crystal-based smart contact lens for continuous intraocular pressure monitoring. Lab Chip 2020, 20, 1740–1750. [Google Scholar] [CrossRef]
- An, H.; Chen, L.; Liu, X.; Zhao, B.; Zhang, H.; Wu, Z. Microfluidic contact lenses for unpowered, continuous and non-invasive intraocular pressure monitoring. Sens. Actuators A Phys. 2019, 295, 177–187. [Google Scholar] [CrossRef]
- Ekinci, G.; Calikoglu, A.; Solak, S.N.; Yalcinkaya, A.D.; Dundar, G.; Torun, H. Split-ring resonator-based sensors on flexible substrates for glaucoma monitoring. Sens. Actuators A Phys. 2017, 268, 32–37. [Google Scholar] [CrossRef]
- Campigotto, A.; Leahy, S.; Zhao, G.; Campbell, R.J.; Lai, Y. Non-invasive Intraocular pressure monitoring with contact lens. Br. J. Ophthalmol. 2020, 104, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, V.; Siddique, R.H.; Lee, J.O.; Kumar, S.; Ndjamen, B.; Du, J.; Hong, N.; Sretavan, D.; Choo, H. Multifunctional biophotonic nanostructures inspired by the longtail glasswing butterfly for medical devices. Nat. Nanotechnol. 2018, 13, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Park, H.; Du, J.; Balakrishna, A.; Chen, O.; Sretavan, D.; Choo, H. A microscale optical implant for continuous in vivo monitoring of intraocular pressure. Microsyst. Nanoeng. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Man, T.; Huang, D.; Li, X.; Zhu, H.; Li, Z. High resolution non-invasive intraocular pressure monitoring by use of graphene woven fabrics on contact lens. Microsyst. Nanoeng. 2019, 5, 39. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.S.; Kim, K.; Ji, S.; Kim, Y.T.; Park, J.; Na, K.; Bae, K.H.; Kyun Kim, H.; et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8, 14997. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, M.J.; Park, K.H.; Jeoung, J.W.; Kim, S.H.; Jang, C.I.; Lee, S.H.; Kim, J.H.; Lee, S.; Kang, J.Y. Preliminary study on implantable inductive-type sensor for continuous monitoring of intraocular pressure. Clin. Exp. Ophthalmol. 2015, 43, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.C.; Huang, Y.C.; Yeh, G.T. A capacitor-based sensor and a contact lens sensing system for intraocular pressure monitoring. J. Micromech. Microeng. 2015, 26, 015001. [Google Scholar] [CrossRef]
- Chen, G.Z.; Chan, I.S.; Leung, L.K.; Lam, D.C. Soft wearable contact lens sensor for continuous intraocular pressure monitoring. Med. Eng. Phys. 2014, 36, 1134–1139. [Google Scholar] [CrossRef]
- Chen, G.Z.; Chan, I.S.; Lam, D.C. Capacitive contact lens sensor for continuous non-invasive intraocular pressure monitoring. Sens. Actuators A: Phys. 2013, 203, 112–118. [Google Scholar] [CrossRef]
- Kouhani, M.H.; Wu, J.; Tavakoli, A.; Weber, A.J.; Li, W. Wireless, passive strain sensor in a doughnut-shaped contact lens for continuous non-invasive self-monitoring of intraocular pressure. Lab Chip 2020, 20, 332–342. [Google Scholar] [CrossRef]
- Jang, C.I.; Shin, K.S.; Kim, M.J.; Yun, K.S.; Park, K.H.; Kang, J.Y.; Lee, S.H. Effects of inner materials on the sensitivity and phase depth of wireless inductive pressure sensors for monitoring intraocular pressure. Appl. Phys. Lett. 2016, 108, 103701. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Elder, M.J. Anatomy and physiology of eyelash follicles: Relevance to lash ablation procedures. Ophthalmic Plast. Reconstr. Surg. 1997, 13, 21–25. [Google Scholar] [CrossRef]
- McCarey, B.E.; Kapik, B.M.; Kane, F.E. Low incidence of iris pigmentation and eyelash changes in 2 randomized clinical trials with unoprostone isopropyl 0.15%. Ophthalmology 2004, 111, 1480–1488. [Google Scholar] [CrossRef]
- Na, J.I.; Kwon, O.S.; Kim, B.J.; Park, W.S.; Oh, J.K.; Kim, K.H.; Cho, K.H.; Eun, H.C. Ethnic characteristics of eyelashes: A comparative analysis in Asian and Caucasian females. Br. J. Dermatol. 2006, 155, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Pitchon, E.M.; Bertsch, A.; Renaud, P.; Mermoud, A. Wireless contact lens sensor for intraocular pressure monitoring: Assessment on enucleated pig eyes. Acta Ophthalmol. 2009, 87, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Opdahl, A.; Kim, S.H.; Koffas, T.S.; Marmo, C.; Somorjai, G.A. Surface mechanical properties of pHEMA contact lenses: Viscoelastic and adhesive property changes on exposure to controlled humidity. J. Biomed. Mater. Res. 2003, 67, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Guozhen, C. Development of Contact Lens Sensor and Wireless Sensing System for Intraocular Pressure Monitoring. Ph.D. Thesis, Hong Kong University of Science and Technology, Hong Kong, China.
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 2011, 26, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Lähdesmäki, I.; Parviz, B.A. A contact lens with an integrated lactate sensor. Sens. Actuators B Chem. 2012, 162, 128–134. [Google Scholar] [CrossRef]
- Vásquez Quintero, A.; Verplancke, R.; De Smet, H.; Vanfleteren, J. Stretchable electronic platform for soft and smart contact lens applications. Adv. Mater. Technol. 2017, 2, 1700073. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raveendran, R.; Prabakaran, L.; Senthil, R.; Yesudhason, B.V.; Dharmalingam, S.; Sathyaraj, W.V.; Atchudan, R. Current Innovations in Intraocular Pressure Monitoring Biosensors for Diagnosis and Treatment of Glaucoma—Novel Strategies and Future Perspectives. Biosensors 2023, 13, 663. https://doi.org/10.3390/bios13060663
Raveendran R, Prabakaran L, Senthil R, Yesudhason BV, Dharmalingam S, Sathyaraj WV, Atchudan R. Current Innovations in Intraocular Pressure Monitoring Biosensors for Diagnosis and Treatment of Glaucoma—Novel Strategies and Future Perspectives. Biosensors. 2023; 13(6):663. https://doi.org/10.3390/bios13060663
Chicago/Turabian StyleRaveendran, Rubiya, Lokesh Prabakaran, Rethinam Senthil, Beryl Vedha Yesudhason, Sankari Dharmalingam, Weslen Vedakumari Sathyaraj, and Raji Atchudan. 2023. "Current Innovations in Intraocular Pressure Monitoring Biosensors for Diagnosis and Treatment of Glaucoma—Novel Strategies and Future Perspectives" Biosensors 13, no. 6: 663. https://doi.org/10.3390/bios13060663
APA StyleRaveendran, R., Prabakaran, L., Senthil, R., Yesudhason, B. V., Dharmalingam, S., Sathyaraj, W. V., & Atchudan, R. (2023). Current Innovations in Intraocular Pressure Monitoring Biosensors for Diagnosis and Treatment of Glaucoma—Novel Strategies and Future Perspectives. Biosensors, 13(6), 663. https://doi.org/10.3390/bios13060663