Electrochemical Characterization Using Biosensors with the Coagulant Moringa oleifera Seed Lectin (cMoL)
Abstract
1. Introduction
2. Materials and Methods
2.1. Lectin Isolation
2.2. Electrochemical Synthesis of MOF [Cu3(BTC)2(H2O)3]n
2.3. Preparation of the Working Electrode
2.4. Potentiometric Measurements
- (1)
- Platinum electrode with MOF (Pt/MOF);
- (2)
- Platinum electrode with Pt/MOF/cMoL with 0.15 M NaCl in the electrolytic medium;
- (3)
- Pt/MOF/cMoL with 0.15 M NaCl and different concentrations of galactose: 10, 15 and 20 mM.
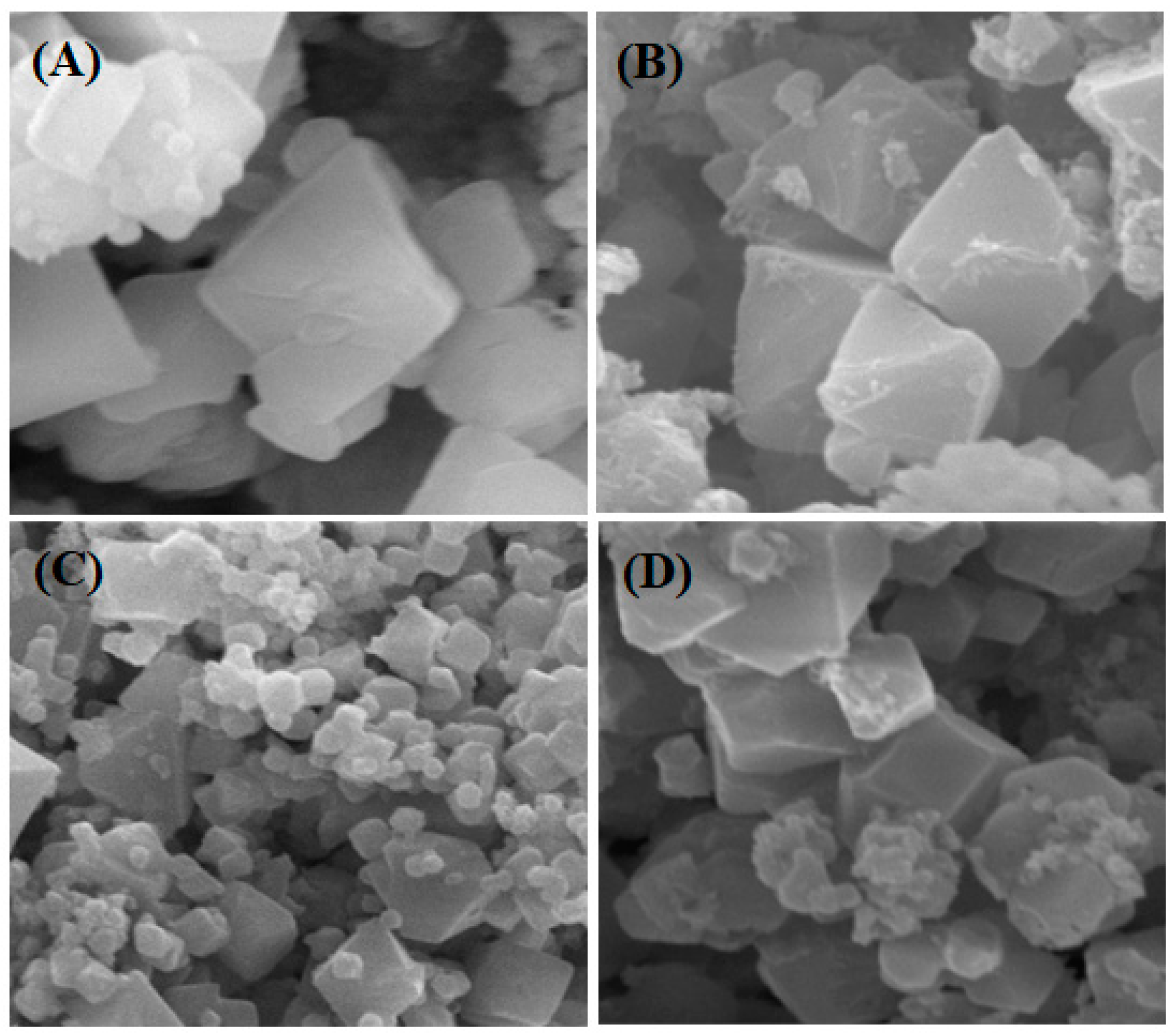
2.5. Scanning Electron Microscopy
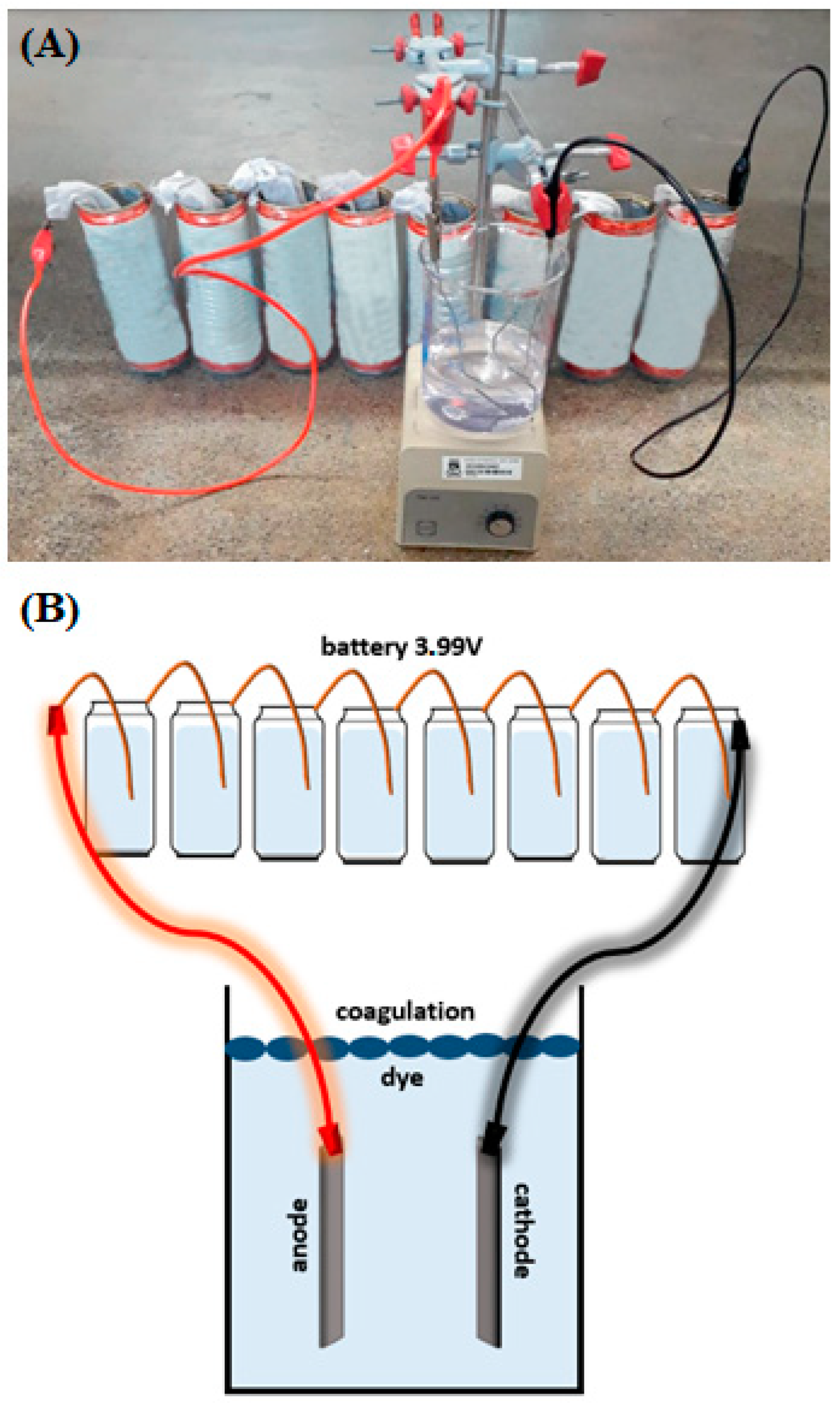
2.6. Electrocoagulation
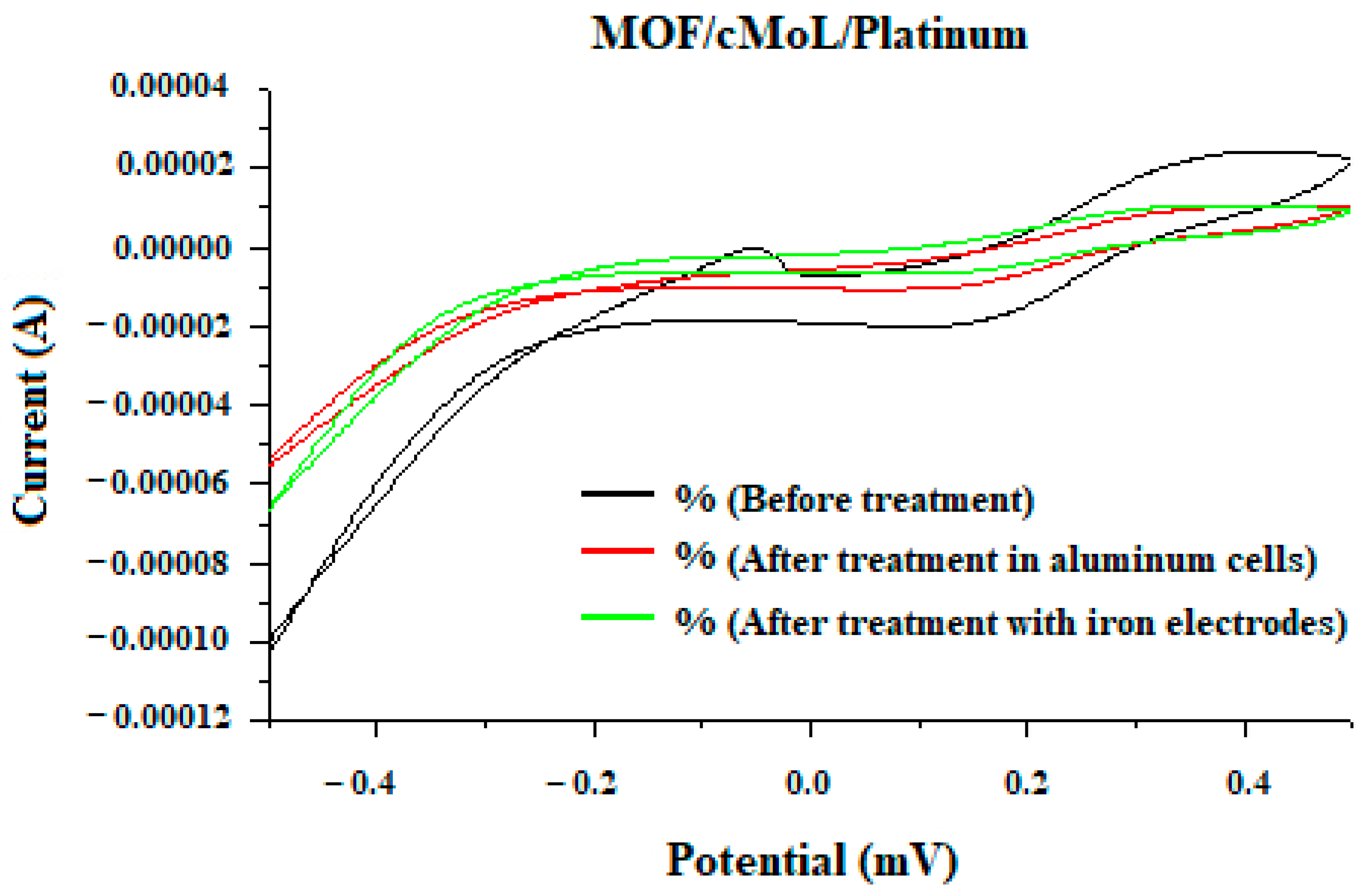
2.7. Cyclic Voltammetry
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chettri, D.; Boro, M.; Sarkar, L.; Verma, A.K. Lectins: Biological significance to biotechnological application. Carbohydr. Res. 2021, 506, 108367. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.C.B.B.; Silva, P.M.; Lima, V.L.; Pontual, E.V.; Paiva, P.M.G.; Napoleão, T.H.; Correia, M.T.S. Lectins, Interconnecting Proteins with Biotechnological/Pharmacological and Therapeutic Applications. Evid. Based Complement. Alternat. Med. 2017, 2017, 1594074. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Bachmann, R.T. A Contemporary Review on Plant-Based Coagulants for Applications in Water Treatment. J. Ind. Eng. Chem. 2019, 72, 281–297. [Google Scholar] [CrossRef]
- Naithani, S.; Komath, S.S.; Nonomura, A.; Govindjee, G. Plant Lectins and Their Many Roles: Carbohydrate-Binding and Beyond. J. Plant. Physiol. 2021, 266, 153531. [Google Scholar] [CrossRef] [PubMed]
- Konozy, E.H.E.; Osman, M.E.M. Plant Lectin: A Promising Future Anti-Tumor Drug. Biochimie 2022, 202, 136–145. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, L.; Zhao, M.; Yang, X. The Hypoglycemic and Hypolipemic Potentials of Moringa oleifera leaf Polysaccharide and Polysaccharide-flavonoid Complex. Int. J. Biol. Macromol. 2022, 210, 518–529. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, M.; Waghmare, R.; Suhag, R.; Gupta, O.P.; Lorenzo, J.M.; Prakash, S.; Radha; Rais, N.; Sampathrajan, V.; et al. Moringa (Moringa oleifera Lam.) Polysaccharides: Extraction, Characterization, Bioactivities, and Industrial Application. Int. J. Biol. Macromol. 2022, 209, 763–778. [Google Scholar] [CrossRef]
- Raman, J.K.; Alves, C.M.; Gnansounou, E. A Review on Moringa Tree and Vetiver Grass-Potential Biorefinery Feedstocks. Bioresour. Technol. 2018, 249, 1044–1051. [Google Scholar] [CrossRef]
- Soliman, M.M.; Aldhahrani, A.; Alkhedaide, A.; Nassan, M.A.; Althobaiti, F.; Mohamed, W.A. The Ameliorative Impacts of Moringa oleifera Leaf Extract Against Oxidative Stress and Methotrexate-Induced Hepato-Renal Dysfunction. Biomed. Pharmacother. 2020, 128, 110259. [Google Scholar] [CrossRef]
- Shan, T.C.; Matar, M.A.; Makky, E.A.; Ali, E.N. The Use of Moringa oleifera Seed as a Natural Coagulant for Wastewater Treatment and Heavy Metals Removal. Appl. Water Sci. 2017, 7, 1369–1376. [Google Scholar] [CrossRef]
- Varsani, V.; Vyas, S.J.; Dudhagara, D.R. Development of Bio-Based Material from the Moringa oleifera and Its Bio-Coagulation Kinetic Modeling-A Sustainable Approach to Treat the Wastewater. Heliyon 2022, 8, e10447. [Google Scholar] [CrossRef]
- Boulaadjoul, S.; Zemmouri, H.; Bendjama, Z.; Drouiche, N. A Novel use of Moringa oleifera Seed Powder in Enhancing the Primary Treatment of Paper Mill Effluent. Chemosphere 2018, 206, 142–149. [Google Scholar] [CrossRef] [PubMed]
- El-Kammah, M.; Elkhatib, E.; Gouveia, S.; Cameselle, C.; Aboukila, E. Enhanced Removal of Indigo Carmine Dye From Textile Effluent Using Green Cost-Efficient Nanomaterial: Adsorption, Kinetics, Thermodynamics and Mechanisms. Sustain. Chem. Pharm. 2022, 29, 100753. [Google Scholar] [CrossRef]
- Vasconcelos, M.W.; Gonçalves, S.; Oliveira, E.C.; Rubert, S.; Ghisi, N.C. Textile Effluent Toxicity Trend: A Scientometric Review. J. Clean. Prod. 2022, 366, 132756. [Google Scholar] [CrossRef]
- Singh, V.; Mishra, A.; Srivastava, P. Textile and Domestic Effluent Treatment Via Co-Cultivation of Diplosphaera mucosa VSPA and Scenedesmus obliquus. Biomass Bioenergy 2023, 172, 106756. [Google Scholar] [CrossRef]
- Castillo-Suárez, L.A.; Sierra-Sánchez, A.G.; Linares-Hernández, I.; Martínez-Miranda, V.; Teutli-Sequeira, E.A. A Critical Review of Textile Industry Wastewater: Green Technologies for the Removal of Indigo Dyes. Int. J. Environ. Sci. Technol. 2023, 20, 1–38. [Google Scholar] [CrossRef]
- Ferreira-Silva, V.; Gusmão, N.B.D.; Gibertoni, T.B.; Silva, L.A.D.O.D. Trametes Lactinea and T. villosa Collected in Brazil Are Able to Discolor Indigo Carmine. Acta Bot. Bras. 2022, 36, 1–7. [Google Scholar] [CrossRef]
- Yao, H.Y.; Guo, H.; Shen, F.; Li, T.; Show, D.Y.; Ling, M.; Yan, Y.G.; Show, K.Y.; Lee, D.J. Anaerobic-Aerobic treatment of High-strength and Recalcitrant Textile Dyeing Effluents. Bioresour. Technol. 2023, 379, 129060. [Google Scholar] [CrossRef]
- Khan, W.U.; Ahmed, S.; Dhoble, Y.; Madhav, S. A Critical Review of Hazardous Waste Generation From Textile Industries and Associated Ecological Impacts. J. Indian Chem. Soc. 2022, 100, 100829. [Google Scholar] [CrossRef]
- Azanaw, A.; Birlie, B.; Teshome, B.; Jemberie, M. Textile Effluent Treatment Methods And Eco-Friendly Resolution Of Textile Wastewater. Case Stud. Therm. Eng. 2022, 6, 100230. [Google Scholar] [CrossRef]
- ABIT—Associação Brasileira da Indústria Têxtil e de Confecção. Available online: https://www.abit.org.br/agenda (accessed on 5 June 2023).
- Santos, A.F.; Luz, L.A.; Argolo, A.C.; Teixeira, J.A.; Paiva, P.M.G.; Coelho, L.C.B.B. Isolation of a Seed Coagulant Moringa oleifera Lectin. Process Biochem. 2009, 44, 504–508. [Google Scholar] [CrossRef]
- Luz, L.A.; Silva, M.C.; Ferreira, R.S.; Santana, L.A.; Silva-Lucca, R.A.; Mentele, R.; Oliva, M.L.; Paiva, P.M.G.; Coelho, L.C.B.B. Structural Characterization of Coagulant Moringa oleifera Lectin and Its Effect on Hemostatic Parameters. Int. J. Biol. Macromol. 2013, 58, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.F.R.; Luz, L.A.; Paiva, P.M.G.; Coelho, L.C.B.B.; Marangoni, S.; Macedo, M.L.R. Evaluation of Seed Coagulant Moringa oleifera Lectin (cMoL) as a Bioinsecticidal Tool with Potential for the Control of Insects. Process Biochem. 2011, 46, 498–504. [Google Scholar] [CrossRef]
- Medeiros, M.L.S.; Alves, R.R.V.; Oliveira, B.F.; Napoleão, T.H.; Paiva, P.M.G.; Coelho, L.C.B.B.; Bezerra, A.C.D.S.; Silva, M.D.C. In vitro effects of Moringa oleifera Seed Lectins on Haemonchus contortus in Larval and Adult Stages. Exp. Parasitol. 2020, 218, 108004. [Google Scholar] [CrossRef]
- Andrade, L.L.; Rossato, F.A.; Costa, R.A.P.E.; Napoleão, T.H.; Paiva, P.M.G.; Coelho, L.C.B.B. Cytotoxicity of the Coagulant Moringa oleifera Lectin (cMoL) to B16-F10 Melanoma Cells. Toxicol. Vitr. 2017, 44, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chao, L.; Pang, J.; Li, Z.; Wan, Y.; Jiang, X.; Mao, Z.; Liu, W.; Chen, X.; Zhang, X. A Novel Way for High Value-Added Application of Lignosulfonate: Producing Lignosulfonate Nanosheets/Graphene Ultrathin Film Electrodes for Electrochemical Capacitors. Int. J. Biol. Macromol. 2020, 161, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.A.; Oliveira, M.D.; Melo, C.P.; Coelho, L.C.B.B.; Correia, M.T.S.; Nogueira, M.L.; Singh, P.R.; Zeng, X. Diagnosis of Dengue Infection Using a Modified Gold Electrode with Hybrid Organic-Inorganic Nanocomposite and Bauhinia monandra Lectin. J. Colloid. Interface. Sci. 2011, 362, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Doménech-Carbó, A.; Villamón, E.; Luna, I.; Ramos, D.; Doménech-Casasús, C.; Cebrián-Torrejón, G. Transmembrane Electrochemistry of Erythrocytes: Direct Electrochemical Test for Detecting Hemolysis in Whole Blood. Sens. Actuators B Chem. 2016, 226, 419–428. [Google Scholar] [CrossRef]
- Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.A.; Soozanipour, A.; Low, Z.X.; Asadnia, M.; Taheri-Kafrani, A.; Razmjou, A. Biosensors for Wastewater Monitoring: A Review. Biosens. Bioelectron. 2018, 118, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, G.; Kim, K. Determination of Equilibrium State and Sn Redox Ratio in Aluminoborosilicate Glass Melts by Potentiometry and Voltammetry. Electrochem. Commun. 2019, 109, 106554. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Kim, Y.J. Recent Developments in the Design and Fabrication of Electrochemical Biosensors Using Functional Materials and Molecules. Biosensors 2023, 13, 424. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent Developments in Biosensors for Healthcare and Biomedical Applications: A Review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Li, M.; Zhan, G.; Boakye, A.; Chai, H.; Qu, L.; Zhang, X. Recent Advances in Metal-Organic Framework-Based Electrochemical Biosensing Applications. Front. Bioeng. Biotechnol. 2021, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Boumya, W.; Taoufik, N.; Achak, M.; Bessbousse, H.; Elhalil, A.; Barka, N. Electrochemical Sensors and Biosensors for the Determination of Diclofenac in Pharmaceutical, Biological and Water Samples. Talanta Open 2021, 3, 100026. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamsshidi-Zanjani, A. A review on Industrial Wastewater Treatment via Electrocoagulation Processes. Curr. Opin. Electrochem. 2020, 22, 159–169. [Google Scholar] [CrossRef]
- Adou, K.E.; Kouakou, A.R.; Ehouman, A.D.; Tyagi, R.D.; Drogui, P.; Adouby, K. Coupling Anaerobic Digestion Process and Electrocoagulation Using Iron and Aluminium Electrodes for Slaughterhouse Wastewater Treatment. Sci. Afr. 2022, 16, e01238. [Google Scholar] [CrossRef]
- Idusuyi, N.; Ajide, O.O.; Abu, R.; Okewole, O.A.; Ibiyemi, O.O. Low Cost Electrocoagulation Process for Treatment of Contaminated Water Using Aluminium Electrodes from Recycled Cans. Mater. Today Proc. 2022, 56, 1712–1716. [Google Scholar] [CrossRef]
- Silva, J.R.; Carvalho, F.; Vicente, C.; Santos, A.D.; Quinta-Ferreira, R.M.; Castro, L.M. Electrocoagulation Treatment of Cork Boiling Wastewater. J. Environ. Chem. Eng. 2022, 10, 107750. [Google Scholar] [CrossRef]
- Ebba, M.; Asaithambi, P.; Alemayehu, E. Development of Electrocoagulation Process for Wastewater Treatment: Optimization by Response Surface Methodology. Heliyon 2022, 8, e09383. [Google Scholar] [CrossRef]
- Kothai, A.; Sathishkumar, C.; Muthupriya, R.; Dharchana, R. Experimental Investigation of Textile Dyeing Wastewater Treatment Using Aluminium in Electro Coagulation Process And Fenton’s Reagent in Advanced Oxidation Process. Mater. Today Proc. 2021, 45, 1411–1416. [Google Scholar] [CrossRef]
- Song, D.; Kadier, A.; Peralta-Hernández, J.M.; Xie, H.; Hao, B.; Ma, P.C. Separation of oil-water emulsions by a novel packed bed electrocoagulation (EC) process using anode from recycled aluminum beverage cans. J. Clean. Prod. 2022, 379, 134693. [Google Scholar] [CrossRef]
- Sohrabi, H.; Maleki, F.; Khaaki, P.; Kadhom, M.; Kudaibergenov, N.; Khataee, A. Electrochemical-Based Sensing Platforms for Detection of Glucose and H2O2 by Porous Metal-Organic Frameworks: A Review of Status and Prospects. Biosensors 2023, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Verpoort, F. Metal-Organic Molecular Cages: Applications of Biochemical Implications. Chem. Soc. Rev. 2015, 44, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2022, 13, 40. [Google Scholar] [CrossRef]
- Green, A.A.; Hughes, W.L. Protein Fractionation on the Basis of Solubility in Aqueous Solutions of Salts and Organic Solvents. Meth. Enzymol. 1955, 1, 67–90. [Google Scholar] [CrossRef]
- Appukuttan, P.S.; Surolia, A.; Bachawat, B.K. Isolation of Two Galactose-Binding Proteins from Ricinus Communis by Affinity Chromatography. Indian J. Biochem. Biophys. 1977, 14, 382–384. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Paiva, P.M.G.; Coelho, L.C.B.B. Purification and Partial Characterization of Two Lectin Isoforms from Cratylia mollis Mart. (camaratu bean). Appl. Biochem. Biotechnol. 1992, 36, 113–118. [Google Scholar] [CrossRef]
- Silva, G.G.; Machado, F.L.A.; Alves Junior, S.; Padrón-Hernández, E. Metal-Organic Framework: Structure and Magnetic Properties of [Cu3(BTC)2 (L)x·(CuO)y]n (L=H2O, DMF). J. Solid State Chem. 2017, 253, 1–5. [Google Scholar] [CrossRef]
- Abreu, D.S.; Sousa, T.P.; Castro, C.B.; Sousa, M.N.; Silva, T.T.; Almeida-Neto, F.W.Q.; Queiros, M.V.A.; Rodrigues, B.S.F.; Oliveira, M.C.F.; Paulo, T.F.; et al. SAM of Gliotoxin on Gold: A Natural Product Platform for Sugar Recognition Based on the Immobilization of Canavalia brasiliensis Lectin (ConBr). Electrochim. Acta 2017, 241, 116–123. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme Immobilized Nanomaterials as Electrochemical Biosensors for Detection of Biomolecules. Enzyme Microb. Technol. 2022, 156, 110006. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez-Cejuela, H.; Calabretta, M.M.; Bocci, V.; D’Elia, M.; Michelini, E. Super-Stable Metal-Organic Framework (MOF)/Luciferase Paper-Sensing Platform for Rapid ATP Detection. Biosensors 2023, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.H.; Araújo, H.D.A.; Silva, R.P.; Correia, M.T.; Freitas, K.C.S.; Souza, S.R.; Coelho, L.C.B.B. Biosensor Characterization from Cratylia mollis Seed Lectin (Cramoll)-MOF and Specific Carbohydrate Interactions in an Electrochemical Model. Chem. Biodivers 2022, 19, e202200515. [Google Scholar] [CrossRef] [PubMed]
- Selzer, S.M.; Vico, R.V.; Ferreyra, N.F. Immobilization of Concanavalin A on Iron Oxide Magnetic Nanoparticles. Effect of Bovine Serum Albumin in the Recognition Interactions of the Lectin. Surf. Interfaces 2022, 30, 101908. [Google Scholar] [CrossRef]
- Abrantes-Coutinho, V.E.; Santos, A.O.; Moura, R.B.; Pereira-Junior, F.N.; Mascaro, L.H.; Morais, S.; Oliveira, T.M.B.F. Systematic Review on Lectin-Based Electrochemical Biosensors for Clinically Relevant Carbohydrates and Glycoconjugates. Colloids Surf. B 2021, 208, 112148. [Google Scholar] [CrossRef]
- Li, W.; Yuan, R.; Chai, Y.; Zhong, H.; Wang, Y. Study of the Biosensor Based on Platinum Nanoparticles Supported on Carbon Nanotubes and Sugar–Lectin Biospecific Interactions for the Determination of Glucose. Electrochim. Acta 2011, 56, 4203–4208. [Google Scholar] [CrossRef]
- Zhao, S.; Shi, C.; Hu, H.; Li, Z.; Xiao, G.; Yang, Q.; Sun, P.; Cheng, L.; Niu, W.; Bi, J.; et al. ISFET and Dex-AgNPs Based Portable Sensor for Reusable and Real-Time Determinations of concanavalin A and Glucose on Smartphone. Biosens. Bioelectron. 2020, 151, 111962. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Aires, A.L.; Soares, C.L.R.; Brito, T.G.S.; Nascimento, W.M.; Martins, M.C.B.; Silva, T.G.; Brayner, F.A.; Alves, L.C.; Silva, N.H.; et al. Usnic acid potassium salt from Cladonia substellata (Lichen): Synthesis, Cytotoxicity and in vitro Anthelmintic Activity and Ultrastructural Analysis Against Adult Worms of Schistosoma mansoni. Acta Trop. 2019, 192, 1–10. [Google Scholar] [CrossRef]
- Cui, H.; Cui, S.; Zhang, S.; Tian, Q.; Liu, Y.; Zhang, P.; Wang, M.; Zhang, J.; Li, X. Cu–MOF/Hemin: A Bionic Enzyme with Excellent Dispersity for the Determination of Hydrogen Peroxide Released from Living Cells. Analyst 2021, 146, 5951–5961. [Google Scholar] [CrossRef]
- Meneses, J.M.D.; Vasconcelos, R.D.F.; Fernandes, T.D.F.; Araújo, G.T.D. Tratamento do Efluente do Biodiesel Utilizando a Eletrocoagulação/Flotação: Investigação dos Parâmetros Operacionais. Química Nova 2012, 35, 235–240. [Google Scholar] [CrossRef]
- Lach, C.E.; Pauli, C.S.; Coan, A.S.; Simionatto, E.L.; Koslowski, L.A.D. Investigating the Process of Electrocoagulation in the Removal of Azo Dye From Synthetic Textile Effluents and the Effects of Acute Toxicity on Daphnia magna Test Organisms. J. Water Process. Eng. 2022, 45, 102485. [Google Scholar] [CrossRef]
- Criado, S.P.; Gonçalves, M.J.; Tavares, L.B.B.; Bertoli, S.L. Optimization of Electrocoagulation Process for Disperse and Reactive Dyes Using the Response Surface Method with Reuse Application. J. Clean. Prod. 2020, 275, 122690. [Google Scholar] [CrossRef]
- Kalivel, P.; Singh, R.P.; Kavitha, S.; Padmanabhan, D.; Krishnan, S.K.; Palanichamy, J. Elucidation of Electrocoagulation Mechanism in the Removal of Blue SI Dye from Aqueous Solution Using Al-Al, Cu-Cu Electrodes—A comparative Study. Ecotoxicol. Environ. Saf. 2020, 201, 110858. [Google Scholar] [CrossRef] [PubMed]
- Hendaoui, K.; Trabelsi-Ayadi, M.; Ayari, F. Optimization and Mechanisms Analysis of Indigo Dye Removal Using Continuous Electrocoagulation. Chin. J. Chem. Eng. 2021, 29, 242–252. [Google Scholar] [CrossRef]
- Özyonar, F.; Gökkuş, Ö.; Sabuni, M. Removal of Disperse and Reactive Dyes from Aqueous Solutions Using Ultrasound-Assisted Electrocoagulation. Chemosphere 2020, 258, 127325. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Tyrosinase-Based Biosensor-A New Tool for Chlorogenic Acid Detection in Nutraceutical Formulations. Materials 2022, 15, 3221. [Google Scholar] [CrossRef]
- Ammar, M.; Yousef, E.; Mahmoud, M.A.; Ashraf, S.; Baltrusaitis, J. A Comprehensive Review of the Developments in Electrocoagulation for the Removal of Contaminants from Wastewater. Separations 2023, 10, 337. [Google Scholar] [CrossRef]

| Samples with Dye | pH |
|---|---|
| Sample without treatment | 5.97 |
| Sample after aluminum electrode treatment | 6.24 |
| Sample after iron electrode treatment | 7.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, B.F.; de Araújo, H.D.A.; Neves, E.F.; Napoleão, T.H.; Paiva, P.M.G.; de Freitas, K.C.S.; de Souza, S.R.; Coelho, L.C.B.B. Electrochemical Characterization Using Biosensors with the Coagulant Moringa oleifera Seed Lectin (cMoL). Biosensors 2023, 13, 655. https://doi.org/10.3390/bios13060655
de Oliveira BF, de Araújo HDA, Neves EF, Napoleão TH, Paiva PMG, de Freitas KCS, de Souza SR, Coelho LCBB. Electrochemical Characterization Using Biosensors with the Coagulant Moringa oleifera Seed Lectin (cMoL). Biosensors. 2023; 13(6):655. https://doi.org/10.3390/bios13060655
Chicago/Turabian Stylede Oliveira, Benny Ferreira, Hallysson Douglas Andrade de Araújo, Eloisa Ferreira Neves, Thiago Henrique Napoleão, Patrícia Maria Guedes Paiva, Katia Cristina Silva de Freitas, Sandra Rodrigues de Souza, and Luana Cassandra Breitenbach Barroso Coelho. 2023. "Electrochemical Characterization Using Biosensors with the Coagulant Moringa oleifera Seed Lectin (cMoL)" Biosensors 13, no. 6: 655. https://doi.org/10.3390/bios13060655
APA Stylede Oliveira, B. F., de Araújo, H. D. A., Neves, E. F., Napoleão, T. H., Paiva, P. M. G., de Freitas, K. C. S., de Souza, S. R., & Coelho, L. C. B. B. (2023). Electrochemical Characterization Using Biosensors with the Coagulant Moringa oleifera Seed Lectin (cMoL). Biosensors, 13(6), 655. https://doi.org/10.3390/bios13060655







