Ultrasensitive Silicon Nanowire Biosensor with Modulated Threshold Voltages and Ultra-Small Diameter for Early Kidney Failure Biomarker Cystatin C
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
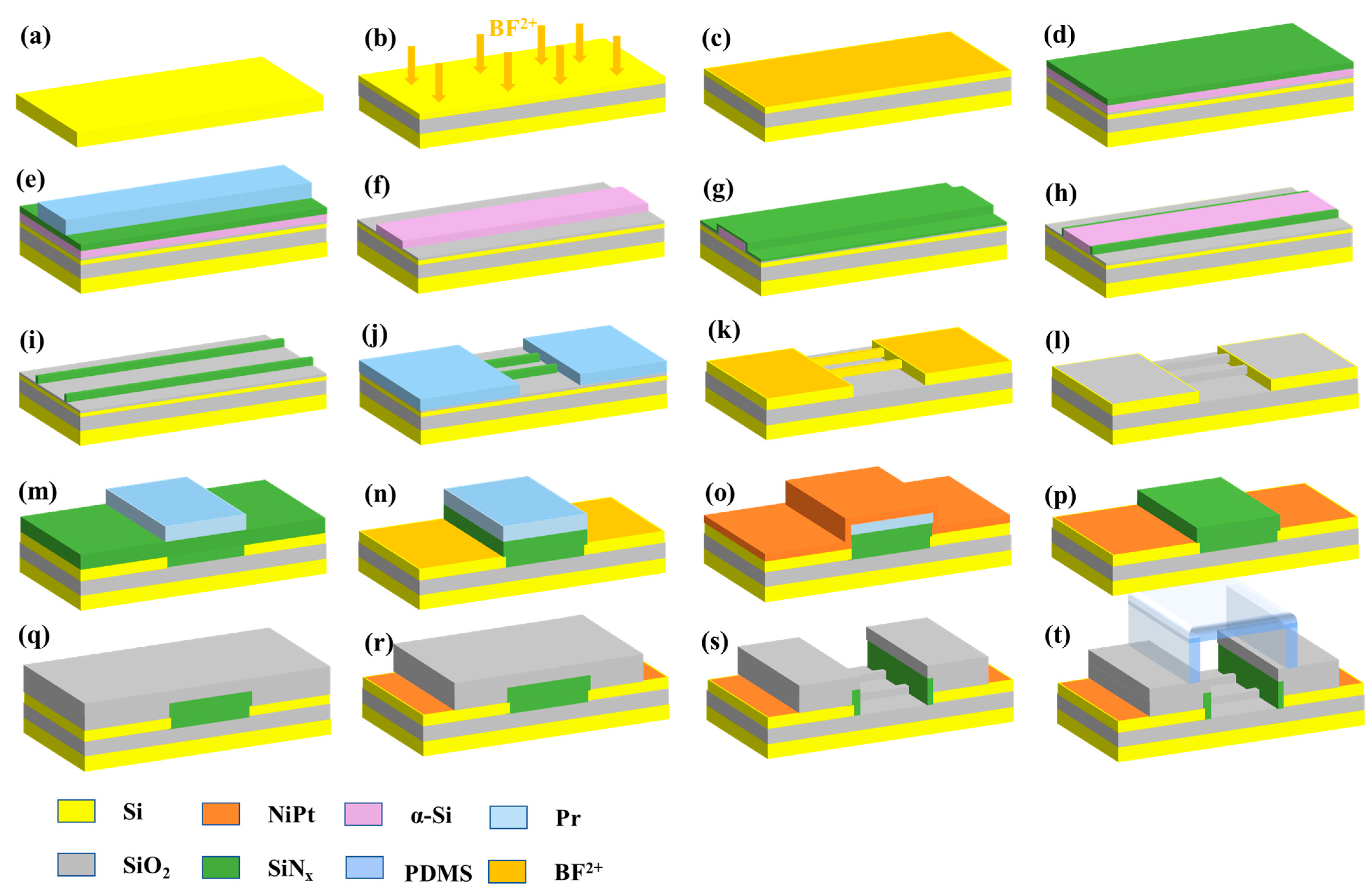
2.2. Fabrication of SiNW FET Biosensor
2.3. Surface Modification
2.4. Working Principles of SiNW FET Biosensors
2.5. Characterization of SiNW FET Biosensors
3. Results and Discussion
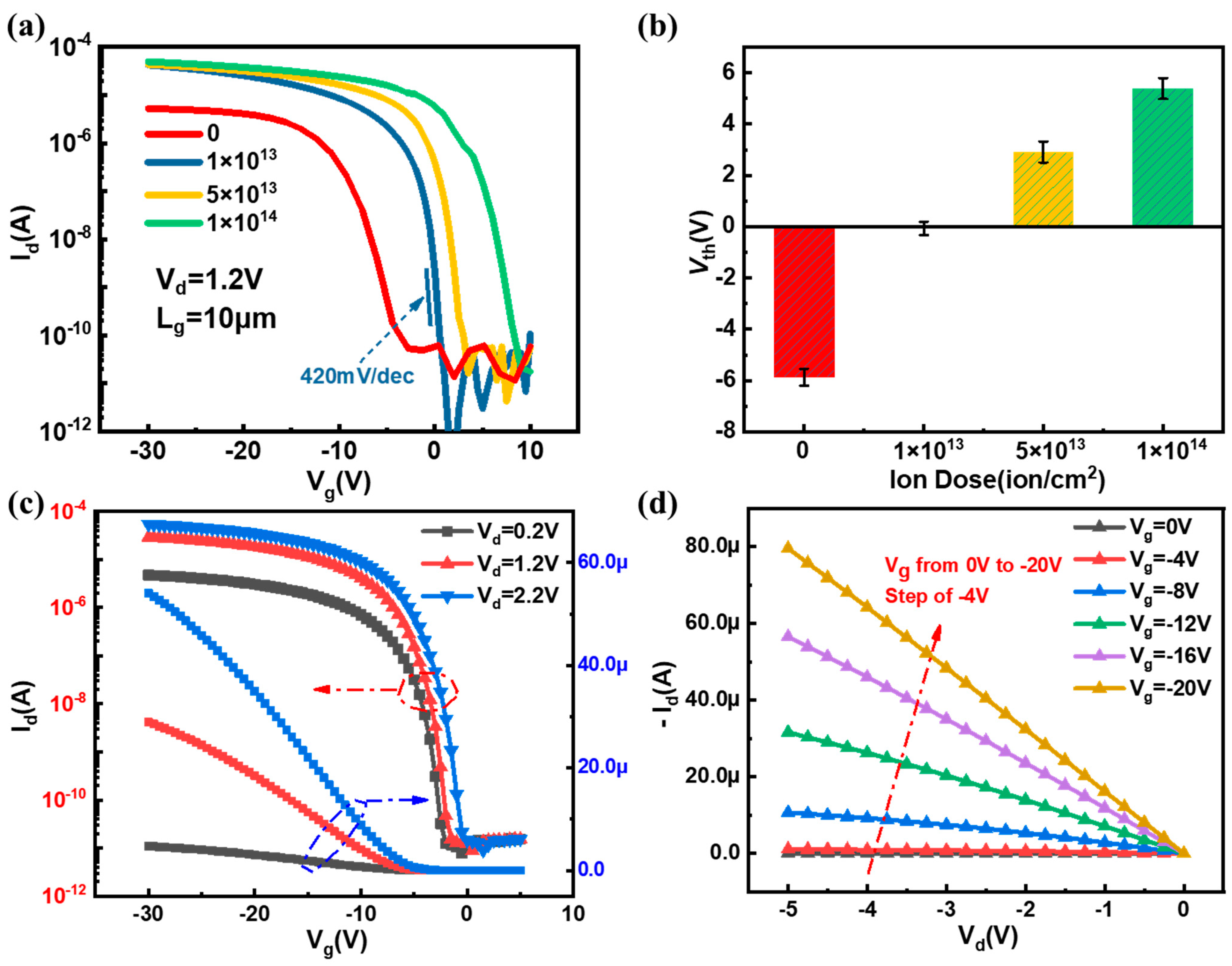
3.1. Structural Characterization and Electrical Characteristics of SiNW FET Biosensor
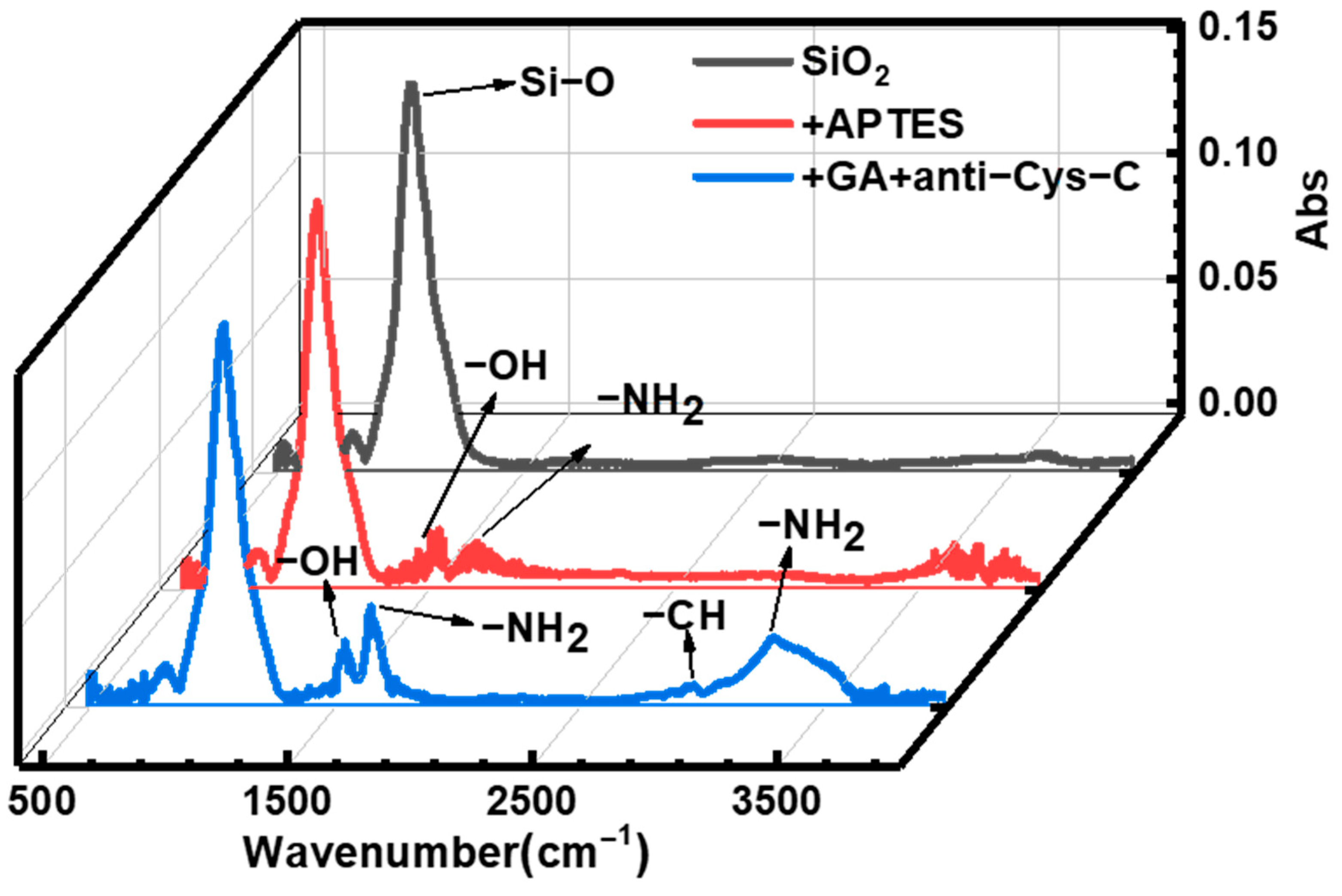
3.2. Surface Modification
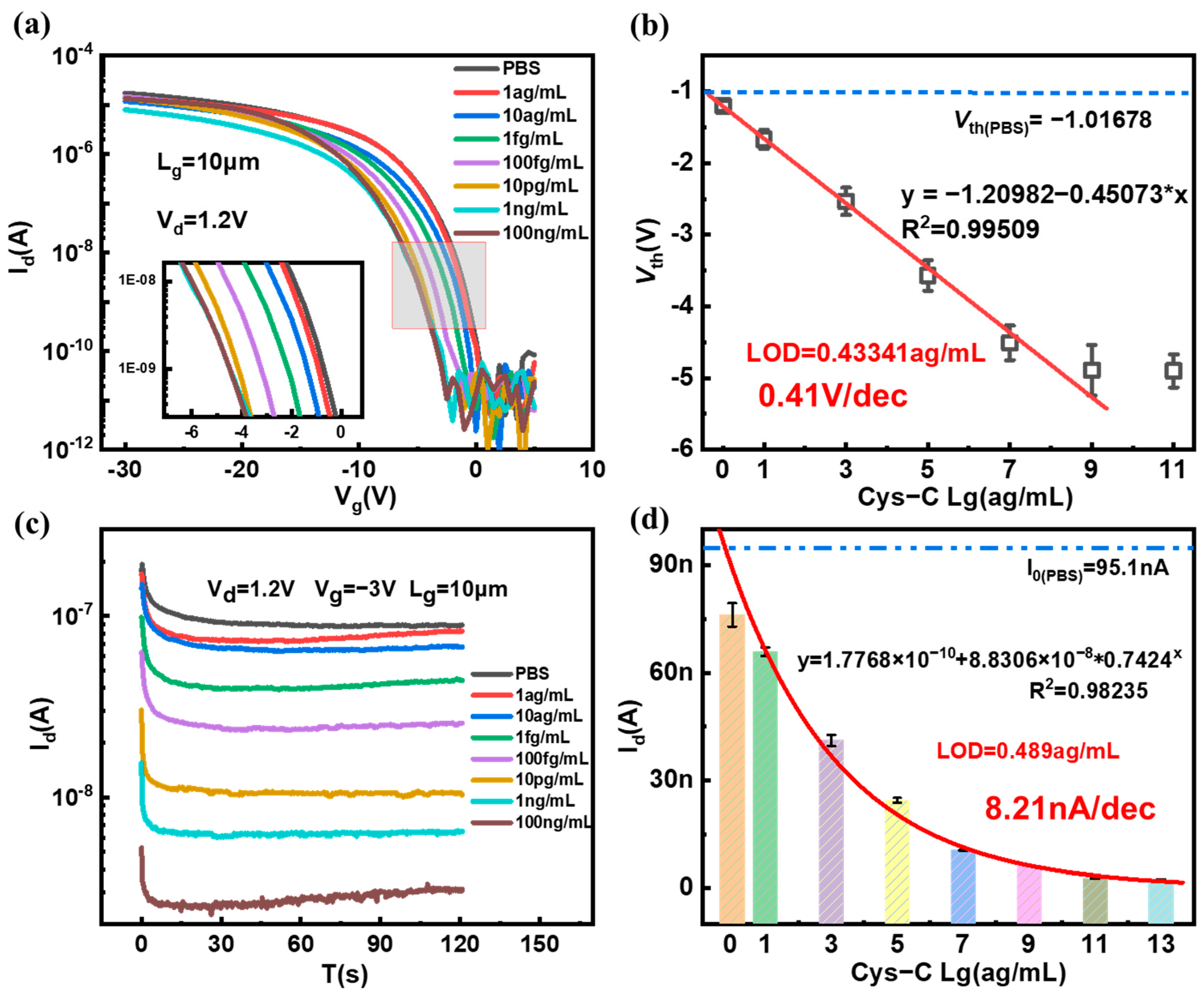
3.3. Detection of Cys-C and Sensitivity
3.4. Repeatability and Specificity of SiNW FET Biosensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lewington, A.J.P.; Cerdá, J.; Mehta, R.L. Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Tidmas, V.; Brazier, J.; Bottoms, L. Ultra-endurance Participation and Acute Kidney Injury: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 16887. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wu, R.; Ji, Y. Identifying Patients at Risk of Acute Kidney Injury among Patients Receiving Immune Checkpoint Inhibitors: A Machine Learning Approach. Diagnostics 2022, 12, 3157. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G. Acute kidney injury. Nat. Rev. Dis. Prim. 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Bell, S.; Forni, L. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Chaudhary, K.; Saha, A. Acute kidney injury in hospitalized patients with COVID-19. medRxiv 2020, 0504, 20090944. [Google Scholar]
- Shlipak, M.G.; Sarnak, M.J.; Katz, R. Cystatin C and the risk of death and cardiovascular events among elderly persons. N. Engl. J. Med. 2005, 352, 2049–2060. [Google Scholar] [CrossRef]
- Stämmler, F.; Grassi, M.; Meeusen, J.W. Estimating glomerular filtration rate from serum myo-inositol, valine, creatinine and cystatin C. Diagnostics 2021, 11, 2291. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Matsushita, K.; Ärnlöv, J. Cystatin C versus creatinine in determining risk based on kidney function. N. Engl. J. Med. 2013, 369, 932–943. [Google Scholar] [CrossRef]
- Randers, E.; Kristensen, H.; Erlandsen, E.J. Serum cystatin C as a marker of the renal function. Scand. J. Clin. Lab. Investig. Suppl. 1998, 58, 585–592. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, H.S.; Kim, M.J. Diagnostic value of multiple serum biomarkers for vancomycin-induced kidney injury. J. Clin. Med. 2021, 10, 5005. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Cavalier, E.; Morel, J. Detection of decreased glomerular filtration rate in intensive care units: Serum cystatin C versusserum creatinine. BMC Nephrol. 2014, 15, 9. [Google Scholar] [CrossRef]
- Pergande, M.; Jung, K. Sandwich enzyme immunoassay of cystatin C in serum with commercially available antibodies. Clin. Chem. 1993, 39, 1885–1890. [Google Scholar] [CrossRef]
- Fonseca, I.; Reguengo, H.; Oliveira, J.C. A triple-biomarker approach for the detection of delayed graft function after kidney transplantation using serum creatinine, cystatin C, and malondialdehyde. Clin. Biochem. 2015, 48, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.; Costa-Rama, E.; Beirão, I. Disposable electrochemical immunosensor for analysis of cystatin C, a CKD biomarker. Talanta 2019, 201, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Zhuo, Y.; Yuan, R. Highly effective protein converting strategy for ultrasensitive electrochemical assay of cystatin C. Anal. Chem. 2016, 88, 5189–5196. [Google Scholar] [CrossRef]
- Mi, L.; Wang, P.; Yan, J. A novel photoelectrochemical immunosensor by integration of nanobody and TiO2 nanotubes for sensitive detection of serum cystatin C. Anal. Chim. Acta 2016, 902, 107–114. [Google Scholar] [CrossRef]
- Desai, D.; Kumar, A.; Bose, D. Ultrasensitive sensor for detection of early stage chronic kidney disease in human. Biosens. Bioelectron. 2018, 105, 90–94. [Google Scholar] [CrossRef]
- Zhao, M.; Bai, L.; Cheng, W. Monolayer rubrene functionalized graphene-based eletrochemiluminescence biosensor for serum cystatin C detection with immunorecognition-induced 3D DNA machine. Biosens. Bioelectron. 2019, 127, 126–134. [Google Scholar] [CrossRef]
- Ferreira, P.A.B.; Araujo, M.C.M.; Prado, C.M. An ultrasensitive Cystatin C renal failure immunosensor based on a PPy/CNT electrochemical capacitor grafted on interdigitated electrode. Colloids Surf. B Biointerfaces 2020, 189, 110834. [Google Scholar] [CrossRef]
- Stasyuk, N.; Smutok, O.; Demkiv, O. Synthesis, catalytic properties and application in biosensorics of nanozymes and electronanocatalysts: A review. Sensors 2020, 20, 4509. [Google Scholar] [CrossRef] [PubMed]
- Torrinha, Á.; Oliveira, T.M.B.F.; Ribeiro, F.W.P. Application of nanostructured carbon-based electrochemical (bio) sensors for screening of emerging pharmaceutical pollutants in waters and aquatic species: A review. Nanomaterials 2020, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Kerry, R.G.; Ukhurebor, K.E.; Kumari, S. A comprehensive review on the applications of nano-biosensor-based approaches for non-communicable and communicable disease detection. Biomater. Sci. 2021, 9, 3576–3602. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, J.; Yosinski, S. A Fast and Label-Free Potentiometric Method for Direct Detection of Glutamine with Silicon Nanowire Biosensors. Biosensors 2022, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lieber, C.M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef]
- Lo Faro, M.J.; Leonardi, A.A.; Priolo, F. Future Prospects of Luminescent Silicon Nanowires Biosensors. Biosensors 2022, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.O.; Krull, U.J. Silicon nanowires as field-effect transducers for biosensor development: A review. Anal. Chim. Acta 2014, 825, 1–25. [Google Scholar] [CrossRef]
- Li, G.; Wei, Q.; Wei, S. Acrylamide Hydrogel-Modified Silicon Nanowire Field-Effect Transistors for pH Sensing. Nanomaterials 2022, 12, 2070. [Google Scholar] [CrossRef]
- Zhou, W.; Dai, X.; Fu, T.M. Long term stability of nanowire nanoelectronics in physiological environments. Nano Lett. 2014, 14, 1614–1619. [Google Scholar] [CrossRef]
- Zhang, Q.; Tu, H.; Yin, H. Si nanowire biosensors using a FinFET fabrication process for real time monitoring cellular ion actitivies. In Proceedings of the 2018 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 1–5 December 2018; pp. 29.6.1–29.6.4. [Google Scholar]
- Shen, M.Y.; Li, B.R.; Li, Y.K. Silicon nanowire field-effect-transistor based biosensors: From sensitive to ultra-sensitive. Biosens. Bioelectron. 2014, 60, 101–111. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Fan, K. A supersensitive silicon nanowire array biosensor for quantitating tumor marker ctDNA. Biosens. Bioelectron. 2021, 181, 113147. [Google Scholar] [CrossRef]
- Yang, X.; Gao, A.; Wang, Y. Wafer-level and highly controllable fabricated silicon nanowire transistor arrays on (111) silicon-on-insulator (SOI) wafers for highly sensitive detection in liquid and gaseous environments. Nano Res. 2018, 11, 1520–1529. [Google Scholar] [CrossRef]
- Su, P.C.; Chen, B.H.; Lee, Y.C. Silicon nanowire field-effect transistor as biosensing platforms for post-translational modification. Biosensors 2020, 10, 213. [Google Scholar] [CrossRef]
- Tang, S.; Yan, J.; Zhang, J. Fabrication of low cost and low temperature poly-silicon nanowire sensor arrays for monolithic three-dimensional integrated circuits applications. Nanomaterials 2020, 10, 2488. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Z.; Zhang, Q. O2 plasma treated biosensor for enhancing detection sensitivity of sulfadiazine in a high-к HfO2 coated silicon nanowire array. Sens. Actuators B Chem. 2020, 306, 127464. [Google Scholar] [CrossRef]
- Nikonov, A.M.; Naumova, O.V.; Generalov, V.M. Surface Preparation as a Step in the Fabrication of Biosensors Based on Silicon Nanowire Field-Effect Transistors. J. Surf. Investig. 2020, 14, 337–346. [Google Scholar] [CrossRef]
- Elfström, N.; Juhasz, R.; Sychugov, I. Surface charge sensitivity of silicon nanowires: Size dependence. Nano Lett. 2007, 7, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.A.; Zheng, G.; Lieber, C.M. Subthreshold regime has the optimal sensitivity for nanowire FET biosensors. Nano Lett. 2010, 10, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, X.; Ma, Z. Multifunctional substrate of label-free electrochemical immunosensor for ultrasensitive detection of cytokeratins antigen 21-1. Sci Rep. 2017, 7, 1023. [Google Scholar] [CrossRef]
- Trindade, E.K.G.; Silva, B.V.M.; Dutra, R.F. A probeless and label-free electrochemical immunosensor for cystatin C detection based on ferrocene functionalized-graphene platform. Biosens. Bioelectron. 2019, 138, 111311. [Google Scholar] [CrossRef] [PubMed]




| Method | Material | Linear Range (LOD) | Reference |
|---|---|---|---|
| Differential pulse Voltammetry (DPV) | Au@Fe3O4 | 0.01 pg/mL~30 ng/mL (3 fg/mL) | Yang et al., 2016 [16] |
| Photocurrent response | TiO2 nanotubes | 0.72 pM~3.6 nM (0.14 pM) | Mi et al., 2016 [17] |
| Square wave voltammetry (SWV) | Prepared poly(thionine)-Au | 100 ng/mL~10 fg/mL (4.6 fg/mL) | Wang et al., 2017 [40] |
| Cyclic voltammetry and differential pulse voltammetry | Multiwalled carbon nanotube (MWCNT) | 0.6~6.6 ng/mL (0.58 pg/mL) | Desai et al., 2018 [18] |
| Linear sweep voltammetry (LSV) | AuNPs | 10~100 ng/mL (6.0 ng/mL) | Lopes et al., 2019 [15] |
| Square wave voltammetry (SWV) | Graphene oxide-ferrocene nanofilm | 0.1~1000 ng/mL (0.03 ng/mL) | Erika et al., 2019 [41] |
| Electrochemiluminescent (ECL) | Graphene composite (G/mRub) | 1.0 fg/mL~10 ng/mL (0.38 fg/mL) | Zhao et al., 2019 [19] |
| Interdigitated electrode (IDE) | Polypyrrole/carbon nanotube | 0~300 ng/mL (28 ng/mL) | Ferreira et al., 2020 [20] |
| Field effect transistor (FET) | Silicon nanowire | 1 ag/mL~1 ng/mL (0.2529 ag/mL) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Li, Y.; Zhang, X.; Wang, Y.; Zhang, J.; Yan, J.; Li, J.; Zhang, Z.; Yin, H.; Wei, Q.; et al. Ultrasensitive Silicon Nanowire Biosensor with Modulated Threshold Voltages and Ultra-Small Diameter for Early Kidney Failure Biomarker Cystatin C. Biosensors 2023, 13, 645. https://doi.org/10.3390/bios13060645
Hu J, Li Y, Zhang X, Wang Y, Zhang J, Yan J, Li J, Zhang Z, Yin H, Wei Q, et al. Ultrasensitive Silicon Nanowire Biosensor with Modulated Threshold Voltages and Ultra-Small Diameter for Early Kidney Failure Biomarker Cystatin C. Biosensors. 2023; 13(6):645. https://doi.org/10.3390/bios13060645
Chicago/Turabian StyleHu, Jiawei, Yinglu Li, Xufang Zhang, Yanrong Wang, Jing Zhang, Jiang Yan, Junjie Li, Zhaohao Zhang, Huaxiang Yin, Qianhui Wei, and et al. 2023. "Ultrasensitive Silicon Nanowire Biosensor with Modulated Threshold Voltages and Ultra-Small Diameter for Early Kidney Failure Biomarker Cystatin C" Biosensors 13, no. 6: 645. https://doi.org/10.3390/bios13060645
APA StyleHu, J., Li, Y., Zhang, X., Wang, Y., Zhang, J., Yan, J., Li, J., Zhang, Z., Yin, H., Wei, Q., Jiang, Q., Wei, S., & Zhang, Q. (2023). Ultrasensitive Silicon Nanowire Biosensor with Modulated Threshold Voltages and Ultra-Small Diameter for Early Kidney Failure Biomarker Cystatin C. Biosensors, 13(6), 645. https://doi.org/10.3390/bios13060645






