Oxidative Stress Sensing System for 8-OHdG Detection Based on Plasma Coupled Electrochemistry by Transparent ITO/AuNTAs/PtNPs Electrode
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Production of ITO Electrode
2.3. Synthesis of AuNTAs
2.4. Plasma-Assembled ITO Electrode
2.5. 8-OHdG Detection
3. Results and Discussion
3.1. Oxidative Stress Sensing System
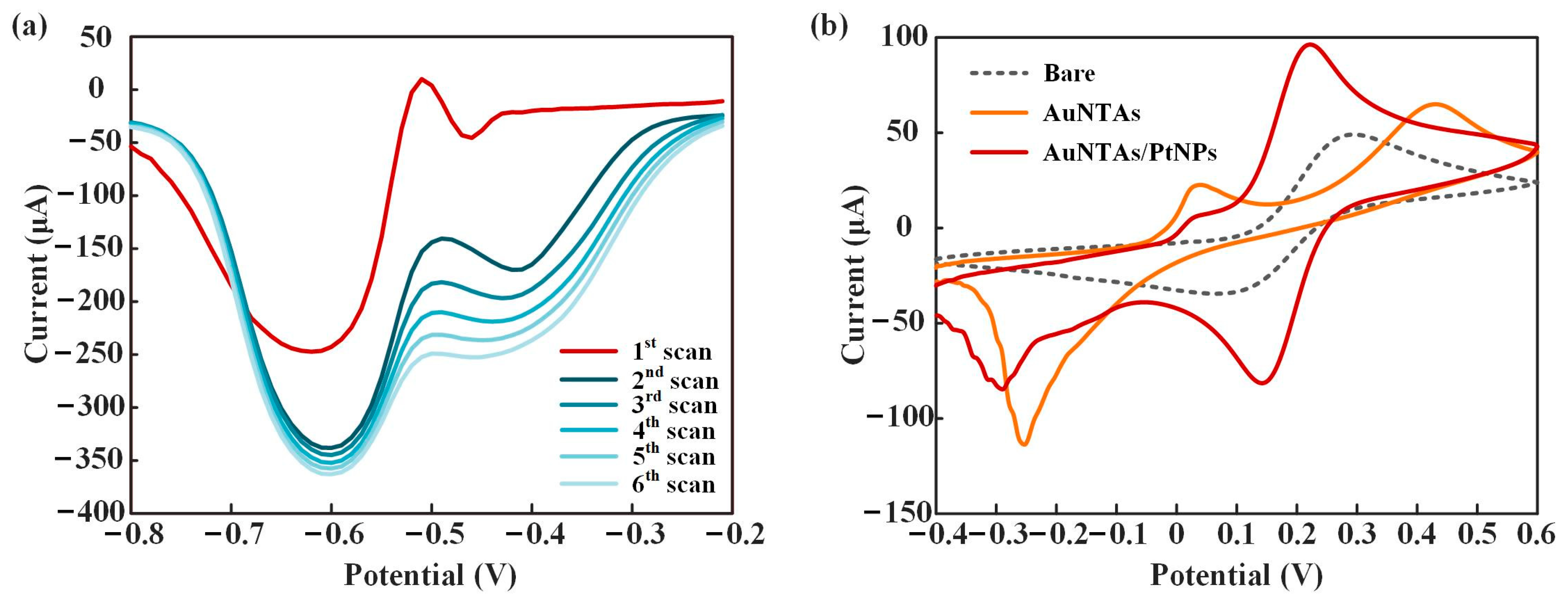
3.2. Characteristics of ITO/AuNTAs/PtNPs Electrode
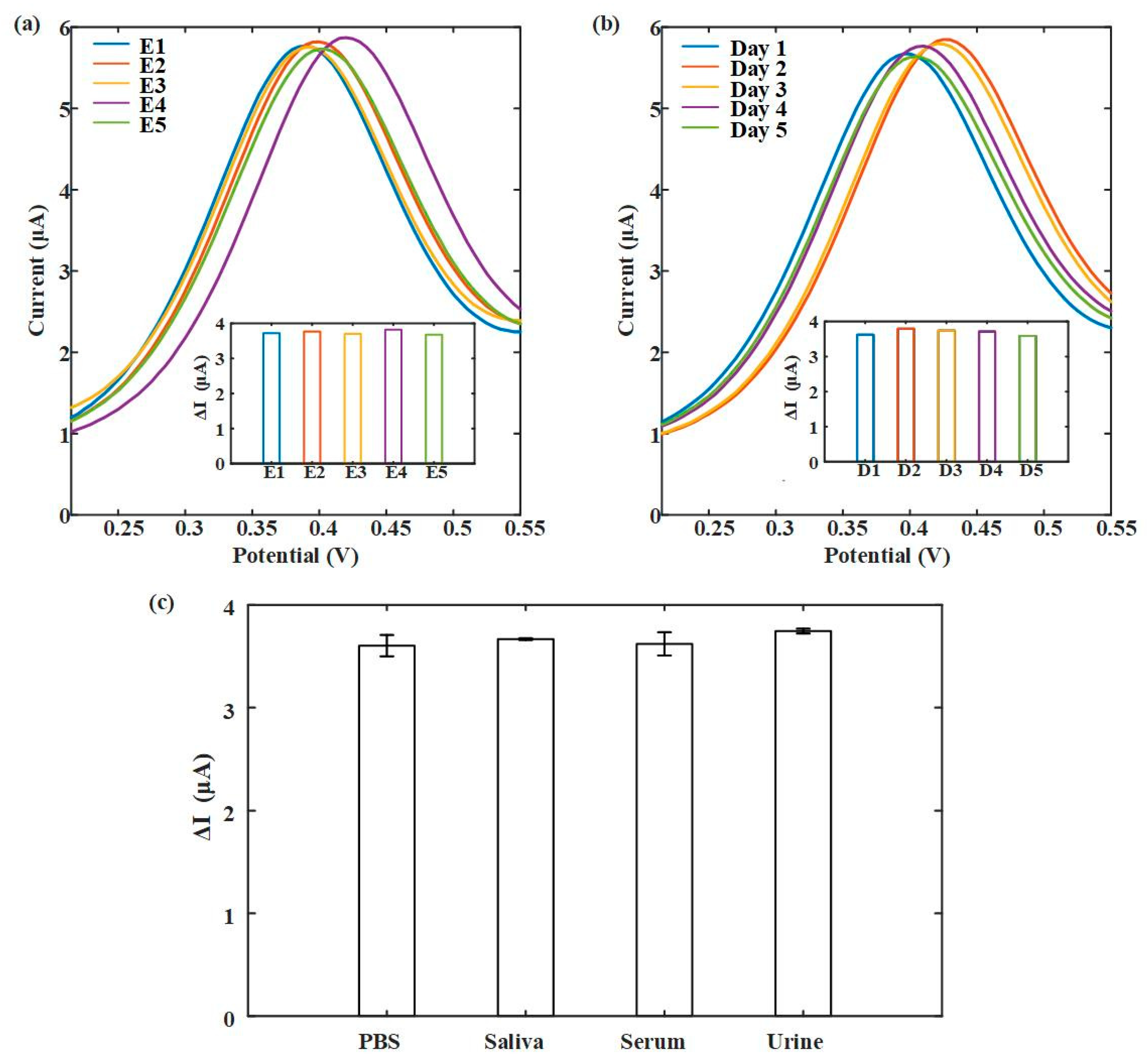
3.3. 8-OhdG Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kafle, R.C.; Kim, D.Y.; Holt, M.M. Gender-specific trends in cigarette smoking and lung cancer incidence: A two-stage age-stratified Bayesian joinpoint model. Cancer Epidemiol. 2023, 84, 102364. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, E.O.-T.; Liu, X.; Lok, V.; Ngai, C.H.; Zhang, L.; Wong, M.C. Global Trends of Prostate Cancer by Age, and Their Associations with Gross Domestic Product (GDP), Human Development Index (HDI), Smoking, and Alcohol Drinking. Clin. Genitourin. Cancer 2023, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; He, J.; Yang, J. Bone marrow microenvironment: Roles and therapeutic implications in obesity-associated cancer. Trends Cancer 2023, 00792, 12. [Google Scholar] [CrossRef]
- Zabransky, D.J.; Jaffee, E.M.; Weeraratna, A.T. Shared genetic and epigenetic changes link aging and cancer. Trends Cell Biol. 2022, 32, 338–350. [Google Scholar] [CrossRef]
- Rezaeian, A.-H.; Dang, F.; Wei, W. The circadian clock, aging and its implications in cancer. Neoplasia 2023, 41, 100904. [Google Scholar] [CrossRef]
- Chatsirisupachai, K.; Lagger, C.; de Magalhães, J.P. Age-associated differences in the cancer molecular landscape. Trends Cancer 2022, 8, 962–971. [Google Scholar] [CrossRef]
- Rossner, P., Jr.; Rossnerova, A.; Sram, R.J. Oxidative stress and chromosomal aberrations in an environmentally exposed population. Mutat. Res. Mol. Mech. Mutagen. 2011, 707, 34–41. [Google Scholar] [CrossRef]
- Barathkumar, S.; Padhi, R.; Parida, P.; Marigoudar, S. In vivo appraisal of oxidative stress response, cell ultrastructural aberration and accumulation in Juvenile Scylla serrata exposed to uranium. Chemosphere 2022, 300, 134561. [Google Scholar] [CrossRef]
- Song, J.Y.; Lim, J.W.; Kim, H.; Morio, T.; Kim, K.H. Oxidative Stress Induces Nuclear Loss of DNA Repair Proteins Ku70 and Ku80 and Apoptosis in Pancreatic Acinar AR42J Cells. J. Biol. Chem. 2003, 278, 36676–36687. [Google Scholar] [CrossRef]
- Than, N.G.; Posta, M.; Györffy, D.; Orosz, L.; Orosz, G.; Rossi, S.W.; Ambrus-Aikelin, G.; Szilágyi, A.; Nagy, S.; Hupuczi, P.; et al. Early pathways, biomarkers, and four distinct molecular subclasses of preeclampsia: The intersection of clinical, pathological, and high-dimensional biology studies. Placenta 2022, 125, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yuan, K.; Chen, L. Molecular biomarkers, network biomarkers, and dynamic network biomarkers for diagnosis and prediction of rare diseases. Fundam. Res. 2022, 2, 894–902. [Google Scholar] [CrossRef]
- Sambiagio, N.; Berthet, A.; Wild, P.; Sauvain, J.J.; Auer, R.; Schoeni, A.; Hopf, N.B. Associations between urinary biomarkers of oxidative stress and biomarkers of tobacco smoke exposure in smokers. Sci. Total Environ. 2022, 852, 158361. [Google Scholar] [CrossRef]
- Peris-Pastor, G.; Alonso-Rodríguez, S.; Benedé, J.L.; Chisvert, A. High-throughput determination of oxidative stress biomarkers in saliva by solvent-assisted dispersive solid-phase extraction for clinical analysis. Adv. Sample Prep. 2023, 6, 100067. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Martins, G.V.; Marques, A.C.; Fortunato, E.; Sales, M.G.F. 8-hydroxy-2′-deoxyguanosine (8-OHdG) biomarker detection down to picoMolar level on a plastic antibody film. Biosens. Bioelectron. 2016, 86, 225–234. [Google Scholar] [CrossRef]
- Bláhová, L.; Janoš, T.; Mustieles, V.; Rodríguez-Carrillo, A.; Fernández, M.F.; Bláha, L. Rapid extraction and analysis of oxidative stress and DNA damage biomarker 8-hydroxy-2′-deoxyguanosine (8-OHdG) in urine: Application to a study with pregnant women. Int. J. Hyg. Environ. Health 2023, 250, 114175. [Google Scholar] [CrossRef]
- Yano, T.; Shoji, F.; Baba, H.; Koga, T.; Shiraishi, T.; Orita, H.; Kohno, H. Significance of the urinary 8-OHdG level as an oxidative stress marker in lung cancer patients. Lung Cancer 2009, 63, 111–114. [Google Scholar] [CrossRef]
- Sova, H.; Jukkolavuorinen, A.; Puistola, U.; Kauppila, S.; Karihtala, P. 8-Hydroxydeoxyguanosine: A new potential independent prognostic factor in breast cancer. Br. J. Cancer 2010, 102, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.; Wang, L.-M.; Tosetto, M.; Sheahan, K.; Hyland, J.; Fennelly, D.; O’Donoghue, D.; Mulcahy, H.; O’Sullivan, J. Nuclear oxidative damage correlates with poor survival in colorectal cancer. Br. J. Cancer 2008, 100, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Zerr, I.; Gawinecka, J.; Gmitterowa, K. P2.028 8-OHdG in cerebrospinal fluid as a marker of oxidative stress in various neurodegenerative diseases. Park. Relat. Disord. 2009, 15, S96. [Google Scholar] [CrossRef]
- Feng, C.; Liu, S.; Zhou, F.; Gao, Y.; Li, Y.; Du, G.; Chen, Y.; Jiao, H.; Feng, J.; Zhang, Y.; et al. Oxidative stress in the neurodegenerative brain following lifetime exposure to lead in rats: Changes in lifespan profiles. Toxicology 2018, 411, 101–109. [Google Scholar] [CrossRef]
- Chen, H.; Sun, C.; Guo, W.; Meng, R.; Du, H.; Qi, Q.; Gu, X.; Li, L.; Zhang, K.; Zhu, D.; et al. AluYb8 insertion in the MUTYH gene is related to increased 8-OHdG in genomic DNA and could be a risk factor for type 2 diabetes in a Chinese population. Mol. Cell. Endocrinol. 2011, 332, 301–305. [Google Scholar] [CrossRef]
- Liu, W.; Wang, B.; Yang, S.; Xu, T.; Yu, L.; Wang, X.; Cheng, M.; Zhou, M.; Chen, W. Associations of propylene oxide exposure with fasting plasma glucose and diabetes: Roles of oxidative DNA damage and lipid peroxidation. Environ. Pollut. 2021, 292, 118453. [Google Scholar] [CrossRef]
- Myoren, T.; Kobayashi, S.; Oda, S.; Nanno, T.; Ishiguchi, H.; Murakami, W.; Okuda, S.; Okada, M.; Takemura, G.; Suga, K.; et al. An oxidative stress biomarker, urinary 8-hydroxy-2′-deoxyguanosine, predicts cardiovascular-related death after steroid therapy for patients with active cardiac sarcoidosis. Int. J. Cardiol. 2016, 212, 206–213. [Google Scholar] [CrossRef]
- Song, J.; Zhu, J.; Tian, G.; Li, H.; Li, H.; An, Z.; Jiang, J.; Fan, W.; Wang, G.; Zhang, Y.; et al. Short time exposure to ambient ozone and associated cardiovascular effects: A panel study of healthy young adults. Environ. Int. 2020, 137, 105579. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Chiou, C.-C.; Chang, P.-Y.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 2003, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, P.; Kosonen, A.-L.; Laakso, J. Oxidative damage marker 8-OHdG in urine of vegetarians and omnivores. Toxicol. Lett. 2015, 238, S72–S73. [Google Scholar] [CrossRef]
- Trachioti, M.G.; Hrbac, J.; Prodromidis, M.I. Determination of 8−hydroxy−2’−deoxyguanosine in urine with “linear” mode sparked graphite screen-printed electrodes. Electrochim. Acta 2021, 399, 139371. [Google Scholar] [CrossRef]
- Ekuni, D.; Tomofuji, T.; Tamaki, N.; Sanbe, T.; Azuma, T.; Yamanaka, R.; Yamamoto, T.; Watanabe, T. Mechanical stimulation of gingiva reduces plasma 8-OHdG level in rat periodontitis. Arch. Oral Biol. 2008, 53, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Sayal, A.; Aydin, A.; Savaser, A.; Erdem, O.; Eken, A.; Arsova-Sarafinovska, Z.; Erten, K. Evaluation of plasma 8-OHdG levels in prostate cancer, benign prostatic hyperplasia patients and healthy individuals. Toxicol. Lett. 2008, 180, S81. [Google Scholar] [CrossRef]
- Yasuda, M.; Ide, H.; Furuya, K.; Yoshii, T.; Nishio, K.; Saito, K.; Isotani, S.; Kamiyama, Y.; Muto, S.; Horie, S. Salivary 8-OHdG: A Useful Biomarker for Predicting Severe ED and Hypogonadism. J. Sex. Med. 2008, 5, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, R.; Reshma, A.P.; Rajeev, V.; Kurra, S.B.; Prince, M.R.J.; Syam, N. Salivary 8-Hydroxydeoxyguanosine—A valuable indicator for oxidative DNA damage in periodontal disease. Saudi J. Dent. Res. 2015, 6, 15–20. [Google Scholar] [CrossRef]
- Mei, S.; Yao, Q.; Wu, C.; Xu, G. Determination of urinary 8-hydroxy-2′-deoxyguanosine by two approaches—Capillary electrophoresis and GC/MS: An assay for in vivo oxidative DNA damage in cancer patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 827, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Ogawa, Y.; Kasai, H. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine values measured by an ELISA correlated well with measurements by high-performance liquid chromatography with electrochemical detection. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1076–1081. [Google Scholar]
- Koide, S.; Kinoshita, Y.; Ito, N.; Kimura, J.; Yokoyama, K.; Karube, I. Determination of human serum 8-hydroxy-2′-deoxyguanosine (8-OHdG) by HPLC-ECD combined with solid phase extraction (SPE). J. Chromatogr. B 2010, 878, 2163–2167. [Google Scholar] [CrossRef]
- Guo, H.; Xue, K.; Yan, L. Resonance Rayleigh scattering spectral method for determination of urinary 8-hydroxy-2′-deoxyguanosine using gold nanoparticles as probe. Sens. Actuators B Chem. 2012, 171, 1038–1045. [Google Scholar] [CrossRef]
- Wu, D.; Liu, B.; Yin, J.; Xu, T.; Zhao, S.; Xu, Q.; Chen, X.; Wang, H. Detection of 8-hydroxydeoxyguanosine (8-OHdG) as a biomarker of oxidative damage in peripheral leukocyte DNA by UHPLC–MS/MS. J. Chromatogr. B 2017, 1064, 1–6. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Gutierrez, F.A.; Eguílaz, M.; González-Domínguez, J.M.; Hernández-Ferrer, J.; Ansón-Casaos, A.; Rivas, G.A. Electrochemical sensing of guanine, adenine and 8-hydroxy-2′-deoxyguanosine at glassy carbon modified with single-walled carbon nanotubes covalently functionalized with lysine. RSC Adv. 2016, 6, 13469–13477. [Google Scholar] [CrossRef]
- Gupta, P.; Oyama, M.; Goyal, R.N. Electrochemical investigations of 8-hydroxydeoxyguanosine and its determination at an edge plane pyrolytic graphite electrode. RSC Adv. 2015, 6, 1722–1728. [Google Scholar] [CrossRef]
- Goyal, R.N. Determination of 8-Hydroxydeoxyguanosine: A potential biomarker of oxidative stress, using carbon-allotropic nanomaterials modified glassy carbon sensor. Talanta 2016, 161, 735–742. [Google Scholar]
- Li, S.; Zhang, J.; Tan, C.S.; Chen, C.; Hu, C.; Bai, Y.; Ming, D. Electrochemical immunosensor based on hybrid MoS2/Pt@Au-nanoprism/PDA for simultaneous detection of free and total prostate specific antigen in serum. Sens. Actuators B-Chem. 2022, 357, 131413. [Google Scholar] [CrossRef]
- Wan, C.; Liu, T.; Wei, S.; Zhang, S. Electrochemical determination of 8-hydroxydeoxyguanosine using a carbon nanotube modified electrode. Russ. J. Electrochem. 2008, 44, 327–331. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Qi, H.; Gao, Q.; Li, C.-Z.; Zhang, C. Highly Sensitive Electrochemical Sensor for the Determination of 8-Hydroxy-2′-deoxyguanosine Incorporating SWCNTs-Nafion Composite Film. J. Sens. 2015, 2015, 504869. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Gutiérrez, S.; García, G.; Galicia, L.; Rivas, G.A. Determinatiom of 8-Hydroxy 2′-Deoxyguanosine Using Electrodes Modified with a Dispersion of Carbon Nanotubes in Polyethylenimine. Electroanalysis 2011, 23, 1221–1228. [Google Scholar] [CrossRef]
- Kumar, N.; Goyal, R.N. A melamine based molecularly imprinted sensor for the determination of 8-hydroxydeoxyguanosine in human urine. Talanta 2017, 166, 215–222. [Google Scholar] [CrossRef] [PubMed]





| Technique | Linear Range | Detection Limit | Real Sample | Reference |
|---|---|---|---|---|
| MWCNT/GCE (CV) | 0.08–5 µM | 9 nM | No | [42] |
| SWCNT-Nafion/GCE (DPV) | 0.03–1.25 µM | 8 nM | No | [43] |
| CNT-PEI/GCE (ASV) | 0.5–30 µM | 100 nM | No | [44] |
| MWCNT/ErGO/GCE (SWV) | 3–75 µM | 35 nM | Yes | [40] |
| MIP Sensor (SWV) | 0.020–3 μM | 3 nM | Yes | [45] |
| ITO/AuNTAs/PtNPs (DPV) | 10–100,000 ng/mL | 10 ng/mL | Yes | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Li, S. Oxidative Stress Sensing System for 8-OHdG Detection Based on Plasma Coupled Electrochemistry by Transparent ITO/AuNTAs/PtNPs Electrode. Biosensors 2023, 13, 643. https://doi.org/10.3390/bios13060643
Bai Y, Li S. Oxidative Stress Sensing System for 8-OHdG Detection Based on Plasma Coupled Electrochemistry by Transparent ITO/AuNTAs/PtNPs Electrode. Biosensors. 2023; 13(6):643. https://doi.org/10.3390/bios13060643
Chicago/Turabian StyleBai, Yongchang, and Shuang Li. 2023. "Oxidative Stress Sensing System for 8-OHdG Detection Based on Plasma Coupled Electrochemistry by Transparent ITO/AuNTAs/PtNPs Electrode" Biosensors 13, no. 6: 643. https://doi.org/10.3390/bios13060643
APA StyleBai, Y., & Li, S. (2023). Oxidative Stress Sensing System for 8-OHdG Detection Based on Plasma Coupled Electrochemistry by Transparent ITO/AuNTAs/PtNPs Electrode. Biosensors, 13(6), 643. https://doi.org/10.3390/bios13060643






