Multi-Probe Nano-Genomic Biosensor to Detect S. aureus from Magnetically-Extracted Food Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
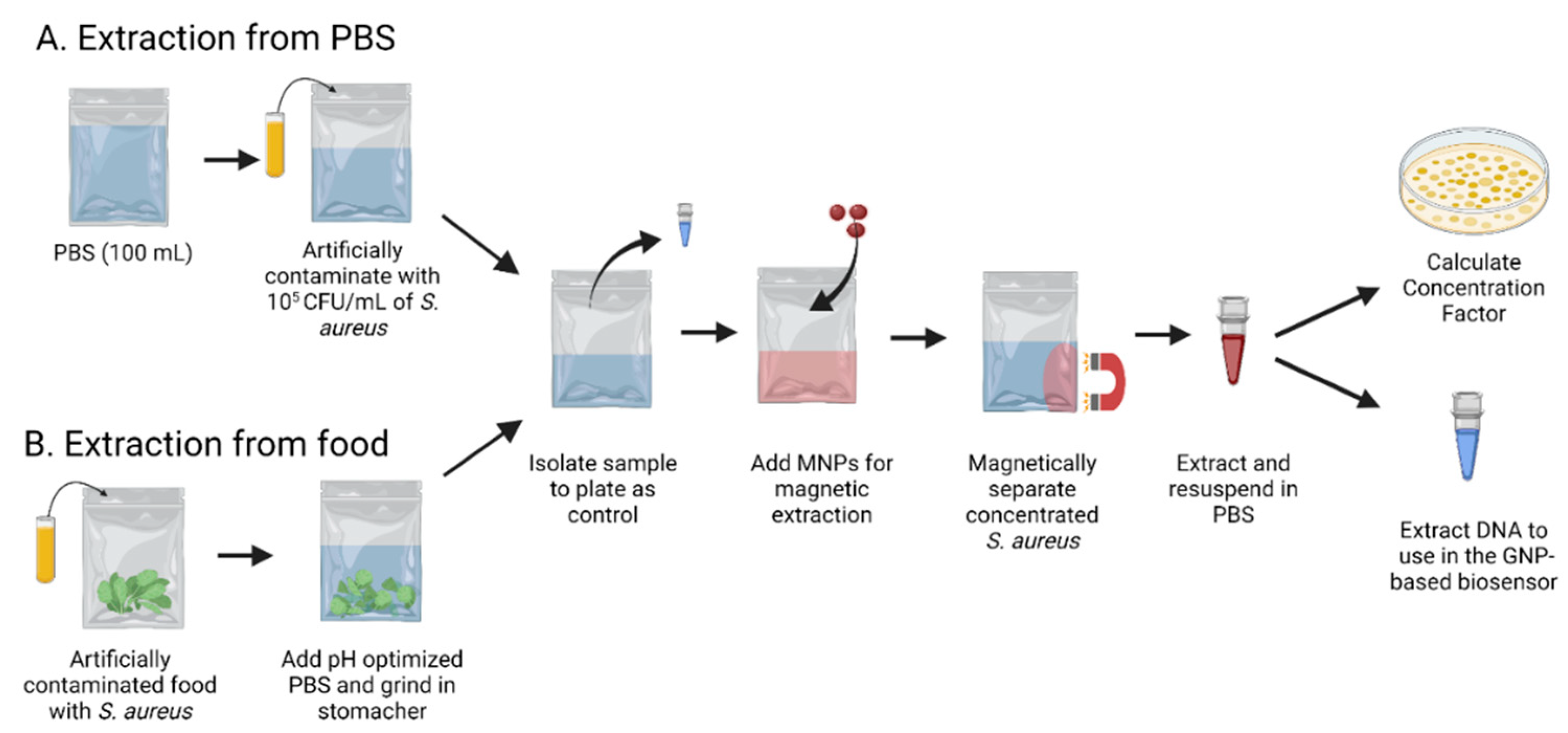
2.2. Magnetic Nanoparticles for Extraction
2.3. Oligonucleotide Probe Designs
2.4. GNP Synthesis and Surface Coating
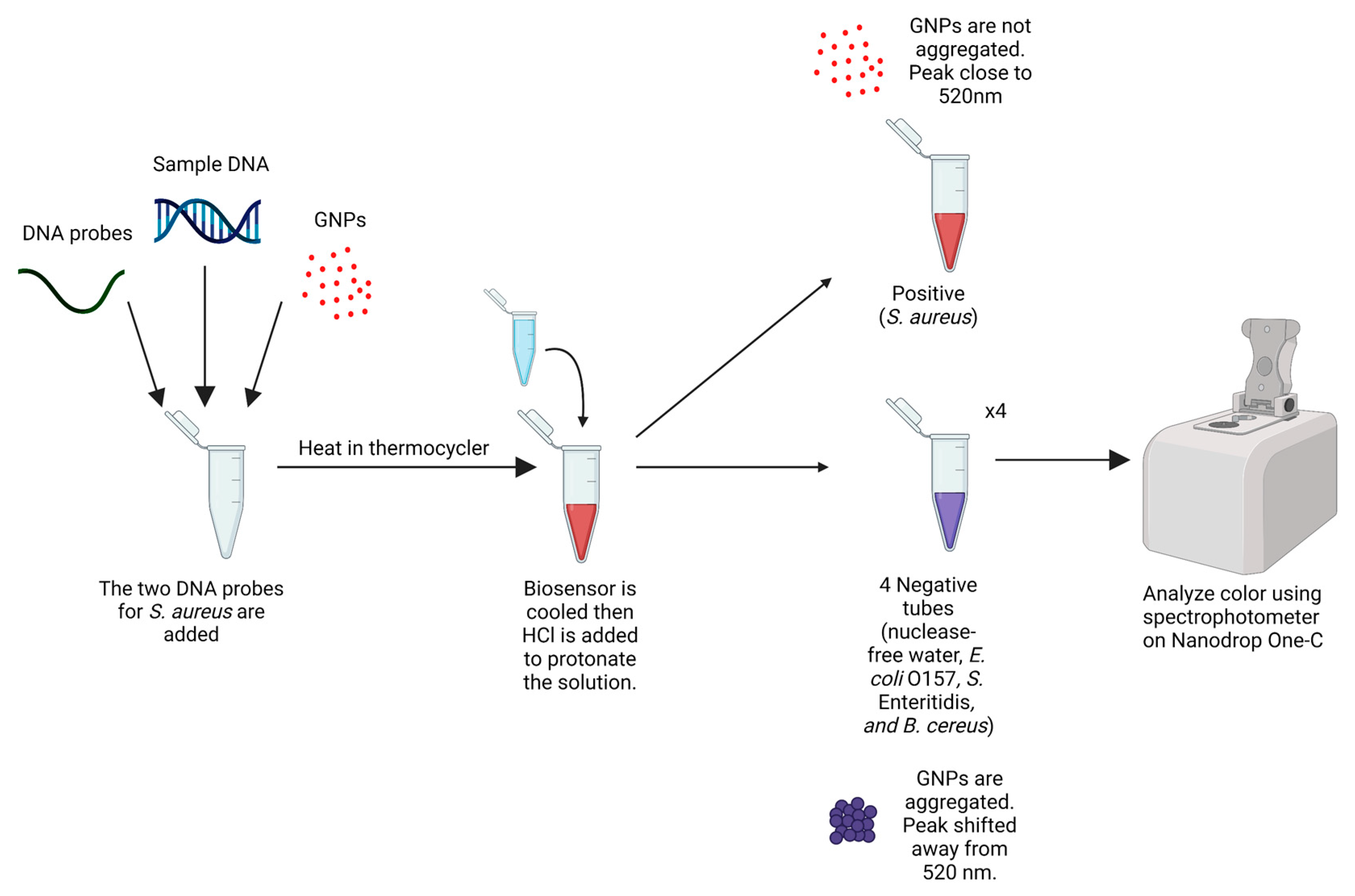
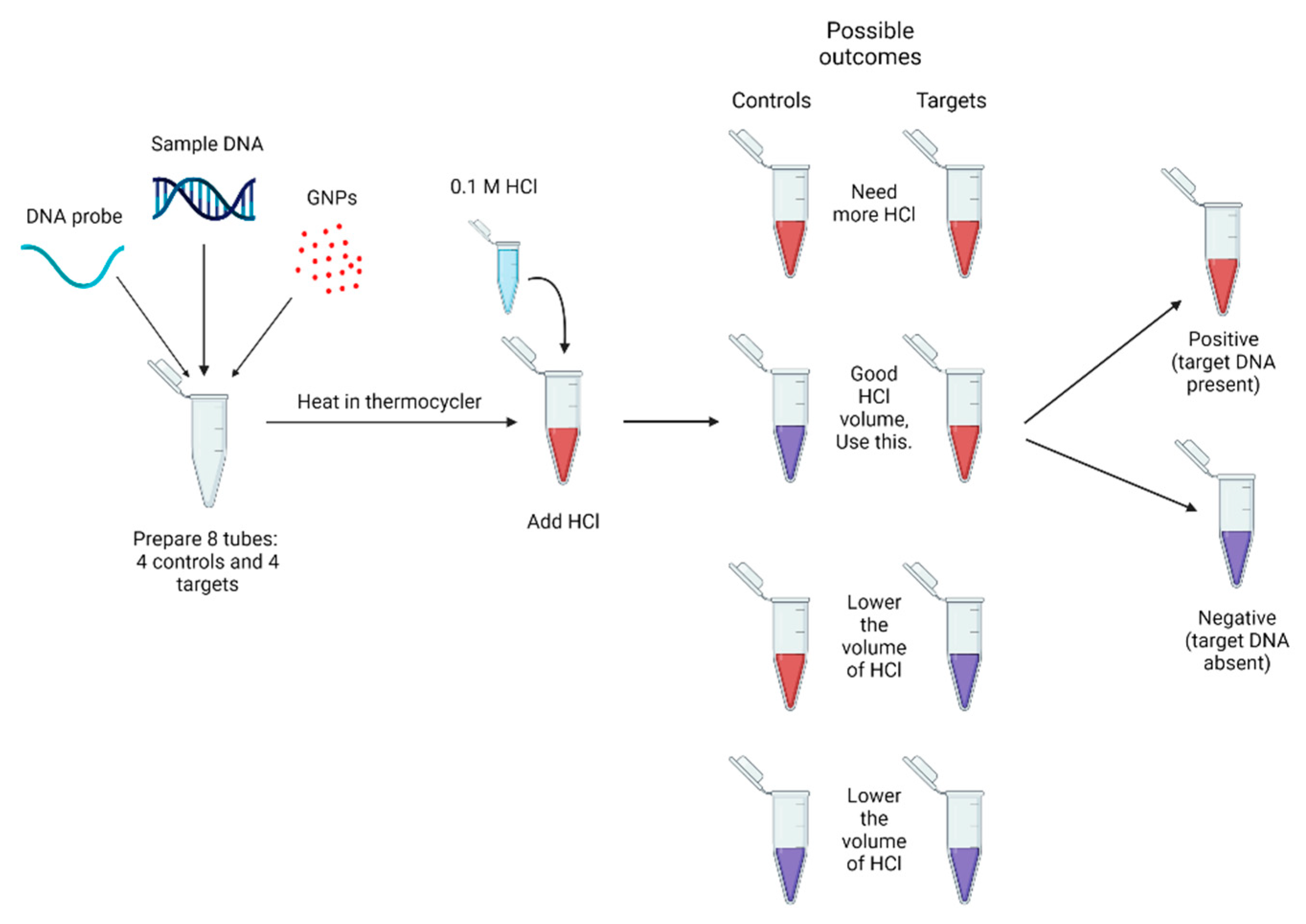
2.5. Biosensor Design and Optimization
2.6. Statistical Analyses
3. Results
3.1. Concentration Factor for the Extraction of S. aureus in Food and PBS
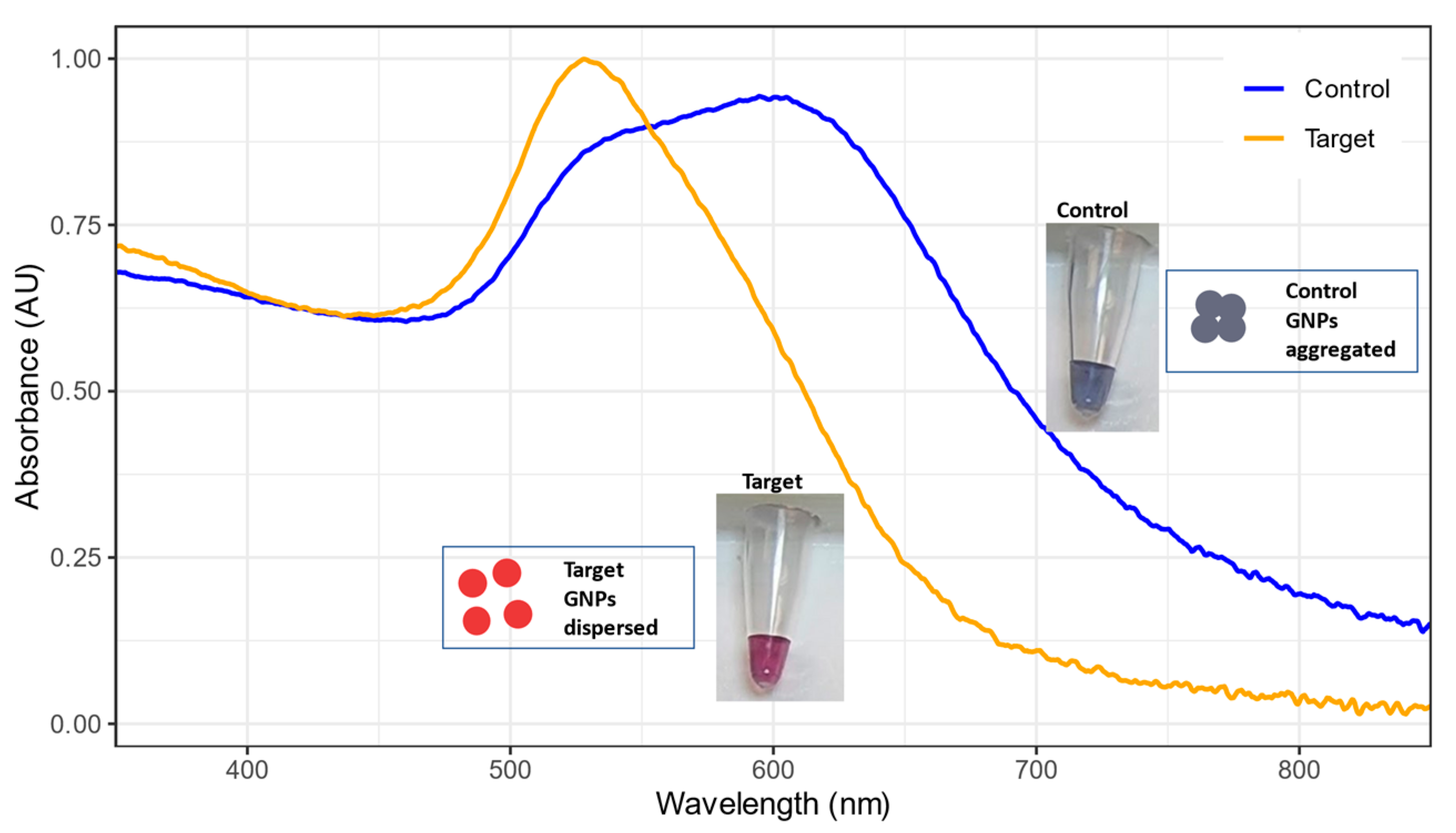
3.2. Peak Shifts and Color Change for GNP Biosensor
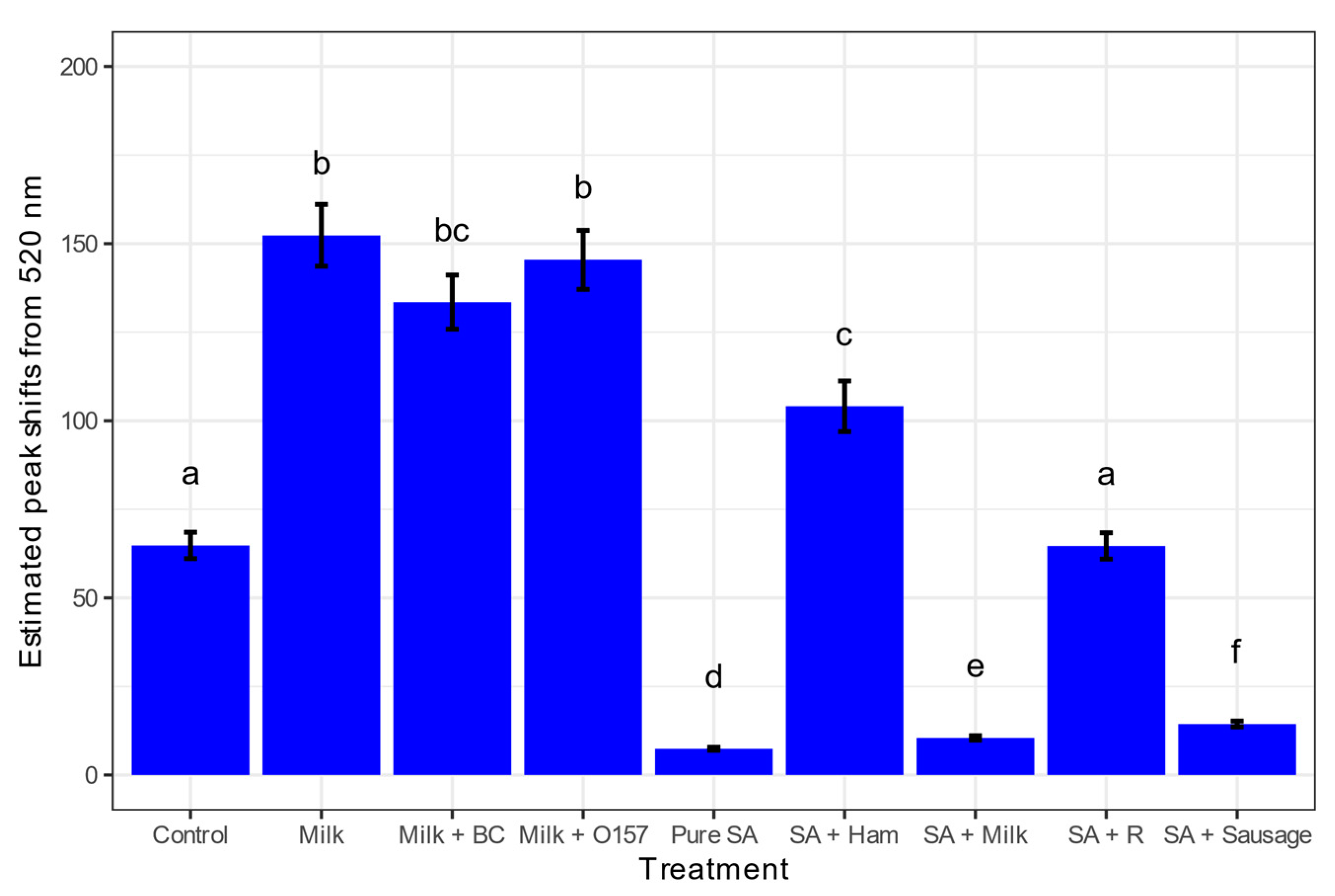
3.3. Detecting S. aureus from Various Food Matrices
3.4. Sensitivity of the S. aureus GNP Biosensor
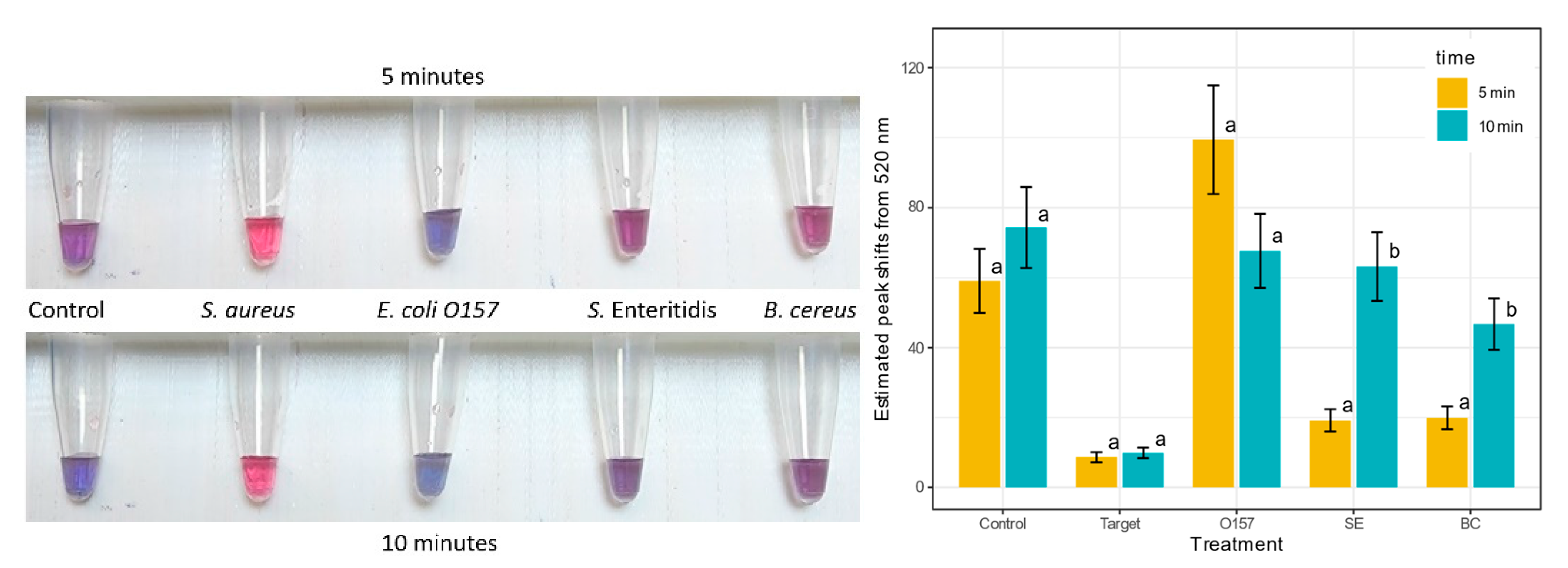
3.5. Specificity of the S. aureus GNP Biosensor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- First Formal Meeting of the Foodborne Disease Burden Epidemiology Reference Group (FERG): Implementing Strategy, Setting Priorities and Assigning the Tasks. Available online: https://www.who.int/publications/i/item/9789241596763 (accessed on 26 July 2022).
- Wadström, T.; Ljungh, Å. Food Associated Pathogens; CRC Press: Boca Raton, FL, USA, 2013; pp. 269–279. [Google Scholar] [CrossRef]
- Hennekinne, J.; Buyser, M.D.; Dragacci, S. Staphylococcus aureus and Its Food Poisoning Toxins: Characterization and Outbreak Investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Asao, T.; Kumeda, Y.; Kawai, T.; Shibata, T.; Oda, H.; Haruki, K.; Nakazawa, H.; Kozaki, S. An Extensive Outbreak of Staphylococcal Food Poisoning Due to Low-Fat Milk in Japan: Estimation of Enterotoxin A in the Incriminated Milk and Powdered Skim Milk. Epidemiol. Infect. 2003, 130, 33–40. [Google Scholar] [CrossRef]
- Pereira, M.L.; Carmo, L.S.D.; Santos, E.J.D.; Pereira, J.L.; Bergdoll, M.S. Enterotoxin H in Staphylococcal Food Poisoning. J. Food Protect. 2016, 59, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Outbreak of Staphylococcal Food Poisoning Associated with Precooked Ham—Florida, 1997. Morb. Mortal. Wkly. Rep. 1997, 46, 1189–1191. [Google Scholar]
- Stevens, K.A.; Jaykus, L.-A. Bacterial Separation and Concentration from Complex Sample Matrices: A Review. Crit. Rev. Microbiol. 2008, 30, 7–24. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Wang, C.; Shao, N.; Dong, P.; Xiao, R.; Wang, S. Magnetically Assisted Surface-Enhanced Raman Spectroscopy for the Detection of Staphylococcus aureus Based on Aptamer Recognition. ACS Appl. Mater. Interfaces 2015, 7, 20919–20929. [Google Scholar] [CrossRef]
- Roda, A.; Mirasoli, M.; Roda, B.; Bonvicini, F.; Colliva, C.; Reschiglian, P. Recent Developments in Rapid Multiplexed Bioanalytical Methods for Foodborne Pathogenic Bacteria Detection. Microchim. Acta 2012, 178, 7–28. [Google Scholar] [CrossRef]
- Kumar, C.G.; Anand, S.K. Significance of Microbial Biofilms in Food Industry: A Review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef]
- Kaclíková, E.; Kuchta, T.; Kay, H.; Gray, D. Separation of Listeria from Cheese and Enrichment Media Using Antibody-Coated Microbeads and Centrifugation. J. Microbiol. Methods 2001, 46, 63–67. [Google Scholar] [CrossRef]
- Pysher, M.D.; Hayes, M.A. Electrophoretic and Dielectrophoretic Field Gradient Technique for Separating Bioparticles. Anal. Chem. 2007, 79, 4552–4557. [Google Scholar] [CrossRef]
- Hahn, M.W.; Stadler, P.; Wu, Q.L.; Pöckl, M. The Filtration–Acclimatization Method for Isolation of an Important Fraction of the Not Readily Cultivable Bacteria. J. Microbiol. Methods 2004, 57, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Song, M.; Cui, Y.; Shi, C.; Wang, D.; Paoli, G.C.; Shi, X. A Rapid Method for the Detection of Foodborne Pathogens by Extraction of a Trace Amount of DNA from Raw Milk Based on Amino-Modified Silica-Coated Magnetic Nanoparticles and Polymerase Chain Reaction. Anal. Chim. Acta 2013, 787, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Wu, Q.; Wang, X.; Zhou, Z.; Oakes, K.D.; Zhang, X.; Huang, Q.; Yang, W. Bacteria Capture, Lysate Clearance, and Plasmid DNA Extraction Using PH-Sensitive Multifunctional Magnetic Nanoparticles. Anal. Biochem. 2010, 398, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Liang, W.; Jin, L.; Tang, Y.; Mou, X.; Shah, M.A.A.; Yang, H.; Deng, Y.; He, N.; Li, Z. Development of Magnetic Nanoparticles Based Nucleic Acid Extraction Method and Application in Hepatitis C Virus Chemiluminescent Detection. Sci. Adv. Mater. 2015, 7, 1233–1240. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors Applications in Medical Field: A Brief Review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Zhu, K.; Dietrich, R.; Didier, A.; Doyscher, D.; Märtlbauer, E. Recent Developments in Antibody-Based Assays for the Detection of Bacterial Toxins. Toxins 2014, 6, 1325–1348. [Google Scholar] [CrossRef]
- Liu, X.; Marrakchi, M.; Xu, D.; Dong, H.; Andreescu, S. Biosensors Based on Modularly Designed Synthetic Peptides for Recognition, Detection and Live/Dead Differentiation of Pathogenic Bacteria. Biosens. Bioelectron. 2016, 80, 9–16. [Google Scholar] [CrossRef]
- Lian, Y.; He, F.; Wang, H.; Tong, F. A New Aptamer/Graphene Interdigitated Gold Electrode Piezoelectric Sensor for Rapid and Specific Detection of Staphylococcus aureus. Biosens. Bioelectron. 2015, 65, 314–319. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, Z.; Chen, Y.; Gao, Z. Rapid Detection of Staphylococcal Enterotoxin B by Two-Dimensional Molecularly Imprinted Film-Coated Quartz Crystal Microbalance. Anal. Lett. 2012, 45, 283–295. [Google Scholar] [CrossRef]
- Suaifan, G.A.R.Y.; Alhogail, S.; Zourob, M. Rapid and Low-Cost Biosensor for the Detection of Staphylococcus aureus. Biosens. Bioelectron. 2017, 90, 230–237. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, J.; Feng, X.; Xie, G.; Zhang, W.; Xu, H. Rapid Enrichment and Detection of Staphylococcus aureus in Milk Using Polyethyleneimine Functionalized Magnetic Nanoparticles. Microchem. J. 2022, 178, 107388. [Google Scholar] [CrossRef]
- Verdoodt, N.; Basso, C.R.; Rossi, B.F.; Pedrosa, V.A. Development of a Rapid and Sensitive Immunosensor for the Detection of Bacteria. Food Chem. 2017, 221, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Dostálek, J.; Chen, S.; Rasooly, A.; Jiang, S.; Yee, S.S. Spectral Surface Plasmon Resonance Biosensor for Detection of Staphylococcal Enterotoxin B in Milk. Int. J. Food Microbiol. 2002, 75, 61–69. [Google Scholar] [CrossRef]
- Dester, E.; Alocilja, E. Current Methods for Extraction and Concentration of Foodborne Bacteria with Glycan-Coated Magnetic Nanoparticles: A Review. Biosensors 2022, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan; Daya, K.S.; Tirumalai, P.S.; Alocilja, E. Impedance and Magnetohydrodynamic Measurements for Label Free Detection and Differentiation of E. Coli and S. Aureus Using Magnetic Nanoparticles. IEEE Trans. NanoBiosci. 2018, 17, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Yim, C.; Kwon, D.; Lee, J.; Shin, H.H.; Cha, H.J.; Jeon, S. A Facile and Sensitive Detection of Pathogenic Bacteria Using Magnetic Nanoparticles and Optical Nanocrystal Probes. Analyst 2012, 137, 3609–3612. [Google Scholar] [CrossRef]
- Bohara, R.A.; Pawar, S.H. Innovative Developments in Bacterial Detection with Magnetic Nanoparticles. Appl. Biochem. Biotech. 2015, 176, 1044–1058. [Google Scholar] [CrossRef]
- Nakamura, N.; Burgess, J.G.; Yagiuda, K.; Kudo, S.; Sakaguchi, T.; Matsunaga, T. Detection and Removal of Escherichia Coli Using Fluorescein Isothiocyanate Conjugated Monoclonal Antibody Immobilized on Bacterial Magnetic Particles. Anal. Chem. 1993, 65, 2036–2039. [Google Scholar] [CrossRef]
- Briceno, R.K.; Sergent, S.R.; Benites, S.M.; Alocilja, E.C. Nanoparticle-Based Biosensing Assay for Universally Accessible Low-Cost TB Detection with Comparable Sensitivity as Culture. Diagnostics 2019, 9, 222. [Google Scholar] [CrossRef]
- Boodoo, C.; Dester, E.; Sharief, S.A.; Alocilja, E.C. Influence of Biological and Environmental Factors in the Extraction and Concentration of Foodborne Pathogens Using Glycan-Coated Magnetic Nanoparticles. J. Food Protect. 2023, 86, 100066. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, P.; Tu, D.; Ma, E.; Zhu, H.; Chen, X. Lanthanide-Doped Upconversion Nano-Bioprobes: Electronic Structures, Optical Properties, and Biodetection. Chem. Soc. Rev. 2014, 44, 1379–1415. [Google Scholar] [CrossRef] [PubMed]
- Sultangaziyev, A.; Bukasov, R. Review: Applications of Surface-Enhanced Fluorescence (SEF) Spectroscopy in Bio-Detection and Biosensing. Sens. Bio-Sens. Res 2020, 30, 100382. [Google Scholar] [CrossRef] [PubMed]
- Multiplex Polymerase Chain Reaction Assay for Simultaneous Detection of Staphylococcus aureus and Streptococcal Causes of Bovine Mastitis. Available online: https://www.journalofdairyscience.org/article/S0022-0302(01)74574-2/pdf (accessed on 29 July 2022).
- Wang, Y.; Alocilja, E.C. Gold Nanoparticle-Labeled Biosensor for Rapid and Sensitive Detection of Bacterial Pathogens. J. Biol. Eng. 2015, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Crosby, H.A.; Hernandez, F.J.; Malone, C.L.; McNamara, J.O.; Horswill, A.R. Staphylococcus aureus Nuc2 Is a Functional, Surface-Attached Extracellular Nuclease. PLoS ONE 2014, 9, e95574. [Google Scholar] [CrossRef]
- Hu, Y.; Meng, J.; Shi, C.; Hervin, K.; Fratamico, P.M.; Shi, X. Characterization and Comparative Analysis of a Second Thermonuclease from Staphylococcus aureus. Microbiol. Res. 2013, 168, 174–182. [Google Scholar] [CrossRef]
- Alarcón, B.; Vicedo, B.; Aznar, R. PCR-based Procedures for Detection and Quantification of Staphylococcus aureus and Their Application in Food. J. Appl. Microbiol. 2006, 100, 352–364. [Google Scholar] [CrossRef]
- Sandel, M.K.; McKillip, J.L. Virulence and Recovery of Staphylococcus aureus Relevant to the Food Industry Using Improvements on Traditional Approaches. Food Control. 2004, 15, 5–10. [Google Scholar] [CrossRef]
- Gearheart, L.A.; Ploehn, H.J.; Murphy, C.J. Oligonucleotide Adsorption to Gold Nanoparticles: A Surface-Enhanced Raman Spectroscopy Study of Intrinsically Bent DNA. J. Phys. Chem. B 2001, 105, 12609–12615. [Google Scholar] [CrossRef]
- Bhusal, N.; Shrestha, S.; Pote, N.; Alocilja, E.C. Nanoparticle-Based Biosensing of Tuberculosis, an Affordable and Practical Alternative to Current Methods. Biosensors 2018, 9, 1. [Google Scholar] [CrossRef]
- Gordillo-Marroquín, C.; Gómez-Velasco, A.; Sánchez-Pérez, H.J.; Pryg, K.; Shinners, J.; Murray, N.; Muñoz-Jiménez, S.G.; Bencomo-Alerm, A.; Gómez-Bustamante, A.; Jonapá-Gómez, L.; et al. Magnetic Nanoparticle-Based Biosensing Assay Quantitatively Enhances Acid-Fast Bacilli Count in Paucibacillary Pulmonary Tuberculosis. Biosensors 2018, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Kamikawa, T.L.; Mikolajczyk, M.G.; Kennedy, M.; Zhong, L.; Zhang, P.; Setterington, E.B.; Scott, D.E.; Alocilja, E.C. Pandemic Influenza Detection by Electrically Active Magnetic Nanoparticles and Surface Plasmon Resonance. IEEE Trans. Nanotechnol. 2012, 11, 88–96. [Google Scholar] [CrossRef] [PubMed]
- NanoDropTM One/OneC Microvolume UV-Vis Spectrophotometer. Available online: https://www.thermofisher.com/order/catalog/product/ND-ONEC-W (accessed on 24 July 2022).
- 3091_NanoDrop One_Help.Book. Available online: https://tools.thermofisher.com/content/sfs/manuals/3091-NanoDrop-One-Help-UG-en.pdf (accessed on 24 July 2022).
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key Role of Teichoic Acid Net Charge in Staphylococcus aureus Colonization of Artificial Surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health. Biomed. Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef]
- Dester, E.; Kao, K.; Alocilja, E.C. Detection of Unamplified E. Coli O157 DNA Extracted from Large Food Samples Using a Gold Nanoparticle Colorimetric Biosensor. Biosensors 2022, 12, 274. [Google Scholar] [CrossRef]
- Staphylococcus aureus (ID 154)—Genome—NCBI. Available online: https://www-ncbi-nlm-nih-gov.proxy1.cl.msu.edu/genome/154 (accessed on 24 July 2022).
- Yrad, F.M.; Castañares, J.M.; Alocilja, E.C. Visual Detection of Dengue-1 RNA Using Gold Nanoparticle-Based Lateral Flow Biosensor. Diagnostics 2019, 9, 74. [Google Scholar] [CrossRef]
- Jin, R.; Wu, G.; Li, Z.; Mirkin, C.A.; Schatz, G.C. What Controls the Melting Properties of DNA-Linked Gold Nanoparticle Assemblies? J. Am. Chem. Soc. 2003, 125, 1643–1654. [Google Scholar] [CrossRef]
- Ways, T.; Lau, W.; Khutoryanskiy, V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Zhang, D.; Huarng, M.C.; Alocilja, E.C. A Multiplex Nanoparticle-Based Bio-Barcoded DNA Sensor for the Simultaneous Detection of Multiple Pathogens. Biosens. Bioelectron. 2010, 26, 1736–1742. [Google Scholar] [CrossRef]
- Vetrone, S.A.; Huarng, M.C.; Alocilja, E.C. Detection of Non-PCR Amplified S. Enteritidis Genomic DNA from Food Matrices Using a Gold-Nanoparticle DNA Biosensor: A Proof-of-Concept Study. Sensors 2012, 12, 10487–10499. [Google Scholar] [CrossRef]
- Baetsen-Young, A.M.; Vasher, M.; Matta, L.L.; Colgan, P.; Alocilja, E.C.; Day, B. Direct Colorimetric Detection of Unamplified Pathogen DNA by Dextrin-Capped Gold Nanoparticles. Biosens. Bioelectron. 2018, 101, 29–36. [Google Scholar] [CrossRef]
- Hao, R.-Z.; Song, H.-B.; Zuo, G.-M.; Yang, R.-F.; Wei, H.-P.; Wang, D.-B.; Cui, Z.-Q.; Zhang, Z.; Cheng, Z.-X.; Zhang, X.-E. DNA Probe Functionalized QCM Biosensor Based on Gold Nanoparticle Amplification for Bacillus Anthracis Detection. Biosens. Bioelectron. 2011, 26, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Carr, D.J.; Alocilja, E.C. Fluorescent Bio-Barcode DNA Assay for the Detection of Salmonella Enterica Serovar Enteritidis. Biosens. Bioelectron. 2009, 24, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Torres-Chavolla, E.; Alocilja, E.C. Nanoparticle Based DNA Biosensor for Tuberculosis Detection Using Thermophilic Helicase-Dependent Isothermal Amplification. Biosens. Bioelectron. 2011, 26, 4614–4618. [Google Scholar] [CrossRef]
- Janell, C.; Lilia, F.; Evangelyn, A.; Ferdinand, S.; Brian, B. Fabrication of a Nanoparticle-Based Sensor for the Detection of Dengue Virus-3 in Aedes Aegypti. Int. J. Sci. Basic Appl. Res. 2016, 26, 138–157. [Google Scholar]
- Fish and Fishery Products Hazards and Controls Guidance. Available online: https://www.fda.gov/files/food/published/Fish-and-Fishery-Products-Hazards-and-Controls-Guidance-Chapter-15-Download.pdf (accessed on 12 April 2023).
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A Microfluidic Colorimetric Biosensor for Rapid Detection of Escherichia Coli O157:H7 Using Gold Nanoparticle Aggregation and Smart Phone Imaging. Biosens. Bioelectron. 2019, 124, 143–149. [Google Scholar] [CrossRef]


| Transducer | Bio-Recognition Element | Preparation | Sample(s) | Assay Time | LOD | Source |
|---|---|---|---|---|---|---|
| Impedimetric | Peptide | Fabrication (<2 h) | Pure culture | 30 min | 102 | [19] |
| Piezoelectric | Aptamer | Gold electrode (7 h) | Milk and pure culture | 1 h | 4.1 × 101, 41 CFU/mL | [20] |
| Piezoelectric | Antibody | Au electrode fabrication (1 day) | Milk | 2 h | 6.1 ng/mL | [21] |
| Colorimetric | Aptamer | Nanobead synthesis (1 day) | Milk, lettuce, turkey sausage, ground beef | 40 min | 40 CFU/mL | [22] |
| Fluorescence | Aptamer | Spike samples, PEI-MNP separation, extraction (30 min) | Skim milk | 1 h | 2.9 × 102 CFU/mL | [23] |
| LSPR | Antibody | GNP preparation (3 h) | Pure culture | 1 min | 120 CFU/mL | [24] |
| SPR direct detection; SPR sandwich assay | Antibody | SPR sensor chip (2 days), incubate S. aureus 10 min | Buffer; Buffer, Milk | ~1 h | 5 ng/mL; 0.5 ng/mL | [25] |
| Sample | Concentration Factor | Standard Error |
|---|---|---|
| PBS | 65.5 | 8.87 |
| Milk | 2.46 | 0.61 |
| Romaine Lettuce | 2.27 | 0.12 |
| Deli Ham | 1.38 | 0.19 |
| Sausage | 1.54 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boodoo, C.; Dester, E.; David, J.; Patel, V.; KC, R.; Alocilja, E.C. Multi-Probe Nano-Genomic Biosensor to Detect S. aureus from Magnetically-Extracted Food Samples. Biosensors 2023, 13, 608. https://doi.org/10.3390/bios13060608
Boodoo C, Dester E, David J, Patel V, KC R, Alocilja EC. Multi-Probe Nano-Genomic Biosensor to Detect S. aureus from Magnetically-Extracted Food Samples. Biosensors. 2023; 13(6):608. https://doi.org/10.3390/bios13060608
Chicago/Turabian StyleBoodoo, Chelsie, Emma Dester, Jeswin David, Vedi Patel, Rabin KC, and Evangelyn C. Alocilja. 2023. "Multi-Probe Nano-Genomic Biosensor to Detect S. aureus from Magnetically-Extracted Food Samples" Biosensors 13, no. 6: 608. https://doi.org/10.3390/bios13060608
APA StyleBoodoo, C., Dester, E., David, J., Patel, V., KC, R., & Alocilja, E. C. (2023). Multi-Probe Nano-Genomic Biosensor to Detect S. aureus from Magnetically-Extracted Food Samples. Biosensors, 13(6), 608. https://doi.org/10.3390/bios13060608






