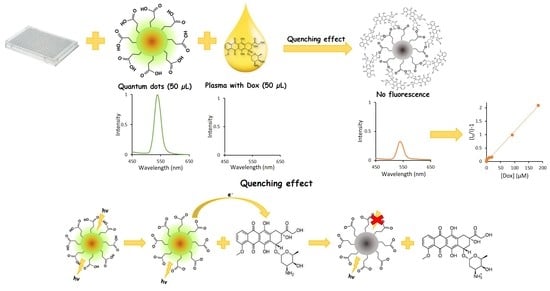
Fluorescent Alloyed CdZnSeS/ZnS Nanosensor for Doxorubicin Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. QD Synthesis and Hydrophilization
2.3. QD Characterization and Spectral Measurements
2.4. Quantum Yield Calculation
- Φx—relative QY of the QDs sample;
- Φst—the relative QY of the reference (coumarin-153);
- Ix—is the integral fluorescence intensity of the QDs sample;
- Ist—integral intensity of the reference (coumarin-153);
- Ax—optical density of the QDs sample;
- Ast—optical density of the reference (coumarin-153);
- nx—the refractive index of the sample (water);
- nst—the refractive index of the reference solvent (ethyl alcohol).
2.5. Optical Measurements
2.6. Analysis Performance in Plasma Samples
3. Results and Discussion
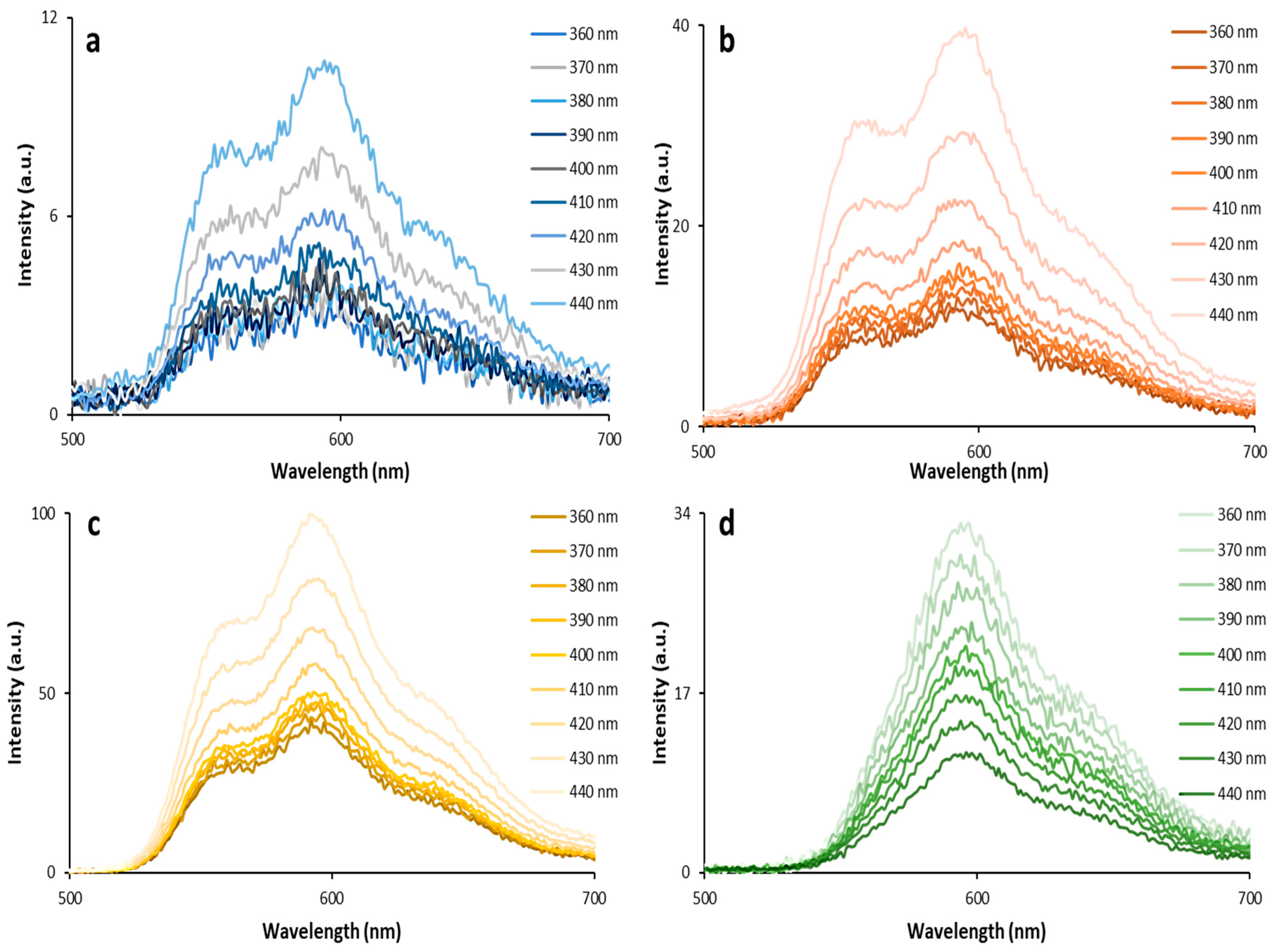
3.1. Optical Properties of QDs
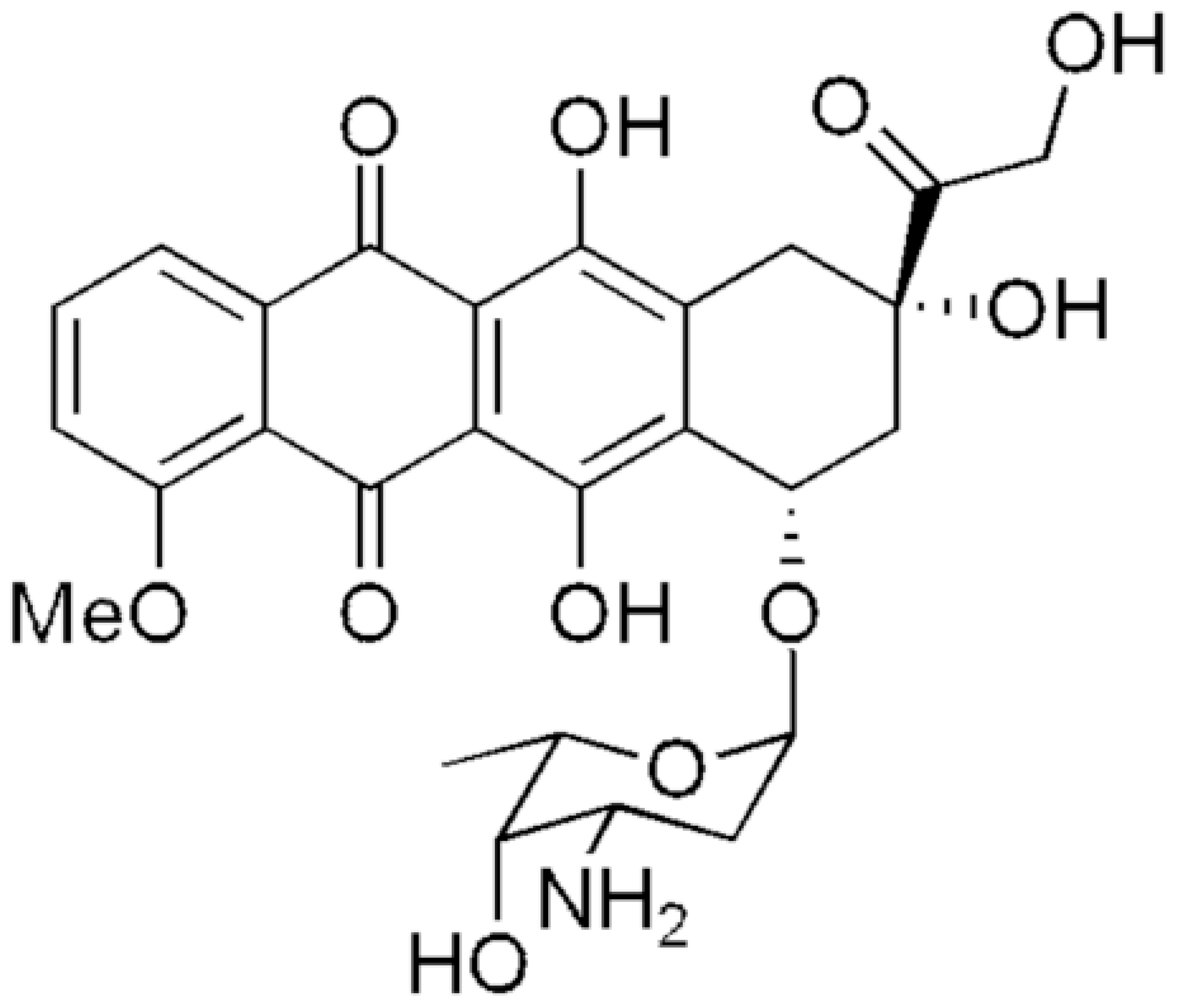
3.2. Optical Properties of DOX
3.3. Influence of QDs on the DOX Fluorescence
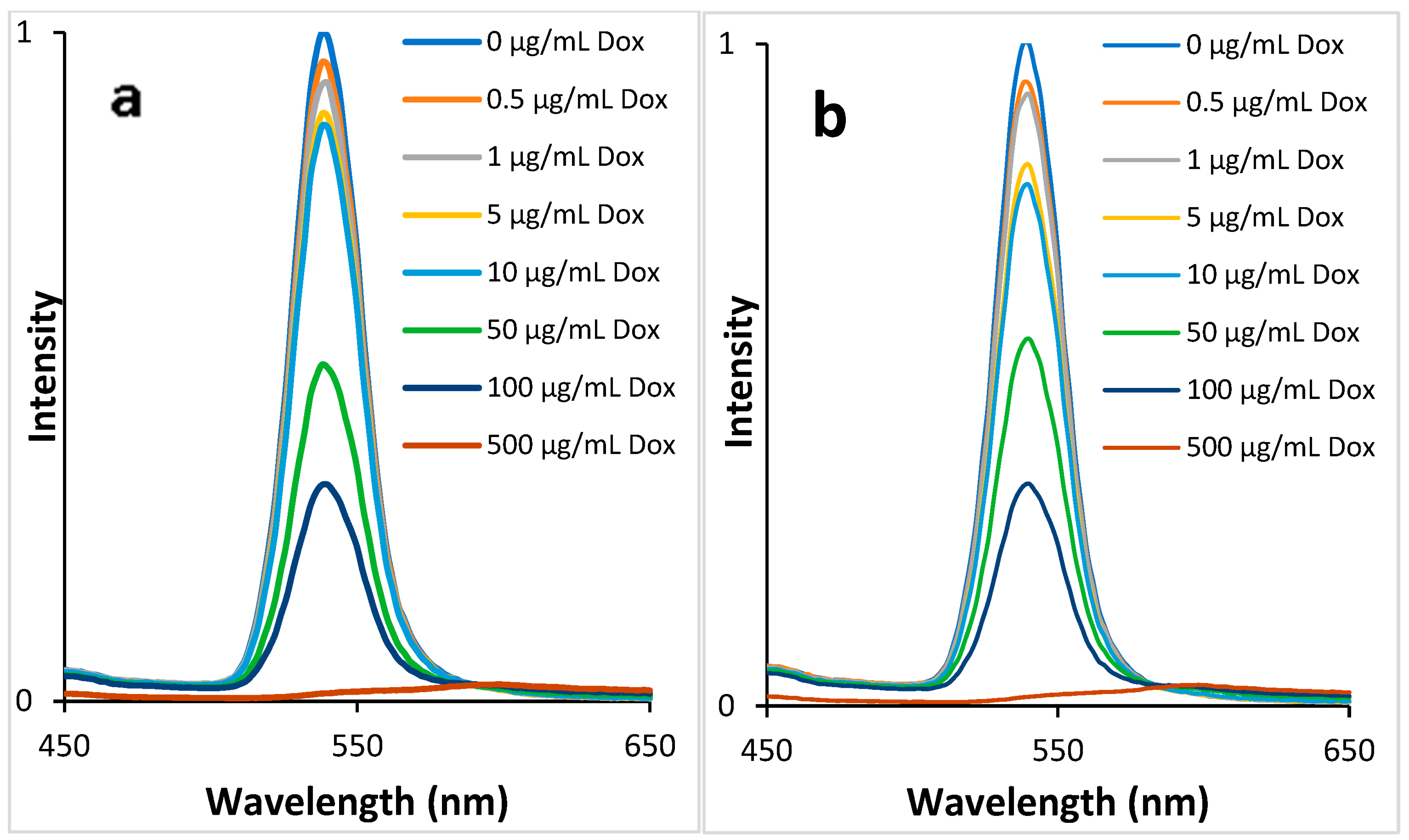
3.4. QDs Fluorescence Quenching by DOX
3.5. Determination of DOX in Human Blood Plasma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-Induced Cardiotoxicity: An Update on the Molecular Mechanism and Novel Therapeutic Strategies for Effective Management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, S.; Sivalingam, N. A Comprehensive Review on Time-Tested Anticancer Drug Doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Sikora, T.; Morawska, K.; Lisowski, W.; Rytel, P.; Dylong, A. Application of Optical Methods for Determination of Concentration of Doxorubicin in Blood and Plasma. Pharmaceuticals 2022, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Gulyaev, A.E.; Gelperina, S.E.; Skidan, I.N.; Antropov, A.S.; Kivman, G.Y.; Kreuter, J. Significant Transport of Doxorubicin into the Brain with Polysorbate 80- Coated Nanoparticles. Pharm. Res. 1999, 16, 1564–1569. [Google Scholar] [CrossRef]
- Álvarez-Cedrón, L.; Sayalero, M.L.; Lanao, J.M. High-Performance Liquid Chromatographic Validated Assay of Doxorubicin in Rat Plasma and Tissues. J. Chromatogr. B Biomed. Sci. Appl. 1999, 721, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Shinozawa, S.; Oda, T. Determination of Adriamycin (Doxorubicin) and Related Fluorescent Compounds in Rat Lymph and Gall by High-Performance Liquid Chromatography. J. Chromatogr. A 1981, 212, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Pinzõn-Daza, M.L.; Garzõn, R.; Couraud, P.O.; Romero, I.; Weksler, B.; Ghigo, D.; Bosia, A.; Riganti, C. The Association of Statins plus LDL Receptor-Targeted Liposome-Encapsulated Doxorubicin Increases in Vitro Drug Delivery across Blood–brain Barrier Cells. Br. J. Pharmacol. 2012, 167, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, H.; Qian, F.; Zhao, C.; Liao, X. Internal Standard Method for the Measurement of Doxorubicin and Daunorubicin by Capillary Electrophoresis with in-Column Double Optical-Fiber LED-Induced Fluorescence Detection. J. Pharm. Biomed. Anal. 2016, 117, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Skalová, Š.; Langmaier, J.; Barek, J.; Vyskočil, V.; Navrátil, T. Doxorubicin Determination Using Two Novel Voltammetric Approaches: A Comparative Study. Electrochim. Acta 2020, 330, 135180. [Google Scholar] [CrossRef]
- Porfireva, A.; Vorobev, V.; Babkina, S.; Evtugyn, G. Electrochemical Sensor Based on Poly(Azure B)-DNA Composite for Doxorubicin Determination. Sensors 2019, 19, 2085. [Google Scholar] [CrossRef]
- Yan, F.; Chen, J.; Jin, Q.; Zhou, H.; Sailjoi, A.; Liu, J.; Tang, W. Fast One-Step Fabrication of a Vertically-Ordered Mesoporous Silica-Nanochannel Film on Graphene for Direct and Sensitive Detection of Doxorubicin in Human Whole Blood. J. Mater. Chem. C 2020, 8, 7113–7119. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Fan, L.; Shen, B.; Ding, X.; Yuan, R.; Li, X.; Chen, W. Target-Driven Rolling Walker Based Electrochemical Biosensor for Ultrasensitive Detection of Circulating Tumor DNA Using Doxorubicin@tetrahedron-Au Tags. Biosens. Bioelectron. 2020, 148, 111826. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Banu, N.; Escobar, E.R.; García, G.R.; Cervantes-Martínez, J.; Villegas, T.C.; Salas, P.; De la Rosa, E. Stealth Modified Bottom up SERS Substrates for Label-Free Therapeutic Drug Monitoring of Doxorubicin in Blood Serum. Talanta 2020, 218, 121138. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Chandra, A.; Kaur, A.; Sabnis, N.; Lacko, A.; Gryczynski, Z.; Fudala, R.; Gryczynski, I. Fluorescence Properties of Doxorubicin in PBS Buffer and PVA Films. J. Photochem. Photobiol. B Biol. 2017, 170, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, R.J.; Schulman, S.G. Electronic Absorption Spectra and Protolytic Equilibria of Doxorubicin: Direct Spectrophotometric Determination of Microconstants. J. Pharm. Sci. 1977, 66, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Changenet-Barret, P.; Gustavsson, T.; Markovitsi, D.; Manet, I.; Monti, S. Unravelling Molecular Mechanisms in the Fluorescence Spectra of Doxorubicin in Aqueous Solution by Femtosecond Fluorescence Spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, X.; Wang, Y.; Zheng, X.; Li, C.M. Aptamer Based Fluorescence Recovery Assay for Aflatoxin B1 Using a Quencher System Composed of Quantum Dots and Graphene Oxide. Microchim. Acta 2014, 182, 571–578. [Google Scholar] [CrossRef]
- Khan, S.; Carneiro, L.S.A.; Vianna, M.S.; Romani, E.C.; Aucelio, R.Q. Determination of Histamine in Tuna Fish by Photoluminescence Sensing Using Thioglycolic Acid Modified CdTe Quantum Dots and Cationic Solid Phase Extraction. J. Lumin. 2017, 182, 71–78. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.Z.; Li, R.S.; Huang, C.Z. Localized Surface Plasmon Resonance of Gold Nanorods and Assemblies in the View of Biomedical Analysis. TrAC Trends Anal. Chem. 2016, 80, 429–443. [Google Scholar] [CrossRef]
- Yang, M.; Yan, Y.; Liu, E.; Hu, X.; Hao, H.; Fan, J. Polyethyleneimine-Functionalized Carbon Dots as a Fluorescent Probe for Doxorubicin Hydrochloride by an Inner Filter Effect. Opt. Mater. (Amst). 2021, 112, 110743. [Google Scholar] [CrossRef]
- Zhu, J.; Chu, H.; Shen, J.; Wang, C.; Wei, Y. Green Preparation of Carbon Dots from Plum as a Ratiometric Fluorescent Probe for Detection of Doxorubicin. Opt. Mater. 2021, 114, 110941. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, R.; Gu, S.; Zhang, L.; Li, N.; Qiao, J. Nitrogen and Phosphorus Co-Doped Carbon Dots as an Effective Fluorescence Probe for the Detection of Doxorubicin and Cell Imaging. Opt. Mater. 2022, 128, 112323. [Google Scholar] [CrossRef]
- Huang, K.Y.; He, H.X.; He, S.B.; Zhang, X.P.; Peng, H.P.; Lin, Z.; Deng, H.H.; Xia, X.H.; Chen, W. Gold Nanocluster-Based Fluorescence Turn-off Probe for Sensing of Doxorubicin by Photoinduced Electron Transfer. Sens. Actuators B Chem. 2019, 296, 126656. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, Z.; Gong, Y.; Yan, G. Phosphorescent Quantum Dots/doxorubicin Nanohybrids Based on Photoinduced Electron Transfer for Detection of DNA. Biosens. Bioelectron. 2014, 59, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Niu, L.; Su, X. Detection of DNA via the Fluorescence Quenching of Mn-Doped ZnSe D-dots/doxorubicin/DNA Ternary Complexes System. J. Fluoresc. 2012, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Raichlin, S.; Sharon, E.; Freeman, R.; Tzfati, Y.; Willner, I. Electron-Transfer Quenching of Nucleic Acid-Functionalized CdSe/ZnS Quantum Dots by Doxorubicin: A Versatile System for the Optical Detection of DNA, Aptamer–substrate Complexes and Telomerase Activity. Biosens. Bioelectron. 2011, 26, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, O.A.; Guhrenz, C.; Schneider, K.; Beloglazova, N.V.; Beloglazova, N.V.; Goryacheva, I.Y.; De Saeger, S.; Gaponik, N. Silanized Luminescent Quantum Dots for the Simultaneous Multicolor Lateral Flow Immunoassay of Two Mycotoxins. ACS Appl. Mater. Interfaces 2020, 12, 24575–24584. [Google Scholar] [CrossRef]
- Goryacheva, O.A.; Wegner, K.D.; Sobolev, A.M.; Häusler, I.; Gaponik, N.; Goryacheva, I.Y.; Resch-Genger, U. Influence of Particle Architecture on the Photoluminescence Properties of Silica-Coated CdSe Core/shell Quantum Dots. Anal. Bioanal. Chem. 2022, 414, 4427–4439. [Google Scholar] [CrossRef] [PubMed]
- Drozd, D.D.; Byzova, N.A.; Pidenko, P.S.; Tsyupka, D.V.; Strokin, P.D.; Goryacheva, O.A.; Zherdev, A.V.; Goryacheva, I.Y.; Dzantiev, B.B. Luminescent Alloyed Quantum Dots for Turn-off Enzyme-Based Assay. Anal. Bioanal. Chem. 2022, 414, 4471–4480. [Google Scholar] [CrossRef]
- Podkolodnaya, Y.A.; Kokorina, A.A.; Goryacheva, I.Y. A Facile Approach to the Hydrothermal Synthesis of Silica Nanoparticle/Carbon Nanostructure Luminescent Composites. Materials 2022, 15, 8469. [Google Scholar] [CrossRef]
- Savla, R.; Taratula, O.; Garbuzenko, O.; Minko, T. Tumor Targeted Quantum Dot-Mucin 1 Aptamer-Doxorubicin Conjugate for Imaging and Treatment of Cancer. J. Control. Release 2011, 153, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bagalkot, V.; Zhang, L.; Levy-Nissenbaum, E.; Jon, S.; Kantoff, P.W.; Langery, R.; Farokhzad, O.C. Quantum Dot-Aptamer Conjugates for Synchronous Cancer Imaging, Therapy, and Sensing of Drug Delivery Based on Bi-Fluorescence Resonance Energy Transfer. Nano Lett. 2007, 7, 3065–3070. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Lipsitz, S.R.; Mone, S.M.; Goorin, A.M.; Sallan, S.E.; Sanders, S.P.; Orav, E.J.; Gelber, R.D.; Colan, S.D. Female Sex and Higher Drug Dose as Risk Factors for Late Cardiotoxic Effects of Doxorubicin Therapy for Childhood Cancer. N. Engl. J. Med. 1995, 332, 1738–1744. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, F.; Liu, R. The Interaction of Bovine Serum Albumin with Doxorubicin-Loaded Superparamagnetic Iron Oxide Nanoparticles: Spectroscope and Molecular Modelling Identification. Nanotoxicology 2013, 7, 97–104. [Google Scholar] [CrossRef]
- Kumar Panigrahi, S.; Kumar Mishra, A. Inner Filter Effect in Fluorescence Spectroscopy: As a Problem and as a Solution. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100318. [Google Scholar] [CrossRef]
- Vale, B.R.C.; Mourão, R.S.; Bettini, J.; Sousa, J.C.L.; Ferrari, J.L.; Reiss, P.; Aldakov, D.; Schiavon, M.A. Ligand Induced Switching of the Band Alignment in Aqueous Synthesized CdTe/CdS Core/shell Nanocrystals. Sci. Rep. 2019, 9, 8332. [Google Scholar] [CrossRef]
- Daramola, O.A.; Siwe Noundou, X.; Nkanga, C.I.; Tseki, P.F.; Krause, R.W.M. Synthesis of pH Sensitive Dual Capped CdTe QDs: Their Optical Properties and Structural Morphology. J. Fluoresc. 2020, 30, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Gołuński, G.; Borowik, A.; Derewońko, N.; Kawiak, A.; Rychłowski, M.; Woziwodzka, A.; Piosik, J. Pentoxifylline as a Modulator of Anticancer Drug Doxorubicin. Part II: Reduction of Doxorubicin DNA Binding and Alleviation of Its Biological Effects. Biochimie 2016, 123, 95–102. [Google Scholar] [CrossRef]
- Hines, A.; Flin, H. Doxorubicin and Ifosfamide Sarcoma PROTOCOL REF: MPHADOXIFO (Version No: 1.0). 2016. Available online: https://www.clatterbridgecc.nhs.uk/application/files/9814/9787/1938/Doxorubicin_and_Ifosfamide_Sarcoma_Protocol_V1.0.pdf (accessed on 1 April 2023).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mescheryakova, S.A.; Matlakhov, I.S.; Strokin, P.D.; Drozd, D.D.; Goryacheva, I.Y.; Goryacheva, O.A. Fluorescent Alloyed CdZnSeS/ZnS Nanosensor for Doxorubicin Detection. Biosensors 2023, 13, 596. https://doi.org/10.3390/bios13060596
Mescheryakova SA, Matlakhov IS, Strokin PD, Drozd DD, Goryacheva IY, Goryacheva OA. Fluorescent Alloyed CdZnSeS/ZnS Nanosensor for Doxorubicin Detection. Biosensors. 2023; 13(6):596. https://doi.org/10.3390/bios13060596
Chicago/Turabian StyleMescheryakova, Svetlana A., Ivan S. Matlakhov, Pavel D. Strokin, Daniil D. Drozd, Irina Yu. Goryacheva, and Olga A. Goryacheva. 2023. "Fluorescent Alloyed CdZnSeS/ZnS Nanosensor for Doxorubicin Detection" Biosensors 13, no. 6: 596. https://doi.org/10.3390/bios13060596
APA StyleMescheryakova, S. A., Matlakhov, I. S., Strokin, P. D., Drozd, D. D., Goryacheva, I. Y., & Goryacheva, O. A. (2023). Fluorescent Alloyed CdZnSeS/ZnS Nanosensor for Doxorubicin Detection. Biosensors, 13(6), 596. https://doi.org/10.3390/bios13060596