Enzymatic Electrochemical/Fluorescent Nanobiosensor for Detection of Small Chemicals
Abstract
1. Introduction
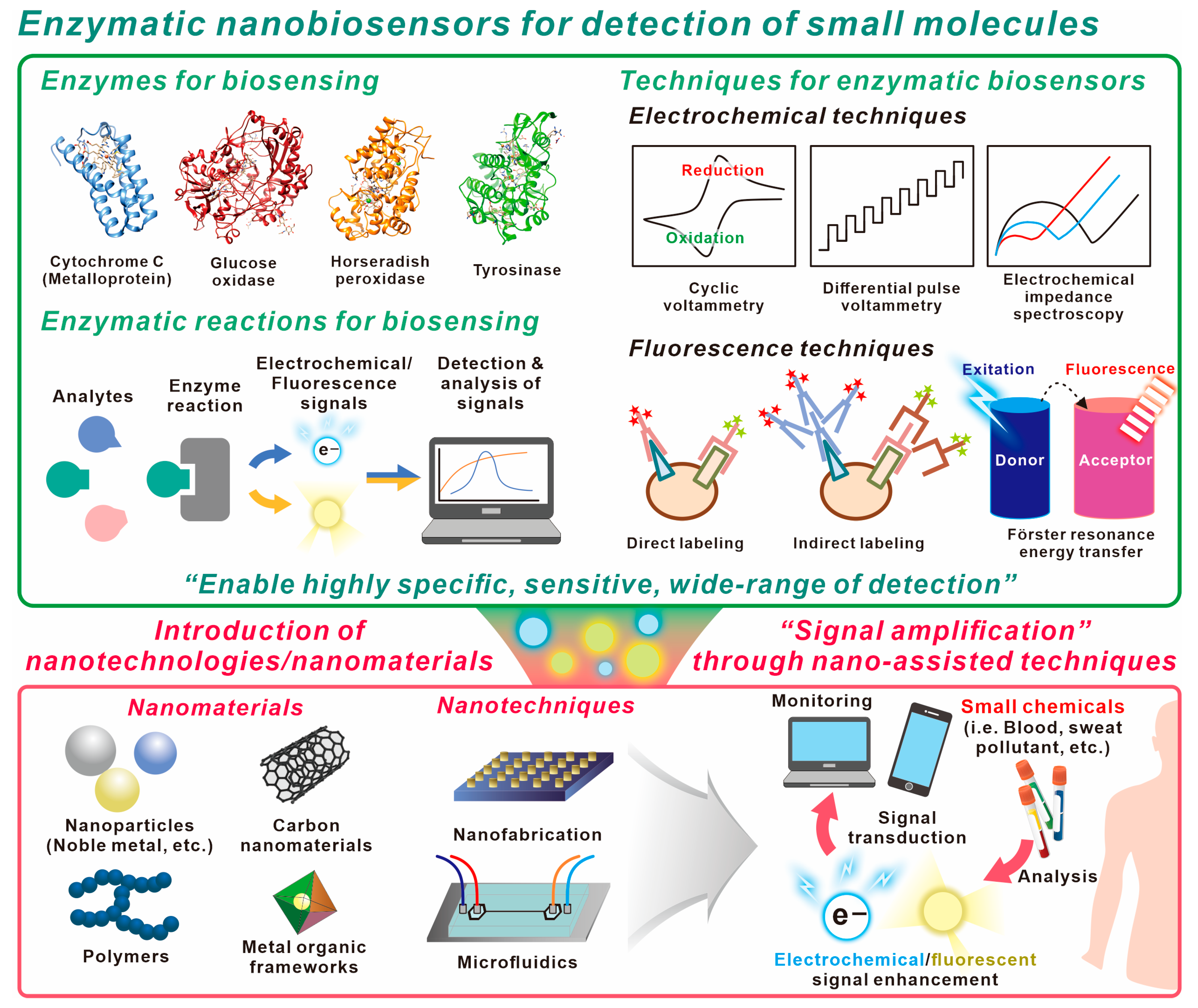
2. Components of Enzymatic Biosensors
2.1. Enzymes
2.2. Enzymatic Reactions for Biosensing Applications

2.3. Techniques Used in Enzymatic Biosensing
2.3.1. Electrochemical Technique
2.3.2. Fluorescence Technique

3. Enzymatic Electrochemical Nanobiosensors
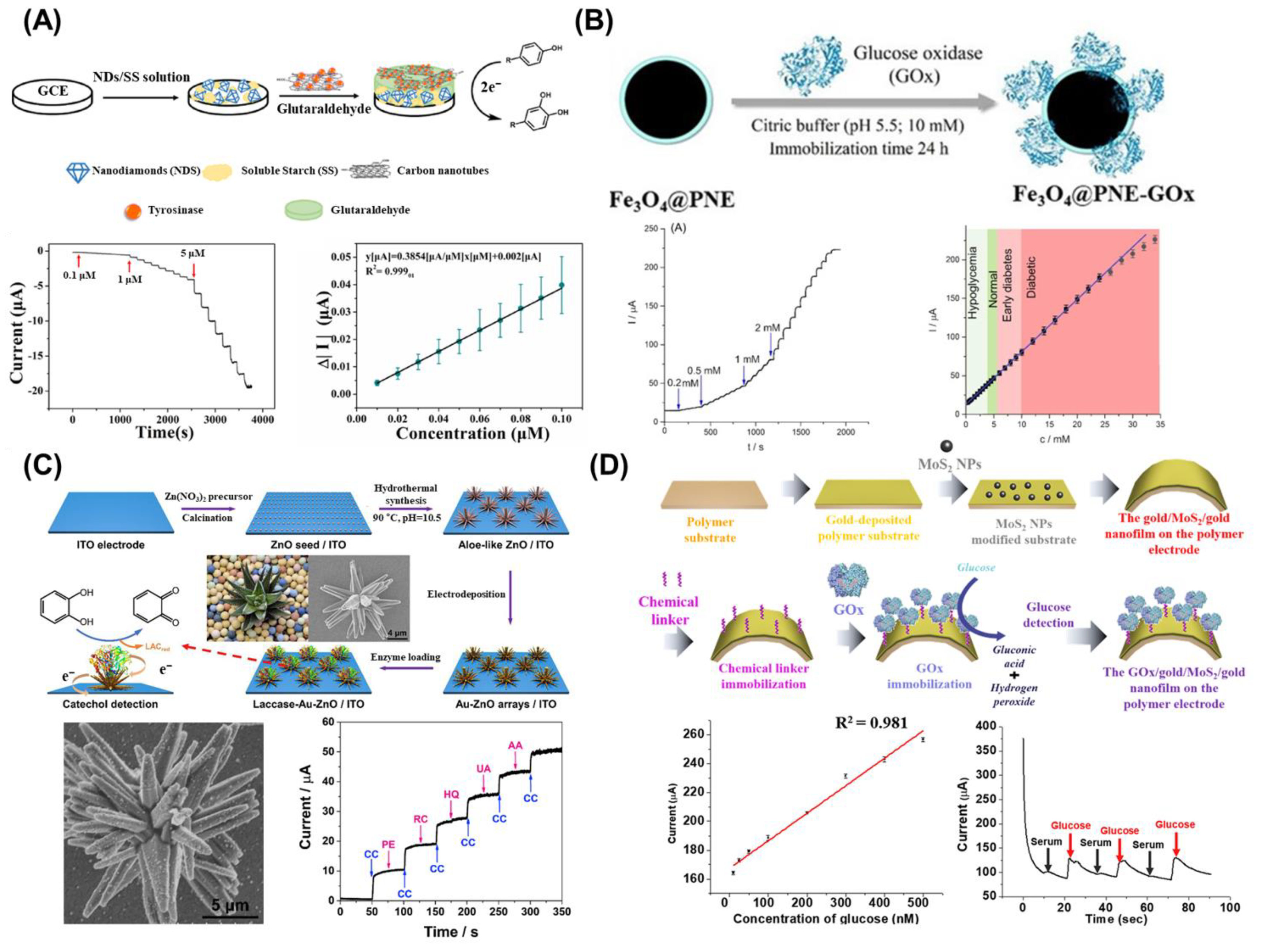
4. Enzymatic Fluorescent Nanobiosensors
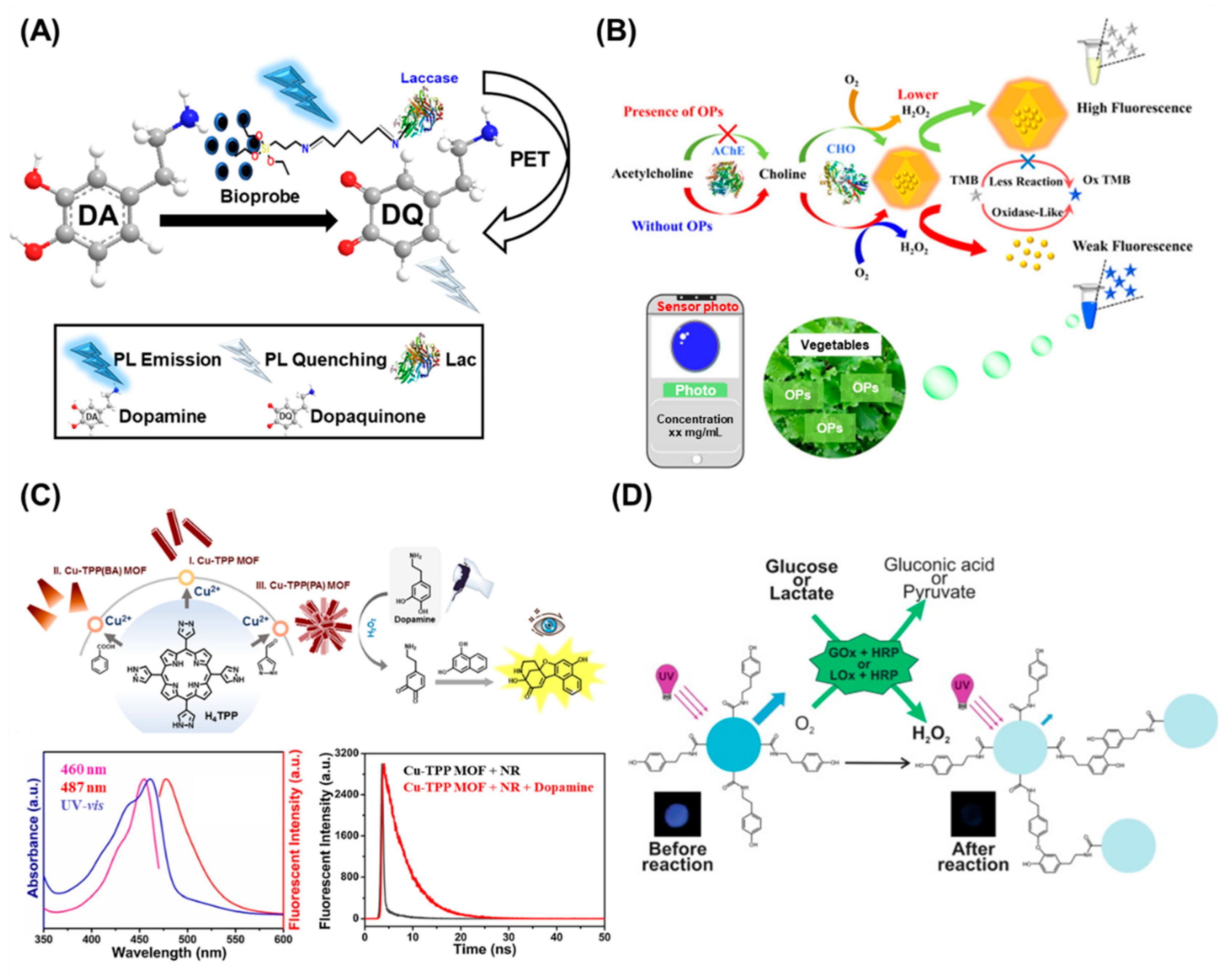
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, L.; Liu, J.; Li, P.; Tang, B.; James, T.D. Two-photon small-molecule fluorescence-based agents for sensing, imaging, and therapy within biological systems. Chem. Soc. Rev. 2021, 50, 702–734. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-H.; Tian, H.; Zang, Y.; Sedgwick, A.C.; Li, J.; Sessler, J.L.; He, X.-P.; James, T.D. Small-molecule fluorescence-based probes for interrogating major organ diseases. Chem. Soc. Rev. 2021, 50, 9391–9429. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Feng, Z.-Y.; Li, D.; Jin, B.; Lan, Y.; Meng, L.-Y. Recent trends in carbon-based microelectrodes as electrochemical sensors for neurotransmitter detection: A review. TrAC Trends Anal. Chem. 2022, 148, 116541. [Google Scholar] [CrossRef]
- Stolz, R.M.; Kolln, A.F.; Rocha, B.C.; Brinks, A.; Eagleton, A.M.; Mendecki, L.; Vashisth, H.; Mirica, K.A. Epitaxial Self-Assembly of Interfaces of 2D Metal–Organic Frameworks for Electroanalytical Detection of Neurotransmitters. ACS Nano 2022, 16, 13869–13883. [Google Scholar] [CrossRef]
- Ryu, J.; Lee, E.; Kang, C.; Lee, M.; Kim, S.; Park, S.; Lee, D.; Kwon, Y. Rapid Screening of Glucocorticoid Receptor (GR) Effectors Using Cortisol-Detecting Sensor Cells. Int. J. Mol. Sci. 2021, 22, 4747. [Google Scholar] [CrossRef]
- Dong, Q.; Ryu, H.; Lei, Y. Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Shamsazar, A.; Asadi, A.; Seifzadeh, D.; Mahdavi, M. A novel and highly sensitive sandwich-type immunosensor for prostate-specific antigen detection based on MWCNTs-Fe3O4 nanocomposite. Sens. Actuators B Chem. 2021, 346, 130459. [Google Scholar] [CrossRef]
- Huang, L.; Zeng, Y.; Liu, X.; Tang, D. Pressure-Based Immunoassays with Versatile Electronic Sensors for Carcinoembryonic Antigen Detection. ACS Appl. Mater. Interfaces 2021, 13, 46440–46450. [Google Scholar] [CrossRef]
- Ding, Q.; Li, C.; Wang, H.; Xu, C.; Kuang, H. Electrochemical detection of heavy metal ions in water. Chem. Commun. 2021, 57, 7215–7231. [Google Scholar] [CrossRef]
- Xianyu, Y.; Lin, Y.; Chen, Q.; Belessiotis-Richards, A.; Stevens, M.M.; Thomas, M.R. Iodide-Mediated Rapid and Sensitive Surface Etching of Gold Nanostars for Biosensing. Angew. Chem. Int. Ed. 2021, 60, 9891–9896. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; de A. Falcão, I.R.; da S. Souza, J.E.; Rocha, T.G.; de Sousa, I.G.; Cavalcante, A.L.G.; de Oliveira, A.L.B.; de Sousa, M.C.M.; dos Santos, J.C.S. Designing of Nanomaterials-Based Enzymatic Biosensors: Synthesis, Properties, and Applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Sohal, N.; Maity, B.; Shetti, N.P.; Basu, S. Biosensors Based on MnO2 Nanostructures: A Review. ACS Appl. Nano Mater. 2021, 4, 2285–2302. [Google Scholar] [CrossRef]
- Yoon, J.; Chung, Y.-H.; Lee, T.; Kim, J.H.; Kim, J.; Choi, J.-W. A biomemory chip composed of a myoglobin/CNT heterolayer fabricated by the protein-adsorption-precipitation-crosslinking (PAPC) technique. Colloids Surf. B Biointerfaces 2015, 136, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lee, T.; Bapurao, G.B.; Jo, J.; Oh, B.-K.; Choi, J.-W. Electrochemical H2O2 biosensor composed of myoglobin on MoS2 nanoparticle-graphene oxide hybrid structure. Biosens. Bioelectron. 2017, 93, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Chung, Y.-H.; Chen, Q.; Min, J.; Choi, J.-W. Fatigue Test of Cytochrome C Self-Assembled on a 11-MUA Layer Based on Electrochemical Analysis for Bioelectronic Device. J. Nanosci. Nanotechnol. 2015, 15, 5537–5542. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Pereira, P.A.; Silva, T.A.; Caetano, F.R.; Ribovski, L.; Zapp, E.; Brondani, D.; Bergamini, M.F.; Marcolino, L.H.; Banks, C.E.; Oliveira, O.N.; et al. Polyphenol oxidase-based electrochemical biosensors: A review. Anal. Chim. Acta 2020, 1139, 198–221. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Soldatkin, O.O.; Kucherenko, D.Y.; Soldatkina, O.V.; Dzyadevych, S.V. Advances in nanomaterial application in enzyme-based electrochemical biosensors: A review. Nanoscale Adv. 2019, 1, 4560–4577. [Google Scholar] [CrossRef] [PubMed]
- Irfan Azizan, M.A.; Taufik, S.; Norizan, M.N.; Abdul Rashid, J.I. A review on surface modification in the development of electrochemical biosensor for malathion. Biosens. Bioelectron. X 2023, 13, 100291. [Google Scholar] [CrossRef]
- Wei, X.; Wang, S.; Zhan, Y.; Kai, T.; Ding, P. Sensitive Identification of Microcystin-LR via a Reagent-Free and Reusable Electrochemical Biosensor Using a Methylene Blue-Labeled Aptamer. Biosensors 2022, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Roslan, M.A.M.; Abd Shukor, M.Y.; Halim, M.; Yasid, N.A.; Abdullah, J.; Md Yasin, I.S.; Wasoh, H. Advances in Aptamer-Based Biosensors and Cell-Internalizing SELEX Technology for Diagnostic and Therapeutic Application. Biosensors 2022, 12, 922. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wei, H.; Han, Z.; Wu, F.; Mao, L. Enzymatic electrochemical biosensors for in situ neurochemical measurement. Curr. Opin. Electrochem. 2020, 19, 162–167. [Google Scholar] [CrossRef]
- Choi, J.-H.; Choi, J.-W. Metal-Enhanced Fluorescence by Bifunctional Au Nanoparticles for Highly Sensitive and Simple Detection of Proteolytic Enzyme. Nano Lett. 2020, 20, 7100–7107. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, D. Exotic carbon nanotube based field effect transistor for the selective detection of sucrose. Mater. Lett. 2020, 268, 127571. [Google Scholar] [CrossRef]
- Si, Y.; Lee, H.J. Carbon nanomaterials and metallic nanoparticles-incorporated electrochemical sensors for small metabolites: Detection methodologies and applications. Curr. Opin. Electrochem. 2020, 22, 234–243. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Ghafori Gorab, M.; Masoud Hashemi, S.; Javanshir, S.; Ahangari Cohan, R.; et al. Applications of carbon-based conductive nanomaterials in biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, D.; Wei, Q. A laccase based biosensor on AuNPs-MoS2 modified glassy carbon electrode for catechol detection. Colloids Surf. B Biointerfaces 2020, 186, 110683. [Google Scholar] [CrossRef]
- He, Y.; Hu, F.; Zhao, J.; Yang, G.; Zhang, Y.; Chen, S.; Yuan, R. Bifunctional Moderator-Powered Ratiometric Electrochemiluminescence Enzymatic Biosensors for Detecting Organophosphorus Pesticides Based on Dual-Signal Combined Nanoprobes. Anal. Chem. 2021, 93, 8783–8790. [Google Scholar] [CrossRef]
- Alizadeh, N.; Ghasemi, S.; Salimi, A.; Sham, T.-K.; Hallaj, R. CuO nanorods as a laccase mimicking enzyme for highly sensitive colorimetric and electrochemical dual biosensor: Application in living cell epinephrine analysis. Colloids Surf. B Biointerfaces 2020, 195, 111228. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, G.; Xu, B. Enzymatic Hydrogelation of Small Molecules. Acc. Chem. Res. 2008, 41, 315–326. [Google Scholar] [CrossRef]
- Singh, H.; Tiwari, K.; Tiwari, R.; Pramanik, S.K.; Das, A. Small Molecule as Fluorescent Probes for Monitoring Intracellular Enzymatic Transformations. Chem. Rev. 2019, 119, 11718–11760. [Google Scholar] [CrossRef]
- Zhang, K.; Cai, R.; Chen, D.; Mao, L. Determination of hemoglobin based on its enzymatic activity for the oxidation of o-phenylenediamine with hydrogen peroxide. Anal. Chim. Acta 2000, 413, 109–113. [Google Scholar] [CrossRef]
- Bickar, D.; Bonaventura, J.; Bonaventura, C. Cytochrome c oxidase binding of hydrogen peroxide. Biochemistry 1982, 21, 2661–2666. [Google Scholar] [CrossRef] [PubMed]
- Brunori, M. Nitric oxide moves myoglobin centre stage. Trends Biochem. Sci. 2001, 26, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.M.; Dean, D.R.; Seefeldt, L.C. Climbing Nitrogenase: Toward a Mechanism of Enzymatic Nitrogen Fixation. Acc. Chem. Res. 2009, 42, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, X.; Li, Y.; Zheng, H.; Liu, Y.; Suye, S.-i. A Glucose Biosensor Based on Direct Electron Transfer of Glucose Oxidase on PEDOT Modified Microelectrode. J. Electrochem. Soc. 2020, 167, 067502. [Google Scholar] [CrossRef]
- Özbek, O.; Berkel, C.; Isildak, Ö.; Isildak, I. Potentiometric urea biosensors. Clin. Chim. Acta 2022, 524, 154–163. [Google Scholar] [CrossRef]
- Baluta, S.; Zając, D.; Szyszka, A.; Malecha, K.; Cabaj, J. Enzymatic Platforms for Sensitive Neurotransmitter Detection. Sensors 2020, 20, 423. [Google Scholar] [CrossRef]
- Matoba, Y.; Kihara, S.; Bando, N.; Yoshitsu, H.; Sakaguchi, M.; Kayama, K.e.; Yanagisawa, S.; Ogura, T.; Sugiyama, M. Catalytic mechanism of the tyrosinase reaction toward the Tyr98 residue in the caddie protein. PLoS Biol. 2019, 16, e3000077. [Google Scholar] [CrossRef]
- Malathi, S.; Pakrudheen, I.; Narayana Kalkura, S.; Webster, T.J.; Balasubramanian, S. Disposable biosensors based on metal nanoparticles. Sens. Int. 2022, 3, 100169. [Google Scholar] [CrossRef]
- Demuru, S.; Huang, C.-H.; Parvez, K.; Worsley, R.; Mattana, G.; Piro, B.; Noël, V.; Casiraghi, C.; Briand, D. All-Inkjet-Printed Graphene-Gated Organic Electrochemical Transistors on Polymeric Foil as Highly Sensitive Enzymatic Biosensors. ACS Appl. Nano Mater. 2022, 5, 1664–1673. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Domínguez-Saldaña, A.; Villa-Manso, A.M.; Gutiérrez-Sánchez, C.; Revenga-Parra, M.; Mateo-Martí, E.; Pariente, F.; Lorenzo, E. Azure A embedded in carbon dots as NADH electrocatalyst: Development of a glutamate electrochemical biosensor. Sens. Actuators B Chem. 2023, 374, 132761. [Google Scholar] [CrossRef]
- Karimian, N.; Campagnol, D.; Tormen, M.; Stortini, A.M.; Canton, P.; Ugo, P. Nanoimprinted arrays of glassy carbon nanoelectrodes for improved electrochemistry of enzymatic redox-mediators. J. Electroanal. Chem. 2023, 932, 117240. [Google Scholar] [CrossRef]
- Rahmawati, I.; Einaga, Y.; Ivandini, T.A.; Fiorani, A. Enzymatic Biosensors with Electrochemiluminescence Transduction. ChemElectroChem 2022, 9, e202200175. [Google Scholar] [CrossRef]
- Chen, J.; Gao, H.; Li, Z.; Li, Y.; Yuan, Q. Ferriporphyrin-inspired MOFs as an artificial metalloenzyme for highly sensitive detection of H2O2 and glucose. Chin. Chem. Lett. 2020, 31, 1398–1401. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Pan, M.; Wei, J.; Jiang, F.; Lu, R.; Liu, X.; Huang, Y.; Huang, F. Chaperonin-Nanocaged Hemin as an Artificial Metalloenzyme for Oxidation Catalysis. ACS Appl. Mater. Interfaces 2017, 9, 25387–25396. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Cheon, H.J.; Nguyen, Q.H.; Kim, M.I. Highly Sensitive Fluorescent Detection of Acetylcholine Based on the Enhanced Peroxidase-Like Activity of Histidine Coated Magnetic Nanoparticles. Nanomaterials 2021, 11, 1207. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.; Shi, D.; Li, F.; Ling, D. Cerium Oxide Nanoparticles-Based Optical Biosensors for Bio-medical Applications. Adv. Sens. Res. 2023, 2, 2200065. [Google Scholar] [CrossRef]
- Wang, L.; Sun, P.; Yang, Y.; Qiao, H.; Tian, H.; Wu, D.; Yang, S.; Yuan, Q.; Wang, J. Preparation of ZIF@ADH/NAD-MSN/LDH Core Shell Nanocomposites for the Enhancement of Coenzyme Catalyzed Double Enzyme Cascade. Nanomaterials 2021, 11, 2171. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, C.C. Recent Advances on Electrochemical Biosensing Strategies toward Universal Point-of-Care Systems. Angew. Chem. Int. Ed. 2019, 58, 12355–12368. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano Biosensors: Properties, applications and electrochemical techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Chupradit, S.; Km Nasution, M.; Rahman, H.S.; Suksatan, W.; Turki Jalil, A.; Abdelbasset, W.K.; Bokov, D.; Markov, A.; Fardeeva, I.N.; Widjaja, G.; et al. Various types of electrochemical biosensors for leukemia detection and therapeutic approaches. Anal. Biochem. 2022, 654, 114736. [Google Scholar] [CrossRef] [PubMed]
- Schachinger, F.; Chang, H.; Scheiblbrandner, S.; Ludwig, R. Amperometric Biosensors Based on Direct Electron Transfer Enzymes. Molecules 2021, 26, 4525. [Google Scholar] [CrossRef] [PubMed]
- Gigli, V.; Tortolini, C.; Capecchi, E.; Angeloni, A.; Lenzi, A.; Antiochia, R. Novel Amperometric Biosensor Based on Tyrosinase/Chitosan Nanoparticles for Sensitive and Interference-Free Detection of Total Catecholamine. Biosensors 2022, 12, 519. [Google Scholar] [CrossRef]
- Harnisch, F.; Freguia, S. A Basic Tutorial on Cyclic Voltammetry for the Investigation of Electroactive Microbial Biofilms. Chem. Asian J. 2012, 7, 466–475. [Google Scholar] [CrossRef]
- Yoon, J.; Conley, B.M.; Shin, M.; Choi, J.-H.; Bektas, C.K.; Choi, J.-W.; Lee, K.-B. Ultrasensitive Electrochemical Detection of Mutated Viral RNAs with Single-Nucleotide Resolution Using a Nanoporous Electrode Array (NPEA). ACS Nano 2022, 16, 5764–5777. [Google Scholar] [CrossRef]
- Baluta, S.; Meloni, F.; Halicka, K.; Szyszka, A.; Zucca, A.; Pilo, M.I.; Cabaj, J. Differential pulse voltammetry and chronoamperometry as analytical tools for epinephrine detection using a tyrosinase-based electrochemical biosensor. RSC Adv. 2022, 12, 25342–25353. [Google Scholar] [CrossRef]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H.; Karimi, F.; Shabani-Nooshabadi, M.; Alizadeh, M.; Al-Othman, A.; Erk, N.; Yegya Raman, P.K.; Karaman, C. Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids. Chemosphere 2022, 287, 132187. [Google Scholar] [CrossRef]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.Z.; Kopechek, J.A.; Priddy, M.C.; Hamorsky, K.T.; Palmer, K.E.; Mittal, N.; Valdez, J.; Flynn, J.; Williams, S.J. Rapid detection of SARS-CoV-2 antibodies using electrochemical impedance-based detector. Biosens. Bioelectron. 2021, 171, 112709. [Google Scholar] [CrossRef] [PubMed]
- Gaviria-Arroyave, M.I.; Cano, J.B.; Peñuela, G.A. Nanomaterial-based fluorescent biosensors for monitoring environmental pollutants: A critical review. Talanta Open 2020, 2, 100006. [Google Scholar] [CrossRef]
- Enander, K.; Choulier, L.; Olsson, A.L.; Yushchenko, D.A.; Kanmert, D.; Klymchenko, A.S.; Demchenko, A.P.; Mély, Y.; Altschuh, D. A Peptide-Based, Ratiometric Biosensor Construct for Direct Fluorescence Detection of a Protein Analyte. Bioconjug. Chem. 2008, 19, 1864–1870. [Google Scholar] [CrossRef]
- Morii, T.; Sugimoto, K.; Makino, K.; Otsuka, M.; Imoto, K.; Mori, Y. A New Fluorescent Biosensor for Inositol Trisphosphate. J. Am. Chem. Soc. 2002, 124, 1138–1139. [Google Scholar] [CrossRef] [PubMed]
- Scheller, F.W.; Wollenberger, U.; Warsinke, A.; Lisdat, F. Research and development in biosensors. Curr. Opin. Biotechnol. 2001, 12, 35–40. [Google Scholar] [CrossRef]
- Bhirde, A.; Xie, J.; Swierczewska, M.; Chen, X. Nanoparticles for cell labeling. Nanoscale 2011, 3, 142–153. [Google Scholar] [CrossRef]
- Yu, W.; Li, Y.; Xie, B.; Ma, M.; Chen, C.; Li, C.; Yu, X.; Wang, Z.; Wen, K.; Tang, B.Z.; et al. An Aggregation-Induced Emission-Based Indirect Competitive Immunoassay for Fluorescence “Turn-On” Detection of Drug Residues in Foodstuffs. Front. Chem. 2019, 7, 228. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Yang, X.; Tang, Y.; Han, S.; Kang, A.; Deng, H.; Chi, Y.; Zhu, D.; Lu, Y. FÖrster resonance energy transfer (FRET)-based biosensors for biological applications. Biosens. Bioelectron. 2019, 138, 111314. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Li, X.; Zhang, J.; Sun, J.; Tong, L.; Zhong, H.; Xia, H.; Hua, R.; Chen, B. Improved LRET-based detection characters of Cu2+ using sandwich structured NaYF4@NaYF4:Er3+/Yb3+@NaYF4 nanoparticles as energy donor. Sens. Actuators B Chem. 2018, 257, 829–838. [Google Scholar] [CrossRef]
- Syshchyk, O.; Skryshevsky, V.A.; Soldatkin, O.O.; Soldatkin, A.P. Enzyme biosensor systems based on porous silicon photoluminescence for detection of glucose, urea and heavy metals. Biosens. Bioelectron. 2015, 66, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ballesta-Claver, J.; Ametis-Cabello, J.; Morales-Sanfrutos, J.; Megía-Fernández, A.; Valencia-Mirón, M.C.; Santoyo-González, F.; Capitán-Vallvey, L.F. Electrochemiluminescent disposable cholesterol biosensor based on avidin–biotin assembling with the electroformed luminescent conducting polymer poly(luminol-biotinylated pyrrole). Anal. Chim. Acta 2012, 754, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, H.; Zhuo, Y.; Song, D.; Li, C.; Zhu, A.; Long, F. Reusable smartphone-facilitated mobile fluorescence biosensor for rapid and sensitive on-site quantitative detection of trace pollutants. Biosens. Bioelectron. 2022, 199, 113863. [Google Scholar] [CrossRef] [PubMed]
- Koveal, D.; Rosen, P.C.; Meyer, D.J.; Díaz-García, C.M.; Wang, Y.; Cai, L.-H.; Chou, P.J.; Weitz, D.A.; Yellen, G. A high-throughput multiparameter screen for accelerated development and optimization of soluble ge-netically encoded fluorescent biosensors. Nat. Commun. 2022, 13, 2919. [Google Scholar] [CrossRef]
- Grazon, C.; Chern, M.; Lally, P.; Baer, R.C.; Fan, A.; Lecommandoux, S.; Klapperich, C.; Dennis, A.M.; Galagan, J.E.; Grinstaff, M.W. The quantum dot vs. organic dye conundrum for ratiometric FRET-based biosensors: Which one would you chose? Chem. Sci. 2022, 13, 6715–6731. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.-W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef]
- Hashem, A.; Hossain, M.A.M.; Marlinda, A.R.; Mamun, M.A.; Simarani, K.; Johan, M.R. Nanomaterials based electrochemical nucleic acid biosensors for environmental monitoring: A review. Appl. Surf. Sci. 2021, 4, 100064. [Google Scholar] [CrossRef]
- Kosri, E.; Ibrahim, F.; Thiha, A.; Madou, M. Micro and Nano Interdigitated Electrode Array (IDEA)-Based MEMS/NEMS as Electrochemical Transducers: A Review. Nanomaterials 2022, 12, 4171. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Fan, Y.; Gao, G.; Zhi, J. Development of a Tyrosinase Amperometric Biosensor Based on Carbon Nanomaterials for the Detection of Phenolic Pollutants in Diverse Environments. ChemElectroChem 2022, 9, e202200861. [Google Scholar] [CrossRef]
- Öndeş, B.; Evli, S.; Şahin, Y.; Uygun, M.; Uygun, D.A. Uricase based amperometric biosensor improved by AuNPs-TiS2 nanocomposites for uric acid determination. Microchem. J. 2022, 181, 107725. [Google Scholar] [CrossRef]
- Haritha, V.S.; Kumar, S.R.S.; Rakhi, R.B. Amperometric cholesterol biosensor based on cholesterol oxidase and Pt-Au/ MWNTs modified glassy carbon electrode. Mater. Today Proc. 2022, 50, 34–39. [Google Scholar] [CrossRef]
- Thakur, D.; Pandey, C.M.; Kumar, D. Highly Sensitive Enzymatic Biosensor Based on Polyaniline-Wrapped Titanium Dioxide Nanohybrid for Fish Freshness Detection. Appl. Biochem. Biotechnol. 2022, 194, 3765–3778. [Google Scholar] [CrossRef] [PubMed]
- Carinelli, S.; Fernández, I.; Luis González-Mora, J.; Salazar-Carballo, P.A. Hemoglobin-modified nanoparticles for electrochemical determination of haptoglobin: Application in bovine mastitis diagnosis. Microchem. J. 2022, 179, 107528. [Google Scholar] [CrossRef]
- Jędrzak, A.; Kuznowicz, M.; Rębiś, T.; Jesionowski, T. Portable glucose biosensor based on polynorepinephrine@magnetite nanomaterial integrated with a smartphone analyzer for point-of-care application. Bioelectrochemistry 2022, 145, 108071. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Wu, Q.; Wei, W.; Liu, X. Photolithographic 3D microarray electrode-based high-performance non-enzymatic H2O2 sensor. Colloids Surf. Physicochem. Eng. Asp. 2021, 628, 127249. [Google Scholar] [CrossRef]
- Cao, L.; Han, G.-C.; Xiao, H.; Chen, Z.; Fang, C. A novel 3D paper-based microfluidic electrochemical glucose biosensor based on rGO-TEPA/PB sensitive film. Anal. Chim. Acta 2020, 1096, 34–43. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Q.; Xie, Y.; Jiang, D.; Chu, Z.; Jin, W. In situ fabrication of aloe-like Au–ZnO micro/nanoarrays for ultrasensitive biosensing of catechol. Biosens. Bioelectron. 2020, 156, 112145. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, H.-Y.; Shin, M.; Choi, H.K.; Lee, T.; Choi, J.-W. Flexible electrochemical biosensors for healthcare monitoring. J. Mater. Chem. B 2020, 8, 7303–7318. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, S.N.; Shin, M.K.; Kim, H.-W.; Choi, H.K.; Lee, T.; Choi, J.-W. Flexible electrochemical glucose biosensor based on GOx/gold/MoS2/gold nanofilm on the polymer electrode. Biosens. Bioelectron. 2019, 140, 111343. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, D.; Xu, C.; Ge, Y.; Liu, X.; Wei, Q.; Huang, L.; Ren, X.; Wang, C.; Wang, Y. Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens. Actuators B Chem. 2020, 320, 128325. [Google Scholar] [CrossRef]
- Muñoz, J.; Pumera, M. 3D-printed biosensors for electrochemical and optical applications. TrAC Trends Anal. Chem. 2020, 128, 115933. [Google Scholar] [CrossRef]
- Sangubotla, R.; Kim, J. Fiber-optic biosensor based on the laccase immobilization on silica-functionalized fluorescent carbon dots for the detection of dopamine and multi-color imaging applications in neuroblastoma cells. Mater. Sci. Eng. C 2021, 122, 111916. [Google Scholar] [CrossRef] [PubMed]
- Osman, D.I.; El-Sheikh, S.M.; Sheta, S.M.; Ali, O.I.; Salem, A.M.; Shousha, W.G.; El-Khamisy, S.F.; Shawky, S.M. Nucleic acids biosensors based on metal-organic framework (MOF): Paving the way to clinical laboratory diagnosis. Biosens. Bioelectron. 2019, 141, 111451. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, J.; Wang, Y.; Liang, Z.; Hu, B.; Xie, J.; Wong, W.-L.; Wong, K.-Y.; Qiu, B.; Peng, W. A new fluorescent biosensor based on inner filter effect and competitive coordination with the europium ion of non-luminescent Eu-MOF nanosheets for the determination of alkaline phosphatase activity in human serum. Sens. Actuators B Chem. 2023, 380, 133379. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.-F.; Lv, Y.-K. Luminescent switch sensors for the detection of biomolecules based on metal–organic frameworks. Analyst 2018, 143, 4221–4229. [Google Scholar] [CrossRef]
- Aggarwal, V.; Solanki, S.; Malhotra, B.D. Applications of metal–organic framework-based bioelectrodes. Chem. Sci. 2022, 13, 8727–8743. [Google Scholar] [CrossRef]
- Lv, M.; Zhou, W.; Tavakoli, H.; Bautista, C.; Xia, J.; Wang, Z.; Li, X. Aptamer-functionalized metal-organic frameworks (MOFs) for biosensing. Biosens. Bioelectron. 2021, 176, 112947. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Ma, J.-G.; Li, H.; Chen, D.-M.; Gu, W.; Yang, G.-M.; Cheng, P. Metal–organic framework biosensor with high stability and selectivity in a bio-mimic environment. Chem. Commun. 2015, 51, 9161–9164. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, H.; Zhou, W.; Qiu, Z.; Chen, C.; Qileng, A.; Li, K.; Liu, Y. Capsulation of AuNCs with AIE Effect into Metal–Organic Framework for the Marriage of a Fluorescence and Colorimetric Biosensor to Detect Organophosphorus Pesticides. Anal. Chem. 2021, 93, 7275–7282. [Google Scholar] [CrossRef]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.-K.; Falcaro, P.; Doonan, C.J. Metal–Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef]
- Huang, S.; Kou, X.; Shen, J.; Chen, G.; Ouyang, G. “Armor-Plating” Enzymes with Metal–Organic Frameworks (MOFs). Angew. Chem. Int. Ed. 2020, 59, 8786–8798. [Google Scholar] [CrossRef] [PubMed]
- Ade, C.; Brodszkij, E.; Thingholm, B.; Gal, N.; Itel, F.; Taipaleenmäki, E.; Hviid, M.J.; Schattling, P.S.; Städler, B. Small Organic Catalase Mimic Encapsulated in Micellar Artificial Organelles as Reactive Oxygen Species Scavengers. ACS Appl. Polym. Mater. 2019, 1, 1532–1539. [Google Scholar] [CrossRef]
- Karim, M.N.; Singh, M.; Weerathunge, P.; Bian, P.; Zheng, R.; Dekiwadia, C.; Ahmed, T.; Walia, S.; Della Gaspera, E.; Singh, S.; et al. Visible-Light-Triggered Reactive-Oxygen-Species-Mediated Antibacterial Activity of Peroxidase-Mimic CuO Nanorods. ACS Appl. Nano Mater. 2018, 1, 1694–1704. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, X.; Wang, S.; Xie, X.; Wang, Y.; Su, X. Fabrication of Bioresource-Derived Porous Carbon-Supported Iron as an Efficient Oxidase Mimic for Dual-Channel Biosensing. Anal. Chem. 2021, 93, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, P.; Chen, J.; Guo, H.; Lu, X. Pyrazolate-based porphyrinic metal-organic frameworks as catechol oxidase mimic enzyme for fluorescent and colorimetric dual-mode detection of dopamine with high sensitivity and specificity. Sens. Actuators B Chem. 2021, 341, 130000. [Google Scholar] [CrossRef]
- Ouyang, Y.; O’Hagan, M.P.; Willner, I. Functional catalytic nanoparticles (nanozymes) for sensing. Biosens. Bioelectron. 2022, 218, 114768. [Google Scholar] [CrossRef]
- Abdel-Lateef, M.A. Utilization of the peroxidase-like activity of silver nanoparticles nanozyme on O-phenylenediamine/H2O2 system for fluorescence detection of mercury (II) ions. Sci. Rep. 2022, 12, 6953. [Google Scholar] [CrossRef]
- Li, X.; He, Z.; Li, C.; Li, P. One-step enzyme kinetics measurement in 3D printed microfluidics devices based on a high-performance single vibrating sharp-tip mixer. Anal. Chim. Acta 2021, 1172, 338677. [Google Scholar] [CrossRef]
- Khandan-Nasab, N.; Askarian, S.; Mohammadinejad, A.; Aghaee-Bakhtiari, S.H.; Mohajeri, T.; Kazemi Oskuee, R. Biosensors, microfluidics systems and lateral flow assays for circulating microRNA detection: A review. Anal. Biochem. 2021, 633, 114406. [Google Scholar] [CrossRef]
- Rossini, E.L.; Milani, M.I.; Lima, L.S.; Pezza, H.R. Paper microfluidic device using carbon dots to detect glucose and lactate in saliva samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119285. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.-Y.; He, W.-M. Surface-enhanced Raman spectroscopy biosensor based on silver nanopar-ticles@metal-organic frameworks with peroxidase-mimicking activities for ultrasensitive monitoring of blood cholesterol. Sens. Actuators B Chem. 2022, 365, 131939. [Google Scholar] [CrossRef]
- Xia, X.; Weng, Y.; Zhang, L.; Tang, R.; Zhang, X. A facile SERS strategy to detect glucose utilizing tandem enzyme activities of Au@Ag nanoparticles. Spectrochim. Acta A Mol. Biomol. 2021, 259, 119889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mai, X.; Hong, X.; Chen, Y.; Li, X. Optical fiber SPR biosensor with a solid-phase enzymatic reaction device for glucose detection. Sens. Actuators B Chem. 2022, 366, 131984. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Wang, Z.; Singh, R.; Marques, C.; Wu, Q.; Kaushik, B.K.; Jha, R.; Zhang, B.; Kumar, S. Lo-calized Plasmon-Based Multicore Fiber Biosensor for Acetylcholine Detection. IEEE Trans. Instrum. Meas. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Avan, A.N.; Demirci-Çekiç, S.; Apak, R. Colorimetric Nanobiosensor Design for Determining Oxidase Enzyme Substrates in Food and Biological Samples. ACS Omega 2022, 7, 44372–44382. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Wang, F.; Gao, T.; Wei, T. Enzyme powered self-assembly of hydrogel biosensor for col-orimetric detection of metabolites. Sens. Actuators B Chem. 2023, 375, 132942. [Google Scholar] [CrossRef]
- You, X.; Pak, J.J. Graphene-based field effect transistor enzymatic glucose biosensor using silk protein for enzyme immobilization and device substrate. Sens. Actuators B Chem. 2014, 202, 1357–1365. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, L.; Rostro-Alanis, M.; Rodríguez-Rodríguez, J.; Sosa-Hernández, J.E.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; Parra-Saldívar, R. Enzyme (Single and Multiple) and Nanozyme Biosensors: Recent Developments and Their Novel Applications in the Water-Food-Health Nexus. Biosensors 2021, 11, 410. [Google Scholar] [CrossRef]
- Pathak, A.; Gupta, B.D. Ultra-selective fiber optic SPR platform for the sensing of dopamine in synthetic cerebrospinal fluid incorporating permselective nafion membrane and surface imprinted MWCNTs-PPy matrix. Biosens. Bioelectron. 2019, 133, 205–214. [Google Scholar] [CrossRef]
- Caldara, M.; Lowdon, J.W.; Rogosic, R.; Arreguin-Campos, R.; Jimenez-Monroy, K.L.; Heidt, B.; Tschulik, K.; Cleij, T.J.; Diliën, H.; Eersels, K.; et al. Thermal Detection of Glucose in Urine Using a Molecularly Imprinted Polymer as a Recognition Element. ACS Sens. 2021, 6, 4515–4525. [Google Scholar] [CrossRef]
- Kim, H.; Seong, W.; Rha, E.; Lee, H.; Kim, S.K.; Kwon, K.K.; Park, K.-H.; Lee, D.-H.; Lee, S.-G. Machine learning linked evolutionary biosensor array for highly sensitive and specific molecular identification. Biosens. Bioelectron. 2020, 170, 112670. [Google Scholar] [CrossRef] [PubMed]
- Mross, S.; Pierrat, S.; Zimmermann, T.; Kraft, M. Microfluidic enzymatic biosensing systems: A review. Biosens. Bioelectron. 2015, 70, 376–391. [Google Scholar] [CrossRef] [PubMed]
| Sensing Technique | Enzymatic Reaction | Nano-Assistance | Target | LoD | Ref. |
|---|---|---|---|---|---|
| Electrochemical | Tyrosinase reaction | Carbon nanotubes Nanodiamonds | Phenolic compounds | 2.9 nM | [79] |
| Cholesterol oxidase reaction | Carbon nanotubes | Cholesterol | 0.5 µM | [81] | |
| Glucose oxidase reaction | Polynorepinephrine grafted on magnetite nanoparticles | Glucose | 6.1 µM | [84] | |
| Laccase reaction | Gold–Zinc oxide micro/nanoarrays | Catechol | 25 nM | [87] | |
| Glucose oxidase reaction | Glucose oxidase/Gold/ Molybdenum disulfide/ Gold nanofilm | Glucose | 10 nM | [89] | |
| Fluorescent | Laccase reaction | Silica-functionalized carbon dots | Dopamine | 41.2 nM | [92] |
| Acetylcholinesterase and choline oxidase reaction | Au nanoclusters modified zeolite-like imidazole framework | Organophosphorus pesticides | 1.79 nM | [99] | |
| Catechol oxidase reaction | Pyrazolate-based porphyrinic metal–organic framework | Dopamine | 2.5 nM | [105] | |
| Peroxidase reaction | Polyvinylpyrrolidone stabilized silver nanoparticles | Mercury (II) ion | 8.9 nM | [107] | |
| Glucose oxidase and lactate oxidase reaction | Carbon dots | Glucose Lactate | 2.6 µM 0.8 µM | [110] | |
| SERS | Peroxidase- mimicking reaction | Silver nanoparticles/metal organic framework | Cholesterol | 0.36 µM | [111] |
| Glucose oxidase- like reaction | Silver/gold nanoparticles | Glucose | 50 nM | [112] | |
| SPR | Glucose oxidase oxidation | Polystyrene nanoparticle with Manganese dioxide | Glucose | 3.1 pM | [113] |
| Acetylcholinesterase reaction | Molybdenum disulfide/gold nanoparticle multicore fiber | Acetylcholine | 14.28 µM | [114] | |
| Colorimetric | Uricase, glucose oxidase, choline oxidase reaction | Magnetic nanoparticles | Uric acid Glucose Choline | 0.34 µM 0.59 µM 0.20 µM | [115] |
| Glucose oxidase reaction | Acrylamide based-copolymer hydrogel | H2O2 Glucose | 8.9 µM 1.6 mM | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.K.; Yoon, J. Enzymatic Electrochemical/Fluorescent Nanobiosensor for Detection of Small Chemicals. Biosensors 2023, 13, 492. https://doi.org/10.3390/bios13040492
Choi HK, Yoon J. Enzymatic Electrochemical/Fluorescent Nanobiosensor for Detection of Small Chemicals. Biosensors. 2023; 13(4):492. https://doi.org/10.3390/bios13040492
Chicago/Turabian StyleChoi, Hye Kyu, and Jinho Yoon. 2023. "Enzymatic Electrochemical/Fluorescent Nanobiosensor for Detection of Small Chemicals" Biosensors 13, no. 4: 492. https://doi.org/10.3390/bios13040492
APA StyleChoi, H. K., & Yoon, J. (2023). Enzymatic Electrochemical/Fluorescent Nanobiosensor for Detection of Small Chemicals. Biosensors, 13(4), 492. https://doi.org/10.3390/bios13040492





