Three-Dimensional Electrochemical Sensors for Food Safety Applications
Abstract
1. Introduction
2. Preparation of Three-Dimensional Electrode
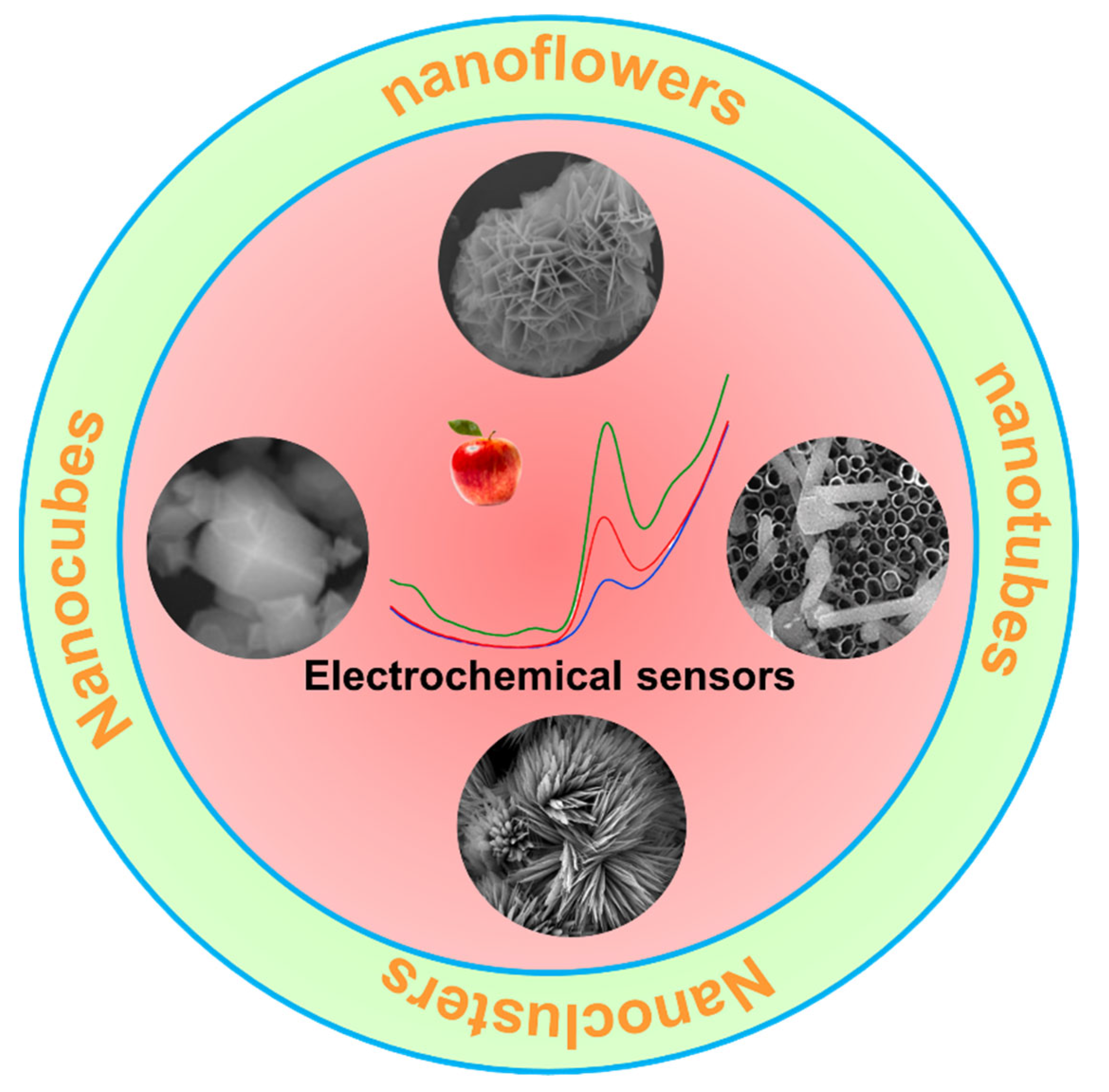
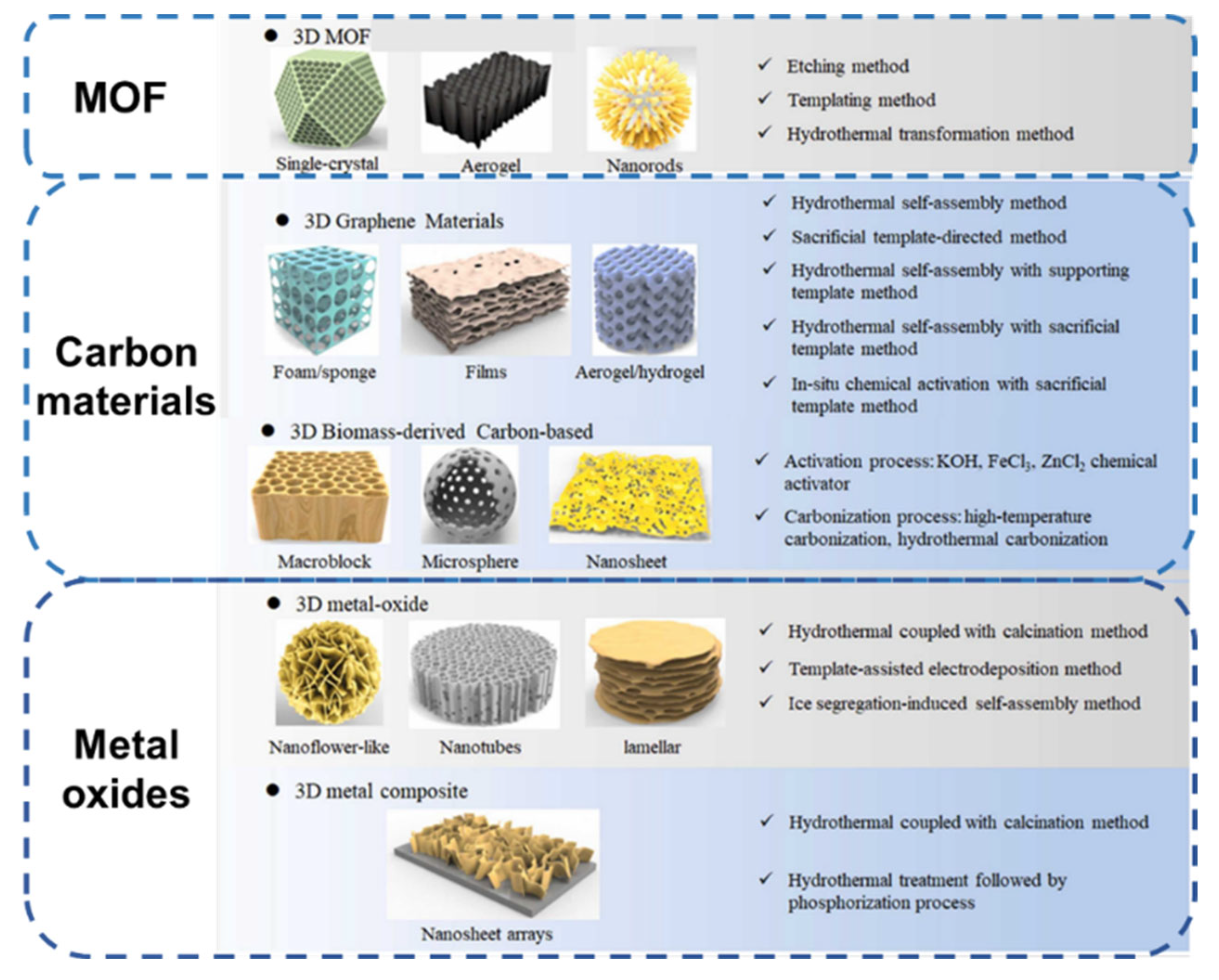
3. Different Kinds of Three-Dimensional Materials and Three-Dimensional Structured Electrodes
3.1. Metal Oxides
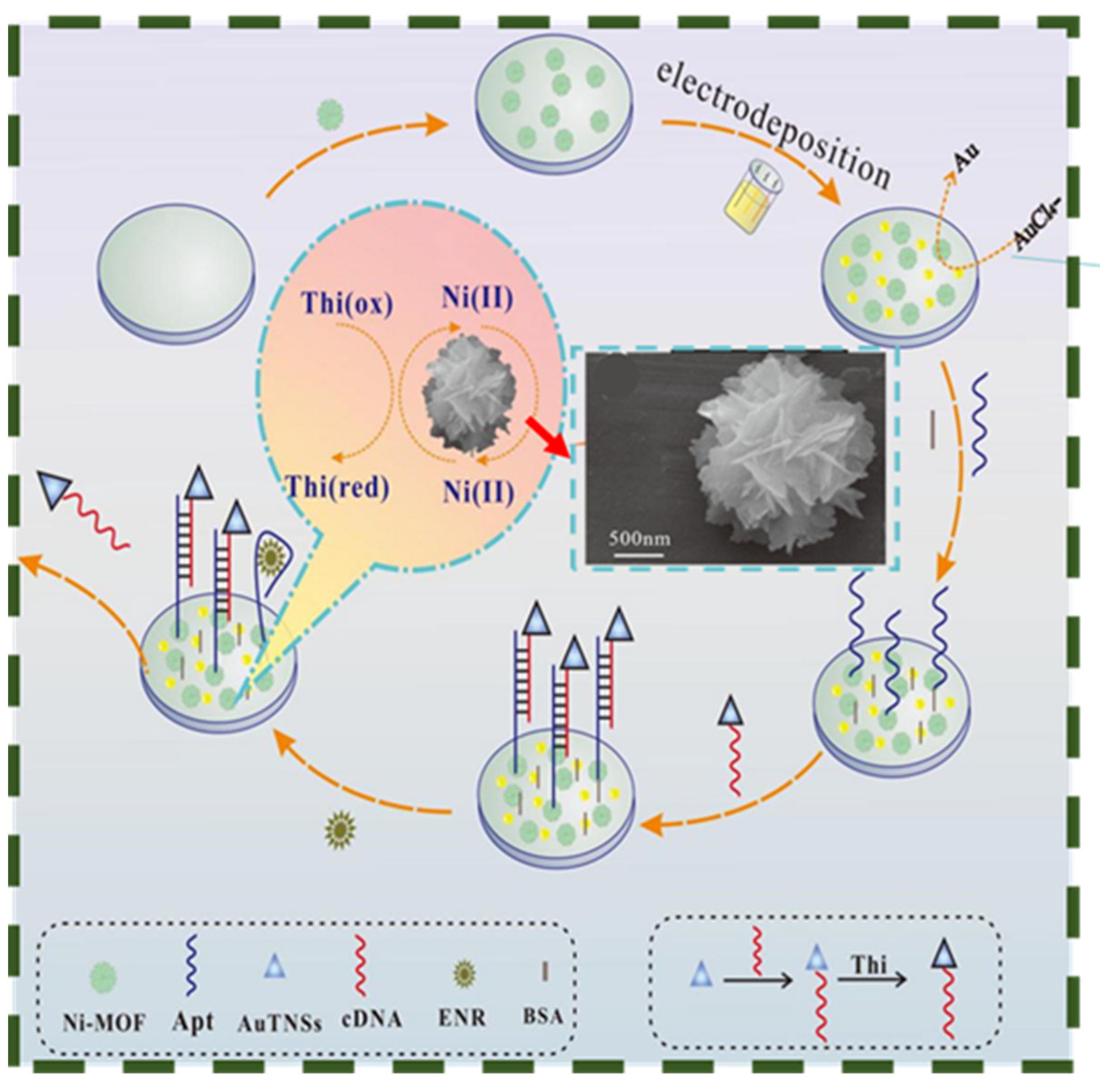
3.2. MOFs
3.3. Carbon Materials
3.4. Alloy and Perovskite-Type Oxides
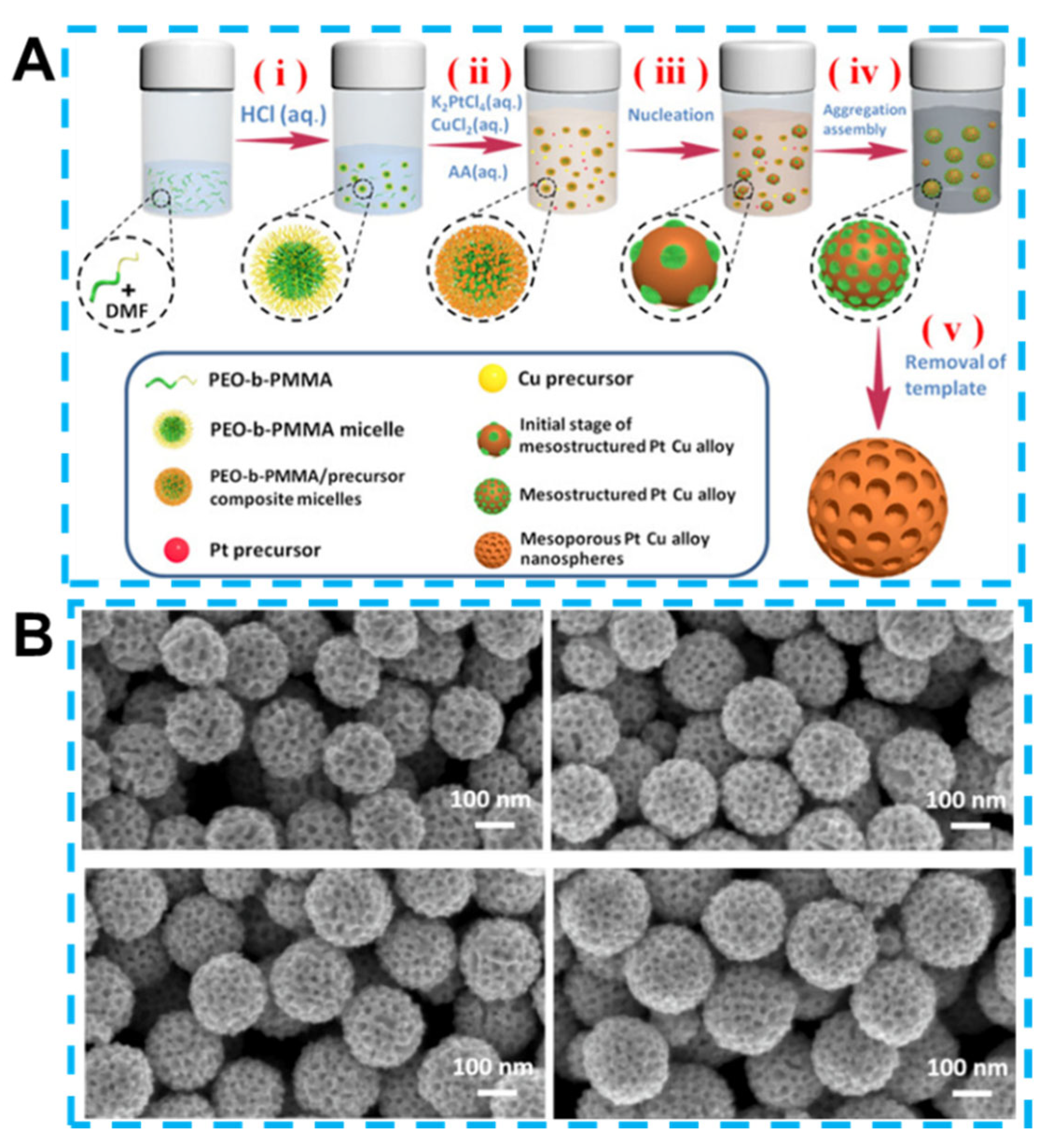
3.5. Three-Dimensional Structured Electrodes
4. Applications of 3D Sensors for Glucose Detection
5. Applications of 3D Sensors for Detections of Bioactive Food Components
6. Applications of 3D Sensors for Detections of Food Additives
7. Applications of 3D Sensors for Detections of Food Preservatives
8. Applications of 3D Sensors for Detections of Emerging Pollutants
8.1. Detection of Heavy Metal Ions
8.2. Detection of Pesticides
8.3. Detection of Antibiotics and Drugs
9. Applications of 3D Sensors for Detections of Foodborne Bacteria
10. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Liao, F.; Guo, T.; Yang, S.; Zeng, C. Synthesis of crystalline silver nanoplates and their application for detection of nitrite in foods. J. Electroanal. Chem. 2012, 664, 135–138. [Google Scholar] [CrossRef]
- Rassaei, L.; Marken, F.; Sillanpää, M.; Amiri, M.; Cirtiu, C.M.; Sillanpää, M. Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 2011, 30, 1704–1715. [Google Scholar] [CrossRef]
- Kaur, H.; Siwal, S.S.; Chauhan, G.; Saini, A.K.; Kumari, A.; Thakur, V.K. Recent advances in electrochemical-based sensors amplified with carbon-based nanomaterials (CNMs) for sensing pharmaceutical and food pollutants. Chemosphere 2022, 304, 135182. [Google Scholar] [CrossRef] [PubMed]
- Curulli, A. Recent Advances in Electrochemical Sensing Strategies for Food Allergen Detection. Biosensors 2022, 12, 503. [Google Scholar] [CrossRef]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S.; Kumar, A. Validation of HPLC Multi-residue Method for Determination of Fluoroquinolones, Tetracycline, Sulphonamides and Chloramphenicol Residues in Bovine Milk. Food Anal. Methods 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Llamas, N.E.; Garrido, M.; Nezio, M.S.D.; Band, B.S.F. Second order advantage in the determination of amaranth, sunset yellow FCF and tartrazine by UV–vis and multivariate curve resolution-alternating least squares. Anal. Chim. Acta 2009, 655, 38–42. [Google Scholar] [CrossRef]
- Pourreza, N.; Fat’hi, M.R.; Hatami, A. Indirect cloud point extraction and spectrophotometric determination of nitrite in water and meat products. Microchem. J. 2012, 104, 22–25. [Google Scholar] [CrossRef]
- Ryvolová, M.; Táborský, P.; Vrábel, P.; Krásenský, P.; Preisler, J. Sensitive determination of erythrosine and other red food colorants using capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. A 2007, 1141, 206–211. [Google Scholar] [CrossRef]
- Rajapaksha, P.; Elbourne, A.; Gangadoo, S.; Brown, R.; Cozzolino, D.; Chapman, J. A review of methods for the detection of pathogenic microorganisms. Analyst 2019, 144, 396–411. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, K.X.; Huang, M.Y.; Zeng, M.M.; Deng, Y.; Li, S.; Chen, H.; Li, W.; Chen, Z. Research progress on detection techniques for point-of-care testing of foodborne pathogens. Front. Bioeng. Biotechnol. 2022, 10, 21. [Google Scholar] [CrossRef]
- Chae, W.; Kim, P.; Hwang, B.J.; Seong, B.L. Universal monoclonal antibody-based influenza hemagglutinin quantitative enzyme-linked immunosorbent assay. Vaccine 2019, 37, 1457–1466. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Arotiba, O.A. Simultaneous determination of cholesterol, ascorbic acid and uric acid as three essential biological compounds at a carbon paste electrode modified with copper oxide decorated reduced graphene oxide nanocomposite and ionic liquid. J. Colloid Interface Sci. 2020, 560, 208–212. [Google Scholar] [CrossRef]
- Fu, J.; An, X.; Yao, Y.; Guo, Y.; Sun, X. Electrochemical aptasensor based on one step co-electrodeposition of aptamer and GO-CuNPs nanocomposite for organophosphorus pesticide detection. Sens. Actuators B Chem. 2019, 287, 503–509. [Google Scholar] [CrossRef]
- Choudhary, M.; Siwal, S.; Nandi, D.; Mallick, K. Single step synthesis of gold–amino acid composite, with the evidence of the catalytic hydrogen atom transfer (HAT) reaction, for the electrochemical recognition of Serotonin. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 77, 72–80. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Yin, H.S.; Li, J.; Li, B.C.; Li, X.; Ai, S.Y.; Zhang, X.S. Electrochemical biosensor for microRNA detection based on poly (U) polymerase mediated isothermal signal amplification. Biosens. Bioelectron. 2016, 79, 79–85. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Huang, K.-J.; Hou, Y.-Y.; Sun, X.; Li, J. Real-Time Biosensor Platform Based on Novel Sandwich Graphdiyne for Ultrasensitive Detection of Tumor Marker. Anal. Chem. 2022, 94, 16980–16986. [Google Scholar] [CrossRef]
- Nikolaus, N.; Strehlitz, B. Amperometric lactate biosensors and their application in (sports) medicine, for life quality and wellbeing. Microchim. Acta 2008, 160, 15–55. [Google Scholar] [CrossRef]
- Li, T.; Shang, D.; Gao, S.; Wang, B.; Kong, H.; Yang, G.; Shu, W.; Xu, P.; Wei, G. Two-Dimensional Material-Based Electrochemical Sensors/Biosensors for Food Safety and Biomolecular Detection. Biosensors 2022, 12, 314. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Heinze, J. Ultramicroelectrodes in Electrochemistry. Angew. Chem. Int. Ed. Engl. 1993, 32, 1268–1288. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Zhang, H.; Zhao, W.; Qu, L.; Chen, S.; Wu, S. Electrospun strong, bioactive, and bioabsorbable silk fibroin/poly (L-lactic-acid) nanoyarns for constructing advanced nanotextile tissue scaffolds. Mater. Today Bio 2022, 14, 100243. [Google Scholar] [CrossRef] [PubMed]
- Valiev, R. Nanomaterial advantage. Nature 2002, 419, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, A.; Pennacchio, A.; Longobardi, S.; Velotta, R.; Giardina, P. Vmh2 hydrophobin as a tool for the development of “self-immobilizing” enzymes for biosensing. Biotechnol. Bioeng. 2017, 114, 46–52. [Google Scholar] [CrossRef]
- Qiu, B.; Xing, M.; Zhang, J. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef]
- Zou, C.E.; Yang, B.; Bin, D.; Wang, J.; Li, S.; Yang, P.; Wang, C.; Shiraishi, Y.; Du, Y. Electrochemical synthesis of gold nanoparticles decorated flower-like graphene for high sensitivity detection of nitrite. J. Colloid Interface Sci. 2017, 488, 135–141. [Google Scholar] [CrossRef]
- Li, X.; Lu, S.; Zhang, G. Three-dimensional structured electrode for electrocatalytic organic wastewater purification: Design, mechanism and role. J. Hazard. Mater. 2023, 445, 130524. [Google Scholar] [CrossRef]
- Zhu, D.; Zhen, Q.; Xin, J.; Ma, H.; Tan, L.; Pang, H.; Wang, X. A free-standing and flexible phosphorus/nitrogen dual-doped three-dimensional reticular porous carbon frameworks encapsulated cobalt phosphide with superior performance for nitrite detection in drinking water and sausage samples. Sens. Actuators B Chem. 2020, 321, 128541. [Google Scholar] [CrossRef]
- Park, S.-W.; Yun, E.-T.; Shin, H.J.; Kim, W.; Lee, J.; Kim, D.-W. Three-dimensional construction of electrode materials using TiC nanoarray substrates for highly efficient electrogeneration of sulfate radicals and molecular hydrogen in a single electrolysis cell. J. Mater. Chem. A 2021, 9, 11705–11717. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Sakthi Priya, T.; Wang, T.-J. Surface Engineering Three-Dimensional Flowerlike Cerium Vanadate Nanostructures Used as Electrocatalysts: Real Time Monitoring of Clioquinol in Biological Samples. ACS Sustain. Chem. Eng. 2019, 7, 16121–16130. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J. Synthesis and characterization of 3D flower-like nickel oxide entrapped on boron doped carbon nitride nanocomposite: An efficient catalyst for the electrochemical detection of nitrofurantoin. Compos. Part B Eng. 2019, 174, 106914. [Google Scholar] [CrossRef]
- Wei, Z.; Zhu, W.; Li, Y.; Ma, Y.; Wang, J.; Hu, N.; Suo, Y.; Wang, J. Conductive Leaflike Cobalt Metal-Organic Framework Nanoarray on Carbon Cloth as a Flexible and Versatile Anode toward Both Electrocatalytic Glucose and Water Oxidation. Inorg. Chem. 2018, 57, 8422–8428. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, H.; Ji, C.; Wu, Q.; Wang, X.; Wu, Y. Sensitive and direct electrochemical detection of bisphenol S based on 1T&2H-MoS2/CNTs-NH2 nanocomposites. New J. Chem. 2022, 46, 8203–8214. [Google Scholar]
- Tan, C.; Zhang, H. Wet-chemical synthesis and applications of non-layer structured two-dimensional nanomaterials. Nat. Commun. 2015, 6, 7873. [Google Scholar] [CrossRef]
- Joshi, R.K.; Schneider, J.J. Assembly of one dimensional inorganic nanostructures into functional 2D and 3D architectures. Synthesis, arrangement and functionality. Chem. Soc. Rev. 2012, 41, 5285–5312. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Su, L.; Gong, L.; Dong, D.; Wu, Z. Self-Assembly/Sacrificial Synthesis of Highly Capacitive Hierarchical Porous Carbon from Longan Pulp Biomass. ChemElectroChem 2020, 7, 4606–4613. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Y.; Xuan, C.; Ma, C.; Wang, J. Three-Dimensional Electrodes for Oxygen Electrocatalysis. ChemElectroChem 2022, 9, e202101522. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef]
- Liu, M.; Dong, H.; Zhang, S.; Chen, X.; Sun, Y.; Gao, S.; Xu, J.; Wu, X.; Yuan, A.; Lu, W. Three-Dimensional Porous TiNb2O7/CNT-KB Composite Microspheres as Lithium-Ion Battery Anode Material. ChemElectroChem 2019, 6, 3959–3965. [Google Scholar] [CrossRef]
- Yu, M.; Qiu, W.; Wang, F.; Zhai, T.; Fang, P.; Lu, X.; Tong, Y. Three dimensional architectures: Design, assembly and application in electrochemical capacitors. J. Mater. Chem. A 2015, 3, 15792–15823. [Google Scholar] [CrossRef]
- Zhang, H.; Ning, H.; Busbee, J.; Shen, Z.; Kiggins, C.; Hua, Y.; Eaves, J.; Davis, J.; Shi, T.; Shao, Y.-T.; et al. Electroplating lithium transition metal oxides. Sci. Adv. 2017, 3, e1602427. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Shen, Z.; Zhong, C.; Zhou, Q.; Liu, J.; Zhu, J.; Zhang, H. Electrodeposition Technologies for Li-Based Batteries: New Frontiers of Energy Storage. Adv. Mater. 2020, 32, 1903808. [Google Scholar] [CrossRef] [PubMed]
- Beidaghi, M.; Gogotsi, Y. Capacitive energy storage in micro-scale devices: Recent advances in design and fabrication of micro-supercapacitors. Energy Environ. Sci. 2014, 7, 867–884. [Google Scholar] [CrossRef]
- Singh, M.; Ghosh, R.; Chen, Y.-S.; Yen, Z.-L.; Hofmann, M.; Chen, Y.-F.; Hsieh, Y.-P. Chemical vapor deposition merges MoS2 grains into high-quality and centimeter-scale films on Si/SiO2. RSC Adv. 2022, 12, 5990–5996. [Google Scholar] [CrossRef]
- Gerard, O.; Numan, A.; Krishnan, S.; Khalid, M.; Subramaniam, R.; Kasi, R. A review on the recent advances in binder-free electrodes for electrochemical energy storage application. J. Energy Storage 2022, 50, 104283. [Google Scholar] [CrossRef]
- Shao, Z.; Chang, Y.; Venton, B.J. Carbon microelectrodes with customized shapes for neurotransmitter detection: A review. Anal. Chim. Acta 2022, 1223, 340165. [Google Scholar] [CrossRef]
- Banciu, C.A.; Nastase, F.; Istrate, A.-I.; Veca, L.M. 3D Graphene Foam by Chemical Vapor Deposition: Synthesis, Properties, and Energy-Related Applications. Molecules 2022, 27, 3634. [Google Scholar] [CrossRef]
- Chen, K.; Chai, Z.; Li, C.; Shi, L.; Liu, M.; Xie, Q.; Zhang, Y.; Xu, D.; Manivannan, A.; Liu, Z. Catalyst-Free Growth of Three-Dimensional Graphene Flakes and Graphene/g-C3N4 Composite for Hydrocarbon Oxidation. ACS Nano 2016, 10, 3665–3673. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Chakrabarti, S.; Schulz, M.J.; Shanov, V. Spinning yarn from long carbon nanotube arrays. J. Mater. Res. 2011, 26, 645–651. [Google Scholar] [CrossRef]
- Kumar, A.; Nanda, D. Chapter 3—Methods and fabrication techniques of superhydrophobic surfaces. In Superhydrophobic Polymer Coatings; Samal, S.K., Mohanty, S., Nayak, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–75. [Google Scholar]
- Hamedani, Y.; Macha, P.; Bunning, T.J.; Naik, R.R.; Vasudev, M.C. Plasma-Enhanced Chemical Vapor Deposition: Where we are and the Outlook for the Future. In Chemical Vapor Deposition; Sudheer, N., Ed.; IntechOpen: Rijeka, Croatia, 2016; Chapter 10. [Google Scholar]
- Vernardou, D. Progress and Challenges in Industrially Promising Chemical Vapour Deposition Processes for the Synthesis of Large-Area Metal Oxide Electrode Materials Designed for Aqueous Battery Systems. Materials 2021, 14, 4177. [Google Scholar] [CrossRef]
- Yu, L.; Wu, H.B.; Lou, X.W. Mesoporous Li4Ti5O12 Hollow Spheres with Enhanced Lithium Storage Capability. Adv. Mater. 2013, 25, 2296–2300. [Google Scholar] [CrossRef]
- Khalily, M.A.; Eren, H.; Akbayrak, S.; Susapto, H.H.; Biyikli, N.; Özkar, S.; Guler, M.O. Facile Synthesis of Three-Dimensional Pt-TiO2 Nano-networks: A Highly Active Catalyst for the Hydrolytic Dehydrogenation of Ammonia–Borane. Angew. Chem. Int. Ed. 2016, 55, 12257–12261. [Google Scholar] [CrossRef]
- Wu, H.B.; Pan, A.; Hng, H.H.; Lou, X.W. Template-Assisted Formation of Rattle-type V2O5 Hollow Microspheres with Enhanced Lithium Storage Properties. Adv. Funct. Mater. 2013, 23, 5669–5674. [Google Scholar] [CrossRef]
- Miao, J.; Lang, Z.; Xue, T.; Li, Y.; Li, Y.; Cheng, J.; Zhang, H.; Tang, Z. Revival of Zeolite-Templated Nanocarbon Materials: Recent Advances in Energy Storage and Conversion. Adv. Sci. 2020, 7, 2001335. [Google Scholar] [CrossRef]
- Sivakumar, M.; Muthukutty, B.; Chen, T.W.; Chen, S.M.; Vivekanandan, A.K.; Chen, S.H.; Hatshan, M.R.; Ali, M.A.; Kumar, M. Electrocatalytic detection of noxious antioxidant diphenylamine in fruit samples with support of Cu@nanoporous carbon modified sensor. Chemosphere 2022, 292, 133400. [Google Scholar] [CrossRef]
- Sohrabi, H.; Salahshour Sani, P.; Orooji, Y.; Majidi, M.R.; Yoon, Y.; Khataee, A. MOF-based sensor platforms for rapid detection of pesticides to maintain food quality and safety. Food Chem. Toxicol. 2022, 165, 113176. [Google Scholar] [CrossRef]
- Chao, D.; Xia, X.; Liu, J.; Fan, Z.; Ng, C.F.; Lin, J.; Zhang, H.; Shen, Z.X.; Fan, H.J. A V2O5/Conductive-Polymer Core/Shell Nanobelt Array on Three-Dimensional Graphite Foam: A High-Rate, Ultrastable, and Freestanding Cathode for Lithium-Ion Batteries. Adv. Mater. 2014, 26, 5794–5800. [Google Scholar] [CrossRef]
- Liu, Y.; Goebl, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar] [CrossRef]
- Wu, B.; Hou, S.; Miao, Z.; Zhang, C.; Ji, Y. Layer-by-Layer Self-Assembling Gold Nanorods and Glucose Oxidase onto Carbon Nanotubes Functionalized Sol-Gel Matrix for an Amperometric Glucose Biosensor. Nanomaterials 2015, 5, 1544–1555. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M. A review of techniques for the application of bioactive coatings on metal-based implants to achieve controlled release of active ingredients. Mater. Des. 2022, 217, 110653. [Google Scholar] [CrossRef]
- He, W.; Liu, P.-J.; He, G.-Q.; Gozin, M.; Yan, Q.-L. Highly Reactive Metastable Intermixed Composites (MICs): Preparation and Characterization. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef] [PubMed]
- Parra-Alfambra, A.M.; Casero, E.; Petit-Domínguez, M.D.; Barbadillo, M.; Pariente, F.; Vázquez, L.; Lorenzo, E. New nanostructured electrochemical biosensors based on three-dimensional (3-mercaptopropyl)-trimethoxysilane network. Analyst 2011, 136, 340–347. [Google Scholar] [CrossRef]
- Yan, D.; Meng, Z.-H.; Qiu, L.-L.; Xue, M. Recent Advances in Preparation and Applications of 3D Transition Metal Oxides Semiconductor Photonic Crystal. Adv. Photonics Res. 2021, 2, 2000191. [Google Scholar] [CrossRef]
- Vinoth Kumar, J.; Karthik, R.; Chen, S.-M.; Chen, K.-H.; Sakthinathan, S.; Muthuraj, V.; Chiu, T.-W. Design of novel 3D flower-like neodymium molybdate: An efficient and challenging catalyst for sensing and destroying pulmonary toxicity antibiotic drug nitrofurantoin. Chem. Eng. J. 2018, 346, 11–23. [Google Scholar] [CrossRef]
- Hou, S.; Ou, Z.; Chen, Q.; Wu, B. Amperometric acetylcholine biosensor based on self-assembly of gold nanoparticles and acetylcholinesterase on the sol–gel/multi-walled carbon nanotubes/choline oxidase composite-modified platinum electrode. Biosens. Bioelectron. 2012, 33, 44–49. [Google Scholar] [CrossRef]
- He, J.; Wang, S.; Jiang, L.; Li, X.; Hong, Q.; Zhu, W.; Sun, J.; Zhang, X.; Xu, Z. Femtosecond Laser One-Step Direct Writing Electrodes with Ag NPs-Graphite Carbon Composites for Electrochemical Sensing. Adv. Mater. Technol. 2022, 7, 2200210. [Google Scholar] [CrossRef]
- Ansari, A.A.; Solanki, P.R.; Malhotra, B.D. Sol-gel derived nanostructured cerium oxide film for glucose sensor. Appl. Phys. Lett. 2008, 92, 263901. [Google Scholar] [CrossRef]
- Zhao, D.-D.; Bao, S.-J.; Zhou, W.-J.; Li, H.-L. Preparation of hexagonal nanoporous nickel hydroxide film and its application for electrochemical capacitor. Electrochem. Commun. 2007, 9, 869–874. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Wang, C.; Du, L.; Xing, X.; Feng, D.; Tian, Y.; Li, Z.; Yang, D. Flexible carbon cloth in-situ assembling WO3 microsheets bunches with Ni dopants for non-enzymatic glucose sensing. Appl. Surf. Sci. 2022, 586, 152822. [Google Scholar] [CrossRef]
- Vinoth, S.; Wang, S.-F. Construction of functionalized carbon nanotube@metal oxide nanocomposite for high-performance electrochemical measurement of antipyretic drug in water samples. Environ. Sci. Pollut. Res. 2023, in press. [Google Scholar] [CrossRef]
- Nguyen, N.S.; Das, G.; Yoon, H.H. Nickel/cobalt oxide-decorated 3D graphene nanocomposite electrode for enhanced electrochemical detection of urea. Biosens. Bioelectron. 2016, 77, 372–377. [Google Scholar] [CrossRef]
- Zhang, K.; Zeng, H.; Feng, J.; Liu, Z.; Chu, Z.; Jin, W. Screen-printing of core-shell Mn3O4@C nanocubes based sensing microchip performing ultrasensitive recognition of allura red. Food Chem. Toxicol. 2022, 162, 112908. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Jiao, T.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based sensors for the detection of food contaminants. TrAC Trends Anal. Chem. 2022, 154, 116642. [Google Scholar] [CrossRef]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal-Organic Framework Nanoparticles. Adv. Mater. 2018, 30, e1800202. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Iftikhar, T.; Aziz, A.; Ashraf, G.; Xu, Y.; Li, G.; Zhang, T.; Asif, M.; Xiao, F.; Liu, H. Engineering MOFs derived metal oxide nanohybrids: Towards electrochemical sensing of catechol in tea samples. Food Chem. 2022, 395, 133642. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Li, J.; Zhang, L.; Zhao, P.; Wang, C.; Fei, J.; Xie, Y. Determination of luteolin in Chrysanthemum tea with a ultra-sensitive electrochemical sensor based on MoO3/poly(3,4-ethylene dioxythiophene)/gama-cyclodextrin metal-organic framework composites. Food Chem. 2022, 397, 133723. [Google Scholar] [CrossRef]
- Hitabatuma, A.; Wang, P.; Su, X.; Ma, M. Metal-Organic Frameworks-Based Sensors for Food Safety. Foods 2022, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Sindoro, M.; Yanai, N.; Jee, A.-Y.; Granick, S. Colloidal-Sized Metal–Organic Frameworks: Synthesis and Applications. Acc. Chem. Res. 2014, 47, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.K.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks—A progress report. Chem. Soc. Rev. 2011, 40, 498–519. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Furukawa, S.; Kondo, M.; Hirai, K.; Horike, N.; Takashima, Y.; Uehara, H.; Louvain, N.; Meilikhov, M.; Tsuruoka, T.; et al. Shape-Memory Nanopores Induced in Coordination Frameworks by Crystal Downsizing. Science 2013, 339, 193–196. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Haque, E.; Khan, N.A.; Park, J.H.; Jhung, S.H. Synthesis of a Metal–Organic Framework Material, Iron Terephthalate, by Ultrasound, Microwave, and Conventional Electric Heating: A Kinetic Study. Chem. Eur. J. 2010, 16, 1046–1052. [Google Scholar] [CrossRef]
- Hermes, S.; Witte, T.; Hikov, T.; Zacher, D.; Bahnmüller, S.; Langstein, G.; Huber, K.; Fischer, R.A. Trapping Metal-Organic Framework Nanocrystals: An in-Situ Time-Resolved Light Scattering Study on the Crystal Growth of MOF-5 in Solution. J. Am. Chem. Soc. 2007, 129, 5324–5325. [Google Scholar] [CrossRef]
- Avci, C.; Ariñez-Soriano, J.; Carné-Sánchez, A.; Guillerm, V.; Carbonell, C.; Imaz, I.; Maspoch, D. Post-Synthetic Anisotropic Wet-Chemical Etching of Colloidal Sodalite ZIF Crystals. Angew. Chem. Int. Ed. 2015, 54, 14417–14421. [Google Scholar] [CrossRef]
- Lu, X.; He, B.; Liang, Y.; Wang, J.; Jiao, Q.; Liu, Y.; Guo, R.; Wei, M.; Jin, H.; Ren, W.; et al. An electrochemical aptasensor based on dual-enzymes-driven target recycling strategy for patulin detection in apple juice. Food Control 2022, 137, 108907. [Google Scholar] [CrossRef]
- Yeung, H.H.M.; Sapnik, A.F.; Massingberd-Mundy, F.; Gaultois, M.W.; Wu, Y.; Fraser, D.A.X.; Henke, S.; Pallach, R.; Heidenreich, N.; Magdysyuk, O.V.; et al. Control of Metal–Organic Framework Crystallization by Metastable Intermediate Pre-equilibrium Species. Angew. Chem. Int. Ed. 2019, 58, 566–571. [Google Scholar] [CrossRef]
- Zavyalova, A.G.; Kladko, D.V.; Chernyshov, I.Y.; Vinogradov, V.V. Large MOFs: Synthesis strategies and applications where size matters. J. Mater. Chem. A 2021, 9, 25258–25271. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Hu, H.; Zhou, X.; You, F.; Yao, C.; Liu, F.J.; Yu, P.; Wu, D.; Yao, J.; et al. Advanced Metal-Organic Frameworks-Based Catalysts in Electrochemical Sensors. Front. Chem. 2022, 10, 881172. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal–organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, B.; Tian, P.; Xie, L.; Wei, W.; He, J.; Lin, M.; Zhu, H.; Chen, H.; He, B. A “signal off” aptasensor based on AuNPs/Ni-MOF substrate-free catalyzed for detection Enrofloxacin. J. Electroanal. Chem. 2022, 911, 116251. [Google Scholar] [CrossRef]
- Xu, Z.; Li, P.; Chen, H.; Zhu, X.; Zhang, Y.; Liu, M.; Yao, S. Picomolar glutathione detection based on the dual-signal self-calibration electrochemical sensor of ferrocene-functionalized copper metal-organic framework via solid-state electrochemistry of cuprous chloride. J. Colloid Interface Sci. 2022, 628, 798–806. [Google Scholar] [CrossRef]
- Khataee, A.; Sohrabi, H.; Ehsani, M.; Agaei, M.; Sisi, A.J.; Abdi, J.; Yoon, Y. State-of-the-art progress of metal-organic framework-based electrochemical and optical sensing platforms for determination of bisphenol A as an endocrine disruptor. Environ. Res. 2022, 212, 113536. [Google Scholar] [CrossRef]
- Peng, C.; Miao, L.; Qiu, D.; Chen, S. Co3O4-chitosan/biomass-derived porous carbon molecularly imprinted polymer integrated electrode for selective detection of glucose. Ceram. Int. 2022, 48, 23137–23144. [Google Scholar] [CrossRef]
- Moradi, O. Electrochemical sensors based on carbon nanostructures for the analysis of bisphenol A—A review. Food Chem. Toxicol. 2022, 165, 113074. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Liang, H.W.; Chen, L.F.; Hu, B.C.; Yu, S.H. Bacterial Cellulose: A Robust Platform for Design of Three Dimensional Carbon-Based Functional Nanomaterials. Acc. Chem. Res. 2016, 49, 96–105. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Three-dimensional graphene architectures. Nanoscale 2012, 4, 5549–5563. [Google Scholar] [CrossRef]
- Dong, X.-C.; Xu, H.; Wang, X.-W.; Huang, Y.-X.; Chan-Park, M.B.; Zhang, H.; Wang, L.-H.; Huang, W.; Chen, P. 3D Graphene–Cobalt Oxide Electrode for High-Performance Supercapacitor and Enzymeless Glucose Detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Deline, A.R.; Frank, B.P.; Smith, C.L.; Sigmon, L.R.; Wallace, A.N.; Gallagher, M.J.; Goodwin, D.G., Jr.; Durkin, D.P.; Fairbrother, D.H. Influence of Oxygen-Containing Functional Groups on the Environmental Properties, Transformations, and Toxicity of Carbon Nanotubes. Chem. Rev. 2020, 120, 11651–11697. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Deng, L.; Wang, Y.; Han, M.; Ding, Y. An electrochemical sensor for the determination of Luteolin using an alizarin red/carboxylic acid group functionalized carbon nanotube. Microchem. J. 2022, 174, 106864. [Google Scholar] [CrossRef]
- Xuan, X.; Kim, J.Y.; Hui, X.; Das, P.S.; Yoon, H.S.; Park, J.-Y. A highly stretchable and conductive 3D porous graphene metal nanocomposite based electrochemical-physiological hybrid biosensor. Biosens. Bioelectron. 2018, 120, 160–167. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, F.; Zeng, B. Fabrication of surface molecularly imprinted electrochemical sensor for the sensitive quantification of chlortetracycline with ionic liquid and MWCNT improving performance. Talanta 2022, 239, 123130. [Google Scholar] [CrossRef]
- Liu, R.; Chang, Y.; Li, F.; Dubovyk, V.; Li, D.; Ran, Q.; Zhao, H. Highly sensitive detection of carbendazim in juices based on mung bean-derived porous carbon@chitosan composite modified electrochemical sensor. Food Chem. 2022, 392, 133301. [Google Scholar] [CrossRef]
- Liu, W.; Yang, X.; Li, M.; Gui, Q.-W.; Jiang, H.; Li, Y.; Shen, Q.; Xia, J.; Liu, X. Sensitive detection of luteolin in peanut shell based on titanium carbide/carbon nanotube composite modified screen-printed electrode. Microchem. J. 2022, 175, 107135. [Google Scholar] [CrossRef]
- Liu, R.; Li, B.; Li, F.; Dubovyk, V.; Chang, Y.; Li, D.; Ding, K.; Ran, Q.; Wang, G.; Zhao, H. A novel electrochemical sensor based on beta-cyclodextrin functionalized carbon nanosheets@carbon nanotubes for sensitive detection of bactericide carbendazim in apple juice. Food Chem. 2022, 384, 132573. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.; Liu, Y.; Zhao, F.; Zeng, B. Kill two birds with one stone: Selective and fast removal and sensitive determination of oxytetracycline using surface molecularly imprinted polymer based on ionic liquid and ATRP polymerization. J. Hazard. Mater. 2022, 434, 128907. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Wang, H.; Li, F.; Zhang, Y.; Zhang, N. Three-dimensional porous reduced graphene oxide modified electrode for highly sensitive detection of trace rifampicin in milk. Anal. Methods 2022, 14, 2304–2310. [Google Scholar] [CrossRef]
- Karuppiah, C.; Babulal, S.M.; Chen, T.-W.; Chen, S.-M.; Hsu, L.-F.; Al Farraj, D.A.; Ramaraj, S.K.; Elshikh, M.S.; Yang, C.-C. A novel ammonium zinc molybdate layered double hydroxide nanoflakes/vapor grown carbon fibers nanomaterials based electrocatalyst for the monitoring of dimetridazole drug in real samples. J. Environ. Chem. Eng. 2022, 10, 108227. [Google Scholar] [CrossRef]
- Priscillal, I.J.D.; Wang, S.F. Synchronously activated strontium aluminate nanoflakes anchored functionalized carbon nanofiber nanocomposite for sensitive amperometric detection of food additive: Propyl gallate. Food Chem. 2022, 389, 133119. [Google Scholar] [CrossRef]
- Lu, L.; Kang, J. Amperometric nonenzymatic sensing of glucose at very low working potential by using a nanoporous PdAuNi ternary alloy. Microchim. Acta 2018, 185, 111. [Google Scholar] [CrossRef]
- Lu, L. Nanoporous noble metal-based alloys: A review on synthesis and applications to electrocatalysis and electrochemical sensing. Mikrochim. Acta 2019, 186, 664. [Google Scholar] [CrossRef]
- Weremfo, A.; Fong, S.T.C.; Khan, A.; Hibbert, D.B.; Zhao, C. Electrochemically roughened nanoporous platinum electrodes for non-enzymatic glucose sensors. Electrochim. Acta 2017, 231, 20–26. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B. Recent advances in porous Pt-based nanostructures: Synthesis and electrochemical applications. Chem. Soc. Rev. 2014, 43, 2439–2450. [Google Scholar] [CrossRef]
- Li, C.; Iqbal, M.; Lin, J.; Luo, X.; Jiang, B.; Malgras, V.; Wu, K.C.W.; Kim, J.; Yamauchi, Y. Electrochemical Deposition: An Advanced Approach for Templated Synthesis of Nanoporous Metal Architectures. Acc. Chem. Res. 2018, 51, 1764–1773. [Google Scholar] [CrossRef]
- Li, C.; Eid, K.; Wang, H.; Deng, Y.; Lu, S.; Li, X.; Wang, L.; Gu, H. One-pot synthesis of bimetallic PdCu nanoframes as an efficient catalyst for the methanol oxidation reaction. New J. Chem. 2018, 42, 798–801. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Lee, S.H.; Kim, N.H.; Lee, J.H. Novel porous gold-palladium nanoalloy network-supported graphene as an advanced catalyst for non-enzymatic hydrogen peroxide sensing. Biosens. Bioelectron. 2016, 85, 669–678. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, C. A nanoporous palladium-nickel alloy with high sensing performance towards hydrogen peroxide and glucose. J. Colloid Interface Sci. 2015, 447, 50–57. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, G.; Tian, K.; Xu, C. A highly sensitive and stable electrochemical sensor for simultaneous detection towards ascorbic acid, dopamine, and uric acid based on the hierarchical nanoporous PtTi alloy. Biosens. Bioelectron. 2016, 82, 119–126. [Google Scholar] [CrossRef]
- Celik Kazici, H.; Caglar, A.; Aydogmus, T.; Aktas, N.; Kivrak, H. Microstructured prealloyed Titanium-Nickel powder as a novel nonenzymatic hydrogen peroxide sensor. J. Colloid Interface Sci. 2018, 530, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Jiang, B.; Alothman, Z.A.; Badjah, A.Y.; Naushad, M.; Habila, M.; Wabaidur, S.; Henzie, J.; Li, H.; Yamauchi, Y. Mesoporous PtCu Alloy Nanoparticles with Tunable Compositions and Particles Sizes Using Diblock Copolymer Micelle Templates. Chem. Eur. J. 2019, 25, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Pandi, K.; Chen, S.-M.; Cheng, Y.-H.; Sakthivel, M. Facile synthesis of perovskite-type NdNiO3 nanoparticles for an effective electrochemical non-enzymatic glucose biosensor. New J. Chem. 2017, 41, 11201–11207. [Google Scholar] [CrossRef]
- Boubezari, I.; Zazoua, A.; Errachid, A.; Jaffrezic-Renault, N. Sensitive Electrochemical Detection of Bioactive Molecules (Hydrogen Peroxide, Glucose, Dopamine) with Perovskites-Based Sensors. Chemosensors 2021, 9, 289. [Google Scholar] [CrossRef]
- Govindasamy, M.; Wang, S.-F.; Huang, C.-H.; Alshgari, R.A.; Ouladsmane, M. Colloidal synthesis of perovskite-type lanthanum aluminate incorporated graphene oxide composites: Electrochemical detection of nitrite in meat extract and drinking water. Microchim. Acta 2022, 189, 210. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhong, H.; Li, X.-M.; Jia, F.-F.; Shi, Y.-X.; Zhang, W.-G.; Cheng, Z.-P.; Zhang, L.-L.; Wang, J.-K. Perovskite LaTiO3–Ag0.2 nanomaterials for nonenzymatic glucose sensor with high performance. Biosens. Bioelectron. 2013, 48, 56–60. [Google Scholar] [CrossRef]
- Ali, S.M.; Al-Otaibi, H.M. The distinctive sensing performance of cobalt ion in LaBO3 perovskite (B = Fe, Mn, Ni, or Cr) for hydrazine electrooxidation. J. Electroanal. Chem. 2019, 851, 113443. [Google Scholar] [CrossRef]
- Tiliakos, A.; Ceaus, C.; Iordache, S.M.; Vasile, E.; Stamatin, I. Morphic transitions of nanocarbons via laser pyrolysis of polyimide films. J. Anal. Appl. Pyrolysis 2016, 121, 275–286. [Google Scholar] [CrossRef]
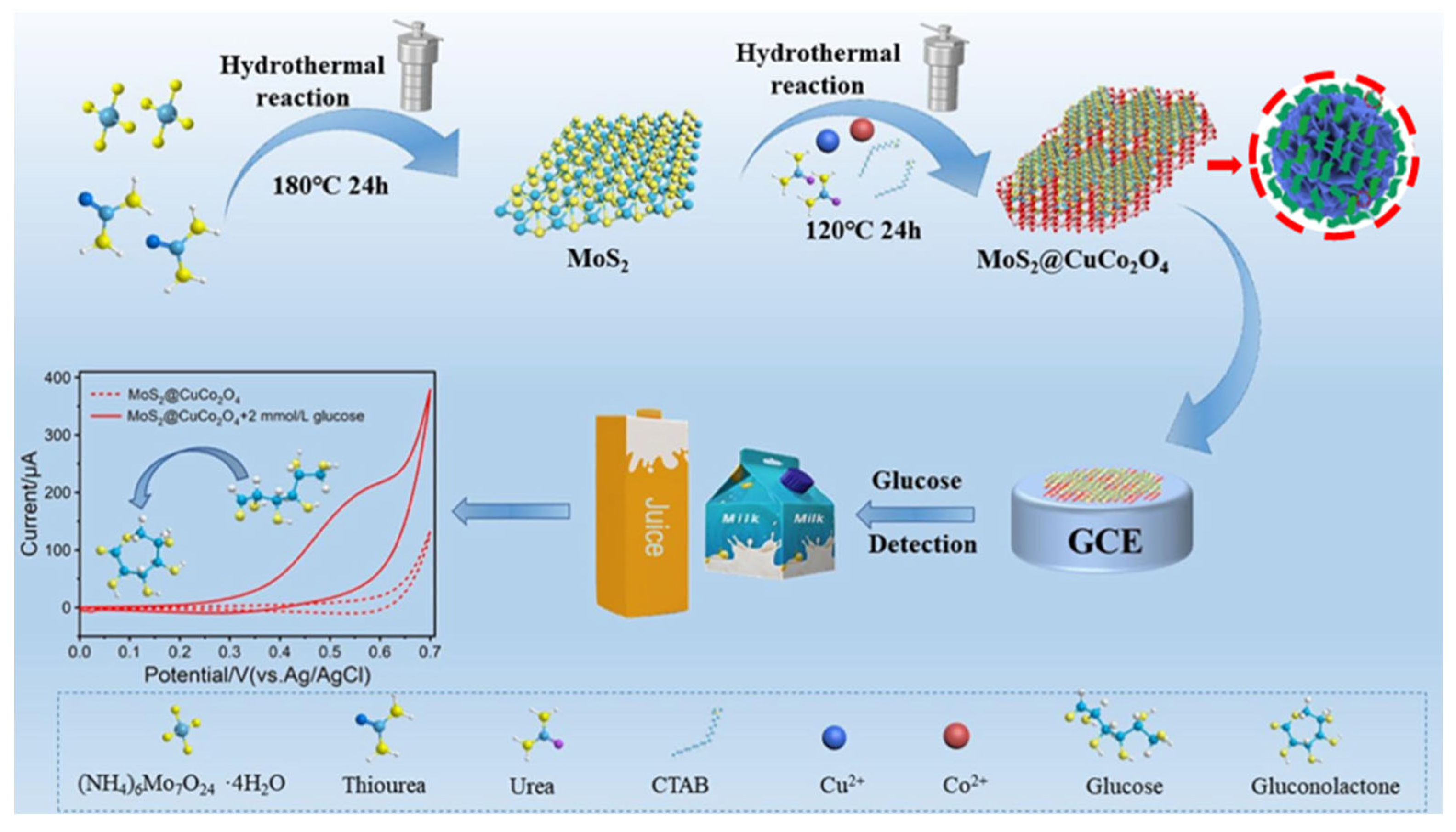
- Wang, H.; Zhu, W.; Xu, T.; Zhang, Y.; Tian, Y.; Liu, X.; Wang, J.; Ma, M. An integrated nanoflower-like MoS2@CuCo2O4 heterostructure for boosting electrochemical glucose sensing in beverage. Food Chem. 2022, 396, 133630. [Google Scholar] [CrossRef]
- Yan, L.; Chu, D.; Chu, X.-Q.; Ge, D.; Chen, X. Co/CoO nanoparticles armored by N-doped nanoporous carbon polyhedrons towards glucose oxidation in high-performance non-enzymatic sensors. New J. Chem. 2022, 46, 15071–15079. [Google Scholar] [CrossRef]
- Li, M.; Fang, L.; Zhou, H.; Wu, F.; Lu, Y.; Luo, H.; Zhang, Y.; Hu, B. Three-dimensional porous MXene/NiCo-LDH composite for high performance non-enzymatic glucose sensor. Appl. Surf. Sci. 2019, 495, 143554. [Google Scholar] [CrossRef]
- Thenrajan, T.; Selvasundarasekar, S.S.; Kundu, S.; Wilson, J. Novel Electrochemical Sensing of Catechins in Raw Green Tea Extract via a Trimetallic Zeolitic Imidazolate Fibrous Framework. ACS Omega 2022, 7, 19754–19763. [Google Scholar] [CrossRef]
- Tang, J.; Hu, T.; Li, N.; Zhu, Y.; Li, J.; Zheng, S.; Guo, J. Ag doped Co/Ni bimetallic organic framework for determination of luteolin. Microchem. J. 2022, 179, 107461. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.Y.; Sebastian, N.; Al-Mubaddel, F.S.; Noman, M.T. Ultrasensitive detection of food colorant sunset yellow using nickel nanoparticles promoted lettuce-like spinel Co3O4 anchored GO nanosheets. Food Chem. Toxicol. 2022, 159, 112725. [Google Scholar] [CrossRef]
- Garkani Nejad, F.; Asadi, M.H.; Sheikhshoaie, I.; Dourandish, Z.; Zaimbashi, R.; Beitollahi, H. Construction of modified screen-printed graphite electrode for the application in electrochemical detection of sunset yellow in food samples. Food Chem. Toxicol. 2022, 166, 113243. [Google Scholar] [CrossRef]
- Chen, Y.; Waterhouse, G.I.N.; Sun, H.; Qiao, X.; Sun, Y.; Xu, Z. Novel ratiometric electrochemical sensing platform with dual-functional poly-dopamine and NiS@HCS signal amplification for sunset yellow detection in foods. Food Chem. 2022, 390, 133193. [Google Scholar] [CrossRef]
- Joseph, X.B.; Sherlin, V.A.; Wang, S.F.; George, M. Integration of iron-manganese layered double hydroxide/tungsten carbide composite: An electrochemical tool for diphenylamine H(*+) analysis in environmental samples. Environ. Res. 2022, 212, 113291. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Gharagozlou, M.; Sohouli, E.; Marzi Khosrowshahi, E. Preparation of an electrochemical sensor based on a HKUST-1/CoFe2O4/SiO2-modified carbon paste electrode for determination of azaperone. Microchem. J. 2022, 175, 107199. [Google Scholar] [CrossRef]
- Xia, Y.; Hu, X.; Liu, Y.; Zhao, F.; Zeng, B. Molecularly imprinted ratiometric electrochemical sensor based on carbon nanotubes/cuprous oxide nanoparticles/titanium carbide MXene composite for diethylstilbestrol detection. Mikrochim. Acta 2022, 189, 137. [Google Scholar] [CrossRef]
- Keerthi, M.; Kumar Panda, A.; Wang, Y.H.; Liu, X.; He, J.H.; Chung, R.J. Titanium nanoparticle anchored functionalized MWCNTs for electrochemical detection of ractopamine in porcine samples with ultrahigh sensitivity. Food Chem. 2022, 378, 132083. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, Y.; Yuan, L.; Li, J. Biochar-supported Cu nanocluster as an electrochemical ultrasensitive interface for ractopamine sensing. Food Chem. X 2022, 15, 100404. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, X.; Li, Y.; Feng, Y.; Ye, B.C. High current flux electrochemical sensor based on nickel-iron bimetal pyrolytic carbon material of paper waste pulp for clenbuterol detection. Talanta 2022, 250, 123756. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Mathiyarasu, J.; Kim, B.-S. Environmental-assisted shape-controlled synthesis and electrocatalytic performance of CuS nanostructures for vanillin detection in commercial food products. Appl. Mater. Today 2022, 27, 101428. [Google Scholar] [CrossRef]
- Kogularasu, S.; Sriram, B.; Wang, S.-F.; Sheu, J.-K. Sea-Urchin-Like Bi2S3 Microstructures Decorated with Graphitic Carbon Nitride Nanosheets for Use in Food Preservation. ACS Appl. Nano Mater. 2022, 5, 2375–2384. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, H.; Zhang, X.; Cheng, X.; Xu, Y.; Zhao, H.; Gao, S.; Huo, L. Non-enzymatic nitrite amperometric sensor fabricated with near-spherical ZnO nanomaterial. Colloids Surf. B Biointerfaces 2022, 211, 112313. [Google Scholar] [CrossRef]
- Somnet, K.; Soravech, P.; Karuwan, C.; Tuantranont, A.; Amatatongchai, M. A compact N-nitrosodiphenylamine imprinted sensor based on a Pd nanoparticles-MIP microsphere modified screen-printed graphene electrode. J. Electroanal. Chem. 2022, 914, 116302. [Google Scholar] [CrossRef]
- Lu, H.; Wang, H.; Yang, L.; Zhou, Y.; Xu, L.; Hui, N.; Wang, D. A sensitive electrochemical sensor based on metal cobalt wrapped conducting polymer polypyrrole nanocone arrays for the assay of nitrite. Mikrochim. Acta 2021, 189, 26. [Google Scholar] [CrossRef]
- Han, S.; Ding, Y.; Teng, F.; Yao, A.; Leng, Q. Molecularly imprinted electrochemical sensor based on 3D-flower-like MoS2 decorated with silver nanoparticles for highly selective detection of butylated hydroxyanisole. Food Chem. 2022, 387, 132899. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.; Liu, T.; Tang, W.; Li, N.; Zheng, S.; Guo, J.; Song, C. N-Doped TiO2–Carbon Composites Derived from NH2-MIL-125(Ti) for Electrochemical Determination of tert-Butylhydroquinone. Food Anal. Methods 2022, 15, 2830–2839. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Y.; Wang, L.; Wang, L.; Chen, S. An ultrafine ZnO/ZnNi2O4@porous carbon@covalent-organic framework for electrochemical detection of paracetamol and tert-butyl hydroquinone. J. Alloys Compd. 2022, 906, 164369. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Al-Mubaddel, F.S.; Noman, M.T. A sensitive and economical electrochemical platform for detection of food additive tert-butylhydroquinone based on porous Co3O4 nanorods embellished chemically oxidized carbon black. Food Control 2022, 136, 108844. [Google Scholar] [CrossRef]
- Ru, J.; Wang, X.; Zhou, Z.; Zhao, J.; Yang, J.; Du, X.; Lu, X. Fabrication of octahedral GO/UiO-67@PtNPs nanocomposites as an electrochemical sensor for ultrasensitive recognition of arsenic (III) in Chinese Herbal Medicine. Anal Chim Acta 2022, 1195, 339451. [Google Scholar] [CrossRef]
- Liu, T.; Lin, B.; Yuan, X.; Chu, Z.; Jin, W. In situ fabrication of urchin-like Cu@carbon nanoneedles based aptasensor for ultrasensitive recognition of trace mercury ion. Biosens. Bioelectron. 2022, 206, 114147. [Google Scholar] [CrossRef]
- Ganesan, M.; Keerthika Devi, R.; Liao, A.H.; Lee, K.Y.; Gopalakrishnan, G.; Chuang, H.C. 3D-flower-like porous neodymium molybdate nanostructure for trace level detection of organophosphorus pesticide in food samples. Food Chem. 2022, 396, 133722. [Google Scholar] [CrossRef]
- Su, X.; Chen, Z.; Wang, H.; Yuan, L.; Zheng, K.; Zhang, W.; Zou, X. Ratiometric immunosensor with DNA tetrahedron nanostructure as high-performance carrier of reference signal and its applications in selective phoxim determination for vegetables. Food Chem. 2022, 383, 132445. [Google Scholar] [CrossRef]
- Mahmoudi-Moghaddam, H.; Akbari Javar, H.; Garkani-Nejad, Z. Fabrication of platinum-doped NiCo2O4 nanograss modified electrode for determination of carbendazim. Food Chem. 2022, 383, 132398. [Google Scholar] [CrossRef]
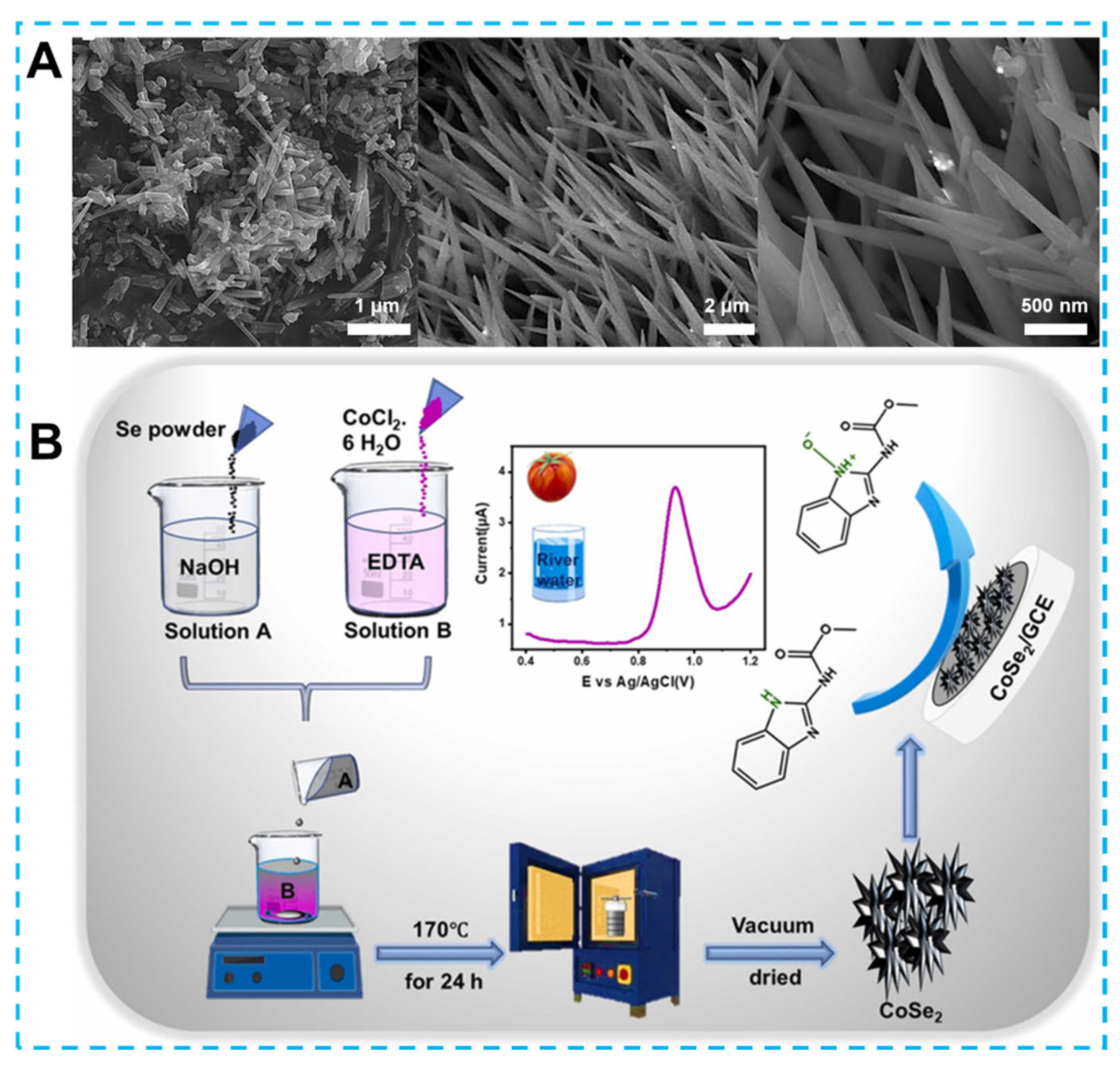
- Tamilalagan, E.; Akilarasan, M.; Chen, S.-M.; Maheshwaran, S.; Huang, Y.-F. Rationally designed urchin-like structured cobalt diselenide (o-CoSe2) for the sensitive voltammetric detection of carbendazim fungicide in vegetables and water samples. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129941. [Google Scholar] [CrossRef]
- Akilarasan, M.; Maheshwaran, S.; Chen, S.-M.; Tamilalagan, E.; Albaqami, M.D.; Alotabi, R.G.; Arumugam, R. In-situ synthesis of bimetallic chalcogenide SrS/Bi2S3 nanocomposites as an efficient electrocatalyst for the selective voltammetric sensing of maleic hydrazide herbicide. Process Saf. Environ. Prot. 2022, 165, 151–160. [Google Scholar] [CrossRef]
- Yamuna, A.; Karikalan, N.; Lee, T.Y. Effect of the Ni3TeO6 phase in a Ni2Te3O8/expanded graphite composite on the electrochemical monitoring of metribuzin residue in soil and water samples. J. Hazard. Mater. 2022, 435, 128988. [Google Scholar] [CrossRef]
- Manjula, N.; Pulikkutty, S.; Chen, S.-M. Hexagonal plate-like NiO/ZnO for highly selective detection of antibiotic drugs in food and biological samples. FlatChem 2022, 34, 100391. [Google Scholar] [CrossRef]
- Li, M.; Zhe, T.; Li, F.; Li, R.; Bai, F.; Jia, P.; Bu, T.; Xu, Z.; Wang, L. Hybrid structures of cobalt-molybdenum bimetallic oxide embedded in flower-like molybdenum disulfide for sensitive detection of the antibiotic drug nitrofurantoin. J. Hazard. Mater. 2022, 435, 129059. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Fu, K.; Jin, C.; Li, M.; Zhang, G.; Zhang, R.; Bai, H. Microwave-prepared surface imprinted magnetic nanoparticles based electrochemical sensor for adsorption and determination of ketamine in sewage. Anal. Chim. Acta 2022, 1217, 340025. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Farokhi, S.; Rahmati, Z. Development of a dual-recognition strategy for the aflatoxin B1 detection based on a hybrid of aptamer-MIP using a Cu2O NCs/GCE. Microchem. J. 2022, 178, 107328. [Google Scholar] [CrossRef]
- Feng, K.; Li, T.; Ye, C.; Gao, X.; Yue, X.; Ding, S.; Dong, Q.; Yang, M.; Huang, G.; Zhang, J. A novel electrochemical immunosensor based on Fe3O4@graphene nanocomposite modified glassy carbon electrode for rapid detection of Salmonella in milk. J. Dairy Sci. 2022, 105, 2108–2118. [Google Scholar] [CrossRef]
- Fatema, K.N.; Areerob, Y.; Meng, Z.-D.; Zhu, L.; Oh, W.-C. Cu- and Sn-Codoped Mesoporous BaTiO3-G-SiO2 Nanocomposite for Bioreceptor-Free, Sensitive, and Quick Electrochemical Sensing of Rhizopus stolonifer Fungus. ACS Appl. Electron. Mater. 2022, 4, 2053–2061. [Google Scholar] [CrossRef]
- Lai, Q.; Niu, Q.; Chen, W.; Zhang, Y.; Long, M.; Liang, B.; Wang, F.; Liu, Z. An ultrasensitive bacteria biosensor using “multilayer cake” silver microelectrode based on local high electric field effect. Appl. Phys. Lett. 2022, 121, 013701. [Google Scholar] [CrossRef]
- Zheng, R.; He, B.; Xie, L.; Yan, H.; Jiang, L.; Ren, W.; Suo, Z.; Xu, Y.; Wei, M.; Jin, H. Molecular Recognition-Triggered Aptazyme Sensor Using a Co-MOF@MCA Hybrid Nanostructure as Signal Labels for Adenosine Triphosphate Detection in Food Samples. Anal. Chem. 2022, 94, 12866–12874. [Google Scholar] [CrossRef]



| Sensor Materials | Structure or Morphology | Analyte | Range of Detection | Detection Limit | Refs. |
|---|---|---|---|---|---|
| CoFe2O4@SiO2/HKUST-1 | approximate octahedron | AZN | 0.05–10,000 nM | 0.01 nM | [141] |
| MIP/CNT/Cu2O NPs/Ti3C2Tx | 3D composite structures | DES | 0.01–70 μM | 6 nM | [142] |
| WC@FeMn-LDH | spheres | DPA | 0.01–183.34 μM | 1.1 nM | [140] |
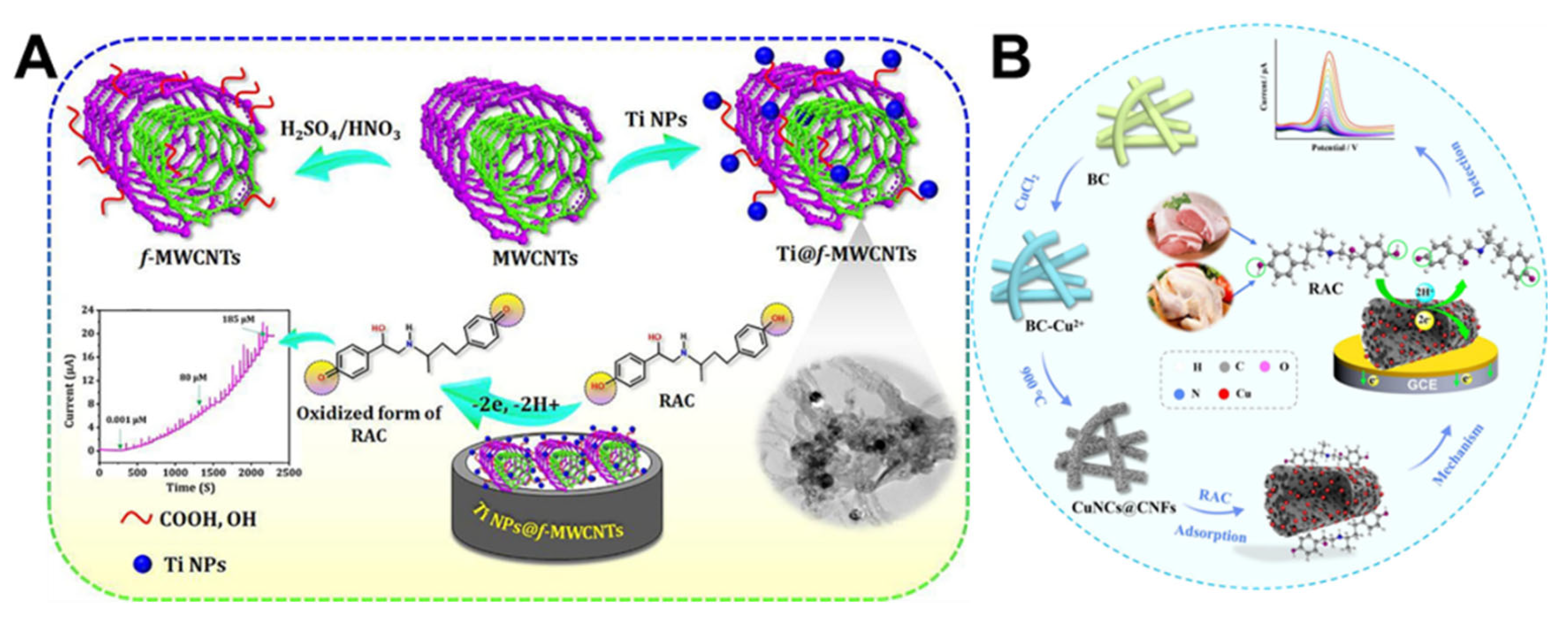
| Ti@f-MWCNTs | 3D composite structures | RAC | 0.01–185 μM | 0.0038 µM | [143] |
| CuNCs@CNFs | 3D network structures | RAC | 0.002–0.025 μM | 0.05 nM | [144] |
| Co3O4 NRs/FCB | nanorods | TBHQ | 0.12–62.2 μM | 1 nM | [154] |
| TiO2/NC | octahedrons | TBHQ | 0.05–100 μM | 4 nM | [152] |
| ZnO/ZnNi2O4 @porous carbon@COFTM | polyhedrons | TBHQ | 47.85 nM–130 μM | 15.95 nM | [153] |
| MoS2/Ag NPs-CS | 3D-flowers | BHA | 1 × 10−9−1 × 10−4 | 7.9 nM | [151] |
| Ni-Co3O4 NPs/GO | 3D composite structures | SY | 0.125–108.5 μM | 0.9 nM | [137] |
| MnO2 NRs/GO | nanorods | SY | 0.01–115.0 μM | 0.008 μM | [138] |
| NiS@HCS | hollow spheres | SY | 0.01–100 μM | 0.003 μM | [139] |
| NiFe2O4–NPCs | 3D network structures | CLB | 10−7–10−5 M and 7 × 10−11–10−7 M | 3.03 × 10−12 M | [145] |
| CuS | hexagons | VN | 0.1–46.5 μM | 53 nM | [146] |
| Co/PPy | nanocones | nitrite | 2–3318 µM | 0.35 µM | [150] |
| g-C3N4/Bi2S3 | 3D sea-urchins | nitrite | 0.001–385.4 μM | 0.4 nM | [147] |
| ZnO | spheres | nitrite | 1.9–800 μM and 1080–5900 μM | 0.89 μM | [148] |
| LaAlO3@GO | 3D composite structures | nitrite | 0.01–1540.5 µM | 0.0041 µM | [128] |
| PdNPs@MIP | microspheres | nitrite | 0.01–0.1 μM and 0.1–100 μM | 0.0013 μM | [149] |
| Ag NPs-Graphite Carbon | 3D micro/nano morphologies by femtosecond Laser | nitrite | 1–4000 × 10−6 M | 0.117 × 10−6 M | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Lai, Q.; Chen, W.; Zhang, Y.; Mo, L.; Liu, Z. Three-Dimensional Electrochemical Sensors for Food Safety Applications. Biosensors 2023, 13, 529. https://doi.org/10.3390/bios13050529
Zhang C, Lai Q, Chen W, Zhang Y, Mo L, Liu Z. Three-Dimensional Electrochemical Sensors for Food Safety Applications. Biosensors. 2023; 13(5):529. https://doi.org/10.3390/bios13050529
Chicago/Turabian StyleZhang, Chi, Qingteng Lai, Wei Chen, Yanke Zhang, Long Mo, and Zhengchun Liu. 2023. "Three-Dimensional Electrochemical Sensors for Food Safety Applications" Biosensors 13, no. 5: 529. https://doi.org/10.3390/bios13050529
APA StyleZhang, C., Lai, Q., Chen, W., Zhang, Y., Mo, L., & Liu, Z. (2023). Three-Dimensional Electrochemical Sensors for Food Safety Applications. Biosensors, 13(5), 529. https://doi.org/10.3390/bios13050529





