Modular Set of Reagents in Lateral Flow Immunoassay: Application for Antibiotic Neomycin Detection in Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Syntheses of Gold Nanoparticles
2.3. Conjugation of Gold Nanoparticles with Goat Anti-Mouse Antibodies
2.4. Transmission Electron Microscopy
2.5. Synthesis of Neomycin-Biotin Conjugate
2.6. Preparation of Immunochromatographic Test Strips
2.7. Honey Samples Preparation
2.8. Immunochromatographic Detection of Neomycin
2.9. Processing of Test Strips Images and Calculation of the Assay Parameters
3. Results and Discussion
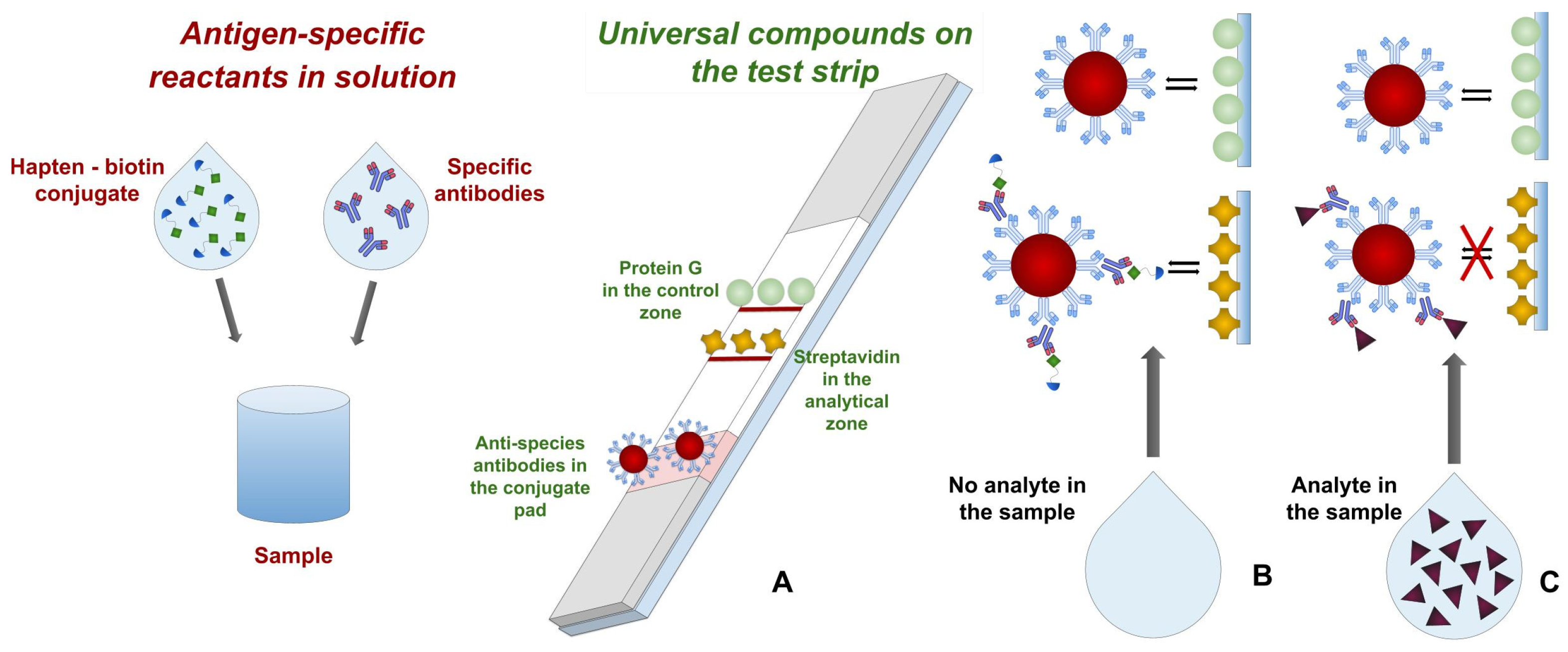
3.1. Assay Principle
3.2. Choice of Reagents Concentrations for the Universal Test Strip
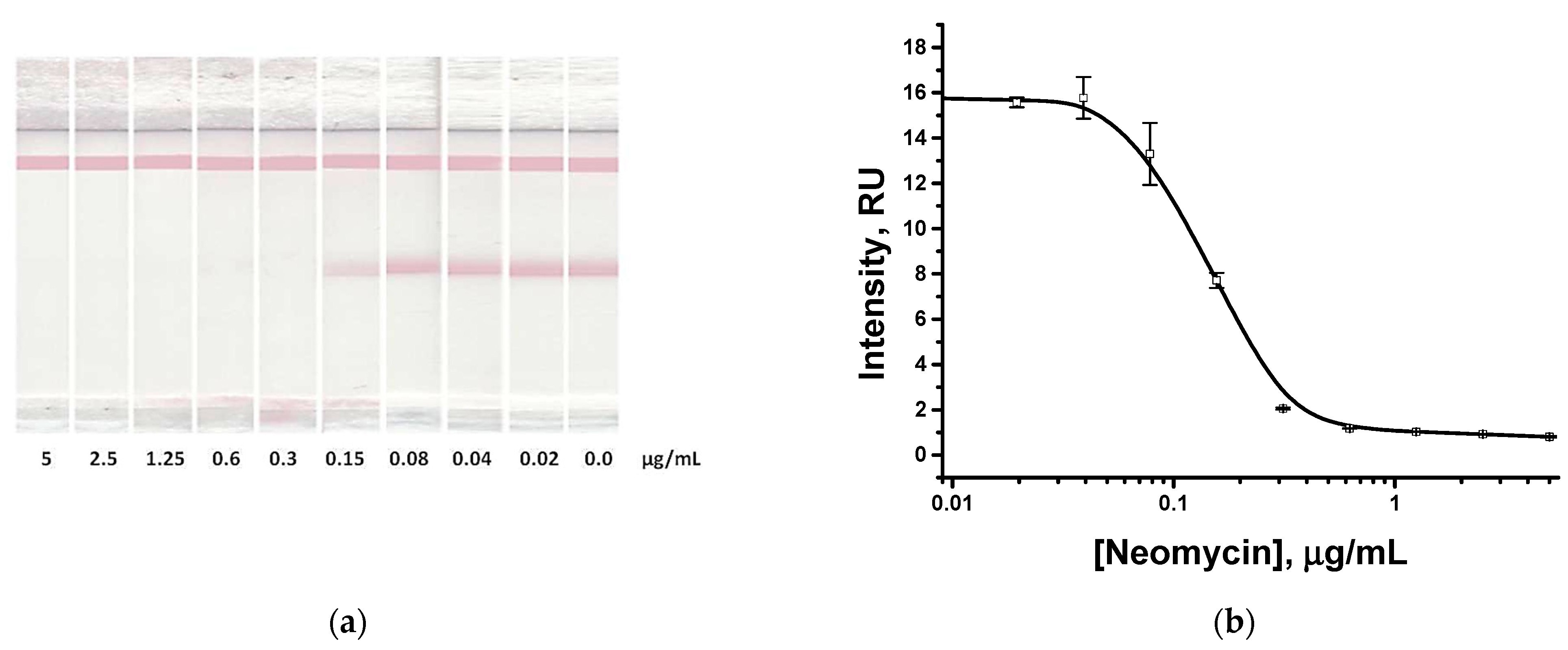
3.3. Choice of Concentrations of Neomycin-Specific Reagents
3.4. Approbation of the Proposed Assay for Honey Testing
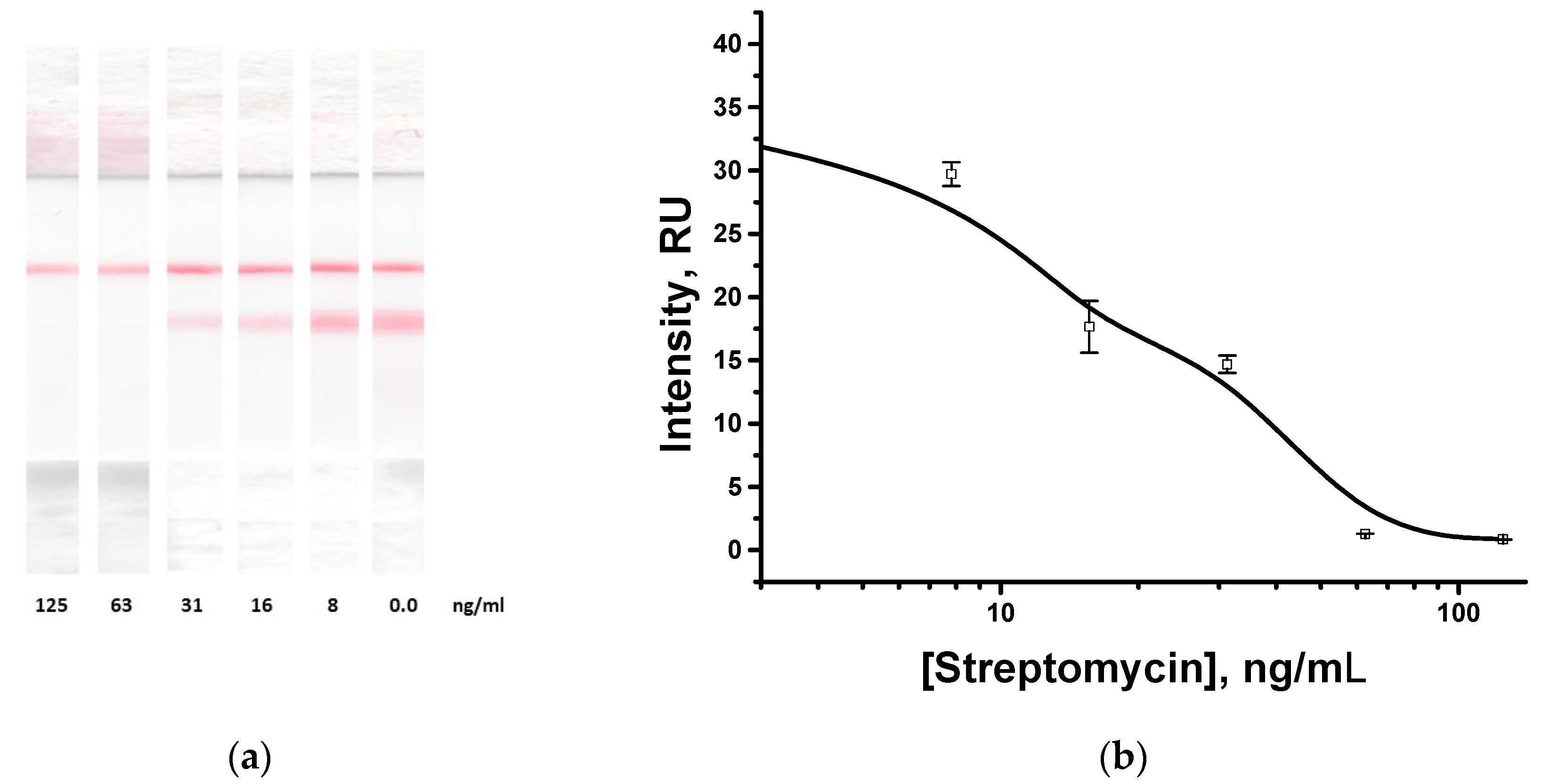
3.5. Demonstration of the Universality of the Proposed Assay for Streptomycin Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten years of lateral flow immunoassay technique applications: Trends, challenges and future perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, P.; Sohrabi, H.; Hejazi, M.; Jahanban-Esfahlan, A.; Baradaran, B.; Tohidast, M.; Majidi, M.R.; Mokhtarzadeh, A.; Tavangar, S.M.; de la Guardia, M. Lateral flow assays (LFA) as an alternative medical diagnosis method for detection of virus species: The intertwine of nanotechnology with sensing strategies. Trends Anal. Chem. 2021, 145, 116460. [Google Scholar] [CrossRef] [PubMed]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Boehringer, H.R.; O’Farrell, B.J. Lateral flow assays in infectious disease diagnosis. Clin. Chem. 2022, 68, 52–58. [Google Scholar] [CrossRef]
- Yeasmin, S.; Ammanath, G.; Onder, A.; Yan, E.; Yildiz, U.H.; Palaniappan, A.; Liedberg, B. Current trends and challenges in point-of-care urinalysis of biomarkers in trace amounts. Trends Anal. Chem. 2022, 157, 116786. [Google Scholar] [CrossRef]
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.; Rivas, L.; Álvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 2020, 15, 3788–3816. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Xu, X.; Guo, L.; Xu, L.; Sun, M.; Hu, S.; Kuang, H.; Xu, C.; Li, A. An overview for the nanoparticles-based quantitative lateral flow assay. Small Methods 2022, 6, 2101143. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Safenkova, I.V.; Zherdev, A.V.; Avdienko, V.G.; Kozlova, I.V.; Babayan, S.S.; Gergert, V.Y.; Dzantiev, B.B. A mechanism of gold nanoparticle aggregation by immunoglobulin G preparation. Appl. Sci. 2020, 10, 475. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Byzova, N.A.; Zherdev, A.V.; Dzantiev, B.B. Retention of activity by antibodies immobilized on gold nanoparticles of different sizes: Fluorometric method of determination and comparative evaluation. Nanomaterials 2021, 11, 3117. [Google Scholar] [CrossRef]
- Tripathi, K.; Driskell, J.D. Quantifying bound and active antibodies conjugated to gold nanoparticles: A comprehensive and robust approach to evaluate immobilization chemistry. ACS Omega 2018, 3, 8253–8259. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.; Li, W.; Zhan, Y.; Yu, L.; Wu, X.; Li, J.; Xu, H.; Yang, G. Label-free and immobilization-free electrochemiluminescent sensing platform for highly sensitive detection of As(III) by combining target-induced strand displacement amplification with polydopamine nanospheres. Sens. Actuators B Chem. 2020, 31115, 127818. [Google Scholar] [CrossRef]
- Liu, Q.; Fei, A.; Wang, K. An immobilization-free and homogeneous electrochemiluminescence assay for detection of environmental pollutant graphene oxide in water. J. Electroanal. Chem. 2021, 89715, 115583. [Google Scholar] [CrossRef]
- Ding, Y.; Cui, P.; Chen, H.; Li, J.; Huang, L.; González-Sapienza, G.; Hammock, B.D.; Wang, M.; Hua, X. “Ready-to-use” immunosensor for the detection of small molecules with fast readout. Biosens. Bioelectron. 2022, 2011, 113968. [Google Scholar] [CrossRef] [PubMed]
- Pfender, N.; Lucassen, R.; Offermann, N.; Schulte-Pelkum, J.; Fooke, M.; Jakob, T. Evaluation of a novel rapid test system for the detection of specific IgE to Hymenoptera Venoms. J. Allergy 2012, 2012, 862023. [Google Scholar] [CrossRef] [PubMed]
- ALFA (Allergy Lateral Flow Assay) Is a Rapid Test for the Determination of Allergen-Specific IgE (sIgE) in Human Serum, Plasma or Whole Blood. Available online: https://www.fooke-labs.de/allergie-diagnostik?lang=en (accessed on 3 March 2023).
- Dzantiev, B.B.; Byzova, N.A.; Urusov, A.E.; Zherdev, A.V. Immunochromatographic methods in food analysis. Trends Anal. Chem. 2014, 55, 81–93. [Google Scholar] [CrossRef]
- Abati, R.; Sampaio, A.R.; Maciel, R.M.A.; Colombo, F.C.; Libardoni, G.; Battisti, L.; Lozano, E.R.; Ghisi, N.C.; Costa-Maia, F.M.; Potrich, M. Bees and pesticides: The research impact and scientometrics relations. Environ. Sci. Pollut. Res. 2021, 28, 32282–32298. [Google Scholar] [CrossRef]
- Neov, B.; Georgieva, A.; Shumkova, R.; Radoslavov, G.; Hristov, P. Biotic and abiotic factors associated with colonies mortalities of managed honey bee (Apis mellifera). Diversity 2019, 11, 237. [Google Scholar] [CrossRef]
- Reybroeck, W. Residues of antibiotics and chemotherapeutics in honey. J. Apic. Res. 2018, 57, 97–112. [Google Scholar] [CrossRef]
- Oliveira, N.A.; Gonçalves, B.L.; Lee, S.H.; Caf, O.; Corassin, C.H. Use of antibiotics in animal production and its impact on human health. J. Food Chem. Nanotechnol. 2020, 6, 40–47. [Google Scholar] [CrossRef]
- van Duijkeren, E.; Schwarz, C.; Bouchard, D.; Catry, B.; Pomba, C.; Baptiste, K.E.; Moreno, M.A.; Rantala, M.; Ružauskas, M.; Sanders, P.; et al. The use of aminoglycosides in animals within the EU: Development of resistance in animals and possible impact on human and animal health: A review. J. Antimicrob. Chemother. 2019, 74, 2480–2496. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Torres, O.B.; Jalah, R.; Rice, K.C.; Li, F.; Antoline, J.F.; Iyer, M.R.; Jacobson, A.E.; Boutaghou, M.N.; Alving, C.R.; Matyas, G.R. Characterization and optimization of heroin hapten-BSA conjugates: Method development for the synthesis of reproducible hapten-based vaccines. Anal. Bioanal. Chem. 2014, 406, 5927–5937. [Google Scholar] [CrossRef] [PubMed]
- Uhrovčík, J. Strategy for determination of LOD and LOQ values—Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Sotnikov, D.V.; Barshevskaya, L.V.; Bartosh, A.V.; Zherdev, A.V.; Dzantiev, B.B. Double competitive immunodetection of small analyte: Realization for highly sensitive lateral flow immunoassay of chloramphenicol. Biosensors 2022, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Use of gold nanoparticle-labeled secondary antibodies to improve the sensitivity of an immunochromatographic assay for aflatoxin B1. Microchim. Acta 2014, 181, 1939–1946. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Byzova, N.A.; Zvereva, E.A.; Zherdev, A.V.; Dzantiev, B.B. Sensitive lateral flow immunoassay of an antibiotic neomycin in foodstuffs. J. Food. Sci. Technol. 2021, 58, 292–301. [Google Scholar] [CrossRef]
- Zvereva, E.A.; Byzova, N.A.; Sveshnikov, P.G.; Zherdev, A.V.; Dzantiev, B.B. Cut-off on demand: Adjustment of the threshold level of an immunochromatographic assay for chloramphenicol. Anal. Methods 2015, 7, 6378–6384. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Byzova, N.A.; Zvereva, E.A.; Bartosh, A.V.; Zherdev, A.V.; Dzantiev, B.B. Mathematical modeling of immunochromatographic test systems in a competitive format: Analytical and numerical approaches. Biochem. Eng. J. 2020, 164, 107763. [Google Scholar] [CrossRef]
- Mao, X.; Yu, B.; Li, Z.; Li, Z.; Shi, G. Comparison of lateral flow immunoassays based on oriented and nonoriented immobilization of antibodies for the detection of aflatoxin B1. Anal. Chim. Acta 2022, 15, 340135. [Google Scholar] [CrossRef]
- Maximum Residue Limits for Neomycin. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/vetdrugs/veterinary-drug-detail/en/?d_id=44 (accessed on 3 March 2023).
- Luong, J.H.t.; Vashist, S.K. Chemistry of Biotin–Streptavidin and the Growing Concern of an Emerging Biotin Interference in Clinical Immunoassays. ACS Omega 2020, 5, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Factors influencing the detection limit of the lateral-flow sandwich immunoassay: A case study with potato virus X. Anal. Bioanal. Chem. 2012, 403, 1595–1605. [Google Scholar] [CrossRef] [PubMed]




| Anti-Neomycin Antibodies, μg/mL | Neomycin-Biotin, µM | Visual Detection Limit, ng/mL | Instrumental Detection Limit, ng/mL |
|---|---|---|---|
| Experiments with varied anti-neomycin antibodies concentration (Figure 4) | |||
| 73 | 0.3 | 2.5 | 0.1 |
| 36.5 | 0.3 | 2.5 | 0.06 |
| 18 | 0.3 | 0.3 | 0.07 |
| 9 | 0.3 | 0.3 | 0.07 |
| 4.5 | 0.3 | 0.3 | 0.06 |
| Experiments with varied neomycin-biotin concentration (Figure 5) | |||
| 18 | 2.3 | 5 | 0.67 |
| 18 | 1.2 | 5 | 0.49 |
| 18 | 0.6 | 5 | 0.32 |
| 18 | 0.3 | 0.3 | 0.07 |
| 18 | 0.15 | 0.3 | 0.06 |
| Neomycin Added, ng/mL | Neomycin Detected, ng/mL | Recovery, % |
|---|---|---|
| 19 | 21 | 110 |
| 39 | 33 | 85 |
| 78 | 88 | 113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barshevskaya, L.V.; Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Modular Set of Reagents in Lateral Flow Immunoassay: Application for Antibiotic Neomycin Detection in Honey. Biosensors 2023, 13, 498. https://doi.org/10.3390/bios13050498
Barshevskaya LV, Sotnikov DV, Zherdev AV, Dzantiev BB. Modular Set of Reagents in Lateral Flow Immunoassay: Application for Antibiotic Neomycin Detection in Honey. Biosensors. 2023; 13(5):498. https://doi.org/10.3390/bios13050498
Chicago/Turabian StyleBarshevskaya, Lyubov V., Dmitriy V. Sotnikov, Anatoly V. Zherdev, and Boris B. Dzantiev. 2023. "Modular Set of Reagents in Lateral Flow Immunoassay: Application for Antibiotic Neomycin Detection in Honey" Biosensors 13, no. 5: 498. https://doi.org/10.3390/bios13050498
APA StyleBarshevskaya, L. V., Sotnikov, D. V., Zherdev, A. V., & Dzantiev, B. B. (2023). Modular Set of Reagents in Lateral Flow Immunoassay: Application for Antibiotic Neomycin Detection in Honey. Biosensors, 13(5), 498. https://doi.org/10.3390/bios13050498








