Recent Advances in Recognition Receptors for Electrochemical Biosensing of Mycotoxins—A Review
Abstract
1. Introduction
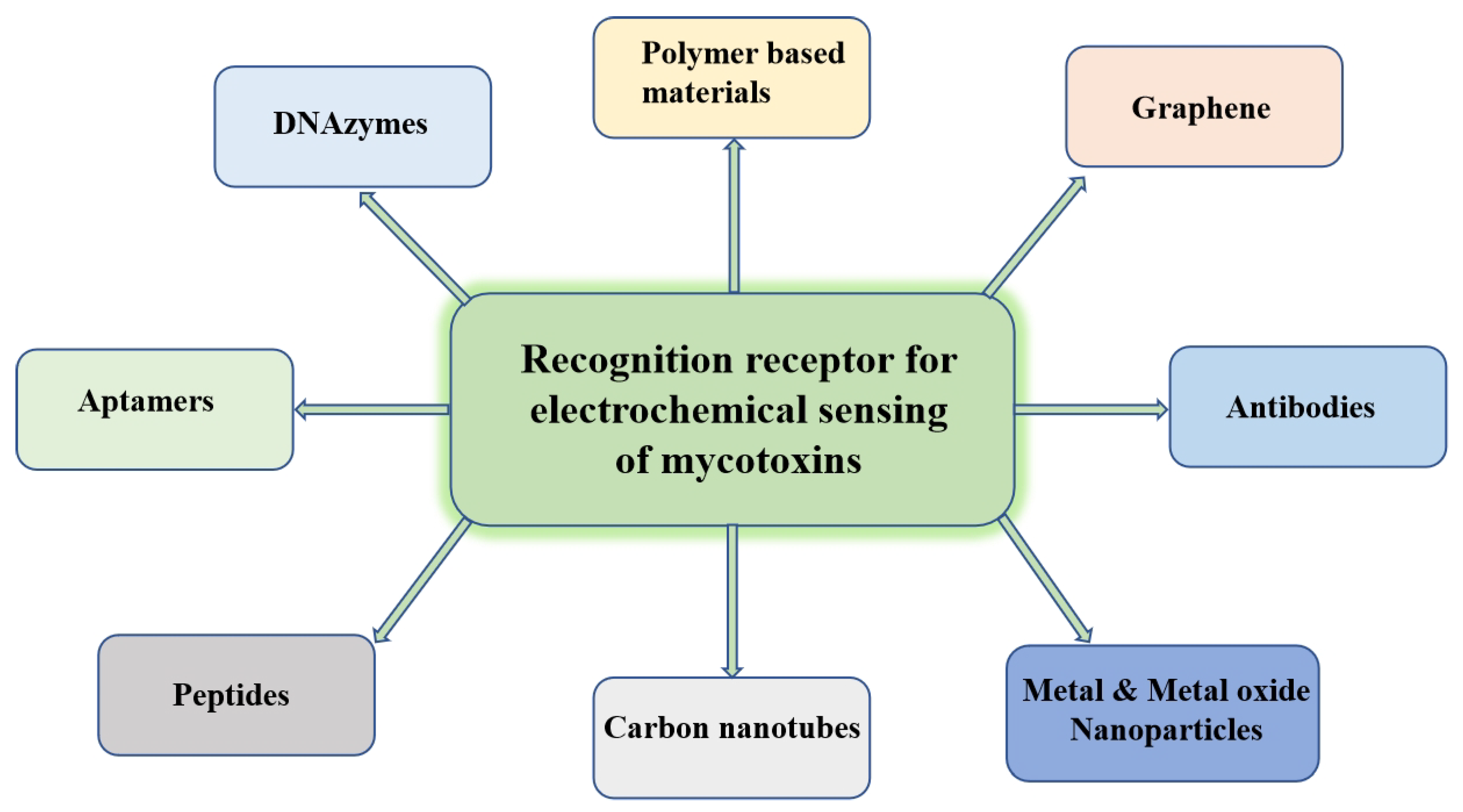
2. Recognition Receptors
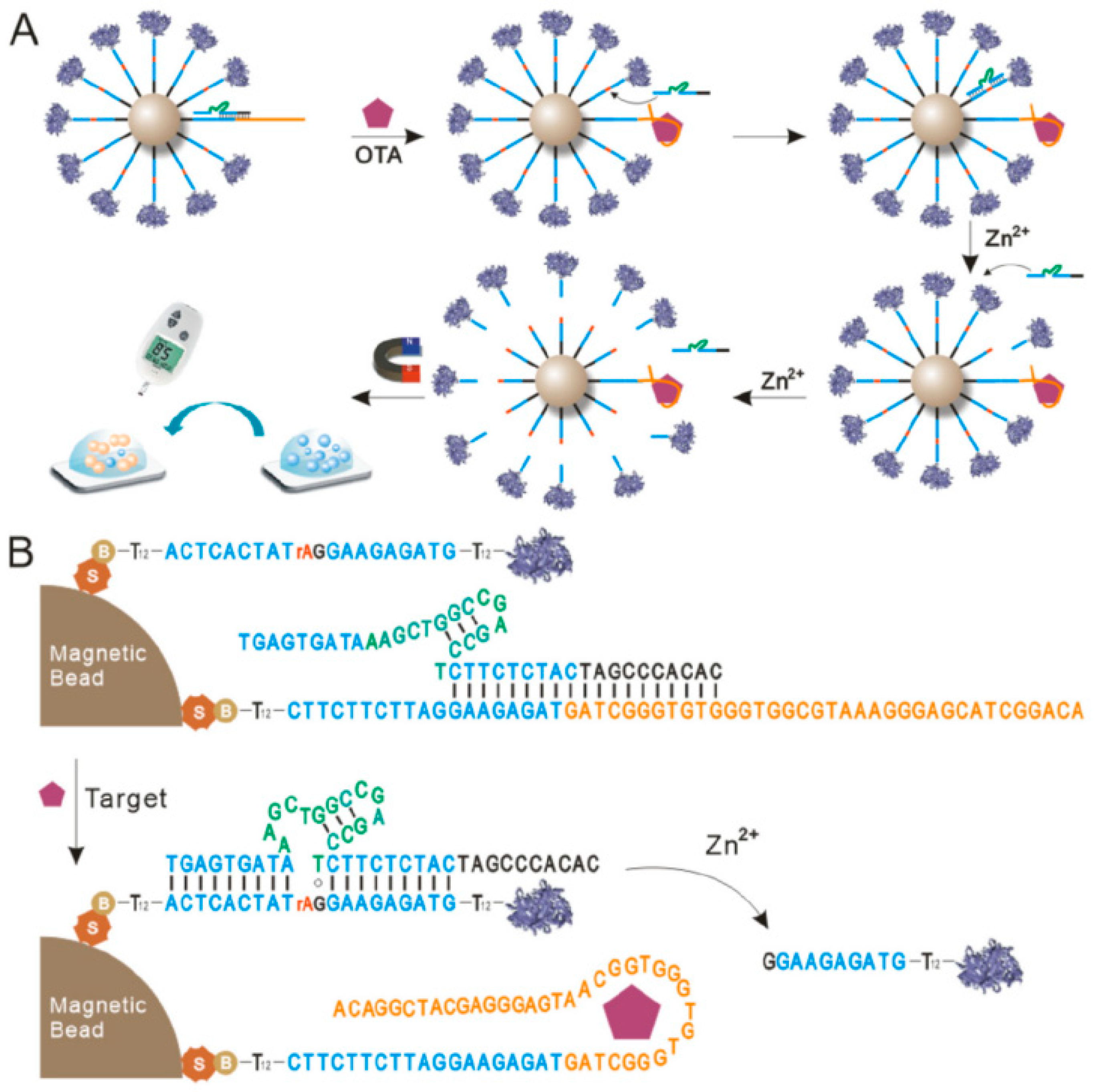
2.1. Aptamers as Recognition Receptors
2.1.1. Immobilization
2.1.2. Classification
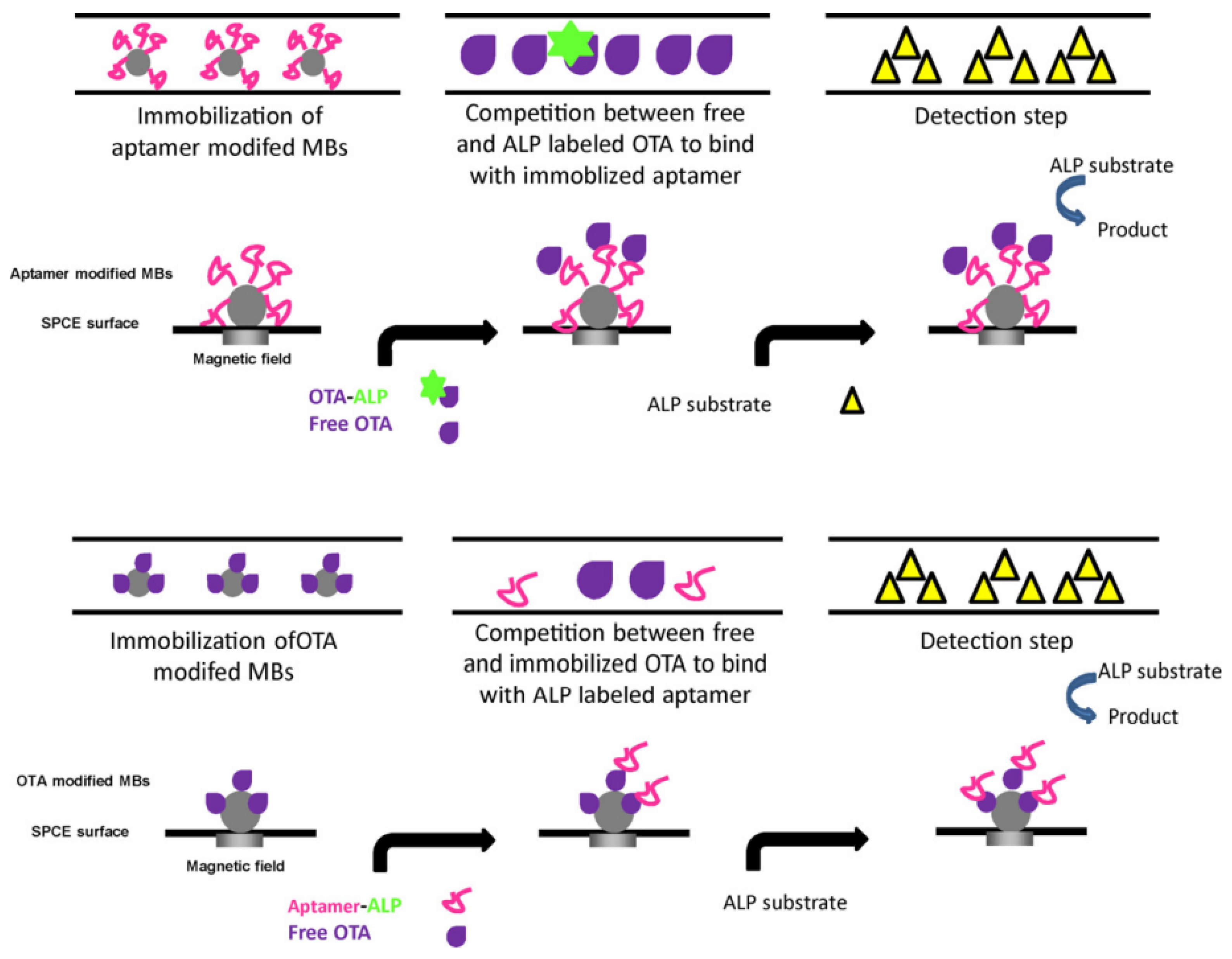
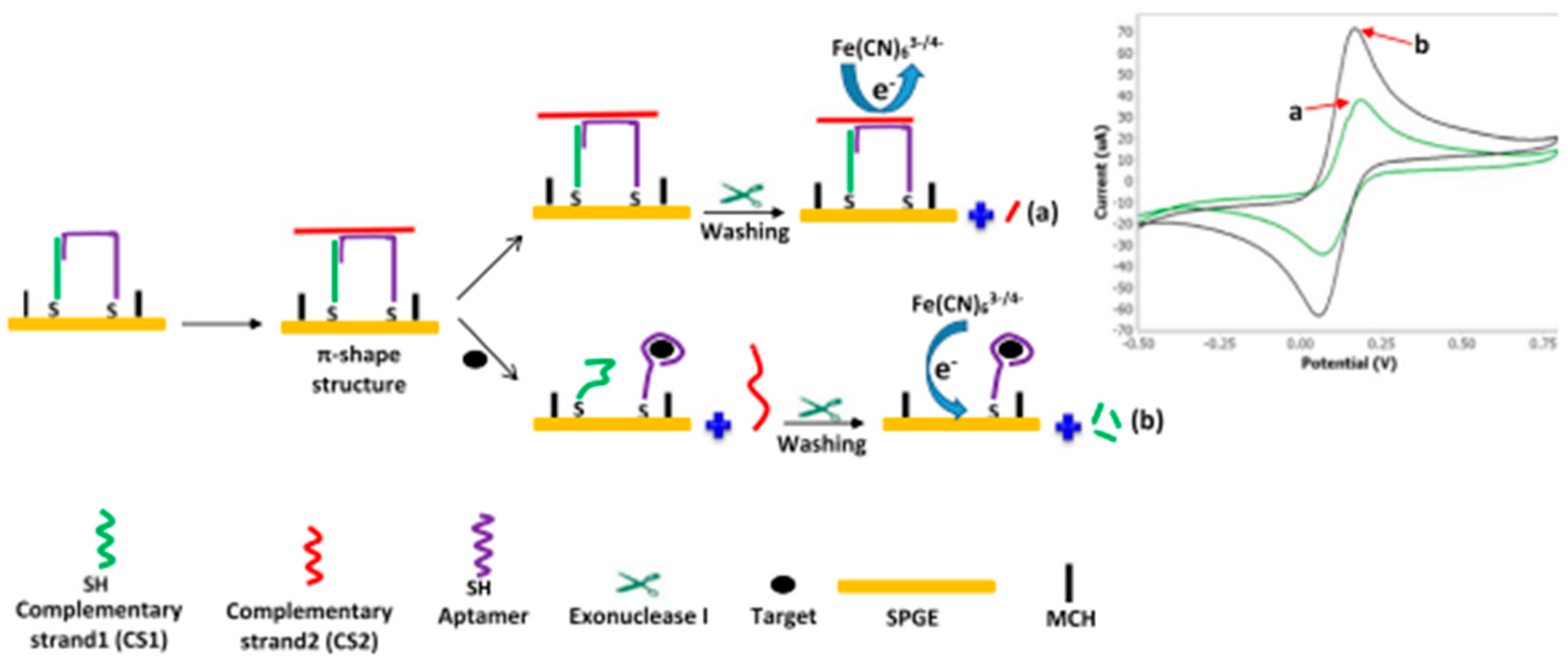
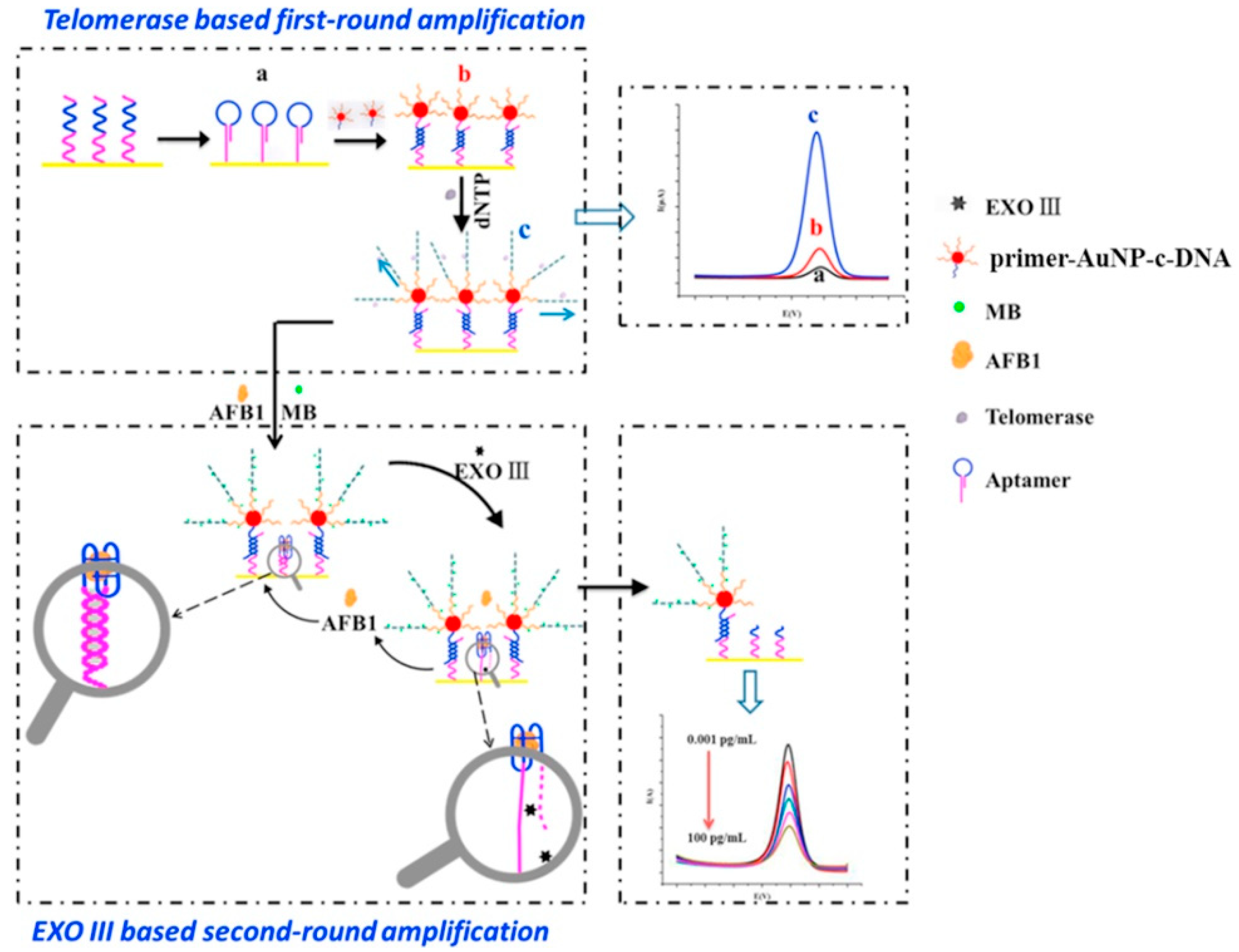
2.1.3. Advances in Aptamer Research
2.2. Nanomaterials as Recognition Receptors
2.2.1. Graphene in Electrochemical Sensors
2.2.2. CNTs in Electrochemical Sensors
2.2.3. Metal and Metal Oxide Nanoparticles in Electrochemical Sensor
2.3. Polymer-Based Materials as Recognition Receptors
3. Conclusions and Future Prospects
| Analyte | Electrode Materials | LOD (ng/mL) | Linear Range (ng/mL) | Analytical Method | Real Samples | Ref. |
|---|---|---|---|---|---|---|
| AFM1 | Fe3O4-GO- CdTe QDs | 0.0003 * | 0.001–0.001 × 105 * | Multifuncional electrochemical analytical system | milk | [78] |
| AFB1 | Apt/NR-TCA/GCE | 0.015 | 0.03–30 | DPV | Beer and wine | [22] |
| Apt-CSs-modified | 0.002 * | 0.007–0.5 | DPV | Human serum, Grape juice | [50] | |
| SPE | 0.6 × 10−7 * | 0.1 × 10−9 to 1 × 10−4 | SWV | Corn | [51] | |
| Screen-printed interdigitated microelectrode (SPIMs) | 10 | 5 to 20 | EIS and CV | Rice | [112] | |
| MPCM@MIP | 0.4 | 5 to 1000 | UV and HPLC Analysis | Soysauce and vinegar | [117] | |
| Thiol-modified aptamer | 0.000034 * | 0.000002 to 2 * | SWV, EIC, and CV | Medicine | [114] | |
| Au NPs | 0.93 × 10−6 * | 0.93 × 10−6 to 0.9 * | CV | Rice | [123] | |
| MIP/Au NPs | 0.001 * | 0.0001 to 10.0 | SPR | Ground corn | [121] | |
| Multi-walled CNTs-Pt | 0.46 * | 0.9–207.95 * | Chronamperomety | Corn, rice, and wheat | [84] | |
| MWCNTs- COOHITO | 0.08 | 0.25– 1.375 | CV | - | [88] | |
| MWCNTs-CS | 0.0001 * | 0.0001– 10 | DPV | Palm Kernel Cake and Feed Samples | [95] | |
| rGO-ITO | 0.12 | 0.125–1.5 | CV | - | [68] | |
| GO-Au NPs | 0.23 | 0.5–5 | CV | - | [69] | |
| rGO-transparent conductive oxidase | 10−10 * | 10−10–10−6 * | CV | Corn | [70] | |
| PtNPs-CoTPP- rGO | 0.0015 * | 0.005–5.0 | DPV | Peanut | [71] | |
| rGO-PPy-PPa | 0.000001 * | 0.000001–0.01 * | EIS | Corn | [72] | |
| G-polymer-Au NPs | 0.3 × 10−6 * | 0.3 × 10−6– 0.910−3 * | CV | Peanut, rice, milk flour, and soyabean | [74] | |
| rGO–Ni NPs- ITO | 0.16 | 1.0–8.0 | CV | - | [76] | |
| Gold electrode-imprinted Protein | 1.97×10−3 * | 1–1000 | CV | Nut | [103] | |
| Gold–PAMAM dendrimers | 0.124 ± 0.03 * | 0.1–10 nM | CV and EIS | Rice, corn | [34] | |
| AFB1 and FuB1 | Polyaniline | 0.0003 and 0.00032 * | 0.001 ng/mLto 500 ng/mL * | CV and DPV | Rice, barley, peanuts, oat | [125] |
| FB1 | Pt electrode | 0.5 × 10−5 * | 0.031 to 3.12 * | DPV and EIS | Maize Sample | [122] |
| SWCNTs-CS | 0.002 * | 0.01–100 | EIS | Corn | [94] | |
| OTA | dsDNA/MCH/ssDNA modified AuE | 0.001 * | 0.005–10.0 | DPV | Mildewed wheat starch sample | [48] |
| Fc and βCD | 0.0004 * | 0.001 to 10 * | CV | Peanut oil | [108] | |
| Au NPs@Co-MOF | 0.000031 * | 0.000001 to 0.00005 * | EIS, CV, and DPV | Red wine | [116] | |
| Au NPs embedded PEI/PVA hydrogel | 0.4 * | Upto 24.2 * | TLN Conductometric biosensor | Olive oil | [118] | |
| MIP/Au NPs/PIL-FMNS/CNT-MoS2/GCE | 5.6 * | 2 × 105–6 × 105 * | EIS and CV | Beer, red wine, and Chinese liquor | [119] | |
| MWCNTs/MIP | 1.7 * | 20.19–403.81 ng/mL * | CV and DPV | Beer and Wine | [120] | |
| CNT-ITO | 0.0025 * | 0.0025–0.06 * | DPV | Serum | [87] | |
| Au NPs-BSA- OTA | 0.86 | 0.3–8.5 | DPV | Wheat | [101] | |
| GO-PAMAM- Mn2+ | 0.0005 * | 0.0001–30 * | EIS | Red wines | [75] | |
| ZEA | AuE | 0.00013 * | 5.0 × 105–50 * | DPV | Corn sample and beer | [52] |
| MIP/Au NPs | 2.5 * | Up to 200 * | EIS and CV | Corn | [125] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, V.; Kaur, M.; Sharma, S. Insight into Peroxidase and Polyphenol Oxidase Mimic Activity of Spinel Ferrite Nanoparticles and Their GO Composites. Mater. Chem. Phys. 2022, 279, 125727. [Google Scholar] [CrossRef]
- Singh, E.; Kaur, M.; Sharma, S. Structural Tuning of CTAB@MgFe2O4 Nanocomposite as Peroxidase Mimic for H2O2 and Glucose Sensing. Mater. Chem. Phys. 2021, 271, 124851. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, G.; Kaur, N.; Singh, N. Organic Cation Receptor for Colorimetric Lateral Flow Device: Detection of Zearalenone in Food Samples. ACS Appl. Mater. Interfaces 2022, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mayank. MOF-Based Electrochemical Sensors for Alkali Metal Cations. In Metal-Organic Frameworks-Based Hybrid Materials for Environmental Sensing and Monitoring; CRC Press: Boca Raton, FL, USA, 2022; ISBN 9781003188. [Google Scholar]
- Khaneghahi Abyaneh, H.; Bahonar, A.; Noori, N.; Yazdanpanah, H.; Shojaee AliAbadi, M.H. The Overall and Variations of Aflatoxin M1 Contamination of Milk in Iran: A Systematic Review and Meta-Analysis Study. Food Chem. 2020, 310, 125848. [Google Scholar] [CrossRef] [PubMed]
- Sengling Cebin Coppa, C.F.; Mousavi Khaneghah, A.; Alvito, P.; Assunção, R.; Martins, C.; Eş, I.; Gonçalves, B.L.; Valganon de Neeff, D.; Sant’Ana, A.S.; Corassin, C.H.; et al. The Occurrence of Mycotoxins in Breast Milk, Fruit Products and Cereal-Based Infant Formula: A Review. Trends Food Sci. Technol. 2019, 92, 81–93. [Google Scholar] [CrossRef]
- Berthiller, F.; Sulyok, M.; Krska, R.; Schuhmacher, R. Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int. J. Food Microbiol. 2007, 119, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.; Munro, I.; Abbot, P.; Baldwin, N.; Lopez-Garcia, R.; Ly, K.; McGirr, L.; Roberts, A.; Socolovsky, S. Review of the Regulation and Safety Assessment of Food Substances in Various Countries and Jurisdictions. Food Addit. Contam. Part A 2013, 30, 1147–1220. [Google Scholar] [CrossRef]
- Hathout, A.S.; Aly, S.E. Biological Detoxification of Mycotoxins: A Review. Ann. Microbiol. 2014, 64, 905–919. [Google Scholar] [CrossRef]
- Ji, C.; Fan, Y.; Zhao, L. Review on Biological Degradation of Mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Xu, J.; Qing, T.; Jiang, Z.; Zhang, P.; Feng, B. Graphene Oxide-Regulated Low-Background Aptasensor for the “Turn on” Detection of Tetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119898. [Google Scholar] [CrossRef]
- Ndiaye, S.; Zhang, M.; Fall, M.; Ayessou, N.M.; Zhang, Q.; Li, P. Current Review of Mycotoxin Biodegradation and Bioadsorption: Microorganisms, Mechanisms, and Main Important Applications. Toxins 2022, 14, 729. [Google Scholar] [CrossRef]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Hernández, S.; Bertolín, J.R.; Cubel, C.; Castillo, J.R. Electrochemical Affinity Biosensors for Detection of Mycotoxins: A Review. Biosens. Bioelectron. 2013, 49, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Kailasa, S.K.; Kumar, V.; Tsang, Y.F.; Lee, S.E.; Gobi, K.V.; Kim, K.-H. Progress on Nanostructured Electrochemical Sensors and Their Recognition Elements for Detection of Mycotoxins: A Review. Biosens. Bioelectron. 2018, 121, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Catanante, G.; Rhouati, A.; Hayat, A.; Marty, J.L. An Overview of Recent Electrochemical Immunosensing Strategies for Mycotoxins Detection. Electroanalysis 2016, 28, 1750–1763. [Google Scholar] [CrossRef]
- Jin, H.; Gui, R.; Yu, J.; Lv, W.; Wang, Z. Fabrication Strategies, Sensing Modes and Analytical Applications of Ratiometric Electrochemical Biosensors. Biosens. Bioelectron. 2017, 91, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.Y.; Pike, A.; Tan, L.L. Recent Advances in Conventional Methods and Electrochemical Aptasensors for Mycotoxin Detection. Foods 2021, 10, 1437. [Google Scholar] [CrossRef]
- Azri, F.A.; Eissa, S.; Zourob, M.; Chinnappan, R.; Sukor, R.; Yusof, N.A.; Raston, N.H.A.; Alhoshani, A.; Jinap, S. Electrochemical Determination of Zearalenone Using a Label-Free Competitive Aptasensor. Microchim. Acta 2020, 187, 266. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Wei, H.; Li, Y. Selection of Aptamers against Pathogenic Bacteria and Their Diagnostics Application. World J. Microbiol. Biotechnol. 2018, 34, 149. [Google Scholar] [CrossRef]
- Hong, P.; Li, W.; Li, J. Applications of Aptasensors in Clinical Diagnostics. Sensors 2012, 12, 1181–1193. [Google Scholar] [CrossRef]
- Xu, R.; Kiarie, E.G.; Yiannikouris, A.; Sun, L.; Karrow, N.A. Nutritional Impact of Mycotoxins in Food Animal Production and Strategies for Mitigation. J. Anim. Sci. Biotechnol. 2022, 13, 69. [Google Scholar] [CrossRef]
- Goud, K.Y.; Catanante, G.; Hayat, A.; Satyanarayana, M.; Gobi, K.V.; Marty, J.L. Disposable and Portable Electrochemical Aptasensor for Label Free Detection of Aflatoxin B1 in Alcoholic Beverages. Sens. Actuators B Chem. 2016, 235, 466–473. [Google Scholar] [CrossRef]
- Nur Topkaya, S.; Cetin, A.E. Electrochemical Aptasensors for Biological and Chemical Analyte Detection. Electroanalysis 2021, 33, 277–291. [Google Scholar] [CrossRef]
- Ning, Y.; Hu, J.; Lu, F. Aptamers Used for Biosensors and Targeted Therapy. Biomed. Pharmacother. 2020, 132, 110902. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Sharma, R.K. Biosensors for the Detection of Mycotoxins. Toxin Rev. 2022, 41, 618–638. [Google Scholar] [CrossRef]
- Evtugyn, G.; Hianik, T. Electrochemical Immuno- and Aptasensors for Mycotoxin Determination. Chemosensors 2019, 7, 10. [Google Scholar] [CrossRef]
- Mukherjee, S.; Meshik, X.; Choi, M.; Farid, S.; Datta, D.; Lan, Y.; Poduri, S.; Sarkar, K.; Baterdene, U.; Huang, C.-E.; et al. A Graphene and Aptamer Based Liquid Gated FET-Like Electrochemical Biosensor to Detect Adenosine Triphosphate. IEEE Trans. Nanobioscience 2015, 14, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.-H.; Marty, J. Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress. Toxins 2017, 9, 349. [Google Scholar] [CrossRef]
- Jarczewska, M.; Górski, Ł.; Malinowska, E. Electrochemical Aptamer-Based Biosensors as Potential Tools for Clinical Diagnostics. Anal. Methods 2016, 8, 3861–3877. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, L.; Tang, D.; Liu, B.; Li, J.; Chen, G. Simultaneous Multiplexed Stripping Voltammetric Monitoring of Marine Toxins in Seafood Based on Distinguishable Metal Nanocluster-Labeled Molecular Tags. J. Agric. Food Chem. 2012, 60, 8974–8982. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Kulikova, T.; Hianik, T. Recent Achievements in Electrochemical and Surface Plasmon Resonance Aptasensors for Mycotoxins Detection. Chemosensors 2021, 9, 180. [Google Scholar] [CrossRef]
- Balamurugan, S.; Obubuafo, A.; Soper, S.A.; Spivak, D.A. Surface Immobilization Methods for Aptamer Diagnostic Applications. Anal. Bioanal. Chem. 2008, 390, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- De Rycke, E.; Foubert, A.; Dubruel, P.; Bol’hakov, O.I.; De Saeger, S.; Beloglazova, N. Recent Advances in Electrochemical Monitoring of Zearalenone in Diverse Matrices. Food Chem. 2021, 353, 129342. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Spinella, K.; Poturnayová, A.; Šnejdárková, M.; Mosiello, L.; Hianik, T. Detection of Aflatoxin B1 by Aptamer-Based Biosensor Using PAMAM Dendrimers as Immobilization Platform. Food Control 2015, 52, 9–18. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Kang, B.H.; Lee, Y.D.; Lee, S.B.; Ju, B.-K. Effect of Fluorine Plasma Treatment with Chemically Reduced Graphene Oxide Thin Films as Hole Transport Layer in Organic Solar Cells. Appl. Surf. Sci. 2013, 287, 91–96. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Gui, R.; Jin, H.; Xia, Y. Carbon Nanomaterials-Based Electrochemical Aptasensors. Biosens. Bioelectron. 2016, 79, 136–149. [Google Scholar] [CrossRef]
- Hosseinzadeh, L.; Mazloum-Ardakani, M. Advances in Aptasensor Technology. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 99, pp. 237–279. ISBN 9780128215609. [Google Scholar]
- Mir, M.; Vreeke, M.; Katakis, I. Different Strategies to Develop an Electrochemical Thrombin Aptasensor. Electrochem. Commun. 2006, 8, 505–511. [Google Scholar] [CrossRef]
- Sumitani, M.; Takagi, S.; Tanamura, Y.; Inoue, H. Oxygen Indicator Composed of an Organic/Inorganic Hybrid Compound of Methylene Blue, Reductant, Surfactant and Saponite. Anal. Sci. 2004, 20, 1153–1157. [Google Scholar] [CrossRef][Green Version]
- Zhu, C.; Liu, D.; Li, Y.; Shen, X.; Ma, S.; Liu, Y.; You, T. Ratiometric Electrochemical Aptasensor for Ultrasensitive Detection of Ochratoxin A Based on a Dual Signal Amplification Strategy: Engineering the Binding of Methylene Blue to DNA. Biosens. Bioelectron. 2020, 150, 111814. [Google Scholar] [CrossRef]
- Chen, W.; Yan, C.; Cheng, L.; Yao, L.; Xue, F.; Xu, J. An Ultrasensitive Signal-on Electrochemical Aptasensor for Ochratoxin A Determination Based on DNA Controlled Layer-by-Layer Assembly of Dual Gold Nanoparticle Conjugates. Biosens. Bioelectron. 2018, 117, 845–851. [Google Scholar] [CrossRef]
- Gupta, R.; Rahi Alhachami, F.; Khalid, I.; Majdi, H.S.; Nisar, N.; Mohamed Hasan, Y.; Sivaraman, R.; Romero Parra, R.M.; Al Mashhadani, Z.I.; Fakri Mustafa, Y. Recent Progress in Aptamer-Functionalized Metal-Organic Frameworks-Based Optical and Electrochemical Sensors for Detection of Mycotoxins. Crit. Rev. Anal. Chem. 2022, 52, 1–22. [Google Scholar] [CrossRef]
- Song, K.-M.; Lee, S.; Ban, C. Aptamers and Their Biological Applications. Sensors 2012, 12, 612–631. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Hayat, A.; Hernandez, D.B.; Meraihi, Z.; Munoz, R.; Marty, J.-L. Development of an Automated Flow-Based Electrochemical Aptasensor for on-Line Detection of Ochratoxin A. Sens. Actuators B Chem. 2013, 176, 1160–1166. [Google Scholar] [CrossRef]
- Dong, N.; Liu, D.; Meng, S.; Liu, S.; You, T. Tetrahedral DNA Nanostructure-Enabled Electrochemical Aptasensor for Ultrasensitive Detection of Fumonisin B1 with Extended Dynamic Range. Sens. Actuators B Chem. 2022, 354, 130984. [Google Scholar] [CrossRef]
- Jafari, M.; Rezaei, M.; Kalantari, H.; Tabarzad, M.; Daraei, B. Optimization of Aflatoxin B1 Aptasensing. J. Toxicol. 2017, 2017, 2461354. [Google Scholar] [CrossRef]
- Jalalian, S.H.; Ramezani, M.; Danesh, N.M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. A Novel Electrochemical Aptasensor for Detection of Aflatoxin M1 Based on Target-Induced Immobilization of Gold Nanoparticles on the Surface of Electrode. Biosens. Bioelectron. 2018, 117, 487–492. [Google Scholar] [CrossRef]
- Suea-Ngam, A.; Howes, P.D.; Stanley, C.E.; deMello, A.J. An Exonuclease I-Assisted Silver-Metallized Electrochemical Aptasensor for Ochratoxin A Detection. ACS Sens. 2019, 4, 1560–1568. [Google Scholar] [CrossRef]
- Tong, P.; Zhang, L.; Xu, J.-J.; Chen, H.-Y. Simply Amplified Electrochemical Aptasensor of Ochratoxin A Based on Exonuclease-Catalyzed Target Recycling. Biosens. Bioelectron. 2011, 29, 97–101. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Alibolandi, M.; Ramezani, M.; Sarreshtehdar Emrani, A.; Zolfaghari, R.; Taghdisi, S.M. A New Amplified π-Shape Electrochemical Aptasensor for Ultrasensitive Detection of Aflatoxin B1. Biosens. Bioelectron. 2017, 94, 374–379. [Google Scholar] [CrossRef]
- Zheng, W.; Teng, J.; Cheng, L.; Ye, Y.; Pan, D.; Wu, J.; Xue, F.; Liu, G.; Chen, W. Hetero-Enzyme-Based Two-Round Signal Amplification Strategy for Trace Detection of Aflatoxin B1 Using an Electrochemical Aptasensor. Biosens. Bioelectron. 2016, 80, 574–581. [Google Scholar] [CrossRef]
- Wei, M.; Xin, L.; Jin, H.; Huang, Y.; Liu, Y. Electrochemical Aptasensor for Zearalenone Based on DNA Assembly and Exonuclease III as Amplification Strategy. Electroanalysis 2021, 33, 1691–1698. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials Based Electrochemical Sensors for Biomedical Applications. Chem. Soc. Rev. 2013, 42, 5425. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, X.; Zhang, W.; Meng, F.; Wang, X.; Qin, Y.; Zhang, M. Carbon-Based Nanocomposite Smart Sensors for the Rapid Detection of Mycotoxins. Nanomaterials 2021, 11, 2851. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Guo, S. Chemically Doped Fluorescent Carbon and Graphene Quantum Dots for Bioimaging, Sensor, Catalytic and Photoelectronic Applications. Nanoscale 2016, 8, 2532–2543. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, Y.; Wang, G.; Mao, J.; Liu, Z.; Sun, T.; Wang, M.; Chen, D.; Yang, Y.; Li, J.; et al. Green, Rapid, and Universal Preparation Approach of Graphene Quantum Dots under Ultraviolet Irradiation. ACS Appl. Mater. Interfaces 2017, 9, 14470–14477. [Google Scholar] [CrossRef]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with Carbon Nanotubes and Graphene: An Outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef]
- Guo, Z.; Ren, J.; Wang, J.; Wang, E. Single-Walled Carbon Nanotubes Based Quenching of Free FAM-Aptamer for Selective Determination of Ochratoxin A. Talanta 2011, 85, 2517–2521. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Marquette, C.A.; Dutta, P.; Rajesh; Sumana, G. Integrated Graphene Quantum Dot Decorated Functionalized Nanosheet Biosensor for Mycotoxin Detection. Anal. Bioanal. Chem. 2020, 412, 7029–7041. [Google Scholar] [CrossRef]
- López Marzo, A.M.; Mayorga-Martinez, C.C.; Pumera, M. 3D-Printed Graphene Direct Electron Transfer Enzyme Biosensors. Biosens. Bioelectron. 2020, 151, 111980. [Google Scholar] [CrossRef]
- Shukla, S.; Haldorai, Y.; Khan, I.; Kang, S.-M.; Kwak, C.H.; Gandhi, S.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K. Bioreceptor-Free, Sensitive and Rapid Electrochemical Detection of Patulin Fungal Toxin, Using a Reduced Graphene Oxide@SnO2 Nanocomposite. Mater. Sci. Eng. C 2020, 113, 110916. [Google Scholar] [CrossRef]
- Scotto, J.; Piccinini, E.; von Bilderling, C.; Coria-Oriundo, L.L.; Battaglini, F.; Knoll, W.; Marmisolle, W.A.; Azzaroni, O. Flexible Conducting Platforms Based on PEDOT and Graphite Nanosheets for Electrochemical Biosensing Applications. Appl. Surf. Sci. 2020, 525, 146440. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical Properties of Graphene and Graphene-Based Nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Wu, S.; He, Q.; Tan, C.; Wang, Y.; Zhang, H. Graphene-Based Electrochemical Sensors. Small 2013, 9, 1160–1172. [Google Scholar] [CrossRef]
- Chen, J.; Liu, D.; Li, S.; Yao, D. Development of an Amperometric Enzyme Electrode Biosensor for Sterigmatocystin Detection. Enzym. Microb. Technol. 2010, 47, 119–126. [Google Scholar] [CrossRef]
- Narayanan, J.; Sharma, M.K.; Ponmariappan, S.; Sarita; Shaik, M.; Upadhyay, S. Electrochemical Immunosensor for Botulinum Neurotoxin Type-E Using Covalently Ordered Graphene Nanosheets Modified Electrodes and Gold Nanoparticles-Enzyme Conjugate. Biosens. Bioelectron. 2015, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kumar, V.; Ali, M.A.; Solanki, P.R.; Srivastava, A.; Sumana, G.; Saxena, P.S.; Joshi, A.G.; Malhotra, B.D. Electrophoretically Deposited Reduced Graphene Oxide Platform for Food Toxin Detection. Nanoscale 2013, 5, 3043. [Google Scholar] [CrossRef]
- Srivastava, S.; Ali, M.A.; Umrao, S.; Parashar, U.K.; Srivastava, A.; Sumana, G.; Malhotra, B.; Pandey, S.S.; Hayase, S. Graphene oxide-based biosensor for food toxin detection. Appl. Biochem. Biotechnol. 2014, 174, 960–970. [Google Scholar] [CrossRef]
- Basu, J.; Datta, S.; RoyChaudhuri, C. A Graphene Field Effect Capacitive Immunosensor for Sub-Femtomolar Food Toxin Detection. Biosens. Bioelectron. 2015, 68, 544–549. [Google Scholar] [CrossRef]
- Shi, Z.-Y.; Zheng, Y.-T.; Zhang, H.-B.; He, C.-H.; Wu, W.-D.; Zhang, H.-B. DNA Electrochemical Aptasensor for Detecting Fumonisins B 1 Based on Graphene and Thionine Nanocomposite. Electroanalysis 2015, 27, 1097–1103. [Google Scholar] [CrossRef]
- Wang, D.; Hu, W.; Xiong, Y.; Xu, Y.; Ming Li, C. Multifunctionalized Reduced Graphene Oxide-Doped Polypyrrole/Pyrrolepropylic Acid Nanocomposite Impedimetric Immunosensor to Ultra-Sensitively Detect Small Molecular Aflatoxin B1. Biosens. Bioelectron. 2015, 63, 185–189. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Zhu, C.; Shen, X.; Liu, Y.; You, T. Sensitivity Programmable Ratiometric Electrochemical Aptasensor Based on Signal Engineering for the Detection of Aflatoxin B1 in Peanut. J. Hazard. Mater. 2020, 387, 122001. [Google Scholar] [CrossRef]
- Linting, Z.; Ruiyi, L.; Zaijun, L.; Qianfang, X.; Yinjun, F.; Junkang, L. An Immunosensor for Ultrasensitive Detection of Aflatoxin B1 with an Enhanced Electrochemical Performance Based on Graphene/Conducting Polymer/Gold Nanoparticles/the Ionic Liquid Composite Film on Modified Gold Electrode with Electrodeposition. Sens. Actuators B Chem. 2012, 174, 359–365. [Google Scholar] [CrossRef]
- Tang, J.; Huang, Y.; Zhang, C.; Liu, H.; Tang, D. Amplified Impedimetric Immunosensor Based on Instant Catalyst for Sensitive Determination of Ochratoxin A. Biosens. Bioelectron. 2016, 86, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kumar, V.; Arora, K.; Singh, C.; Ali, M.A.; Puri, N.K.; Malhotra, B.D. Antibody Conjugated Metal Nanoparticle Decorated Graphene Sheets for a Mycotoxin Sensor. RSC Adv. 2016, 6, 56518–56526. [Google Scholar] [CrossRef]
- Krittayavathananon, A.; Sawangphruk, M. Impedimetric Sensor of Ss-HSDNA/Reduced Graphene Oxide Aerogel Electrode toward Aflatoxin B1 Detection: Effects of Redox Mediator Charges and Hydrodynamic Diffusion. Anal. Chem. 2017, 89, 13283–13289. [Google Scholar] [CrossRef]
- Gan, N.; Zhou, J.; Xiong, P.; Hu, F.; Cao, Y.; Li, T.; Jiang, Q. An Ultrasensitive Electrochemiluminescent Immunoassay for Aflatoxin M1 in Milk, Based on Extraction by Magnetic Graphene and Detection by Antibody-Labeled CdTe Quantumn Dots-Carbon Nanotubes Nanocomposite. Toxins 2013, 5, 865–883. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms, and Remedies of Toxicity: Miniperspective. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Wei, F. Horizontally Aligned Carbon Nanotube Arrays: Growth Mechanism, Controlled Synthesis, Characterization, Properties and Applications. Chem. Soc. Rev. 2017, 46, 3661–3715. [Google Scholar] [CrossRef]
- An, K.H.; Jeong, S.Y.; Hwang, H.R.; Lee, Y.H. Enhanced Sensitivity of a Gas Sensor Incorporating Single-Walled Carbon Nanotube–Polypyrrole Nanocomposites. Adv. Mater. 2004, 16, 1005–1009. [Google Scholar] [CrossRef]
- Bai, L.; Yuan, R.; Chai, Y.; Yuan, Y.; Mao, L.; Wang, Y. Platinum–Gold Alloy Nanoparticles and Horseradish Peroxidase Functionalized Nanocomposite as a Trace Label for Ultrasensitive Electrochemical Detection of Thrombin. Anal. Chim. Acta 2011, 698, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Cao, H.; Yao, D.; Liu, D. Amperometric Biosensor for Aflatoxin B1 Based on Aflatoxin-Oxidase Immobilized on Multiwalled Carbon Nanotubes. Food Control 2011, 22, 43–49. [Google Scholar] [CrossRef]
- Wang, S.G.; Zhang, Q.; Wang, R.; Yoon, S.F.; Ahn, J.; Yang, D.J.; Tian, J.Z.; Li, J.Q.; Zhou, Q. Multi-Walled Carbon Nanotubes for the Immobilization of Enzyme in Glucose Biosensors. Electrochem. Commun. 2003, 5, 800–803. [Google Scholar] [CrossRef]
- Yao, D.; Cao, H.; Wen, S.; Liu, D.; Bai, Y.; Zheng, W. A Novel Biosensor for Sterigmatocystin Constructed by Multi-Walled Carbon Nanotubes (MWNT) Modified with Aflatoxin–Detoxifizyme (ADTZ). Bioelectrochemistry 2006, 68, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Solanki, P.R.; Pandey, M.K.; Kaneto, K.; Ahmad, S.; Malhotra, B.D. Carbon Nanotubes—Chitosan Nanobiocomposite for Immunosensor. Thin Solid Film. 2010, 519, 1160–1166. [Google Scholar] [CrossRef]
- Singh, C.; Srivastava, S.; Ali, M.A.; Gupta, T.K.; Sumana, G.; Srivastava, A.; Mathur, R.B.; Malhotra, B.D. Carboxylated Multiwalled Carbon Nanotubes Based Biosensor for Aflatoxin Detection. Sens. Actuators B Chem. 2013, 185, 258–264. [Google Scholar] [CrossRef]
- Fang, Y.-S.; Chen, S.-Y.; Huang, X.-J.; Wang, L.-S.; Wang, H.-Y.; Wang, J.-F. Simple Approach for Ultrasensitive Electrochemical Immunoassay of Clostridium Difficile Toxin B Detection. Biosens. Bioelectron. 2014, 53, 238–244. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.J.; Liu, Y.Q.; Yang, H.M.; Guo, Q.L. Determination of Aflatoxin M1 in Milk by Triple Quadrupole Liquid Chromatography-Tandem Mass Spectrometry. Food Addit. Contam. Part A 2010, 27, 1261–1265. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, J.; Xu, C.; Ding, L.; Ju, H. Carbon Nanohorn Sensitized Electrochemical Immunosensor for Rapid Detection of Microcystin-LR. Anal. Chem. 2010, 82, 1117–1122. [Google Scholar] [CrossRef]
- Viswanathan, S.; Wu, L.; Huang, M.-R.; Ho, J.A. Electrochemical Immunosensor for Cholera Toxin Using Liposomes and Poly(3,4-Ethylenedioxythiophene)-Coated Carbon Nanotubes. Anal. Chem. 2006, 78, 1115–1121. [Google Scholar] [CrossRef]
- Queirós, R.B.; Guedes, A.; Marques, P.V.S.; Noronha, J.P.; Sales, M.G.F. Recycling Old Screen-Printed Electrodes with Newly Designed Plastic Antibodies on the Wall of Carbon Nanotubes as Sensory Element for in Situ Detection of Bacterial Toxins in Water. Sens. Actuators B Chem. 2013, 189, 21–29. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, X.; Zhang, X.; Qing, Y.; Luo, M.; Liu, X.; Li, C.; Li, Y.; Xia, H.; Qiu, J. A Highly Sensitive Electrochemical Immunosensor for Fumonisin B 1 Detection in Corn Using Single-Walled Carbon Nanotubes/Chitosan. Electroanalysis 2015, 27, 2679–2687. [Google Scholar] [CrossRef]
- Azri, F.; Selamat, J.; Sukor, R. Electrochemical Immunosensor for the Detection of Aflatoxin B1 in Palm Kernel Cake and Feed Samples. Sensors 2017, 17, 2776. [Google Scholar] [CrossRef]
- Pumera, M. The Electrochemistry of Carbon Nanotubes: Fundamentals and Applications. Chem. Eur. J. 2009, 15, 4970–4978. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, I.; Unwin, P.R.; Macpherson, J.V. Electrochemistry at Carbon Nanotubes: Perspective and Issues. Chem. Commun. 2009, 45, 6886. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Gu, H.; Rapole, S.B.; Wang, Q.; Luo, Z.; Haldolaarachchige, N.; Young, D.P.; Guo, Z. One-Pot Synthesis of Magnetic Graphene Nanocomposites Decorated with Core@Double-Shell Nanoparticles for Fast Chromium Removal. Environ. Sci. Technol. 2012, 46, 977–985. [Google Scholar] [CrossRef]
- Nasir, M.; Nawaz, M.H.; Latif, U.; Yaqub, M.; Hayat, A.; Rahim, A. An Overview on Enzyme-Mimicking Nanomaterials for Use in Electrochemical and Optical Assays. Microchim Acta 2017, 184, 323–342. [Google Scholar] [CrossRef]
- Vidal, J.C.; Bonel, L.; Duato, P.; Castillo, J.R. Improved Electrochemical Competitive Immunosensor for Ochratoxin A with a Biotinylated Monoclonal Antibody Capture Probe and Colloidal Gold Nanostructuring. Anal. Methods 2011, 3, 977. [Google Scholar] [CrossRef]
- Bonel, L.; Vidal, J.C.; Duato, P.; Castillo, J.R. Ochratoxin A Nanostructured Electrochemical Immunosensors Based on Polyclonal Antibodies and Gold Nanoparticles Coupled to the Antigen. Anal. Methods 2010, 2, 335. [Google Scholar] [CrossRef]
- Gutierrez, R.A.V.; Hedström, M.; Mattiasson, B. Screening of Self-Assembled Monolayer for Aflatoxin B1 Detection Using Immune-Capacitive Sensor. Biotechnol. Rep. 2015, 8, 144–151. [Google Scholar] [CrossRef][Green Version]
- Gutierrez, R.A.V.; Hedström, M.; Mattiasson, B. Bioimprinting as a Tool for the Detection of Aflatoxin B1 Using a Capacitive Biosensor. Biotechnol. Rep. 2016, 11, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Rossini, G.P.; Hess, P. Phycotoxins: Chemistry, Mechanisms of Action and Shellfish Poisoning. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Birkhäuser: Basel, Switzerland, 2010; Volume 100, pp. 65–122. ISBN 9783764383374. [Google Scholar]
- Tang, D.; Tang, J.; Su, B.; Chen, G. Gold Nanoparticles-Decorated Amine-Terminated Poly(Amidoamine) Dendrimer for Sensitive Electrochemical Immunoassay of Brevetoxins in Food Samples. Biosens. Bioelectron. 2011, 26, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- MacEwan, S.R.; Chilkoti, A. Applications of Elastin-like Polypeptides in Drug Delivery. J. Control. Release 2014, 190, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Deng, W.; Li, Y.; Huang, Z.; Meng, Y.; Xie, Q.; Ma, M.; Yao, S. Polymeric Bionanocomposite Cast Thin Films with in Situ Laccase-Catalyzed Polymerization of Dopamine for Biosensing and Biofuel Cell Applications. J. Phys. Chem. B 2010, 114, 5016–5024. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, S.; Zhao, D.; Rong, Y.; Zhou, Y.; Ding, J.; He, C.; Chen, X. Effect of Polymer Topology and Residue Chirality on Biodegradability of Polypeptide Hydrogels. ACS Biomater. Sci. Eng. 2022, 8, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.Y.; Peng, J.; Shan, S.; Liu, D.F.; Huang, Y.N.; Lai, W.H. Green Enzyme-Linked Immunosorbent Assay Based on the Single-Stranded Binding Protein-Assisted Aptamer for the Detection of Mycotoxin. Anal. Chem. 2020, 92, 8422–8426. [Google Scholar] [CrossRef]
- Zhang, S.; Luan, Y.; Xiong, M.; Zhang, J.; Lake, R.; Lu, Y. DNAzyme Amplified Aptasensing Platform for Ochratoxin A Detection Using a Personal Glucose Meter. ACS Appl. Mater. Interfaces 2021, 13, 9472–9481. [Google Scholar] [CrossRef]
- Hu, H.; Cao, L.; Li, Q.; Ma, K.; Yan, P.; Kirk, D.W. Fabrication and Modeling of an Ultrasensitive Label Free Impedimetric Immunosensor for Aflatoxin B1 Based on Poly(o-Phenylenediamine) Modified Gold 3D Nano Electrode Ensembles. RSC Adv. 2015, 5, 55209–55217. [Google Scholar] [CrossRef]
- Li, Z.; Ye, Z.; Fu, Y.; Xiong, Y.; Li, Y. A Portable Electrochemical Immunosensor for Rapid Detection of Trace Aflatoxin B1 in Rice. Anal. Methods 2016, 8, 548–553. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, J.; Chen, L.; Ma, L.; Yang, H.; Zhang, Y.; Miao, M. A Signal-off Aptamer Sensor Based on Competition with Complementary DNA and Click Polymerization for Electrochemical Detection of AFB1. Microchem. J. 2022, 181, 107775. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Z.; Zhou, S.; Cui, X.; Liu, Y. Highly Sensitive Detection of Aflatoxin B1 by in Situ Growth of Electroactive Polymers via Ring-Opening Metathesis Polymerization. Sens. Actuators B Chem. 2022, 372, 132619. [Google Scholar] [CrossRef]
- Joshi, S.; Segarra-Fas, A.; Peters, J.; Zuilhof, H.; van Beek, T.A.; Nielen, M.W.F. Multiplex Surface Plasmon Resonance Biosensing and Its Transferability towards Imaging Nanoplasmonics for Detection of Mycotoxins in Barley. Analyst 2016, 141, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Si, P.B.; Yang, T.; Wu, Y.; Yang, Y.H.; Hu, R. A Novel Method for Detection of Ochratoxin A in Foods—Co-MOFs Based Dual Signal Ratiometric Electrochemical Aptamer Sensor Coupled with DNA Walker. Food Chem. 2022, 403, 134316. [Google Scholar] [CrossRef] [PubMed]
- Dridi, F.; Marrakchi, M.; Gargouri, M.; Garcia-Cruz, A.; Dzyadevych, S.; Vocanson, F.; Saulnier, J.; Jaffrezic-Renault, N.; Lagarde, F. Thermolysin Entrapped in a Gold Nanoparticles/Polymer Composite for Direct and Sensitive Conductometric Biosensing of Ochratoxin A in Olive Oil. Sens. Actuators B Chem. 2015, 221, 480–490. [Google Scholar] [CrossRef]
- Wang, S.; Shao, R.; Li, W.; Li, X.; Sun, J.; Jiao, S.; Dai, S.; Dou, M.; Xu, R.; Li, Q.; et al. Three-Dimensional Ordered Macroporous Magnetic Inverse Photonic Crystal Microsphere-Based Molecularly Imprinted Polymer for Selective Capture of Aflatoxin B1. ACS Appl. Mater. Interfaces 2022, 14, 18845–18853. [Google Scholar] [CrossRef]
- Hu, X.; Xia, Y.; Liu, Y.; Chen, Y.; Zeng, B. An Effective Ratiometric Electrochemical Sensor for Highly Selective and Reproducible Detection of Ochratoxin A: Use of Magnetic Field Improved Molecularly Imprinted Polymer. Sens. Actuators B Chem. 2022, 359, 131582. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Castro, M.; Machado, S.; Barroso, M.F.; Nouws, H.P.A.; Delerue-Matos, C. Molecularly Imprinted Electrochemical Sensor for Ochratoxin A Detection in Food Samples. Sens. Actuators B Chem. 2015, 215, 107–112. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. SPR Nanosensor Based on Molecularly Imprinted Polymer Film with Gold Nanoparticles for Sensitive Detection of Aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef]
- Munawar, H.; Garcia-Cruz, A.; Majewska, M.; Karim, K.; Kutner, W.; Piletsky, S.A. Electrochemical Determination of Fumonisin B1 Using a Chemosensor with a Recognition Unit Comprising Molecularly Imprinted Polymer Nanoparticles. Sens. Actuators B Chem. 2020, 321, 128552. [Google Scholar] [CrossRef]
- Jiang, M.; Braiek, M.; Florea, A.; Chrouda, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Zhang, A.; Jaffrezic-Renault, N. Aflatoxin B1 Detection Using a Highly-Sensitive Molecularly-Imprinted Electrochemical Sensor Based on an Electropolymerized Metal Organic Framework. Toxins 2015, 7, 3540–3553. [Google Scholar] [CrossRef]
- Singh, A.K.; Lakshmi, G.B.V.S.; Fernandes, M.; Sarkar, T.; Gulati, P.; Singh, R.P.; Solanki, P.R. A Simple Detection Platform Based on Molecularly Imprinted Polymer for AFB1 and FuB1 Mycotoxins. Microchem. J. 2021, 171, 106730. [Google Scholar] [CrossRef]
- Radi, A.E.; Eissa, A.; Wahdan, T. Molecularly Imprinted Impedimetric Sensor for Determination of Mycotoxin Zearalenone. Electroanalysis 2020, 32, 1788–1794. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, M.; Gaba, J.; Singh, K.; Bhatia, Y.; Singh, A.; Singh, N. Recent Advances in Recognition Receptors for Electrochemical Biosensing of Mycotoxins—A Review. Biosensors 2023, 13, 391. https://doi.org/10.3390/bios13030391
Kaur M, Gaba J, Singh K, Bhatia Y, Singh A, Singh N. Recent Advances in Recognition Receptors for Electrochemical Biosensing of Mycotoxins—A Review. Biosensors. 2023; 13(3):391. https://doi.org/10.3390/bios13030391
Chicago/Turabian StyleKaur, Manpreet, Jyoti Gaba, Komal Singh, Yashika Bhatia, Anoop Singh, and Narinder Singh. 2023. "Recent Advances in Recognition Receptors for Electrochemical Biosensing of Mycotoxins—A Review" Biosensors 13, no. 3: 391. https://doi.org/10.3390/bios13030391
APA StyleKaur, M., Gaba, J., Singh, K., Bhatia, Y., Singh, A., & Singh, N. (2023). Recent Advances in Recognition Receptors for Electrochemical Biosensing of Mycotoxins—A Review. Biosensors, 13(3), 391. https://doi.org/10.3390/bios13030391






