Development of a New Lab-on-Paper Microfluidics Platform Using Bi-Material Cantilever Actuators for ELISA on Paper
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Bi-Material Cantilever
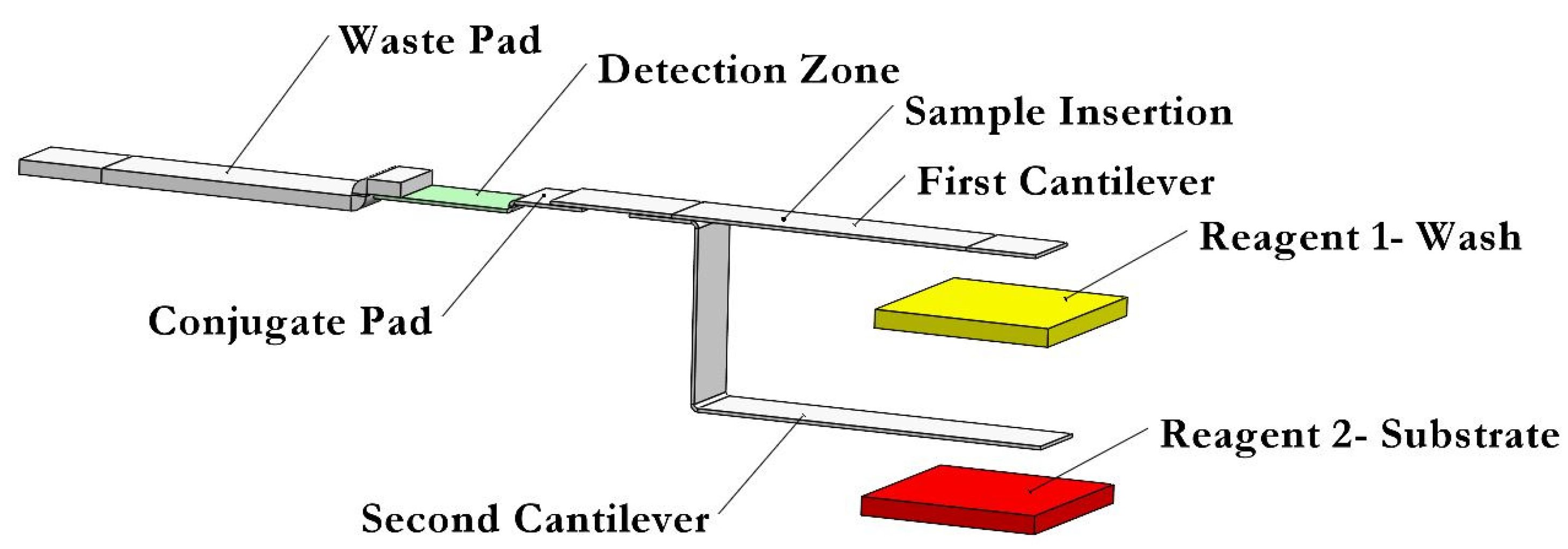
2.3. Dual Bi-Material Cantilevers System
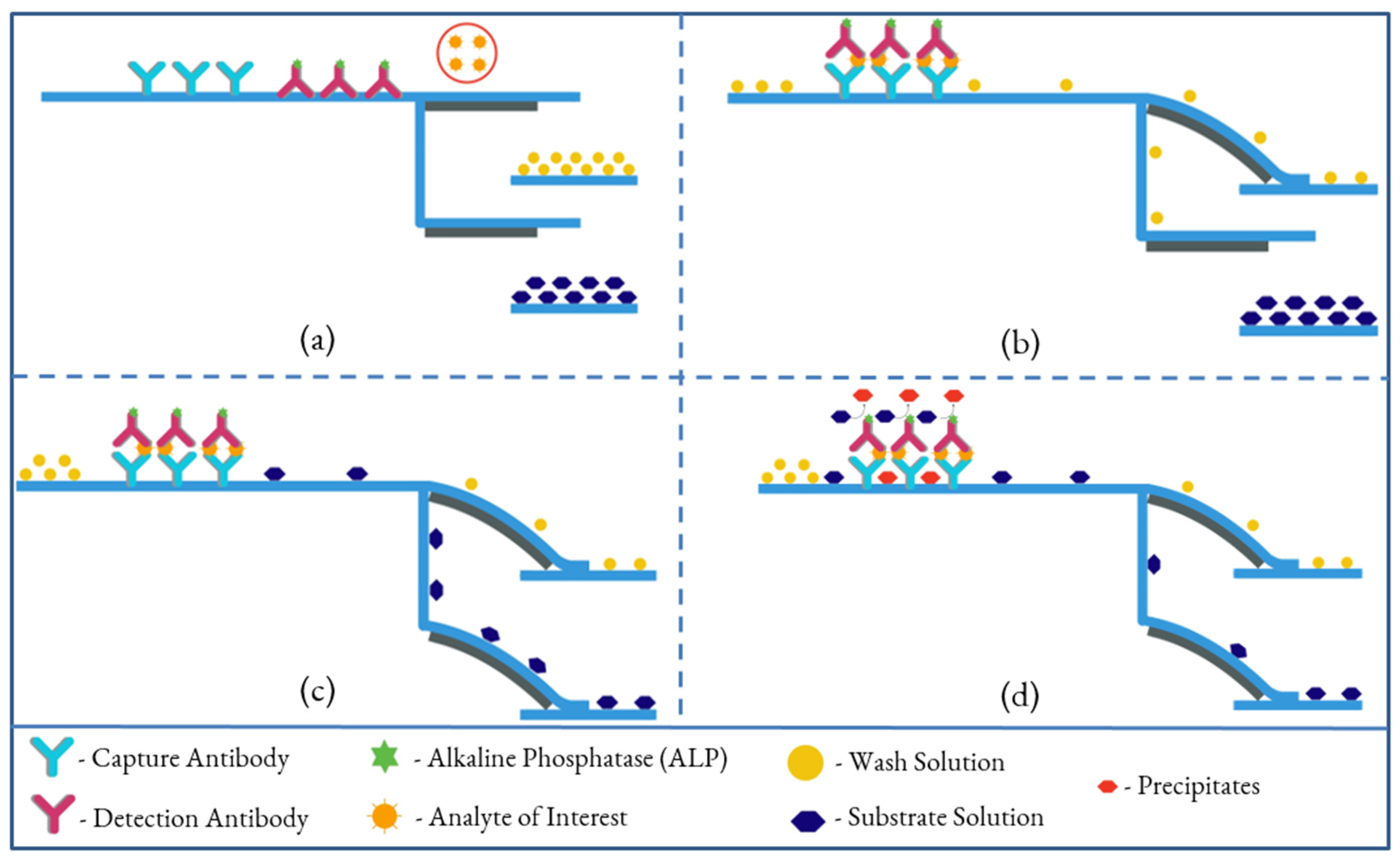
2.4. Dual Bi-Material Cantilevers System for Performing an ELISA
2.5. Storing and Releasing Reagents Mechanism
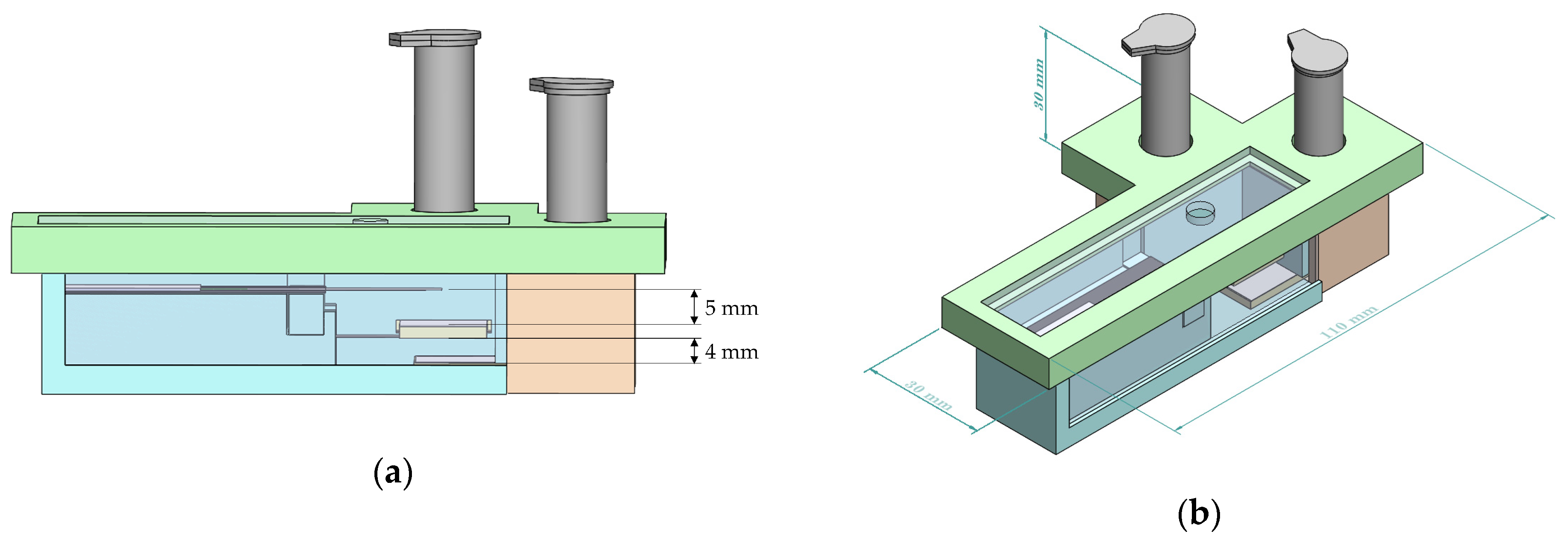
2.6. Platform Housing
2.7. Conjugate Pad
2.8. Assay Preparation
2.9. Assay Procedure
3. Results and Discussion
3.1. Hole Size of Release Mechanism
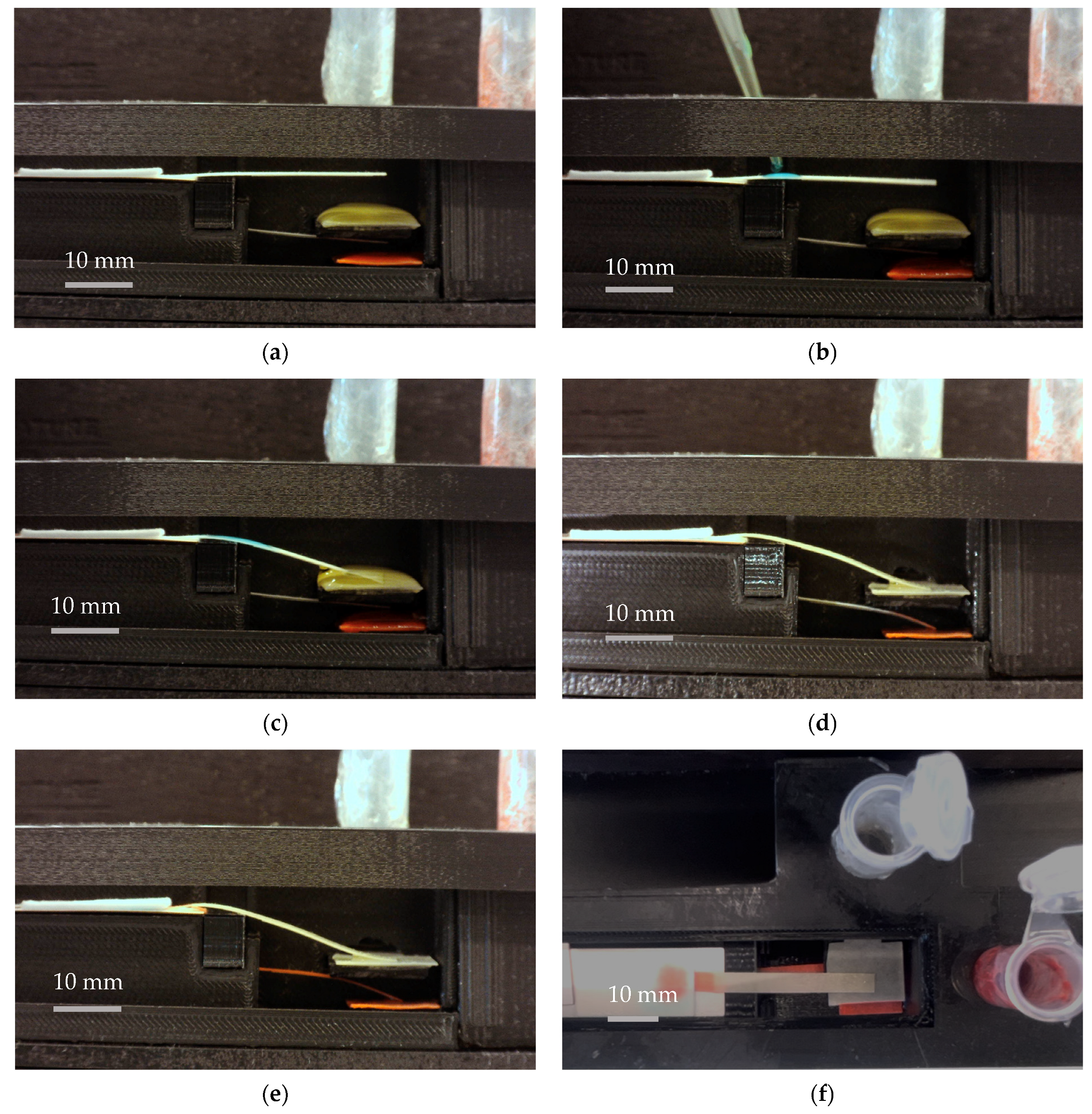
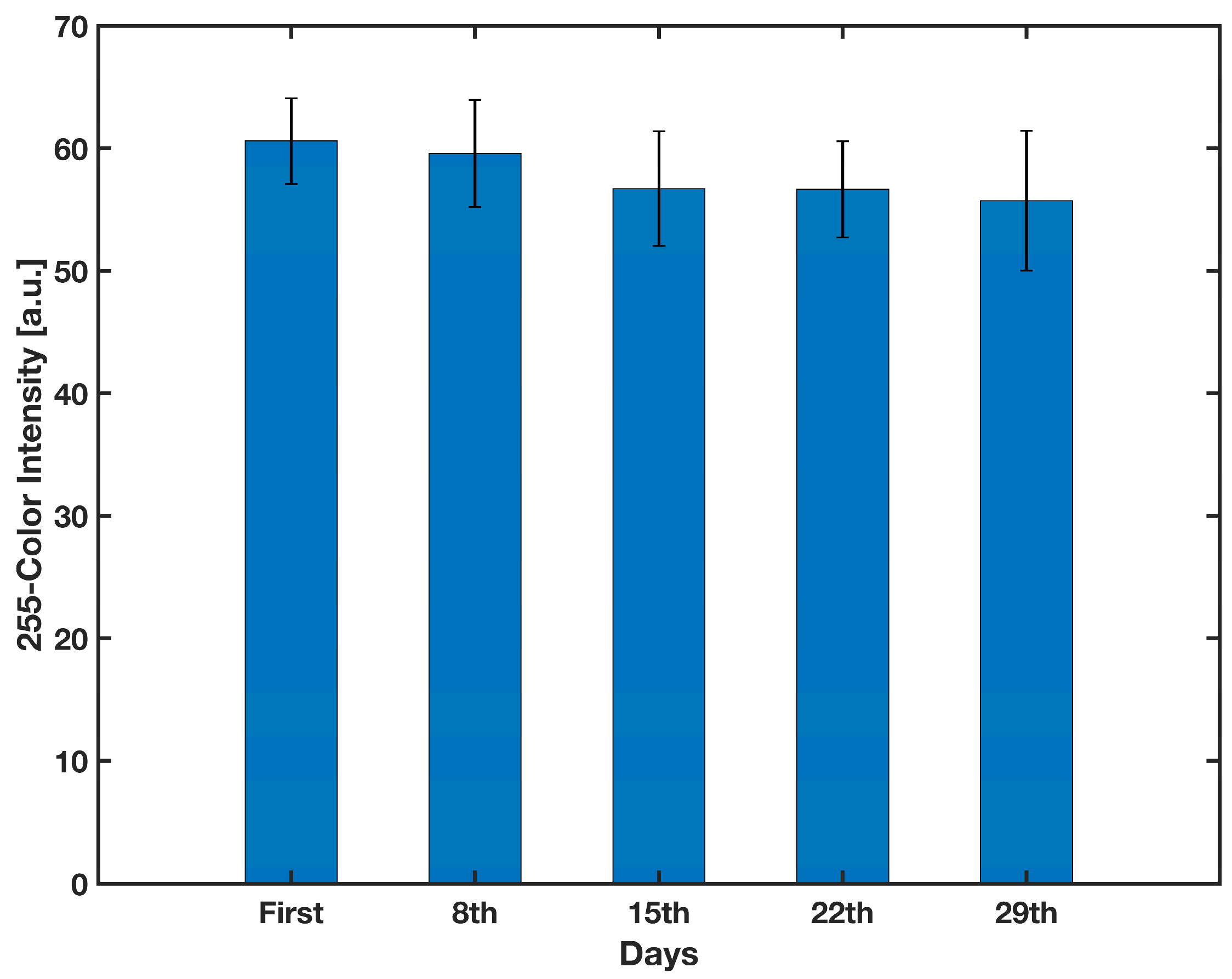
3.2. Performance of the Platform with Food Color Reagents
3.3. Shelf Life of Conjugate Pad
3.4. Rabbit IgG Detection
3.5. Comparison of Results with Those in the Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, T.; Ramana, L.N.; Sharma, T.K. Current Advancements and Future Road Map to Develop ASSURED Microfluidic Biosensors for Infectious and Non-Infectious Diseases. Biosensors 2022, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Curtin, K.; Fike, B.J.; Binkley, B.; Godary, T.; Li, P. Recent Advances in Digital Biosensing Technology. Biosensors 2022, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, S.; Faqah, O.; Asghar, W.; Ilyas, A. Microfluidic Devices for HIV Diagnosis and Monitoring at Point-of-Care (POC) Settings. Biosensors 2022, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef]
- Struss, A.; Pasini, P.; Ensor, C.M.; Raut, N.; Daunert, S. Paper Strip Whole Cell Biosensors: A Portable Test for the Semiquantitative Detection of Bacterial Quorum Signaling Molecules. Anal. Chem. 2010, 82, 4457–4463. [Google Scholar] [CrossRef]
- Wang, S.; Ge, L.; Song, X.; Yu, J.; Ge, S.; Huang, J.; Zeng, F. Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens. Bioelectron. 2012, 31, 212–218. [Google Scholar] [CrossRef]
- Gerbers, R.; Foellscher, W.; Chen, H.; Anagnostopoulos, C.; Faghri, M. A new paper-based platform technology for point-of-care diagnostics. Lab. Chip. 2014, 14, 4042–4049. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef]
- Lim, W.Y.; Goh, B.T.; Khor, S.M. Microfluidic paper-based analytical devices for potential use in quantitative and direct detection of disease biomarkers in clinical analysis. J. Chromatogr. B 2017, 1060, 424–442. [Google Scholar] [CrossRef]
- Ma, L.; Nilghaz, A.; Choi, J.R.; Liu, X.; Lu, X. Rapid detection of clenbuterol in milk using microfluidic paper-based ELISA. Food Chem. 2018, 246, 437–441. [Google Scholar] [CrossRef]
- Kim, T.H.; Hahn, Y.K.; Kim, M.S. Recent Advances of Fluid Manipulation Technologies in Microfluidic Paper-Based Analytical Devices (μPADs) toward Multi-Step Assays. Micromachines 2020, 11, 269. [Google Scholar] [CrossRef]
- Gong, F.; Wei, H.; Qi, J.; Ma, H.; Liu, L.; Weng, J.; Zheng, X.; Li, Q.; Zhao, D.; Fang, H.; et al. Pulling-Force Spinning Top for Serum Separation Combined with Paper-Based Microfluidic Devices in COVID-19 ELISA Diagnosis. ACS Sens. 2021, 6, 2709–2719. [Google Scholar] [CrossRef]
- Lin, D.; Li, B.; Fu, L.; Qi, J.; Xia, C.; Zhang, Y.; Chen, J.; Choo, J.; Chen, L. A novel polymer-based nitrocellulose platform for implementing a multiplexed microfluidic paper-based enzyme-linked immunosorbent assay. Microsyst. Nanoeng. 2022, 8, 53. [Google Scholar] [CrossRef]
- Toda, H.; Iwasaki, W.; Morita, N.; Motomura, T.; Takemura, K.; Nagano, M.; Nakanishi, Y.; Nakashima, Y. Reversible Thermo-Responsive Valve for Microfluidic Paper-Based Analytical Devices. Micromachines 2022, 13, 690. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Cheng, C.M.; Martinez, A.W.; Mirica, K.A.; Li, X.J.; Phillips, S.T.; Mascarenas, M.; Whitesides, G.M. A portable microfluidic paper-based device for ELISA. In Proceedings of the 2011 IEEE 24th International Conference on Micro Electro Mechanical Systems, Cancun, Mexico, 23–27 January 2011; pp. 75–78. [Google Scholar]
- Smith, S.; Sewart, R.; Becker, H.; Roux, P.; Land, K. Blister pouches for effective reagent storage on microfluidic chips for blood cell counting. Microfluid. Nanofluidics 2016, 20, 163. [Google Scholar] [CrossRef]
- Czurratis, D.; Beyl, Y.; Grimm, A.; Brettschneider, T.; Zinober, S.; Lärmer, F.; Zengerle, R. Liquids on-chip: Direct storage and release employing micro-perforated vapor barrier films. Lab. Chip 2015, 15, 2887–2895. [Google Scholar] [CrossRef] [PubMed]
- Bwambok, D.K.; Christodouleas, D.C.; Morin, S.A.; Lange, H.; Phillips, S.T.; Whitesides, G.M. Adaptive Use of Bubble Wrap for Storing Liquid Samples and Performing Analytical Assays. Anal. Chem. 2014, 86, 7478–7485. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Gordon, R.; Varjani, S.; Vinayak, V. Employing newly developed plastic bubble wrap technique for biofuel production from diatoms cultivated in discarded plastic waste. Sci. Total Environ. 2022, 823, 153667. [Google Scholar] [CrossRef]
- Gordon, R. Optimisation of Microalgal Diatom Farming Using Bubble Plastic Wrap As A Sealant in A Closed Photobioreactor For Harvesting Biofuel. Res. Sq. 2021, 1–17. [Google Scholar] [CrossRef]
- Fairclough, S.M.; Giannetti, C.; Wagner, I.; Shakeel, H. Colorimetric sensor for pH monitoring of liquid samples using bubble wrap and mobile phone camera. In Proceedings of the 2020 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 16–19 August 2020; pp. 1–4. [Google Scholar]
- Gonzalez-Suarez, A.M.; Long, A.; Huang, X.; Revzin, A. A Compact Control System to Enable Automated Operation of Microfluidic Bioanalytical Assays. Biosensors 2022, 12, 1160. [Google Scholar] [CrossRef]
- Chen, H.; Cogswell, J.; Anagnostopoulos, C.; Faghri, M. A fluidic diode, valves, and a sequential-loading circuit fabricated on layered paper. Lab Chip 2012, 12, 2909. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Luan, X.; Li, X.; Jiang, J.; Fan, Y.; Li, M.; Huang, C.; Zhang, L.; Zhao, Y. A 3D Capillary-Driven Multi-Micropore Membrane-Based Trigger Valve for Multi-Step Biochemical Reaction. Biosensors 2022, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Peshin, S.; Madou, M.; Kulinsky, L. Microvalves for Applications in Centrifugal Microfluidics. Sensors 2022, 22, 8955. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.; Nguyen, T.; Shen, W. Paper-Based Microfluidic Devices by Plasma Treatment. Anal. Chem. 2008, 80, 9131–9134. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi-Anbuhi, S.; Chavan, P.; Sicard, C.; Leung, V.; Hossain, S.M.Z.; Pelton, R.; Brennan, J.D.; Filipe, C.D.M. Creating fast flow channels in paper fluidic devices to control timing of sequential reactions. Lab Chip 2012, 12, 5079. [Google Scholar] [CrossRef] [PubMed]
- Han, K.N.; Choi, J.-S.; Kwon, J. Three-dimensional paper-based slip device for one-step point-of-care testing. Sci. Rep. 2016, 6, 25710. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.M.; Wong, W.S.; Liu, L.; Dewar, R.; Klapperich, C.M. A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab Chip 2016, 16, 753–763. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Nie, Z.; Cheng, C.-M.; Carrilho, E.; Wiley, B.J.; Whitesides, G.M. Programmable diagnostic devices made from paper and tape. Lab Chip 2010, 10, 2499. [Google Scholar] [CrossRef]
- Fu, H.; Song, P.; Wu, Q.; Zhao, C.; Pan, P.; Li, X.; Li-Jessen, N.Y.K.; Liu, X. A paper-based microfluidic platform with shape-memory-polymer-actuated fluid valves for automated multi-step immunoassays. Microsyst. Nanoeng. 2019, 5, 50. [Google Scholar] [CrossRef]
- Tran, B.T.; Rijiravanich, P.; Puttaraksa, N.; Surareungchai, W. Wax gates in laminated microfluidic paper-based immunosensors. Microchem. J. 2022, 178, 107343. [Google Scholar] [CrossRef]
- Li, X.; Zwanenburg, P.; Liu, X. Magnetic timing valves for fluid control in paper-based microfluidics. Lab Chip 2013, 13, 2609. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Heidari-Bafroui, H.; Charbaji, A.; Rahmani, N.; Anagnostopoulos, C.; Faghri, M. Numerical and Experimental Modeling of Paper-Based Actuators. In Proceedings of the The 1st International Electronic Conference on Chemical Sensors and Analytical Chemistry, Virtual, 1–15 July 2021; MDPI: Basel, Switzerland, 2021; p. 15. [Google Scholar]
- Heidari-Bafroui, H.; Kumar, A.; Charbaji, A.; Smith, W.; Rahmani, N.; Anagnostopoulos, C.; Faghri, M. A Parametric Study on a Paper-Based Bi-Material Cantilever Valve. Micromachines 2022, 13, 1502. [Google Scholar] [CrossRef] [PubMed]
- Charbaji, A.; Heidari-Bafroui, H.; Kumar, A.; Rahmani, N.; Anagnostopoulos, C.; Faghri, M. Characterization and Modeling of Paper-based Bi-Material Actuator Cantilever; Application in Phosphate Detection. In Proceedings of the Innovations in Microfluidics, Boston, MA, USA, 18–19 March 2021. [Google Scholar]
- Smith, W.; Rahmani, N.; Charbaji, A.; Lemos, N.C.; Anagnostopoulos, M.; Faghri, C.H. A Fluidically Controlled Bi-Material Actuator For Automation of Paper-based Assay. In Proceedings of the International Symposium on Thermal Effects in Gas flows In Microscale, Karlsruhe, Germany, 24–25 October 2019. [Google Scholar]
- Qin, Z.; Huang, Z.; Pan, P.; Pan, Y.; Zuo, R.; Sun, Y.; Liu, X. A Nitrocellulose Paper-Based Multi-Well Plate for Point-of-Care ELISA. Micromachines 2022, 13, 2232. [Google Scholar] [CrossRef] [PubMed]
- Janik-Karpinska, E.; Ceremuga, M.; Niemcewicz, M.; Podogrocki, M.; Stela, M.; Cichon, N.; Bijak, M. Immunosensors—The Future of Pathogen Real-Time Detection. Sensors 2022, 22, 9757. [Google Scholar] [CrossRef]
- Kasetsirikul, S.; Shiddiky, M.J.A.; Nguyen, N.-T. Challenges and perspectives in the development of paper-based lateral flow assays. Microfluid. Nanofluidics 2020, 24, 17. [Google Scholar] [CrossRef]
- Heidari-Bafroui, H.; Ribeiro, B.; Charbaji, A.; Anagnostopoulos, C.; Faghri, M. Portable infrared lightbox for improving the detection limits of paper-based phosphate devices. Measurement 2021, 173, 108607. [Google Scholar] [CrossRef]
- Van Stralen, K.J.; Stel, V.S.; Reitsma, J.B.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Diagnostic methods I: Sensitivity, specificity, and other measures of accuracy. Kidney Int. 2009, 75, 1257–1263. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Konishi, I.; Tanaka, M.; Sawa, M.; Yamamoto, R.; Endo, I.; Handharyani, E.; Sari, D.; Takama, K.; Fukuda, K.; et al. Development of neutralization antibodies against highly pathogenic H5N1 avian influenza virus using ostrich (Struthio camelus) yolk. Mol. Med. Rep. 2008, 1, 203–209. [Google Scholar] [CrossRef]
- Kuo, Z.-K.; Chang, T.-H.; Chen, Y.-S.; Cheng, C.-M.; Tsai, C.-Y. Two Potential Clinical Applications of Origami-Based Paper Devices. Diagnostics 2019, 9, 203. [Google Scholar] [CrossRef]






| Parameter | Value |
|---|---|
| Paper type | Whatman filter paper grade 41 |
| Paper direction | Cross-machine direction |
| Cantilever width | 4 mm |
| Tape type | Scotch® tape 600 |
| Sample volume | 8–12 µL |
| Activation time | 3.1 ± 0.4 s |
| Device | Sample Size (μL) | Autonomously | Signal Detection | LOD (pM) | Ref. |
|---|---|---|---|---|---|
| Movable paper-based strip | 2 | No | Low-cost desktop scanner | 330 | [31] |
| 3D lateral flow device | 130 | Yes | Low-cost desktop scanner | 36.7 | [7] |
| Nitrocellulose paper-based multi-well plate | 5 | No | Low-cost desktop scanner | 59.2 | [38] |
| 3D folding paper-based device | 3 | No | Low-cost desktop scanner | 201 | [44] |
| Standard plate-based ELISA | 100 | No | Expensive microplate reader | 0.3 | [44] |
| Dual B-MaCs platform | 10 | Yes | Low-cost desktop scanner | 15.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari-Bafroui, H.; Kumar, A.; Hahn, C.; Scholz, N.; Charbaji, A.; Rahmani, N.; Anagnostopoulos, C.; Faghri, M. Development of a New Lab-on-Paper Microfluidics Platform Using Bi-Material Cantilever Actuators for ELISA on Paper. Biosensors 2023, 13, 310. https://doi.org/10.3390/bios13030310
Heidari-Bafroui H, Kumar A, Hahn C, Scholz N, Charbaji A, Rahmani N, Anagnostopoulos C, Faghri M. Development of a New Lab-on-Paper Microfluidics Platform Using Bi-Material Cantilever Actuators for ELISA on Paper. Biosensors. 2023; 13(3):310. https://doi.org/10.3390/bios13030310
Chicago/Turabian StyleHeidari-Bafroui, Hojat, Ashutosh Kumar, Cameron Hahn, Nicholas Scholz, Amer Charbaji, Nassim Rahmani, Constantine Anagnostopoulos, and Mohammad Faghri. 2023. "Development of a New Lab-on-Paper Microfluidics Platform Using Bi-Material Cantilever Actuators for ELISA on Paper" Biosensors 13, no. 3: 310. https://doi.org/10.3390/bios13030310
APA StyleHeidari-Bafroui, H., Kumar, A., Hahn, C., Scholz, N., Charbaji, A., Rahmani, N., Anagnostopoulos, C., & Faghri, M. (2023). Development of a New Lab-on-Paper Microfluidics Platform Using Bi-Material Cantilever Actuators for ELISA on Paper. Biosensors, 13(3), 310. https://doi.org/10.3390/bios13030310







