Framing of Poly(arylene-ethynylene) around Carbon Nanotubes and Iodine Doping for the Electrochemical Detection of Dopamine
Abstract
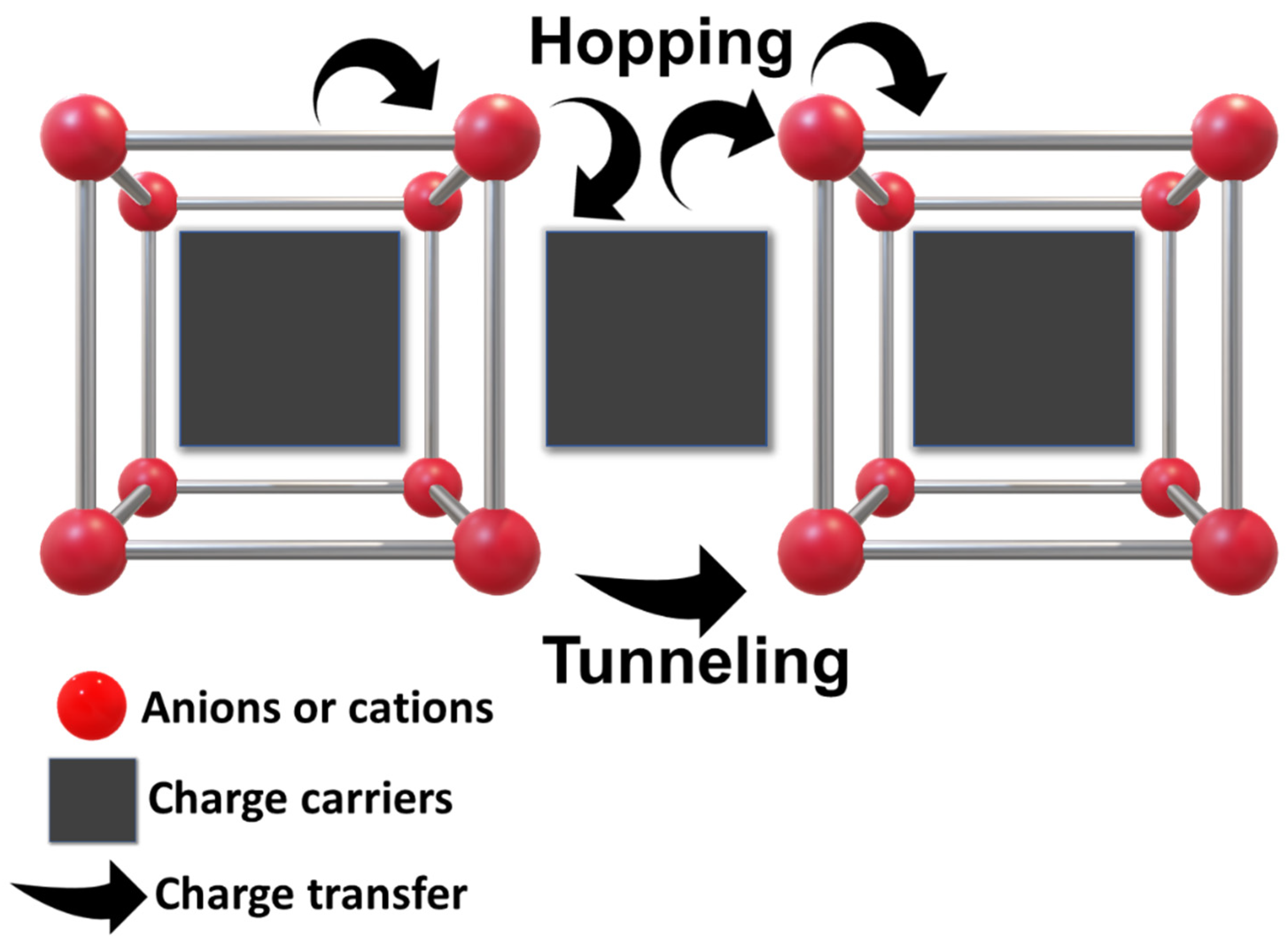
1. Introduction
2. Experimental Section
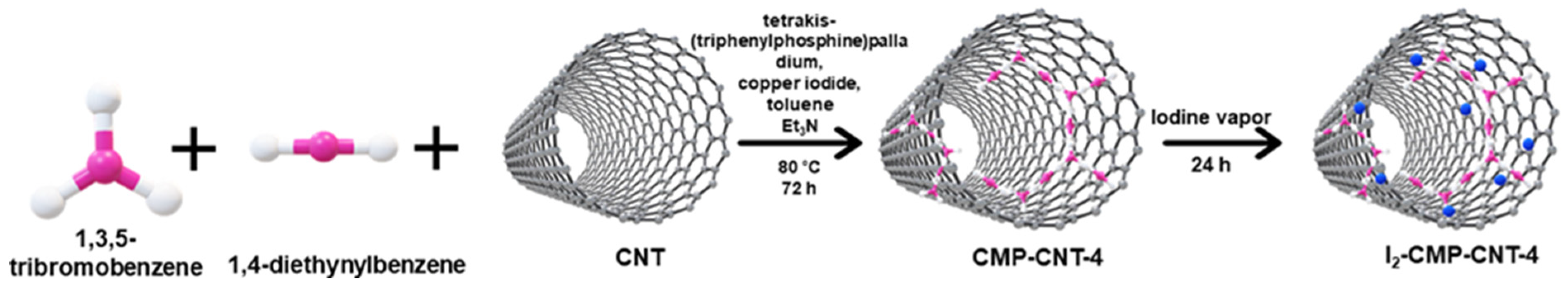
2.1. Preparation of CMP-4
2.2. Preparation of CMP-CNT-4
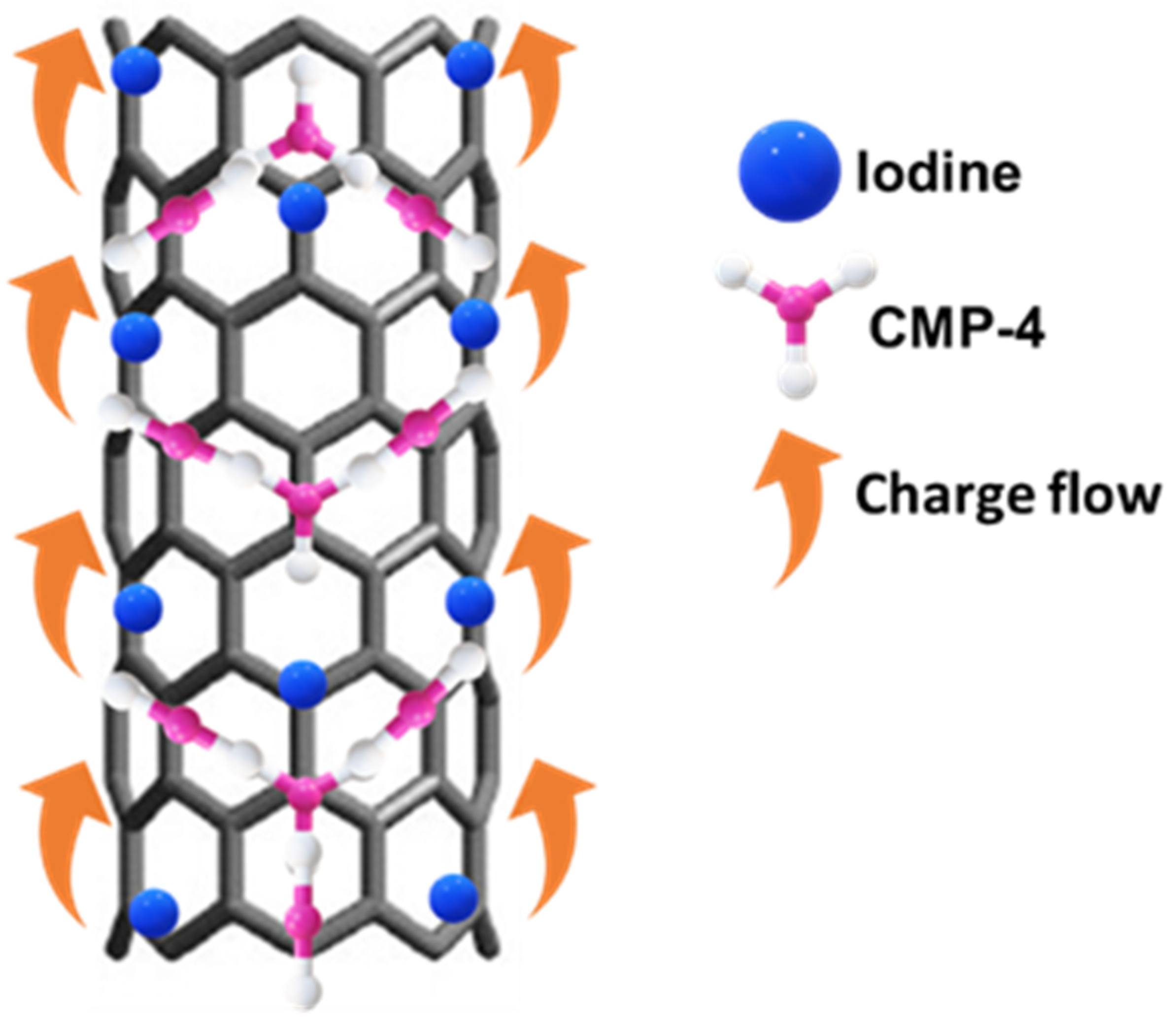
2.3. Synthesis of I2-CMP-CNT-4
3. Results and Discussion
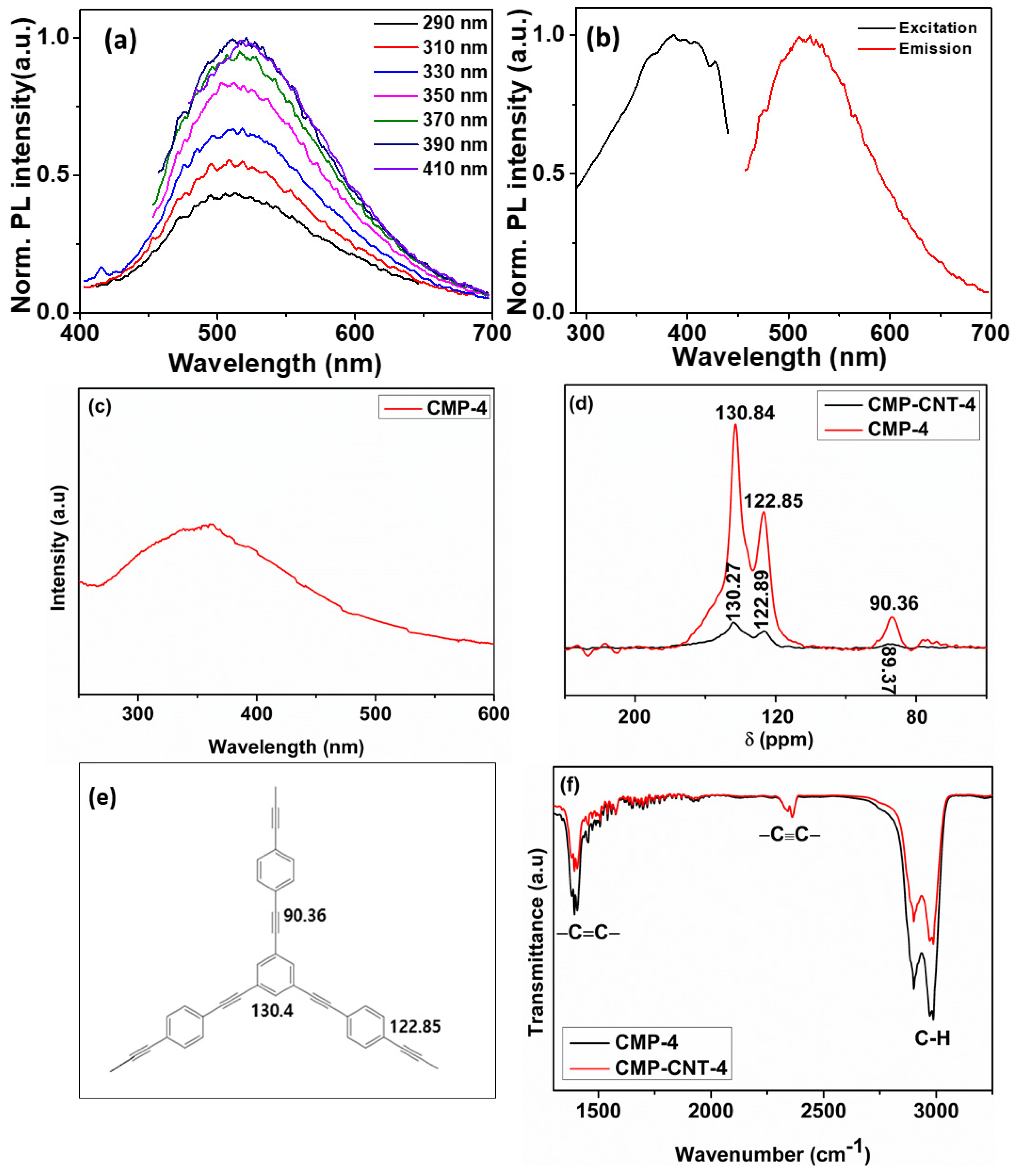
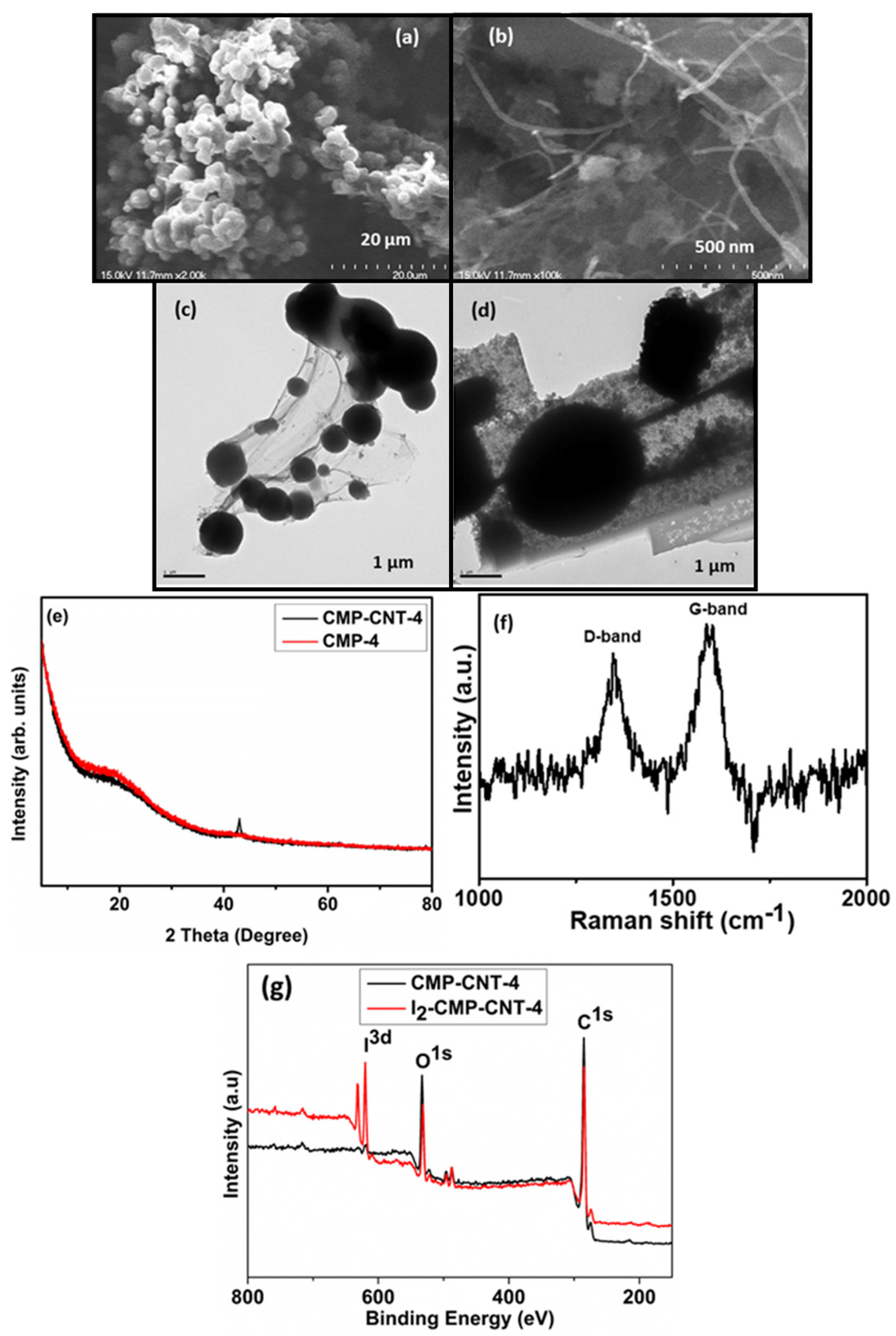
3.1. Physical and Chemical Characterization of the CMP-4 and CMP-CNT-4
3.2. Morphological Characterization of the CMP-CNT-4
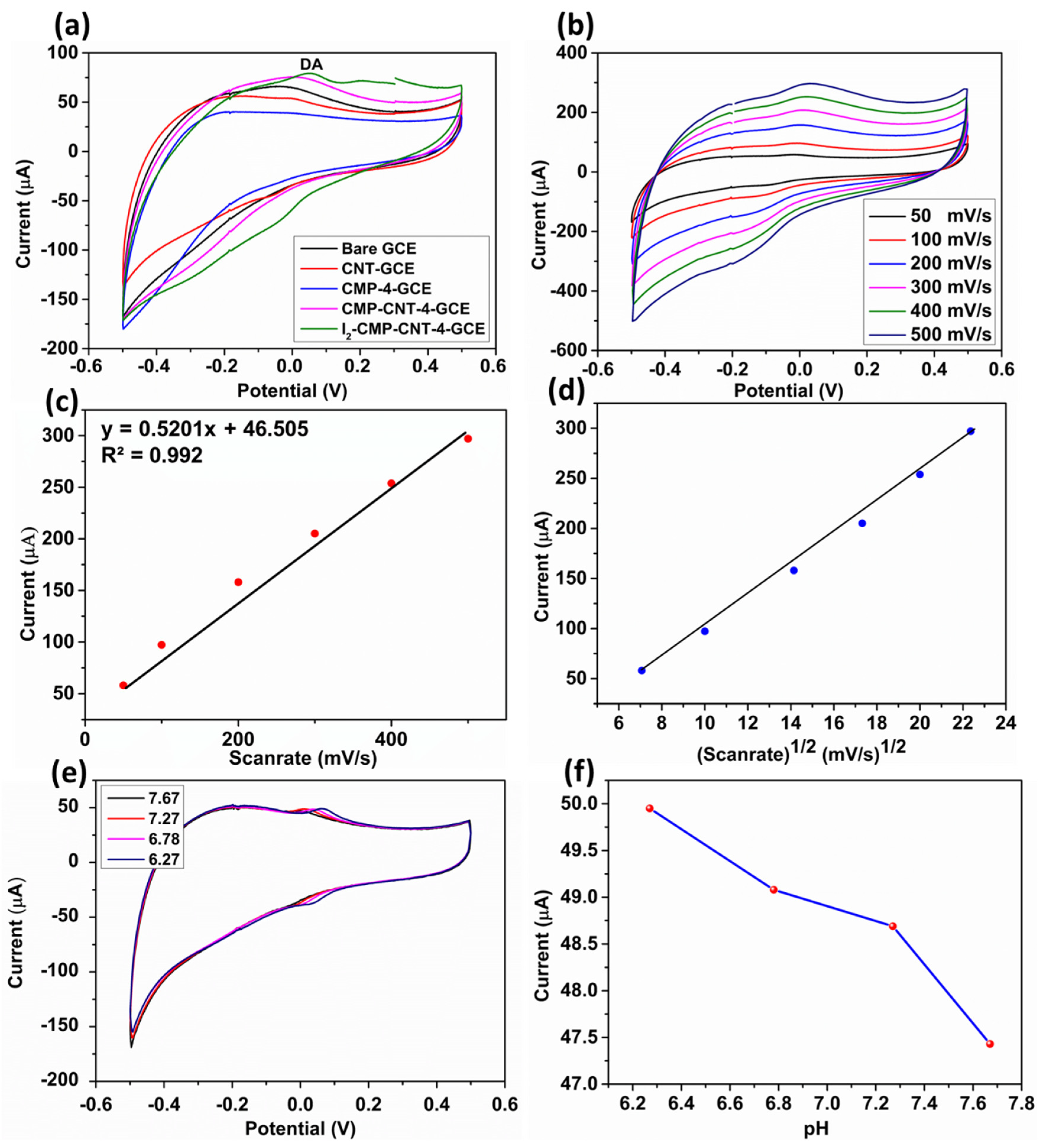
3.3. Electrochemical Behavior of a Modified GCE
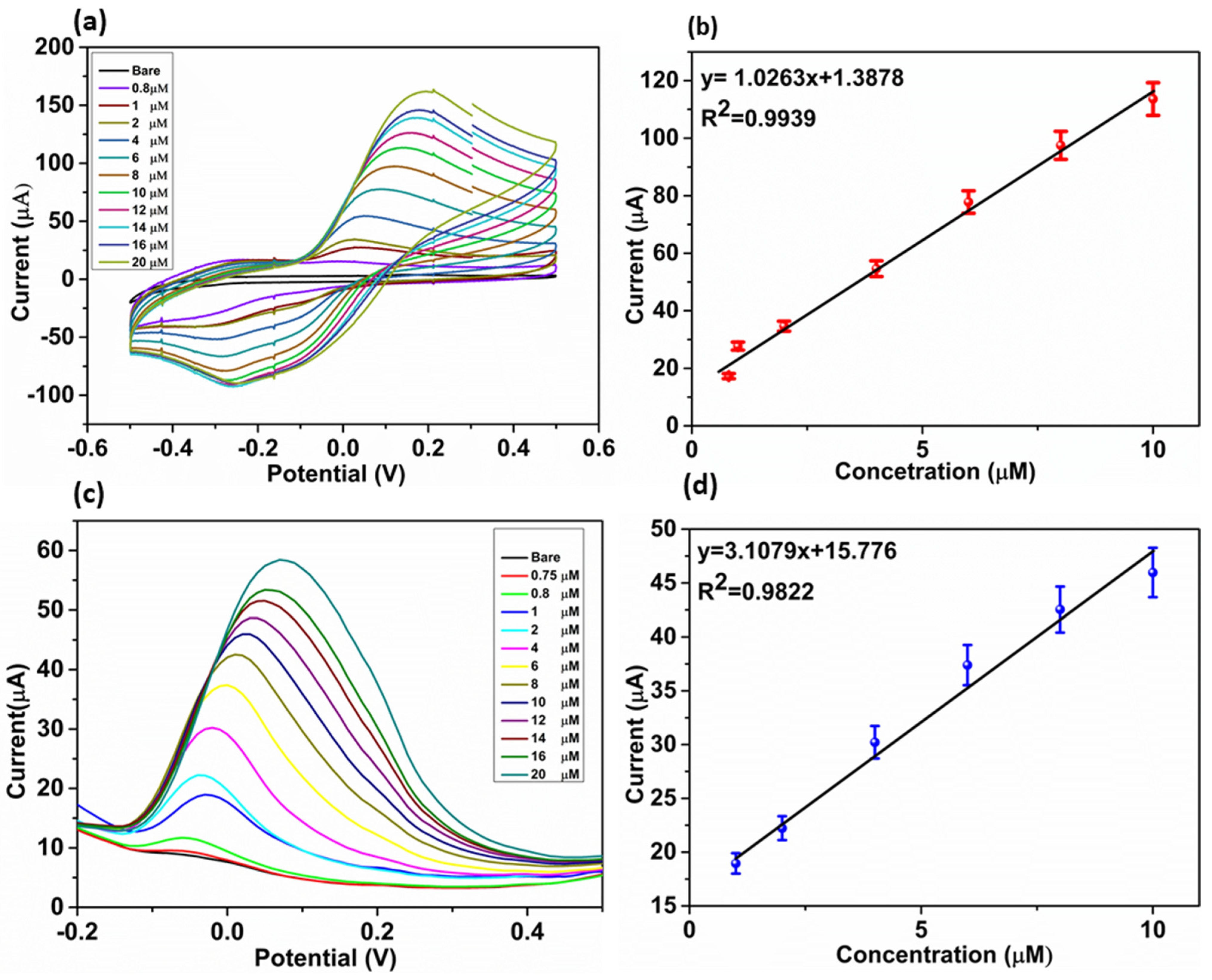
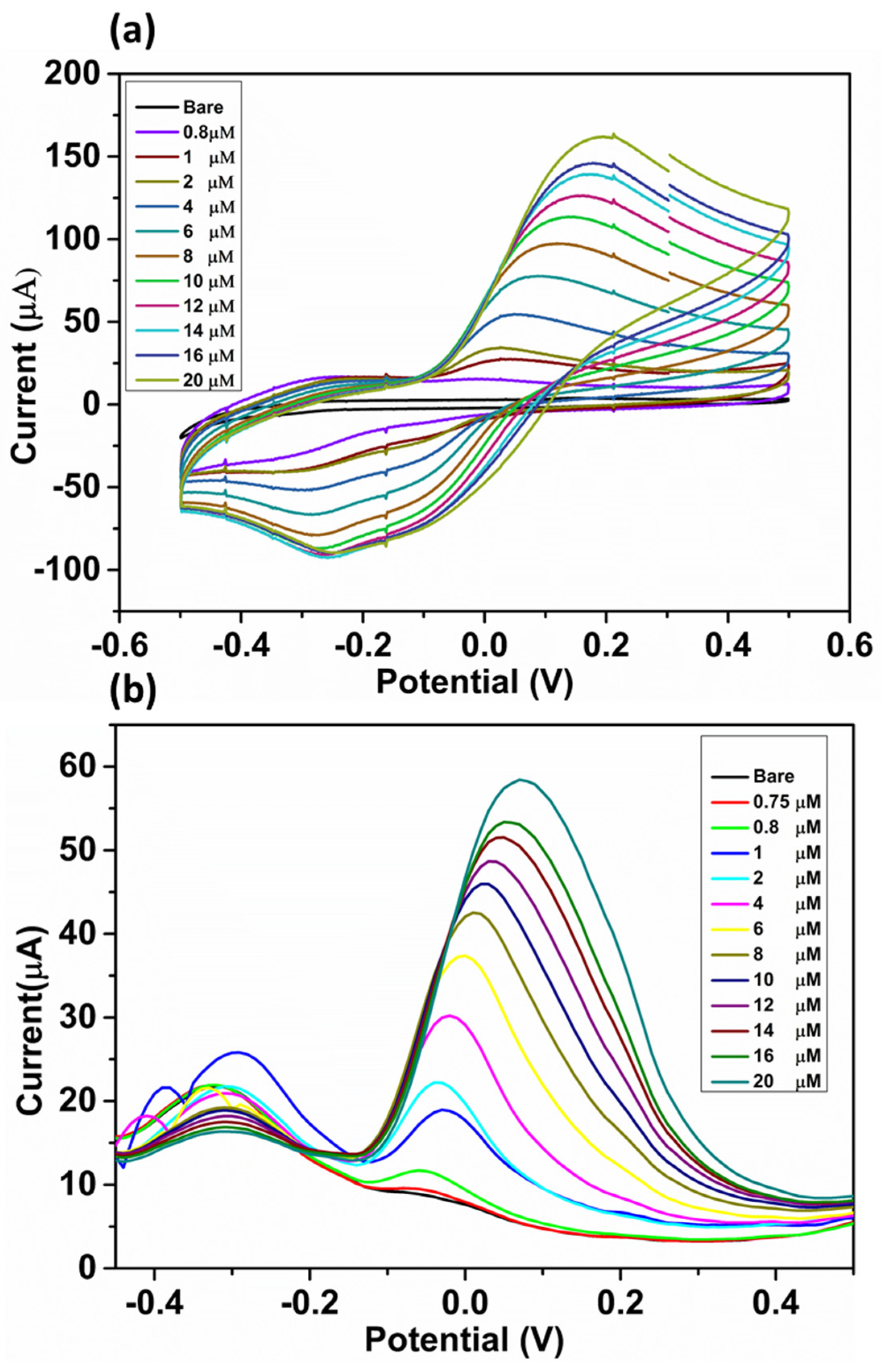
3.4. Electrochemical Detection of DA and Sensing Mechanism
3.5. Interference, Stability, and Reproducibility Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Benes, F.M.; Berretta, S. GABAergic Interneurons: Implications for Understanding Schizophrenia and Bipolar Disorder. Neuropsychopharmacology 2001, 25, 1–27. [Google Scholar] [CrossRef]
- Carlsson, A.; Lindqvist, M.; Magnusson, T. 3,4-Dihydroxyphenylalanine and 5-Hydroxytryptophan as Reserpine Antago-nists. Nature 1957, 180, 1200. Available online: https://www.nature.com/articles/1801200a0 (accessed on 8 January 2023). [CrossRef] [PubMed]
- Li, H.; Yang, M.; Liu, J.; Zhang, Y.; Yang, Y.; Huang, H.; Liu, Y.; Kang, Z. A Practical and Highly Sensitive C3N4-TYR Fluorescent Probe for Convenient Detection of Dopamine. Nanoscale 2015, 7, 12068–12075. [Google Scholar] [CrossRef] [PubMed]
- Florescu, M.; David, M. Tyrosinase-Based Biosensors for Selective Dopamine Detection. Sensors 2017, 17, 1314. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, J.H.; Oh, B.K.; Choi, J.W. Localized Surface Plasmon Resonance-Based Label-Free Biosensor for Highly Sensitive Detection of Dopamine. J. Nanosci. Nanotechnol. 2014, 14, 5658–5661. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, G.; Song, Q.; Tang, T.; Wang, X.; Wei, F.; Hu, Q. Highly Sensitive Determination of Dopamine by a Turn-on Fluorescent Biosensor Based on Aptamer Labeled Carbon Dots and Nano-Graphite. Sens. Actuators B Chem. 2016, 231, 506–512. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and Sensors for Dopamine Detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Yang, X. Aptamer-Based Colorimetric Biosensing of Dopamine Using Unmodified Gold Nanoparticles. Sens. Actuators B Chem. 2011, 156, 95–99. [Google Scholar] [CrossRef]
- Lin, T.Y.; Wei, K.C.; Ju, S.P.; Huang, C.Y.; Yang, H.W. Diagnosis by Simplicity: An Aptachip for Dopamine Capture and Accurate Detection with a Dual Colorimetric and Fluorometric System. J. Mater. Chem. B 2018, 6, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, S.; Liu, Z.; Shi, Y.; Yue, W.; Yi, C. Rapid Determination of Dopamine in Human Plasma Using a Gold Nanoparticle-Based Dual-Mode Sensing System. Mater. Sci. Eng. C 2016, 61, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martos, I.; Ferapontova, E.E. A DNA Sequence Obtained by Replacement of the Dopamine RNA Aptamer Bases Is Not an Aptamer. Biochem. Biophys. Res. Commun. 2017, 489, 381–385. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Cai, N.; Long, S.; Yu, F. Negatively Charged Gold Nanoparticles as an Intrinsic Peroxidase Mimic and Their Applications in the Oxidation of Dopamine. J. Mater. Sci. 2014, 49, 7143–7150. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-Induced Dopamine Polymerization for Multifunctional Coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Sarkar, B.; Banerjee, A.; Das, A.K.; Nag, S.; Kaushalya, S.K.; Tripathy, U.; Shameem, M.; Shukla, S.; Maiti, S. Label-Free Dopamine Imaging in Live Rat Brain Slices. ACS Chem. Neurosci. 2014, 5, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Maity, B.K.; Maiti, S. Label-Free Imaging of Neurotransmitters in Live Brain Tissue by Multi-Photon Ultraviolet Microscopy. Neuronal Signal. 2018, 2, NS20180132. [Google Scholar] [CrossRef] [PubMed]
- Lakard, S.; Pavel, I.A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Ucar, A.; González-Fernández, E.; Staderini, M.; Avlonitis, N.; Murray, A.F.; Bradley, M.; Mount, A.R. Miniaturisation of a Peptide-Based Electrochemical Protease Activity Sensor Using Platinum Microelectrodes. Analyst 2020, 145, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Ibau, C.; Md Arshad, M.K.; Gopinath, S.C.B.; Nuzaihan, M.; Fathil, M.F.M.; Estrela, P. Gold Interdigitated Triple-Microelectrodes for Label-Free Prognosticative Aptasensing of Prostate Cancer Biomarker in Serum. Biosens. Bioelectron. 2019, 136, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.A.V.; Wightman, R.M. Detecting Subsecond Dopamine Release with Fast-Scan Cyclic Voltammetry in Vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Cogan, S.F.; Troyk, P.R.; Ehrlich, J.; Plante, T.D.; Detlefsen, D.E. Potential-Biased, Asymmetric Waveforms for Charge-Injection with Activated Iridium Oxide (AIROF) Neural Stimulation Electrodes. IEEE Trans. Biomed. Eng. 2006, 53, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Liu, S.F.; Swager, T.M.; Dincə, M. Chemiresistive Sensor Arrays from Conductive 2D Metal-Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 13780–13783. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Mirica, K. Two-dimensional d-π conjugated metal-organic framework based on hexahydroxytrinaphthylene. Nano Res. 2021, 14, 369–375. [Google Scholar] [CrossRef]
- Lin, X.; Deng, Y.; He, Y.; Chen, J.; Hu, S. Construction of Hydrophilic N, O-Rich Carboxylated Triazine-Covalent Organic Frameworks for the Application in Selective Simultaneous Electrochemical Detection. Appl. Surf. Sci. 2021, 545, 149047. [Google Scholar] [CrossRef]
- Apollo, N.V.; Maturana, M.I.; Tong, W.; Nayagam, D.A.X.; Shivdasani, M.N.; Foroughi, J.; Wallace, G.G.; Prawer, S.; Ibbotson, M.R.; Garrett, D.J. Soft, Flexible Freestanding Neural Stimulation and Recording Electrodes Fabricated from Reduced Graphene Oxide. Adv. Funct. Mater. 2015, 25, 3551–3559. [Google Scholar] [CrossRef]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal Organic Frameworks for Electrochemical Sensor Applications: A Review. Environ. Res. 2022, 204, 112320. [Google Scholar] [CrossRef]
- Lee, J.S.M.; Cooper, A.I. Advances in Conjugated Microporous Polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated Microporous Poly(Aryleneethynylene) Networks. Angew. Chem.-Int. Ed. 2007, 46, 8574–8578. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, B.; Liu, G.; Zhang, D. Electrochemical Sensors Based on Covalent Organic Frameworks: A Critical Review. Front. Chem. 2020, 8, 601044. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Gándara, F.; Asano, A.; Furukawa, H.; Saeki, A.; Dey, S.K.; Liao, L.; Ambrogio, M.W.; Botros, Y.Y.; Duan, X.; et al. Covalent Organic Frameworks with High Charge Carrier Mobility. Chem. Mater. 2011, 23, 4094–4097. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, D.; Zhang, Z.; Matsushita, M.M.; Awaga, K. Electron Highways into Nanochannels of Covalent Organic Frameworks for High Electrical Conductivity and Energy Storage. ACS Appl. Mater. Interfaces 2019, 11, 7661–7665. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Ge, X.; Liu, R.; Xu, M.; Yang, J. Covalent Organic Frameworks on Reduced Graphene Oxide with Enhanced Electrochemical Performance. Microporous Mesoporous Mater. 2019, 287, 65–70. [Google Scholar] [CrossRef]
- Wang, L.; Pumera, M. Electrochemical Catalysis at Low Dimensional Carbons: Graphene, Carbon Nanotubes and beyond—A Review. Appl. Mater. Today 2016, 5, 134–141. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Hu, H.; Sun, Y.; Wang, J.; Wang, C.; Wang, C.; Zhang, G.; Wang, B.; Xu, W.; et al. A Tetrathiafulvalene-Based Electroactive Covalent Organic Framework. Chem.-A Eur. J. 2014, 20, 14614–14618. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, B.; Gao, X.; Xu, D. A Review of the Interfacial Characteristics of Polymer Nanocomposites Containing Carbon Nanotubes. RSC Adv. 2018, 8, 28048–28085. [Google Scholar] [CrossRef] [PubMed]
- Joao, A.F.; Matias, T.; Gomes, J.S.; Guimaraes, R.R.; Sousa, R.M.F.; Munoz, R.A.A. An environmentally friendly three-dimensional printed graphene integrated polylactic acid electrochemical sensor for the quality control of biofuels. ACS Sustain. Chem. Eng. 2021, 9, 16052–16062. [Google Scholar] [CrossRef]
- Jackowska, K.; Krysinski, P. New Trends in the Electrochemical Sensing of Dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar] [CrossRef]
- Lakshmi, V.; Liu, C.H.; Rajeswara Rao, M.; Chen, Y.; Fang, Y.; Dadvand, A.; Hamzehpoor, E.; Sakai-Otsuka, Y.; Stein, R.S.; Perepichka, D.F. A Two-Dimensional Poly(Azatriangulene) Covalent Organic Framework with Semiconducting and Paramagnetic States. J. Am. Chem. Soc. 2020, 142, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Guan, C.Z.; Wang, D.; Wan, L.J. Graphene-like Single-Layered Covalent Organic Frameworks: Synthesis Strategies and Application Prospects. Adv. Mater. 2014, 26, 6912–6920. [Google Scholar] [CrossRef]
- Song, X.; Han, B.; Yu, X.; Hu, W. The Analysis of Charge Transport Mechanism in Molecular Junctions Based on Current-Voltage Characteristics. Chem. Phys. 2020, 528, 110514. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dincǎ, M. Electrically Conductive Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef]
- Gutzler, R.; Perepichka, D.F. Π-Electron Conjugation in Two Dimensions. J. Am. Chem. Soc. 2013, 135, 16585–16594. [Google Scholar] [CrossRef]
- Meddings, N.; Heinrich, M.; Overney, F.; Lee, J.S.; Ruiz, V.; Napolitano, E.; Seitz, S.; Hinds, G.; Raccichini, R.; Gaberšček, M.; et al. Application of Electrochemical Impedance Spectroscopy to Commercial Li-Ion Cells: A Review. J. Power Sources 2020, 480, 228742. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Schindler, S.; Bechtold, T. Mechanistic Insights into the Electrochemical Oxidation of Dopamine by Cyclic Voltammetry. J. Electroanal. Chem. 2019, 836, 94–101. [Google Scholar] [CrossRef]
- Sabaté del Río, J.; Henry, O.Y.F.; Jolly, P.; Ingber, D.E. An Antifouling Coating That Enables Affinity-Based Electrochemical Biosensing in Complex Biological Fluids. Nat. Nanotechnol. 2019, 14, 1143–1149. [Google Scholar] [CrossRef]
- Ponnada, S.; Gorle, D.B.; Kiai, M.S.; Rajagopal, S.; Sharma, R.K.; Nowduri, A. A Facile, Cost-Effective, Rapid, Single-Step Synthesis of Ag-Cu Decorated ZnO Nanoflower-like Composites (NFLCs) for Electrochemical Sensing of Dopamine. Mater. Adv. 2021, 2, 5986–5996. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M.; Pournaghi-Azar, M.H. Simultaneous Determination of Acetaminophen and Dopamine Using SWCNT Modified Carbon-Ceramic Electrode by Differential Pulse Voltammetry. Electrochim. Acta 2011, 56, 2888–2894. [Google Scholar] [CrossRef]
- Garg, A.K.; Dalal, C.; Kaushik, J.; Anand, S.R.; Sonkar, S.K. Selective Sensing of Explosive Nitrophenol Compounds by Using Hydrophobic Carbon Nanoparticles. Mater. Today Sustain. 2022, 20, 100202. [Google Scholar] [CrossRef]
- Dhiman, N.; Ghosh, S.; Mishra, Y.K.; Tripathi, K.M. Prospects of Nano-Carbons as Emerging Catalysts for Enzyme-Mimetic Applications. Mater. Adv. 2022, 3, 3101–3122. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lee, J.J. Electrochemical Dopamine Sensors Based on Graphene. J. Electrochem. Sci. Technol. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R.; Dar, M.A. One-Pot Synthesis of Au-Cu2O/RGO Nanocomposite Based Electrochemical Sensor for Selective and Simultaneous Detection of Dopamine and Uric Acid. J. Alloys Compd. 2018, 741, 1130–1141. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Keeley, G.P.; McEvoy, N.; Nolan, H.; Kumar, S.; Rezvani, E.; Holzinger, M.; Cosnier, S.; Duesberg, G.S. Simultaneous Electrochemical Determination of Dopamine and Paracetamol Based on Thin Pyrolytic Carbon Films. Anal. Methods 2012, 4, 2048–2053. [Google Scholar] [CrossRef]
- Yin, H.; Shang, K.; Meng, X.; Ai, S. Voltammetric sensing of paracetamol, dopamine and 4-aminophenol at a glassy carbon electrode coated with gold nanoparticles and an organophillic layered double hydroxide. Microchim. Acta 2011, 175, 39–46. [Google Scholar] [CrossRef]
- Huang, H.; Bai, J.; Li, J.; Lei, L.; Zhang, W.; Yan, S.; Li, Y. Fluorescence detection of dopamine based on the polyphenol oxidase–mimicking enzyme. Anal. Bioanal. Chem. 2020, 412, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, D.; Wang, Y.; Fu, C. Facile fabrication of tungsten disulfide quantum dots (WS2 QDs) as effective probes for fluorescence detection of dopamine (DA). Mater. Chem. Phys. 2018, 207, 130–134. [Google Scholar] [CrossRef]
- Sivakumar, P.; Priyatharshni, S.; Kumar, K. Fluorescent silver nanoparticles for sensitive and selective detection of dopamine. Mater. Chem. Phys. 2020, 240, 122167. [Google Scholar] [CrossRef]
- Palanisamy, S.; Zhang, X.; He, T. Simple colorimetric detection of dopamine using modified silver nanoparticles. Sci. China Chem. 2016, 59, 387–393. [Google Scholar] [CrossRef]
- Zhang, C.; You, X.; Li, Y.; Zuo, Y.; Wang, W.; Li, D.D.; Huang, S.W.; Hu, H.; Yuan, F.; Shao, F.; et al. A novel electrochemical aptasensor for serum dopamine detection based on methylene blue-integrated m-PdNFs signal material. Sens. Actuators B Chem. 2022, 354, 131233–131242. [Google Scholar] [CrossRef]
| Modified Electrode | The Detection Limit (μM) | Detection Method | References |
|---|---|---|---|
| Au/Pt/GCE | 24 | DPV | [47] |
| GCE/Au-Cu2O/rGO | 3.9 | DPV | [51] |
| PDA-RGO/Au | 3.2 | LSV | [52] |
| PyC | 2.3 | CV, DPV | [53] |
| GC/SDSLDH/AuNP | Not given | DPV | [54] |
| Pdots@AMP-Cu | 4 | Fluorescence | [55] |
| WS2 QDs | 3.3 | Fluorescence | [56] |
| Ag@MPBA-ABCDE | 5.9 | Fluorescence | [57] |
| Ag NP | 6 | Colorimetric | [58] |
| I2-CMP-CNT-4 | 1, 1.7 | CV, DPV | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, J.; Moniruzzaman, M.; Kim, J. Framing of Poly(arylene-ethynylene) around Carbon Nanotubes and Iodine Doping for the Electrochemical Detection of Dopamine. Biosensors 2023, 13, 308. https://doi.org/10.3390/bios13030308
Paul J, Moniruzzaman M, Kim J. Framing of Poly(arylene-ethynylene) around Carbon Nanotubes and Iodine Doping for the Electrochemical Detection of Dopamine. Biosensors. 2023; 13(3):308. https://doi.org/10.3390/bios13030308
Chicago/Turabian StylePaul, Jose, Md Moniruzzaman, and Jongsung Kim. 2023. "Framing of Poly(arylene-ethynylene) around Carbon Nanotubes and Iodine Doping for the Electrochemical Detection of Dopamine" Biosensors 13, no. 3: 308. https://doi.org/10.3390/bios13030308
APA StylePaul, J., Moniruzzaman, M., & Kim, J. (2023). Framing of Poly(arylene-ethynylene) around Carbon Nanotubes and Iodine Doping for the Electrochemical Detection of Dopamine. Biosensors, 13(3), 308. https://doi.org/10.3390/bios13030308




