Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications
Abstract
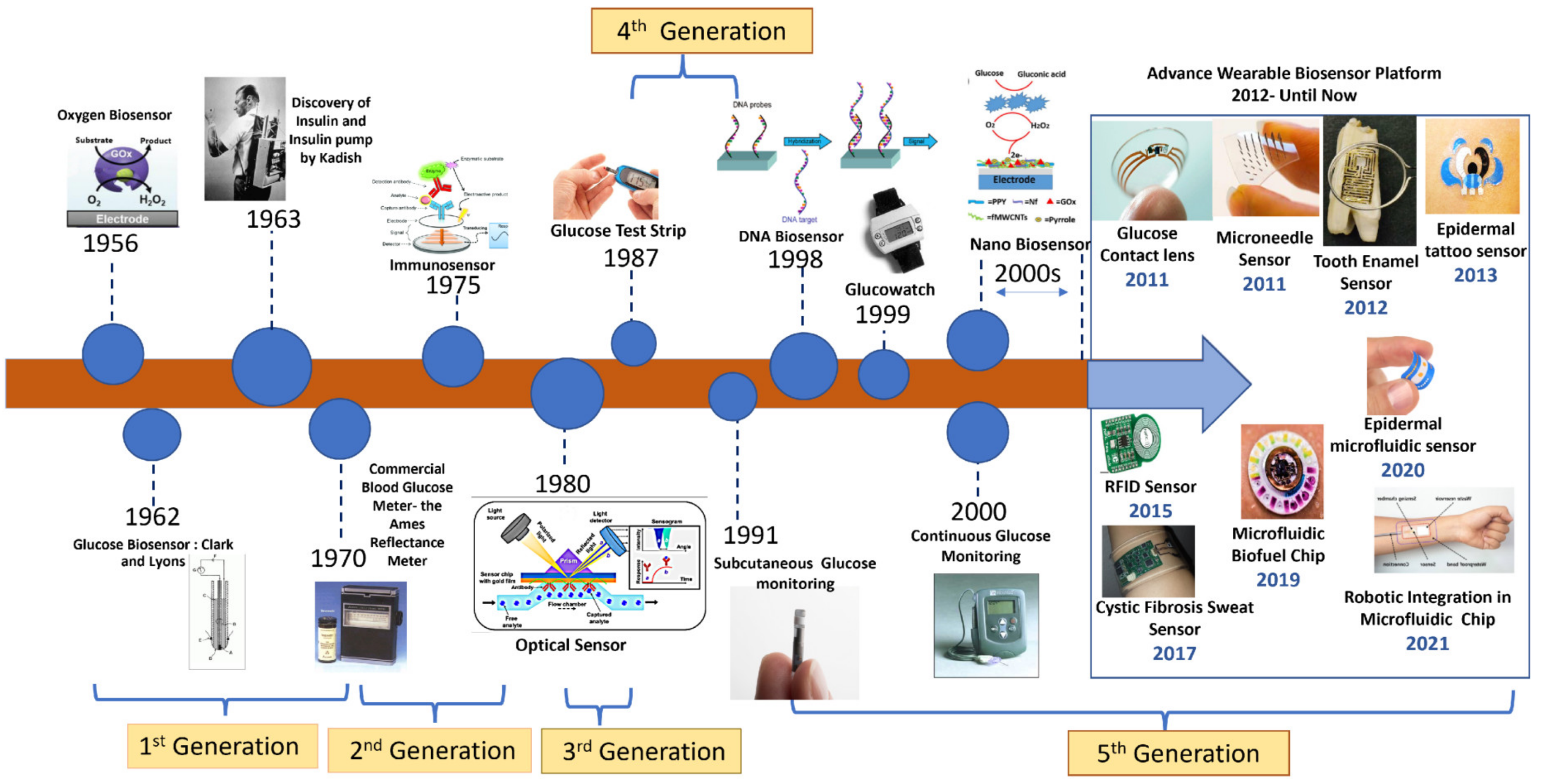
1. Introduction
2. Nanotechnology—An Overview
- Categorization—Signals obtained from the sensing data are classified into different types using various algorithms depending on the type of target analyte.
- Anomaly detection—The operating conditions of a biosensor and the sample matrix significantly affect the functions of a biosensor and the contaminations interfere with the sensing signal if the biosensor is deployed in the target site. In such cases, the ML algorithm checks the correctness of the obtained signal, and if found incorrect, the signals which are interfered by the biofouling are corrected to improve the sensor performance.
- Reduction of noise—Sensed signals are commonly embedded with noise. If the sensed signal is interfered with by electrical noise, the signal changes in a few seconds or minutes which makes it shift to a sub-second timeline. If ML models are trained to detect noise, the sensing signal accuracy can be substantially improved.
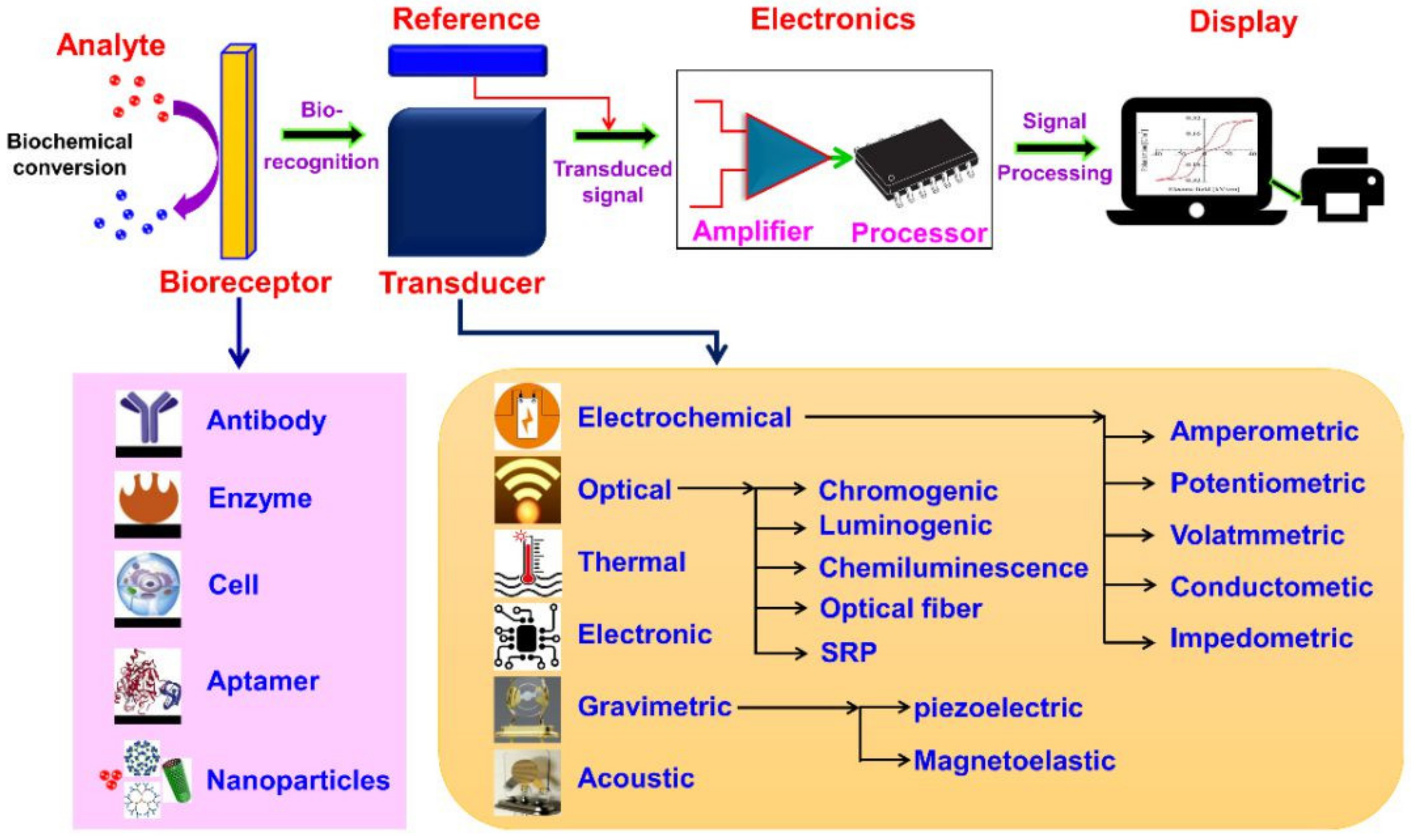
3. Fundamentals
3.1. Biosensors
3.2. Nanobiosensors
4. Architectural Design
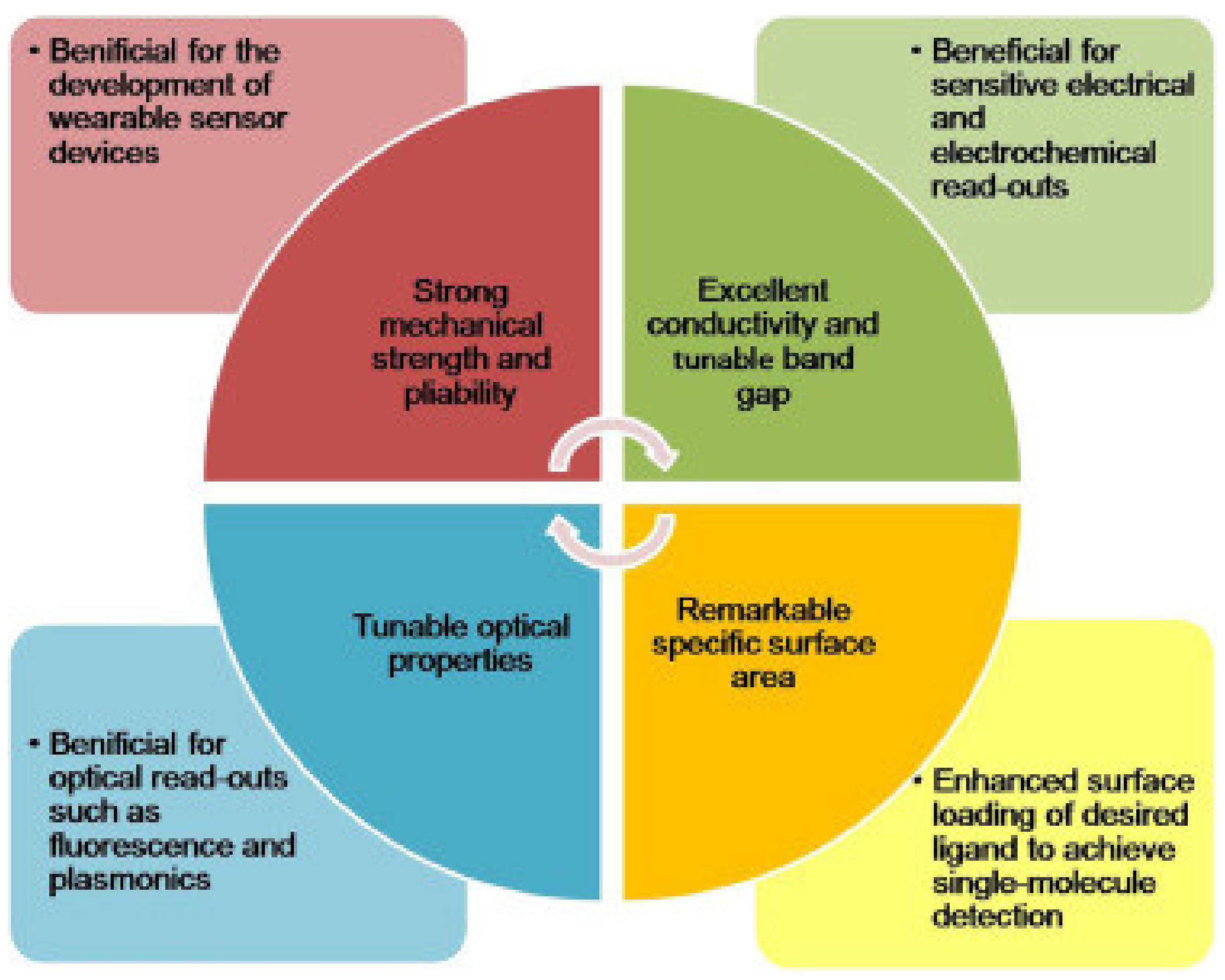
5. Nanomaterials for Biosensors
5.1. Graphene-Based Biosensors
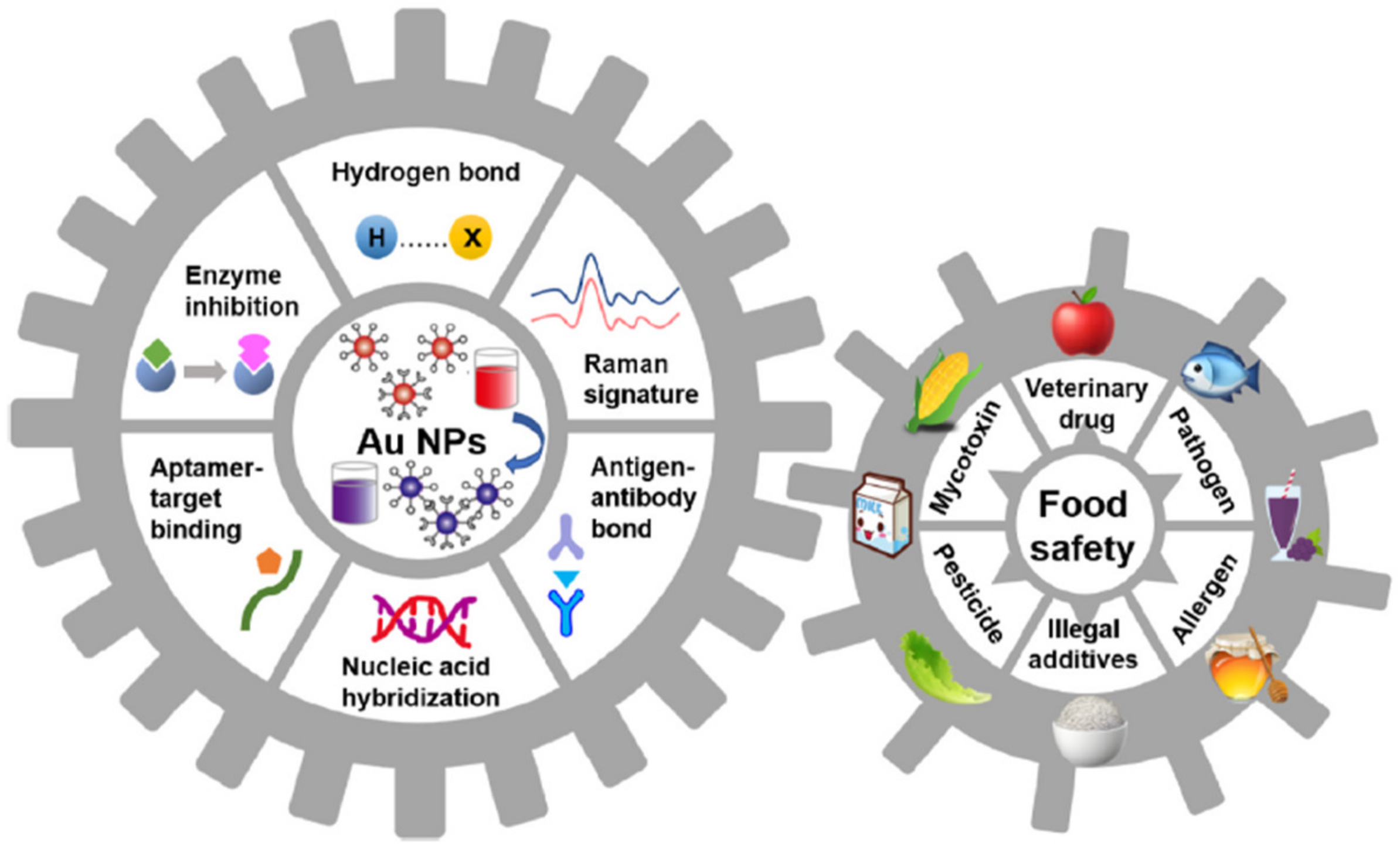
5.2. Gold Nanoparticles (GNPs) in Biosensors
5.3. CNTs in Biosensors
5.4. QD-Based Biosensors
5.5. Cell-Based Biosensors
5.6. COF- and MOF-Based Biosensors
5.7. Other Nano-Materials in Biosensors
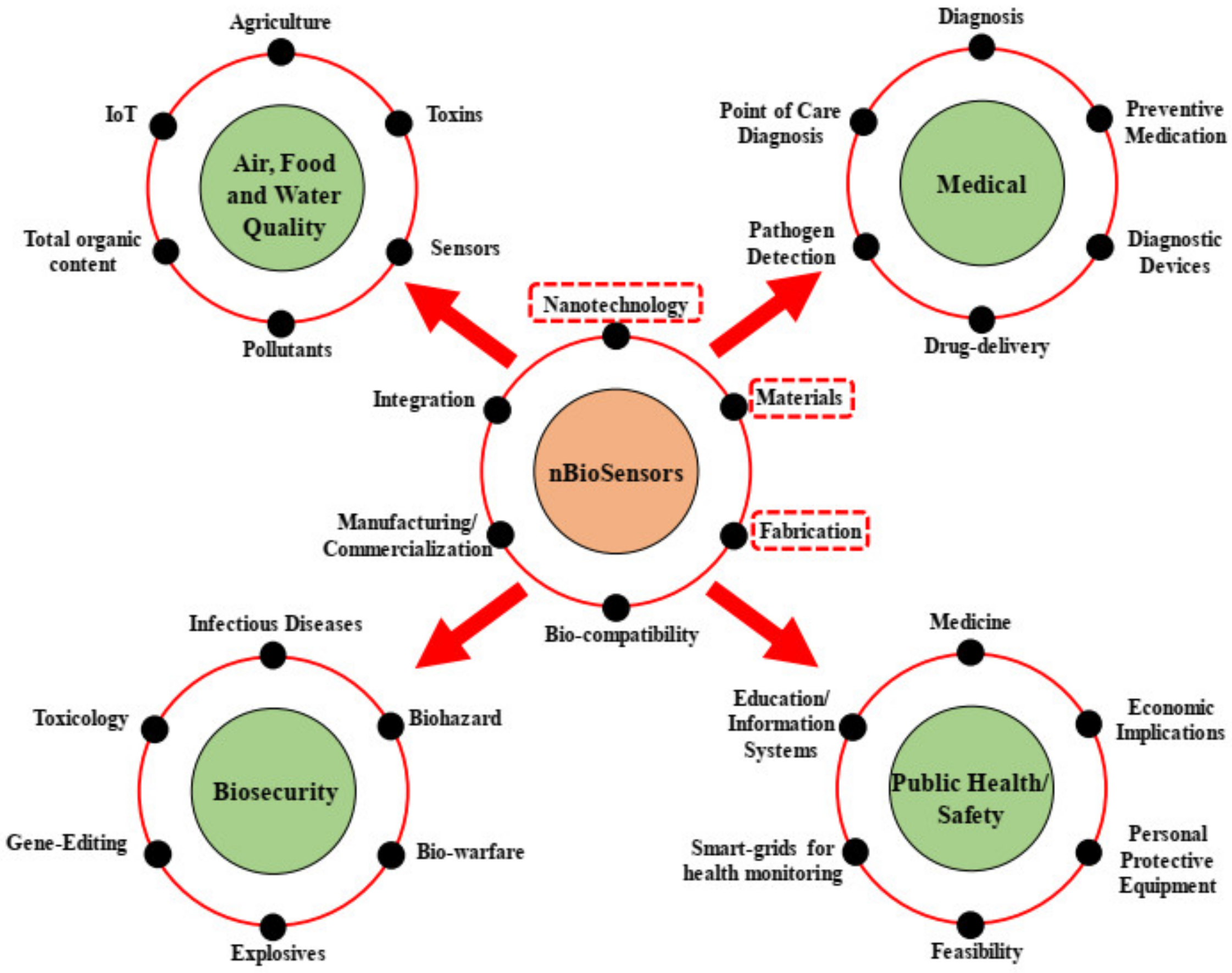
6. Applications of Nanotechnology-Based Biosensors
6.1. Biomedical Applications
6.2. Cancer and Bone Disease
6.3. Tissue Engineering Applications
6.4. Microfluidic Systems
6.5. Diagnostics
6.6. Porphyrin and Phthalocyanine
6.7. Detection of Glucose
6.8. Detection of DNA and Protein
7. Challenges and Prospects
8. Summary and Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naresh, V.; Lee, N.A. Review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Maity, S.; Mastrangelo, C.H. Nanostructures for biosensing, with a brief overview on cancer detection, IoT, and the role of machine learning in smart biosensors. Sensors 2021, 21, 1253. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, S.; Maity, S.; Yazhini, D.; Ghosh, A. Surface-directed disparity in self-assembled structures of small-peptide l-glutathione on gold and silver nanoparticles. Langmuir 2020, 36, 11255–11261. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Leng, B.; Yang, N.; Yang, B.; Liu, L.; Huang, N.; Jiang, X. Rational Construction of 3D-Networked Carbon Nanowalls/Diamond Supporting CuO Architecture for High-Performance Electrochemical Biosensors. Small 2019, 15, 1901527. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.F.D.; Norena, N.; Kaushik, A.; Bhansali, S. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens. Bioelectron. 2014, 62, 249–254. [Google Scholar] [CrossRef]
- Kaushik, A.; Kumar, R.; Huey, E.; Bhansali, S.; Nair, N.; Nair, M. Silica nanowires: Growth, integration, and sensing applications. Microchim. Acta 2014, 181, 1759–1780. [Google Scholar] [CrossRef]
- Kaushik, A.; Mujawar, M.A. Point of care sensing devices: Better care for everyone. Sensors 2018, 18, 4303. [Google Scholar] [CrossRef]
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014, 53, 499–512. [Google Scholar] [CrossRef]
- Shakeel, A.; Rizwan, K.; Farooq, U.; Iqbal, S.; Altaf, A.A. Advanced polymeric/inorganic nanohybrids: An integrated platform for gas sensing applications. Chemosphere 2022, 294, 133772. [Google Scholar] [CrossRef]
- Tyagi, M.; Chandran, A.; Joshi, T.; Prakash, J.; Agrawal, V.V.; Biradar, A.M. Self-assembled monolayer-based liquid crystal biosensor for free cholesterol detection. Appl. Phys. Lett. 2014, 104, 154104. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Z. Beyond displays: The recent progress of liquid crystals for bio/chemical detections. Chin. Sci. Bull. 2013, 58, 2557–2562. [Google Scholar] [CrossRef]
- Kaushik, A.; Vabbina, P.K.; Atluri, V.; Shah, P.; Vashist, A.; Jayant, R.D.; Yandart, A.; Nair, M. Electrochemical monitoring-on-chip (E-MoC) of HIV-infection in presence of cocaine and therapeutics. Biosens. Bioelectron. 2016, 86, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Popov, P.; Mann, E.K.; Jákli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mater. Chem. B 2017, 5, 5061–5078. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Parveen, A.; Mishra, Y.K.; Kaushik, A. Nanotechnology-assisted liquid crystals-based biosensors: Towards fundamental to advanced applications. Biosens. Bioelectron. 2020, 168, 112562. [Google Scholar] [CrossRef]
- Mao, W.; He, H.; Sun, P.; Ye, Z.; Huang, J. Three-dimensional porous nickel frameworks anchored with cross-linked Ni(OH)2 nanosheets as a highly sensitive nonenzymatic glucose sensor. ACS Appl. Mater. Interfaces 2018, 10, 15088–15095. [Google Scholar] [CrossRef]
- Zhu, X.; Ju, Y.; Chen, J.; Liu, D.; Liu, H. Nonenzymatic wearable sensor for electrochemical analysis of perspiration glucose. ACS Sens. 2018, 3, 1135–1141. [Google Scholar] [CrossRef]
- Karikalan, N.; Velmurugan, M.; Chen, S.M.; Karuppiah, C. Modern approach to the synthesis of Ni(OH)2 decorated sulfur doped carbon nanoparticles for the nonenzymatic glucose sensor. ACS Appl. Mater. Interfaces 2016, 8, 22545–22553. [Google Scholar] [CrossRef]
- Rizwan, K.; Rahdar, A.; Bilal, M.; Iqbal, H.M. MXene-based electrochemical and biosensing platforms to detect toxic elements and pesticides pollutants from environmental matrices. Chemosphere 2022, 291, 132820. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Rahighi, R. Toward single-DNA electrochemical biosensing by graphene nanowalls. ACS Nano 2012, 6, 2904–2916. [Google Scholar] [CrossRef]
- Chandra, S.; Mayer, M.; Baeumner, A.J. PAMAM dendrimers: A multifunctional nanomaterial for ECL biosensors. Talanta 2017, 168, 126–129. [Google Scholar] [CrossRef]
- Deepa, C.; Rajeshkumar, L.; Ramesh, M. Preparation, synthesis, properties and characterization of graphene-based 2D nano-materials for biosensors and bioelectronics. J. Mater. Res. Technol. 2022, 19, 2657–2694. [Google Scholar] [CrossRef]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing biosensors with machine learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef] [PubMed]
- Schackart, K.E., III; Yoon, J.Y. Machine learning enhances the performance of bioreceptor-free biosensors. Sensors 2021, 21, 5519. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hu, N.; Wei, X.; Gong, L.; Zhang, B.; Wan, H.; Wang, P. 3D cell-based biosensor for cell viability and drug assessment by 3D electric cell/matrigel-substrate impedance sensing. Biosens. Bioelectron. 2019, 130, 344–351. [Google Scholar] [CrossRef]
- Justino, C.I.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Santos, T.A.R. Recent developments in recognition elements for chemical sensors and biosensors. TrAC Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Reinholds, I.; Bartkevics, V.; Silvis, I.C.; van Ruth, S.M.; Esslinger, S. Analytical techniques combined with chemometrics for authentication and determination of contaminants in condiments: A review. J. Food Compos. Anal. 2015, 44, 56–72. [Google Scholar] [CrossRef]
- Panchuk, V.; Yaroshenko, I.; Legin, A.; Semenov, V.; Kirsanov, D. Application of chemometric methods to XRF-data—A tutorial review. Anal. Chim. Acta 2018, 1040, 19–32. [Google Scholar] [CrossRef]
- Villa, J.E.; Afonso, M.A.; Dos Santos, D.P.; Mercadal, P.A.; Coronado, E.A.; Poppi, R.J. Colloidal gold clusters formation and chemometrics for direct SERS determination of bioanalytes in complex media. Spectrochim. Part A Mol. Biomol. Spectrosc. 2020, 224, 117380. [Google Scholar] [CrossRef]
- Coroş, M.; Pruneanu, S.; Stefan-van Staden, R.I. Recent progress in the graphene-based electrochemical sensors and biosensors. J. Electrochem. Soc. 2019, 167, 037528. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral. Biol. Craniofacial. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Chamorro-Garcia, A.; Merkoçi, A. Nanobiosensors in diagnostics. Nanobiomedicine 2016, 3, 1849543516663574. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Tsai, T.T.; Huang, T.H.; Chen, C.A.; Ho, N.Y.J.; Chou, Y.J.; Chen, C.F. Development a stacking pad design for enhancing the sensitivity of lateral flow immunoassay. Sci. Rep. 2018, 8, 17319. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.; O’Riordan, A. Electrochemical nanosensors: Advances and applications. Rep. Electrochem. 2016, 6, 1–14. [Google Scholar]
- Yousefi, S.R.; Alshamsi, H.A.; Amiri, O.; Salavati-Niasari, M. Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J. Mol. Liq. 2021, 337, 116405. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wei, X.; Xue, Y.; Wan, H.; Wang, P. Recent advances in acoustic wave biosensors for the detection of disease-related biomarkers: A review. Anal. Chim. Acta 2021, 1164, 338321. [Google Scholar] [CrossRef]
- Parker, R.N.; Grove, T.Z. Designing repeat proteins for biosensors and medical imaging. Biochem. Soc. Trans. 2015, 43, 856–860. [Google Scholar] [CrossRef]
- Pollard, T.D.; Ong, J.J.; Goyanes, A.; Orlu, M.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Electrochemical biosensors: A nexus for precision medicine. Drug Discov. Today 2021, 26, 69–79. [Google Scholar] [CrossRef]
- Alhadrami, H.A. Biosensors: Classifications, medical applications, and future prospective. Biotechnol. Appl. Biochem. 2018, 65, 497–508. [Google Scholar] [CrossRef]
- Saylan, Y.; Erdem, Ö.; Ünal, S.; Denizli, A. An alternative medical diagnosis method: Biosensors for virus detection. Biosensors 2019, 9, 65. [Google Scholar] [CrossRef]
- Chauhan, N.; Maekawa, T.; Kumar, D.N.S. Graphene based biosensors—Accelerating medical diagnostics to new-dimensions. J. Mater. Res. 2017, 32, 2860–2882. [Google Scholar] [CrossRef]
- Mowbray, S.E.; Amiri, A.M. A brief overview of medical fiber optic biosensors and techniques in the modification for enhanced sensing ability. Diagnostics 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.V.; Le, P.M.L. Nanoflake manganese oxide and nickel-manganese oxide synthesized by electrode position for electrochemical capacitor. J. Nanomater. 2015, 2015, 230. [Google Scholar] [CrossRef]
- Munawar, A.; Ong, Y.; Schirhagl, R.; Tahir, M.A.; Khan, W.S.; Bajwa, S.Z. Nanosensors for diagnosis with optical, electric and mechanical transducers. RSC Adv. 2019, 9, 6793–6803. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, S.K.; Singh, J.; Srivastava, S.; Sachan, S.; Singh, S.K. Biomedical perspective of electrochemical nanobiosensor. Nano-Micro Lett. 2016, 8, 193–203. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Adeel, M.; Rizwan, K.; Bilal, M.; Iqbal, H.M. Carbon nanotubes-based cues: A pathway to future sensing and detection of hazardous pollutants. J. Mol. Liq. 2019, 292, 111425. [Google Scholar] [CrossRef]
- Kargozar, S.; Hoseini, S.J.; Milan, P.B.; Hooshmand, S.; Kim, H.W.; Mozafari, M. Quantum dots: A review from concept to clinic. Biotechnol. J. 2020, 15, 2000117. [Google Scholar] [CrossRef]
- Solaimuthu, A.; Vijayan, A.N.; Murali, P.; Korrapati, P.S. Nano-biosensors and their relevance in tissue engineering. Curr. Opin. Biomed. Eng. 2020, 13, 84–93. [Google Scholar] [CrossRef]
- Sheervalilou, R.; Shahraki, O.; Hasanifard, L.; Shirvaliloo, M.; Mehranfar, S.; Lotfi, H.; Pilehvar-Soltanahmadi, Y.; Bahmanpour, Z.; Zadeh, S.S.; Nazarlou, Z.; et al. Electrochemical nano-biosensors as novel approach for the detection of lung cancer-related MicroRNAs. Curr. Mol. Med. 2020, 20, 13–35. [Google Scholar] [CrossRef]
- Wang, N.; Hang, T.; Ling, H.; Hu, A.; Li, M. High-performance Si-based 3D Cu nanostructured electrode assembly for rechargeable lithium batteries. J. Mater. Chem. A 2015, 3, 11912–11919. [Google Scholar] [CrossRef]
- Shahzad, S.; Rizwan, K.; Zubair, M. Organic-Inorganic Nanohybrids-Based Sensors for Gases, Humidity, UV and Others. In Hybrid Nanomaterials; Springer: Singapore, 2022; pp. 227–246. [Google Scholar]
- Malik, P.; Katyal, V.; Malik, V.; Asatkar, A.; Inwati, G.; Mukherjee, T.K. Nanobiosensors: Concepts and variations. Int. Sch. Res. Not. 2013, 2013, 327435. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Tian, Y.; Liu, X.Q.; Niu, Z.; Yang, Q.Z.; Ramamurthy, V.; Tung, C.H.; Chen, Y.Z.; Wu, L.Z. Luminescent supramolecular polymer nanoparticles for ratiometric hypoxia sensing, imaging and therapy. Mater. Chem. Front. 2018, 2, 1893–1899. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Nguyen, T.K. Thanh Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Jeedigunta, S.; Kumar, A. Fabrication and characterization of ZnO nanowire arrays with an investigation into electrochemical sensing capabilities. J. Nanomater. 2008, 2008, 638523. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting polymers in the design of biosensors and biofuel cells. Polymers 2020, 13, 49. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Kaseta, V.; Voronovic, J.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of cytotoxicity of polypyrrole nanoparticles synthesized by oxidative polymerization. J. Hazard. Mater. 2013, 250, 167–174. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Zamani, F.G.; Moulahoum, H.; Ak, M.; Demirkol, D.O.; Timur, S. Current trends in the development of conducting polymers-based biosensors. TrAC Trends Anal. Chem. 2019, 118, 264–276. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of enzymatic formation of polyaniline nanoparticles. Polymer 2017, 115, 211–216. [Google Scholar] [CrossRef]
- Krikstolaityte, V.; Kuliesius, J.; Ramanaviciene, A.; Mikoliunaite, L.; Kausaite-Minkstimiene, A.; Oztekin, Y.; Ramanavicius, A. Enzymatic polymerization of polythiophene by immobilized glucose oxidase. Polymer 2014, 55, 1613–1620. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Y.; Guo, M.; Lin, B.; Zhang, L. A sensitive determination of albumin in urine by molecularly imprinted electrochemical biosensor based on dual-signal strategy. Sens. Actuators B Chem. 2019, 288, 564–570. [Google Scholar] [CrossRef]
- Tretjakov, A.; Syritski, V.; Reut, J.; Boroznjak, R.; Volobujeva, O.; Öpik, A. Surface molecularly imprinted polydopamine films for recognition of immunoglobulin G. Microchim. Acta 2013, 180, 1433–1442. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Bochenkov, V.E.; Runager, K.; Aslan, H.; Dong, M.; Enghild, J.J.; De Freitas, V.; Ferreira Sales, M.G.; Sutherland, D.S. Molecular imprinting of complex matrices at localized surface plasmon resonance biosensors for screening of global interactions of polyphenols and proteins. ACS Sens. 2016, 1, 258–264. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Progress and insights in the application of MXenes as new 2D nano-materials suitable for biosensors and biofuel cell design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, K.; Kovářík, T.; Pasha, S.K. State of the art recent progress in two dimensional MXenes based gas sensors and biosensors: A comprehensive review. Coord. Chem. Rev. 2020, 424, 213514. [Google Scholar] [CrossRef]
- Eklund, P.; Rosen, J.; Persson, P.O.Å. Layered ternary M n+ 1AX n phases and their 2D derivative MXene: An overview from a thin-film perspective. J. Phys. D Appl. Phys. 2017, 50, 113001. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Banerjee, A.; Khan, S.U.H.; Broadbent, S.; Bulbul, A.; Kim, K.H.; Noh, S.; Kim, H. Molecular bridge-mediated ultralow-power gas sensing. Microsyst. Nanoeng. 2021, 7, 27. [Google Scholar] [CrossRef]
- Girigoswami, K.; Akhtar, N. Nanobiosensors and fluorescence based biosensors: An overview. Int. J. Nano Dimens. 2019, 10, 1–17. [Google Scholar]
- Szunerits, S.; Boukherroub, R. Graphene-based biosensors. Interface Focus 2018, 8, 20160132. [Google Scholar] [CrossRef]
- Zamora-Galvez, A.; Morales-Narváez, E.; Mayorga-Martinez, C.C.; Merkoçi, A. Nanomaterials connected to antibodies and molecularly imprinted polymers as bio/receptors for bio/sensor applications. Appl. Mater. Today 2017, 9, 387–401. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Graphene-based field-effect transistor biosensors for the rapid detection and analysis of viruses: A perspective in view of COVID-19. Carbon Trends 2021, 2, 100011. [Google Scholar] [CrossRef]
- Afsahi, S.; Lerner, M.B.; Goldstein, J.M.; Lee, J.; Tang, X.; Bagarozzi, D.A.; Pan, D.; Locascio, L.; Walker, A.; Barron, F.; et al. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens. Bioelectron. 2018, 100, 85–88F. [Google Scholar] [CrossRef] [PubMed]
- Schedin, F.; Geim, A.; Morozov, S.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.R. Eco-friendly production of high quality low cost graphene and its application in lithium ion batteries. Green Chem. 2016, 18, 1952. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiab, G.; Dua, M.; Lua, Y.; Xua, H. Scotch-tape-like exfoliation effect of graphene quantum dots for efficient preparation of graphene nanosheets in water. Appl. Surf. Sci. 2019, 483, 52–59. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Jin, X. Sulfuric acid intercalated graphite oxide for graphene preparation. Sci. Rep. 2013, 3, 3439. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid exfoliation of defect-free graphene. Acc. Chem. Res. 2013, 46, 14–22. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Xu, T.; Zhang, T.; Mo, Y.; Liu, J.; Yan, L.; Xing, F. Ultra-sensitive and ultra-fast detection of whole unlabeled living cancer cell responses to paclitaxel with a graphene-based biosensor. Sens. Actuators B Chem. 2018, 263, 417–425. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qia, Q.; Jinga, Q.; Ao, S.; Zhang, Z.; Ding, M.C.; Wu, M.; Liu, K.; Wang, W.; Ling, Y.; et al. Electrical probing of COVID-19 spike protein receptor binding domain via a graphene field-effect transistor. arXiv 2020, arXiv:2003.12529. [Google Scholar]
- Aspermair, P.; Mishyn, V.; Bintinger, J.; Happy, H.; Bagga, K.; Subramanian, P.; Knoll, W.; Boukherroub, R.; Szunerits, S. Reduced graphene oxide–based field effect transistors for the detection of E7 protein of human papillomavirus in saliva. Anal. Bioanal. Chem. 2021, 413, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold Nanoparticle-Based Colorimetric Biosensors. Nanoscale 2017, 10, 18–33. [Google Scholar] [CrossRef]
- Xu, G.; Li, H.; Ma, X.; Jia, X.; Dong, J.; Qian, W. A cuttlebone-derived matrix substrate for hydrogen peroxide/glucose detection. Biosens. Bioelectron. 2009, 25, 362. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Teles, F.R.R.; Fonseca, L.P. Trends in DNA biosensors. Talanta 2008, 77, 606–623. [Google Scholar] [CrossRef]
- Yuan, D.; Fang, X.; Liu, Y.; Kong, J.; Chen, Q. A hybridization chain reaction coupled with gold nanoparticles for allergen gene detection in peanut, soybean and sesame DNAs. Analyst 2019, 144, 3886–3891. [Google Scholar] [CrossRef]
- Karakus, E.; Erdemir, E.; Demirbilek, N.; Liv, L. Colorimetric and electrochemical detection of SARS-CoV-2 spike antigen with a gold nanoparticle-based biosensor. Anal. Chim. Acta 2021, 1182, 338939. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Fu, D.; Zhao, D.; Wang, Y.; Yuan, Q.; Zhu, Y.; Yang, J.; Yang, F. Gold nanoparticles amplified microcantilever biosensor for detecting protein biomarkers with high sensitivity. Sens. Actuators A Phys. 2021, 321, 112563. [Google Scholar] [CrossRef]
- Walters, F.; Rozhko, S.; Buckley, D.; Ahmadi1, E.D.; Ali, M.; Tehrani, Z.; Mitchell, J.; Burwell, G.; Liu, Y.; Kazakova, O.; et al. Real-time detection of hepatitis B surface antigen using a hybrid graphene-gold nanoparticle biosensor. 2D Mater. 2020, 7, 024009. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Luo, X.; Shi, W.; Yu, H.; Xie, Y.; Li, K.; Cui, Y. Wearable carbon nanotube-based biosensors on gloves for lactate. Sensors 2018, 18, 3398. [Google Scholar] [CrossRef]
- Skaria, E.; Patel, B.A.; Flint, M.S.; Ng, K.W. Poly(lactic acid)/Carbon Nanotube Composite Microneedle Arraysfor Dermal Biosensing. Anal. Chem. 2019, 91, 4436–4443. [Google Scholar] [CrossRef]
- Farzin, M.A.; Abdoos, H. A critical review on quantum dots: From synthesis toward applications in electrochemical biosensors for determination of disease-related biomolecules. Talanta 2020, 224, 121828. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, P.; Liu, T.; Pu, H.; Sun, D.W. A fluorescence biosensor based on single-stranded DNA and carbonquantum dots for acrylamide detection. Food Chem. 2021, 356, 129668. [Google Scholar] [CrossRef]
- Kamaci, U.D.; Kamaci, M. Selective and sensitive ZnO quantum dots based fluorescent biosensor for detection of cysteine. J. Fluoresc. 2021, 31, 401–414. [Google Scholar] [CrossRef]
- Kalkal, A.; Pradhan, R.; Kadian, S.; Manik, G.; Packirisamy, G. Biofunctionalized graphene quantum dots based fluorescent biosensor towards efficient detection of small cell lung cancer. ACS Appl. Bio Mater. 2020, 3, 4922–4932. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, Y.; Shen, L.; Ma, H.; Sun, T.; Lv, F.; Liu, Y.; Liu, J.; Zhu, N. Oil-water self-assembly engineering of Prussian blue/quantum dots decorated graphene film for wearable textile biosensors and photoelectronic unit. Chem. Eng. J. 2022, 427, 131824. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, H.; Sun, X. Recent progress on cell-based biosensors for analysis of food safety and quality control. Biosens. Bioelectron. 2019, 126, 389–404. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.A.; Kumari, D.; Banerjee, R. Nanocomposite biosensors for point-of-care—Evaluation of food quality and safety. In Nanobiosensors; Academic Press: Cambridge, MA, USA, 2017; pp. 629–676. [Google Scholar]
- Fan, C.; Zhang, D.; Mo, Q.; Yuan, J. Engineering Saccharomyces cerevisiae-based biosensors for copper detection. Microb. Biotechnol. 2022, 15, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, E.L.; Coniglio, M.A.; Corso, D.; van der Meer, J.R.; Acerbi, F.; Gola, A.; Libertino, S. Biosensors in Monitoring Water Quality and Safety: An Example of a Miniaturizable Whole-Cell Based Sensor for Hg2+ Optical Detection in Water. Water 2019, 11, 1986. [Google Scholar] [CrossRef]
- Yildirim, O.; Derkus, B. Triazine-based 2D covalent organic frameworks improve the electrochemical performance of enzymatic biosensors. J. Mater. Sci. 2020, 55, 3034–3044. [Google Scholar] [CrossRef]
- Wang, L.; Xie, H.; Lin, Y.; Wang, M.; Sha, L.; Yu, X.; Yang, J.; Zhao, J.; Li, G. Covalent organic frameworks (COFs)-based biosensors for the assay of disease biomarkers with clinical applications. Biosens. Bioelectron. 2022, 217, 114668. [Google Scholar] [CrossRef]
- Singh, S.; Arshid, N.; Cinti, S. Electrochemical nano biosensors for the detection of extracellular vesicles exosomes: From the benchtop to everywhere? Biosens. Bioelectron. 2022, 216, 114635. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Amperometric galectin-3 immunosensor-based gold nanoparticle-functionalized graphitic carbon nitride nanosheets and core–shell Ti-MOF@ COFs composites. Nanoscale 2020, 12, 19824–19832. [Google Scholar] [CrossRef]
- Boyacıoğlu, H.; Yola, B.B.; Karaman, C.; Karaman, O.; Atar, N.; Yola, M.L. A novel electrochemical kidney injury molecule-1 (KIM-1) immunosensor based covalent organic frameworks-gold nanoparticles composite and porous NiCo2S4@ CeO2 microspheres: The monitoring of acute kidney injury. Appl. Surf. Sci. 2022, 578, 152093. [Google Scholar] [CrossRef]
- Rasheed, T.; Rizwan, K. Metal-organic frameworks based hybrid nanocomposites as state-of–the-art analytical tools for electrochemical sensing applications. Biosens. Bioelectron. 2022, 199, 113867. [Google Scholar] [CrossRef]
- Carrasco, S. Metal-organic frameworks for the development of biosensors: A current overview. Biosensors 2018, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Nangare, S.N.; Sangale, P.M.; Patil, A.G.; Boddu, S.H.; Deshmukh, P.K.; Jadhav, N.R.; Tade, R.S.; Patil, D.R.; Pandey, A.; Mutalik, S.; et al. Surface architectured metal organic frameworks-based biosensor for ultrasensitive detection of uric acid: Recent advancement and future perspectives. Microchem. J. 2021, 169, 106567. [Google Scholar] [CrossRef]
- Osman, D.I.; El-Sheikh, S.M.; Sheta, S.M.; Ali, O.I.; Salem, A.M.; Shousha, W.G.; El-Khamisy, S.F.; Shawky, S.M. Nucleic acids biosensors based on metal-organic framework (MOF): Paving the way to clinical laboratory diagnosis. Biosens. Bioelectron. 2019, 141, 111451. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Kim, K.H.; Deep, A. MOF–bacteriophage biosensor for highly sensitive and specific detection of staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 33589–33598. [Google Scholar] [CrossRef] [PubMed]
- Augustine, S.; Kumar, P.; Malhotra, B.D. Amine-Functionalized MoO3@RGO Nanohybrid-Based Biosensor for Breast Cancer Detection. ACS Appl. Bio Mater. 2019, 2, 5366–5378. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M. A Comprehensive Review of Glucose Biosensors Based on Nanostructured Metal-Oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Tian, J.; Li, Y.; Dong, J.; Huang, M.; Lu, J. Photoelectrochemical TiO2 nanotube arrays biosensor for asulam determination based on in-situ generation of quantum dots. Biosens. Bioelectron. 2018, 110, 1–7. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahn, M.S.; Hahn, Y.B. ZnO nanorods array based field-effect transistor biosensor for phosphate detection. J. Colloid Interface Sci. 2017, 498, 292–297. [Google Scholar] [CrossRef]
- Kailasa, S.; Rani, B.G.; Reddy, M.S.B.; Jayarambabu, N.; Munindra, P.; Sharma, S.; Rao, K.V. NiO nanoparticles-decorated conductive polyaniline nanosheets for amperometric glucose biosensor. Mater. Chem. Phys. 2020, 242, 122524. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Dolez, P. Nanomaterials definitions, classifications, and applications. In Nanoengineering, 1st ed.; Dolez, P., Ed.; Elsevier: Cambridge, MA, USA, 2015; pp. 3–40. [Google Scholar]
- Karim, R.A.; Reda, Y.; Fattah, A.A. Review—Nanostructured materials-based nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- Ondes, B.; Akpınar, F.; Uygun, M.; Muti, M.; Uygun, D.A. High stability potentiometric urea biosensor based on enzyme attached nanoparticles. Microchem. J. 2021, 160, 105667. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. Gold nanobipyramids integrated ultrasensitive optical and electrochemical biosensor for Aflatoxin B1 detection. Talanta 2021, 222, 121578. [Google Scholar] [CrossRef]
- Kaur, H.; Shorie, M. Nanomaterial based aptasensors for clinical and environmental diagnostic applications. Nanoscale Adv. 2019, 1, 2123–2138. [Google Scholar] [CrossRef] [PubMed]
- Berepiki, A.; Kent, R.; Machado, L.F.M.; Dixon, N. Development of high-performance whole cell biosensors aided by statistical modeling. ACS Synth. Biol. 2020, 9, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M. Potentiometric biosensors: Concept and analytical applications—An editorial. Biochem. Anal. Biochem. 2016, 5, 19–20. [Google Scholar] [CrossRef]
- Vasuki, S.; Varsha, V.; Mithra, R.; Dharshni, R.A.; Abinaya, S.; Dharshini, R.D.; Sivarajasekar, N. Thermal biosensors and their applications. Am. Int. J. Res. Sci. Technol. Eng. Math. 2019, 1, 262–264. [Google Scholar]
- Chalklen, T.; Jing, Q.; Kar-Narayan, S. Biosensors based on mechanical and electrical detection techniques. Sensors 2020, 20, 5605. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, S.; Zhao, M.; Zhang, P.; Xin, Z.; Tao, J.; Bai, L. Amperometric DNA biosensor for Mycobacterium tuberculosis detection using flower-like carbon nanotubes-polyaniline nanohybrid and enzyme-assisted signal amplification strategy. Biosens. Bioelectron. 2018, 119, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Lee, S.S. Sensitive detection of microRNA using QCM biosensors: Sandwich hybridization and signal amplification by TiO2 nanoparticles. Anal. Methods 2020, 12, 5103–5109. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Kamil, Y.M.; Ebtisyam, W.M.; Daniyal, M.M.; Sadrohosseini, A.R.; Mahdi, M.A. Sensitive detection of dengue virus type 2 E-proteins signals using self-assembled monolayers/reduced graphene oxide-PAMAM dendrimer thin film-SPR optical sensor. Sci. Rep. 2020, 10, 2374. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Liu, T.; Pang, J.; Chu, Z.; Jin, W. Facile preparation of porous Co3O4nanocubes for directly screen-printing an ultrasensitive glutamate biosensor microchip. Sens. Actuators B Chem. 2020, 306, 127587. [Google Scholar] [CrossRef]
- Perdomo, S.A.; Tejada, J.S.M.; Botero, A.J. Review—Bio-Nanosensors: Fundamentals and Recent Applications. J. Electrochem. Soc. 2021, 168, 107506. [Google Scholar] [CrossRef]
- Shin, J.; Yan, Y.; Bai, W.; Xue, Y.; Gamble, P.; Tian, L.; Kandela, I.; Haney, C.R.; Spees, W.; Lee, Y.; et al. Bioresorbable pressure sensors protected with thermally grown silicon dioxide for the monitoring of chronic diseases and healing processes. Nat. Biomed. Eng. 2019, 3, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Teo, S.; Liu, J. Liquid-metal-painted stretchable capacitor sensors for wearable healthcare electronics. J. Med. Biolog. Eng. 2016, 36, 265–272. [Google Scholar] [CrossRef]
- Pandey, G.; Chaudhari, R.; Joshi, B.; Choudhary, S.; Kaur, J.; Joshi, A. Fluorescent biocompatible platinum-porphyrin-doped polymeric hybrid particles for oxygen and glucose biosensing. Sci. Rep. 2019, 22, 5029. [Google Scholar] [CrossRef]
- Hasan, M.R.; Ahommed, M.S.; .Daizy, M.; Bacchuad, M.S.; Aliad, M.R.; Al-Mamunad, M.R.; Aly Saad Aly, M.; Khanad, M.Z.H.; Hossain, S.I. Recent development in electrochemical biosensors for cancer biomarkers detection. Biosens. Bioelectron. 2021, 8, 100075. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Tang, C.; Deng, Y.; Huang, H.; He, Z. Recent advances in biosensor for detection of lung cancer biomarkers. Biosens. Bioelectron. 2019, 141, 111416. [Google Scholar] [CrossRef]
- Mostufa, S.; Akib, T.B.A.; Rana, M.M.; Islam, M.R. Highly Sensitive TiO2/Au/graphene layer-based surface plasmon resonance biosensor for cancer detection. Biosensors 2022, 12, 603. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.G.; Lee, S.H.; Kim, W.; Bang, A.; Moon, S.W.; Song, J.; Shin, J.H.; Yu, J.S.; Cho, S. Label-Free surface-enhanced Raman spectroscopy biosensor for on-site breast cancer detection using human tears. ACS Appl. Mater. Interfaces 2020, 12, 7897–7904. [Google Scholar] [CrossRef]
- Mobed, A.; Dolatia, S.; Shakouri, S.K.; Eftekharsadat, B.; Izadseresht, B. Recent advances in biosensors for detection of osteoarthritis and rheumatoid arthritis biomarkers. Sens. Actuators A 2021, 331, 112975. [Google Scholar] [CrossRef]
- Hu, F.; Xu, J.; Chen, Y. Surface plasmon resonance imaging detection ofsub-femtomolar microRNA. Anal. Chem. 2017, 89, 10071–10077. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Colak, B.; Zhang, D.-W.; Gibbs, M.J.; Watkinson, M.; Becer, C.R.; Gautrot, J.E.; Krause, S. Peptide Cross-Linked Poly (Ethylene Glycol) Hydrogel Filmsas Biosensor Coatings for the Detection of Collagenase. Sensors 2019, 19, 1677. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Zuncheddu, D.; Bella, E.D.; Schwab, A.; Petta, D.; Rocchitta, G.; Generelli, S.; Kurth, F.; Parrilli, A.; Verrier, S.; Rau, J.V.; et al. Quality control methods in musculoskeletal tissue engineering: From imaging to biosensors. Bone Res. 2021, 9, 46. [Google Scholar] [CrossRef]
- Kieninger, J.; Tamari, Y.; Enderle, B.; Jobst, G.; Sandvik, J.A.; Pettersen, E.O.; Urban, G.A. Sensor access to the cellular microenvironment using the sensing cell culture flask. Biosensors 2018, 8, 44. [Google Scholar] [CrossRef]
- Ilinoiu, E.C.; Manea, F.; Serra, P.A.; Pode, R. Simultaneous/selective detection of dopamine and ascorbic acid at synthetic zeolite-modified/graphite-epoxy composite macro/quasi-microelectrodes. Sensors 2013, 13, 7296–7307. [Google Scholar] [CrossRef]
- Kumar, A.; Furtado, V.L.; Gonçalves, J.M.; Fernandes, R.B.; Netto, L.E.S.; Arakia, K.; Bertotti, M. Amperometric microsensor based on nanoporous gold for ascorbic acid detection in highly acidic biological extracts. Anal. Chim. Acta 2020, 1095, 61–70. [Google Scholar] [CrossRef]
- Bazzu, G.; Puggioni, G.G.M.; Dedola, S.; Calia, G.; Rocchitta, G.; Migheli, R.; Desole, M.S.; Lowry, J.P.; O’Neill, R.D.; Serra, P.A. Real-time monitoring of brain tissue oxygen using a miniaturized biotelemetric device implanted in freely moving rats. Anal. Chem. 2009, 81, 2235–2241. [Google Scholar] [CrossRef]
- Sanna, D.; Rocchitta, G.; Serra, M.; Abbondio, M.; Serra, P.A.; Migheli, R.; De Luca, L.; Garribba, E.; Porcheddu, A. Synthesis of nitric oxide donors derived from Piloty’s acid and study of their effects on dopamine secretion from PC12 cells. Pharmaceuticals 2017, 10, 74. [Google Scholar] [CrossRef]
- Holmes, D.; Gawad, S. The application of microfluidics in biology. Methods Mol. Biol. 2010, 583, 55–80. [Google Scholar] [PubMed]
- Huang, C.-C.; Kuo, Y.-H.; Chen, Y.-S.; Huang, P.-C.; Lee, G.B. A miniaturized, DNA-FET biosensor-based microfluidic systemfor quantification of two breast cancer biomarkers. Microfluid. Nanofluid. 2021, 25, 33. [Google Scholar] [CrossRef]
- Funari, R.; Chu, K.-Y.; Shen, A.Q. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens. Bioelectron. 2020, 169, 112578. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yuan, H.; Zhou, C.; Mao, M.; Liu, Q.; Shen, H.; Cen, Y.; Qin, Z.; Ma, L.; Song Li, L. Multiplexed detection of influenza A virus subtype H5 and H9 via quantum dot-based immunoassay. Biosens. Bioelectron. 2016, 77, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Lee, H.-B.; Lee, N.-E. A durable, stretchable, and disposable electrochemical biosensor on three-dimensional micro-patterned stretchable substrate. Sens. Actuators B Chem. 2019, 283, 312–320. [Google Scholar] [CrossRef]
- Karbelkar, A.A.; Furst, A.L. Electrochemical diagnostics for bacterial infectious diseases. ACS Infect. Dis. 2020, 6, 1567–1571. [Google Scholar] [CrossRef]
- Cimafonte, M.; Fulgione, A.; Gaglione, R.; Papaianni, M.; Capparelli, R.; Arciello, A.; Bolletti Censi, S.; Borriello, G.; Velotta, R.; Della Ventura, B. Screen printed based impedimetric immunosensor for rapid detection of escherichia coli in drinking water. Sensors 2020, 20, 274. [Google Scholar] [CrossRef]
- Marin, M.J.; Rashid, A.; Rejzek, M.; Fairhurst, S.A.; Wharton, S.A.; Martin, S.R.; McCauley, J.W.; Wileman, T.; Field, R.A.; Russell, D.A. Glyconanoparticles for the plasmonic detection and discrimination between human and avian influenza virus. Org. Biomol. Chem. 2013, 11, 7101–7107. [Google Scholar] [CrossRef] [PubMed]
- Brangel, P.; Sobarzo, A.; Parolo, C.; Miller, B.S.; Howes, P.D.; Gelkop, S.; Lutwama, J.J.; Dye, J.M.; McKendry, R.A.; Lobel, L.; et al. A Serological Point-of-Care Test for the Detection of IgG Antibodies against Ebola Virus in Human Survivors. ACS Nano 2018, 12, 63–73. [Google Scholar] [CrossRef]
- Attia, M.S.; Ali, K.; El-Kemary, M.; Darwish, W.M. Phthalocyanine-doped polystyrene fluorescent nanocomposite as a highly selective biosensor for quantitative determination of cancer antigen 125. Talanta 2019, 201, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Khan, M.A.; Tariq, M. Emerging applications of porphyrins and metalloporphyrins in biomedicine and diagnostic magnetic resonance imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, M.; Ma, L.; Cui, Z.; Liu, Z.; Yang, H.; Liu, Y. Carboxyl porphyrin as signal molecule for sensitive fluorescent detection of aflatoxin Bvia ARGET-ATRP. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 280, 121535. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jin, C.; Chen, M.; Zhu, X.; Fu, M.; Liu, Z.; Gao, L.; Liu, Q. Preparation of porphyrin modified Co9S8 nanocomposites and application for colorimetric biosensing of H2O2. J. Porphyr. Phthalocyanines 2018, 22, 935–943. [Google Scholar] [CrossRef]
- Welch, E.C.; Welch, E.C.; Powell, J.M.; Clevinger, T.B.; Fairman, A.E.; Shukla, A. Advances in Biosensors and Diagnostic Technologies Using Nanostructures and Nanomaterials. Adv. Funct. Mater. 2021, 31, 2104126. [Google Scholar] [CrossRef]
- Chakraborty, P.; Deka, N.; Patra, D.C.; Debnath, K. Salivary glucose sensing using highly sensitive and selective non-enzymatic porous NiO nanostructured electrodes. Surf. Interfaces 2021, 26, 101324. [Google Scholar] [CrossRef]
- Ahmad, R.; Khan, M.; Mishra, P.; Jahan, N.; Ahsan, M.A.; Ahmad, I.; Khan, M.R.; Watanabe, Y.R.; Syed, M.A.; Furukawa, H. Engineered hierarchical CuO nanoleaves based electrochemical nonenzymatic biosensor for glucose detection. J. Electrochem. Soc. 2021, 168, 017501. [Google Scholar] [CrossRef]
- Baek, S.H.; Roha, J.; Park, C.Y.; Kim, M.W.; Shi, R.; Kailasa, S.K.; Park, T.J. Cu-nanoflower decorated gold nanoparticles-graphene oxide nanofiber as electrochemical biosensor for glucose detection. Mater. Sci. Eng. C 2020, 107, 110273. [Google Scholar] [CrossRef]
- Rashtabri, S.; Dehghan, G.; Amini, M. An ultrasensitive label-free colorimetric biosensor for the detection of glucose based on glucose oxidase-like activity of nanolayered manganese-calcium oxide. Anal. Chim. Acta 2020, 1110, 98–108. [Google Scholar] [CrossRef]
- Irrera, A.; Leonardi, A.A.; Franco, C.D.; Faro, M.J.L.; Palazzo, G.; D’Andrea, C.; Manoli, K.; Franzò, G.; Musumeci, P.; Fazio, B.; et al. New generation of ultrasensitive label-free optical Si nanowire-based biosensors. ACS Photonics 2018, 5, 471–479. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Suryani, A.A.; Agustina, M.; Anggraeni, A. A gold nanoparticle–DNA bioconjugate–based electrochemical biosensor for detection of susscrofamtDNA in raw and processed meat. Food Anal. Methods 2019, 12, 2591–2600. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, C.; Chiu, L.Y.; Abbasi, K.; Tolbert, B.S.; Sauvé, G.; Yen, Y.; Liu, C.C. Application of bioconjugation chemistry on biosensor fabrication for detection of TAR-DNA binding Protein 43. Biosens. Bioelectron. 2018, 117, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Azmi, M.A.M.; Tehrani, Z.; Lewis, R.P.; Walker, K.A.; Jones, D.R.; Daniels, D.R.; Doak, S.H.; Guy, O.J. Highly sensitive covalently functionalised integrated silicon nanowire biosensor devices for detection of cancer risk biomarker. Biosens. Bioelectron. 2014, 52, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Cao, X.; Gao, N.; Jina, Q.; Sanjay, S.T.; Pabon, G.H.; Li, X.J. A low-cost nanomaterial-based electrochemical immunosensor on paper for high-sensitivity early detection of pancreatic cancer. Sens. Actuators B Chem. 2020, 305, 127516. [Google Scholar] [CrossRef]
- Yadav, V.; Roy, S.; Singh, P.; Khan, Z.; Jaiswal, A. 2D MoS2-based nanomaterials for therapeutic, bioimaging, and biosensing applications. Small 2018, 15, 1803706. [Google Scholar] [CrossRef] [PubMed]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, X.; Jin, D.; Wu, S.; Wang, H.; Yin, M.; Aldalbahi, A.; El-Newehy, M.; Mo, X.; Wu, J. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020, 108, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive review on wearable sweat-glucose sensors for continuous glucose monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Tîlmaciu, C.M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]

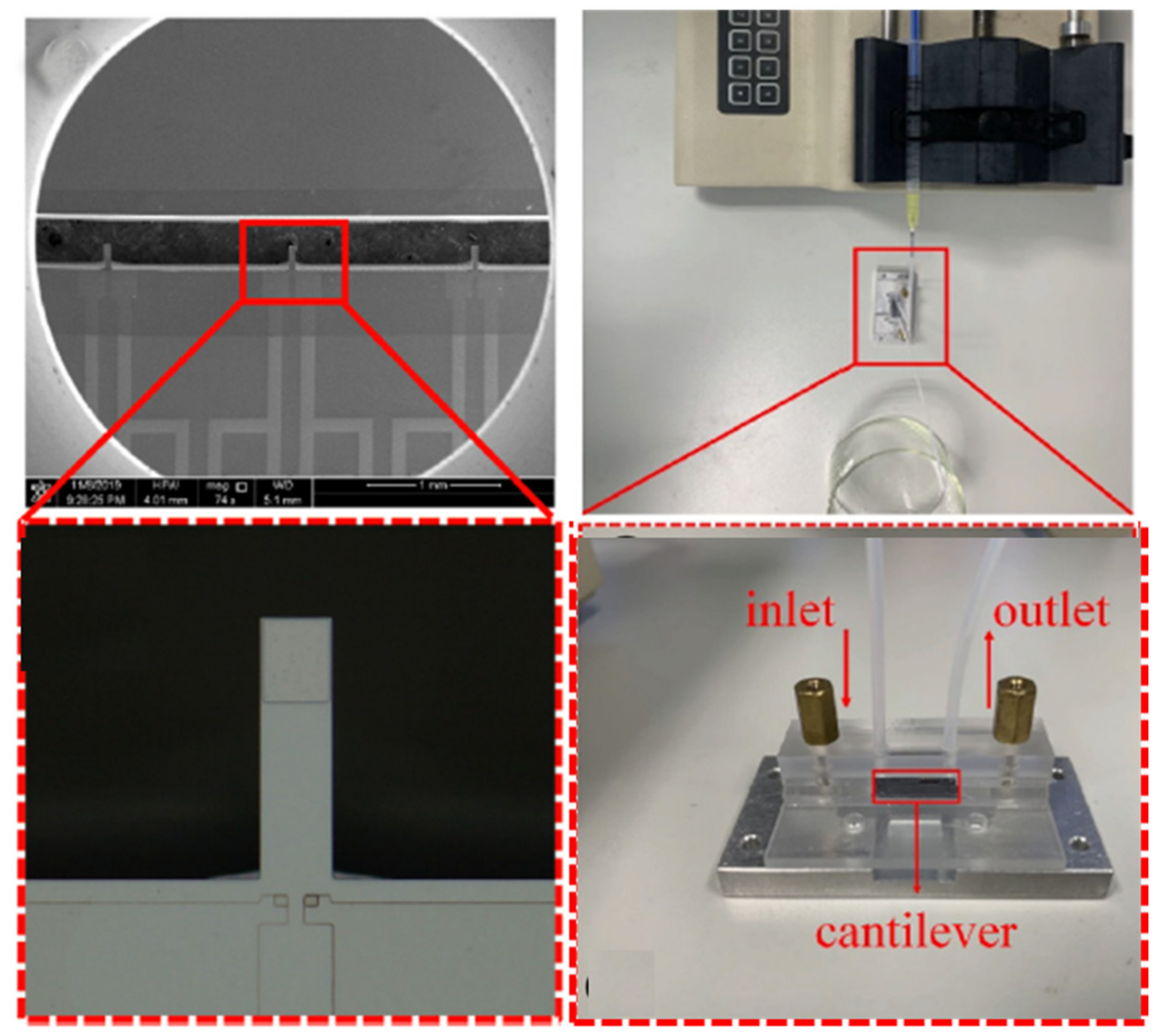

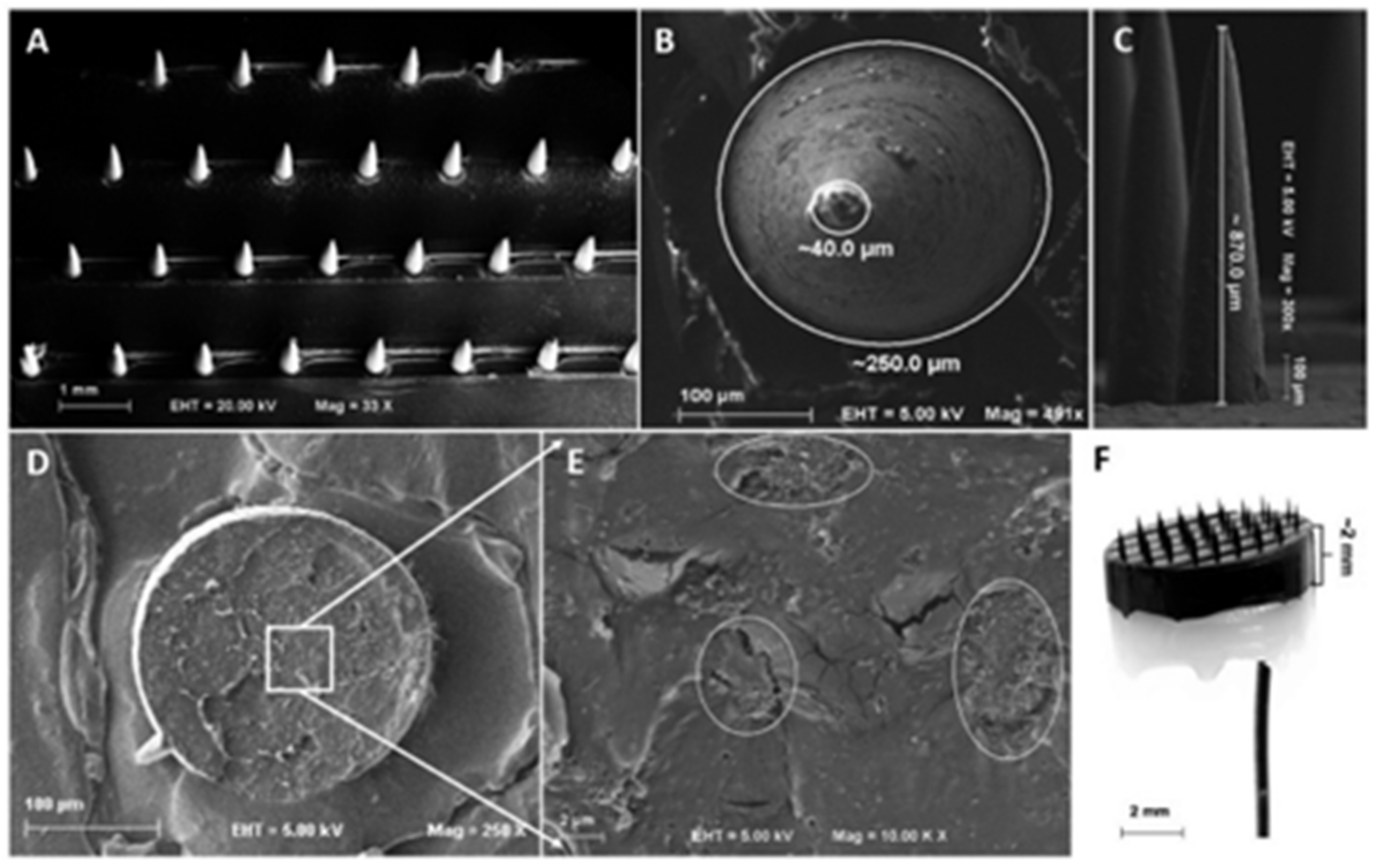
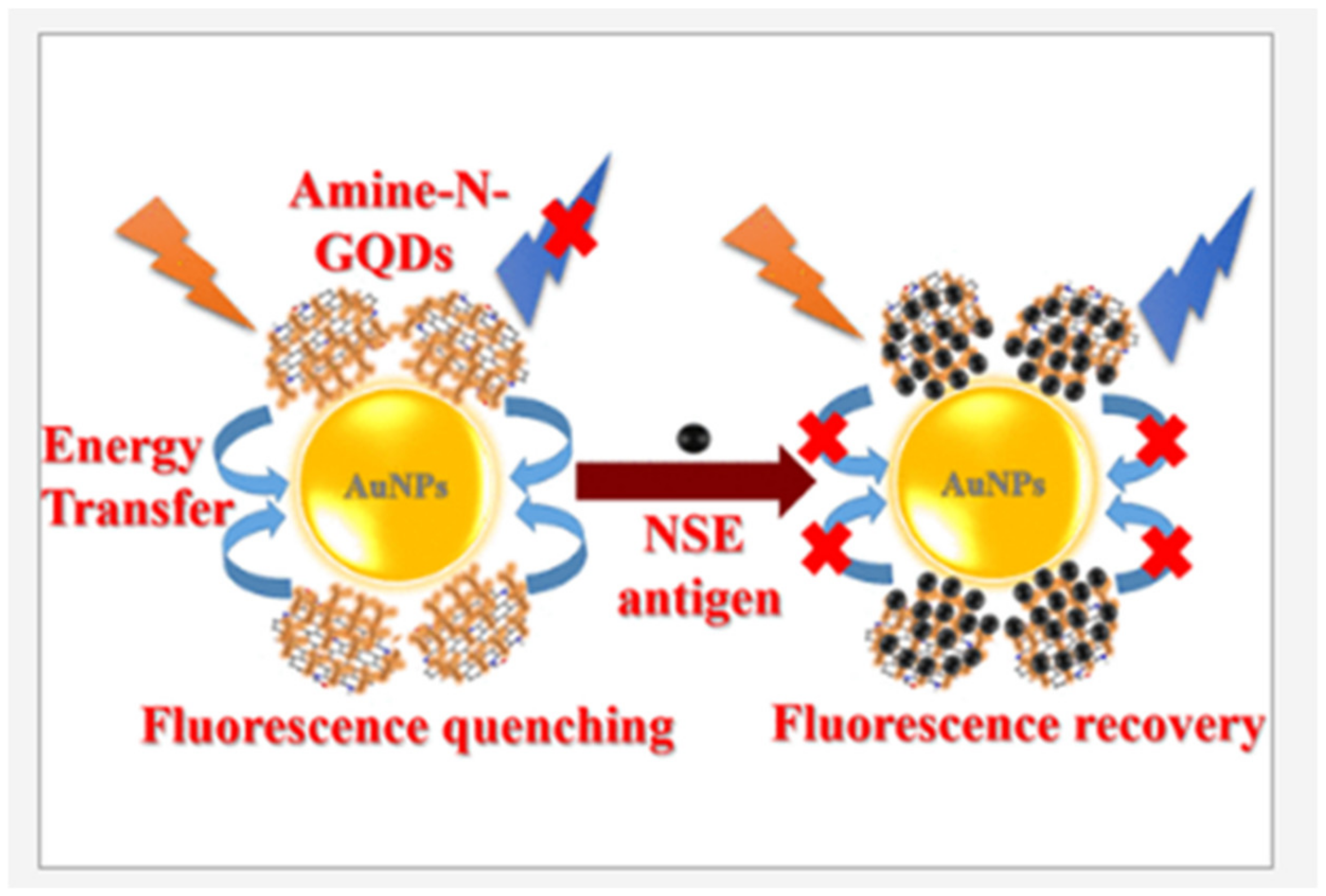
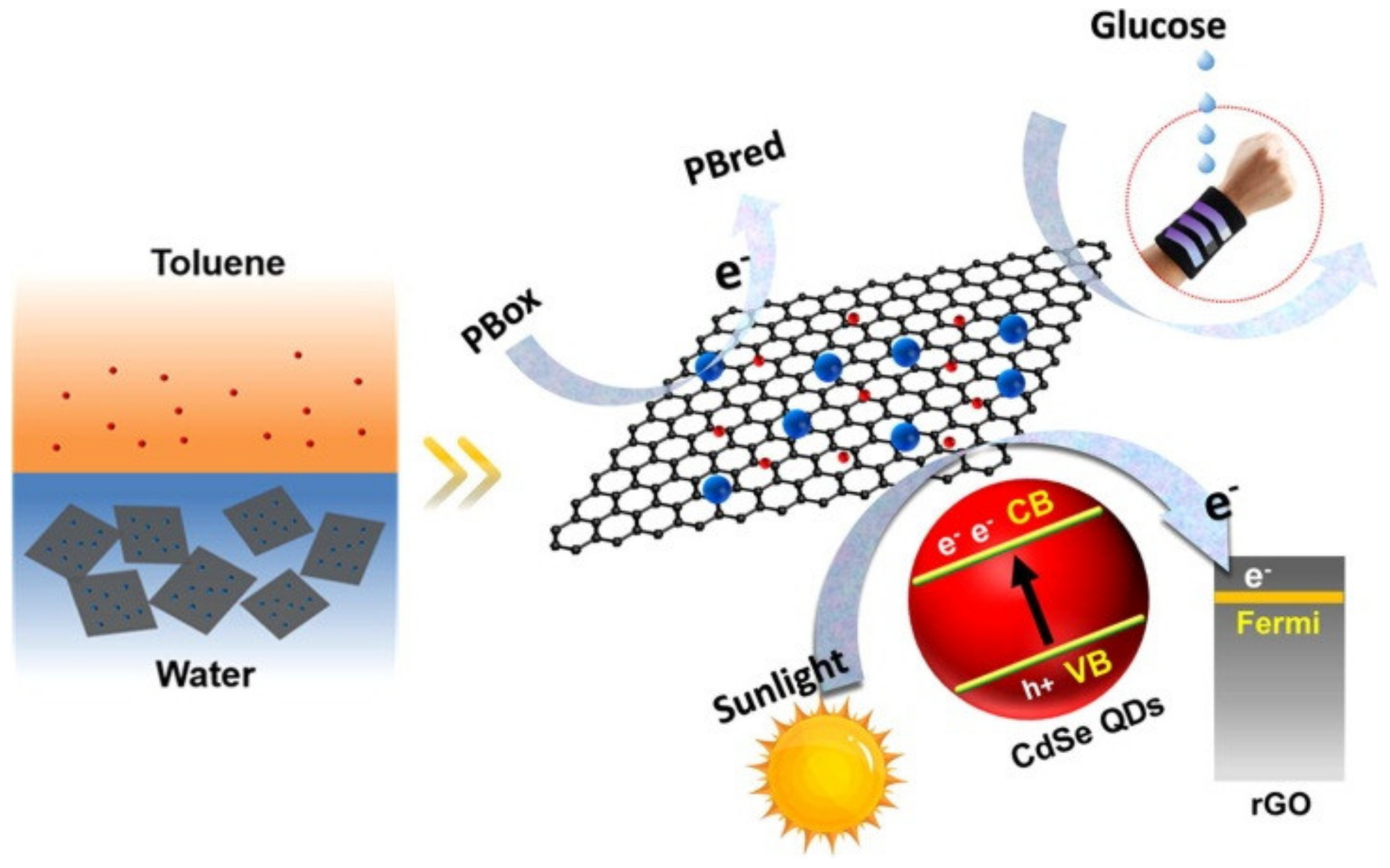
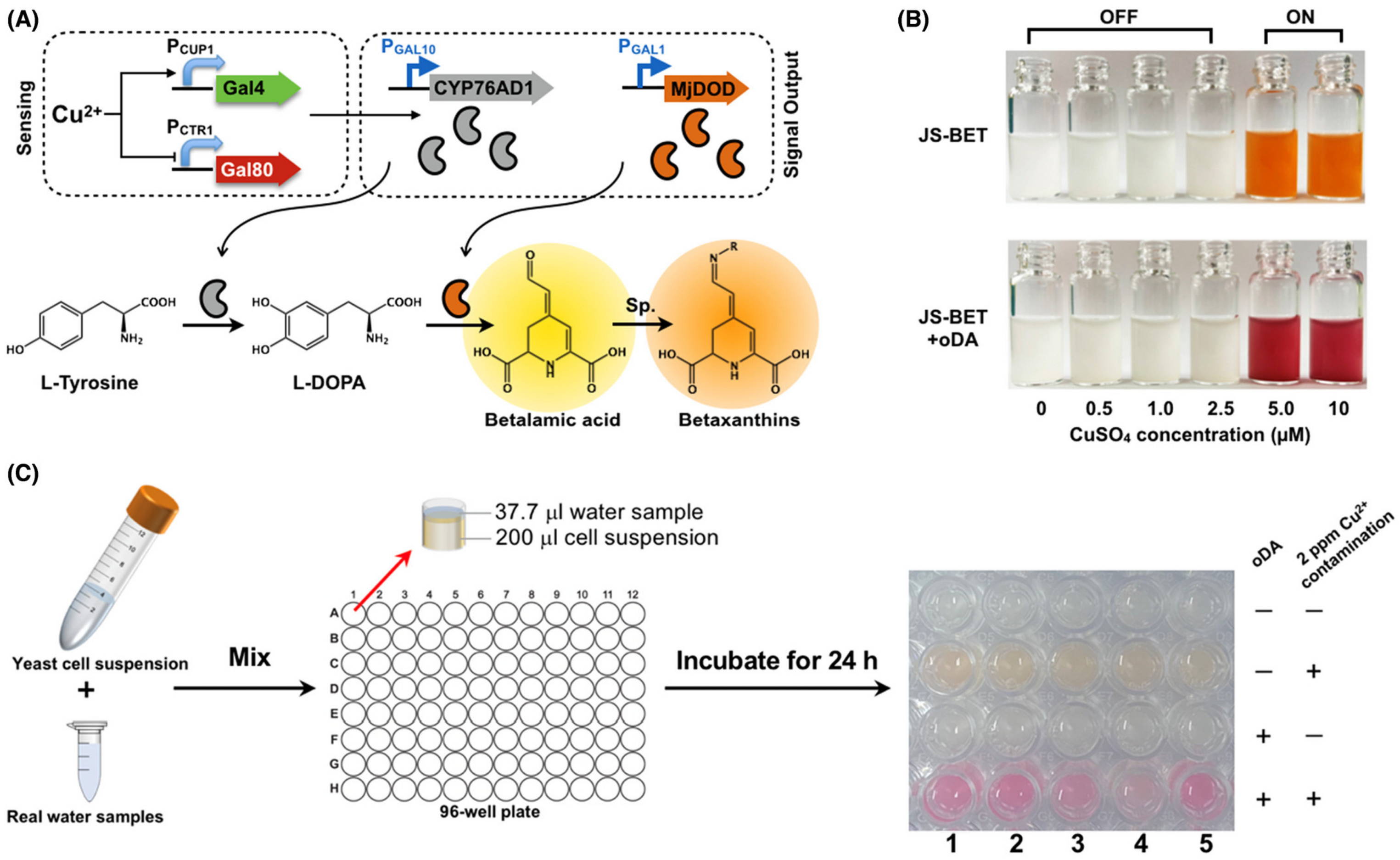
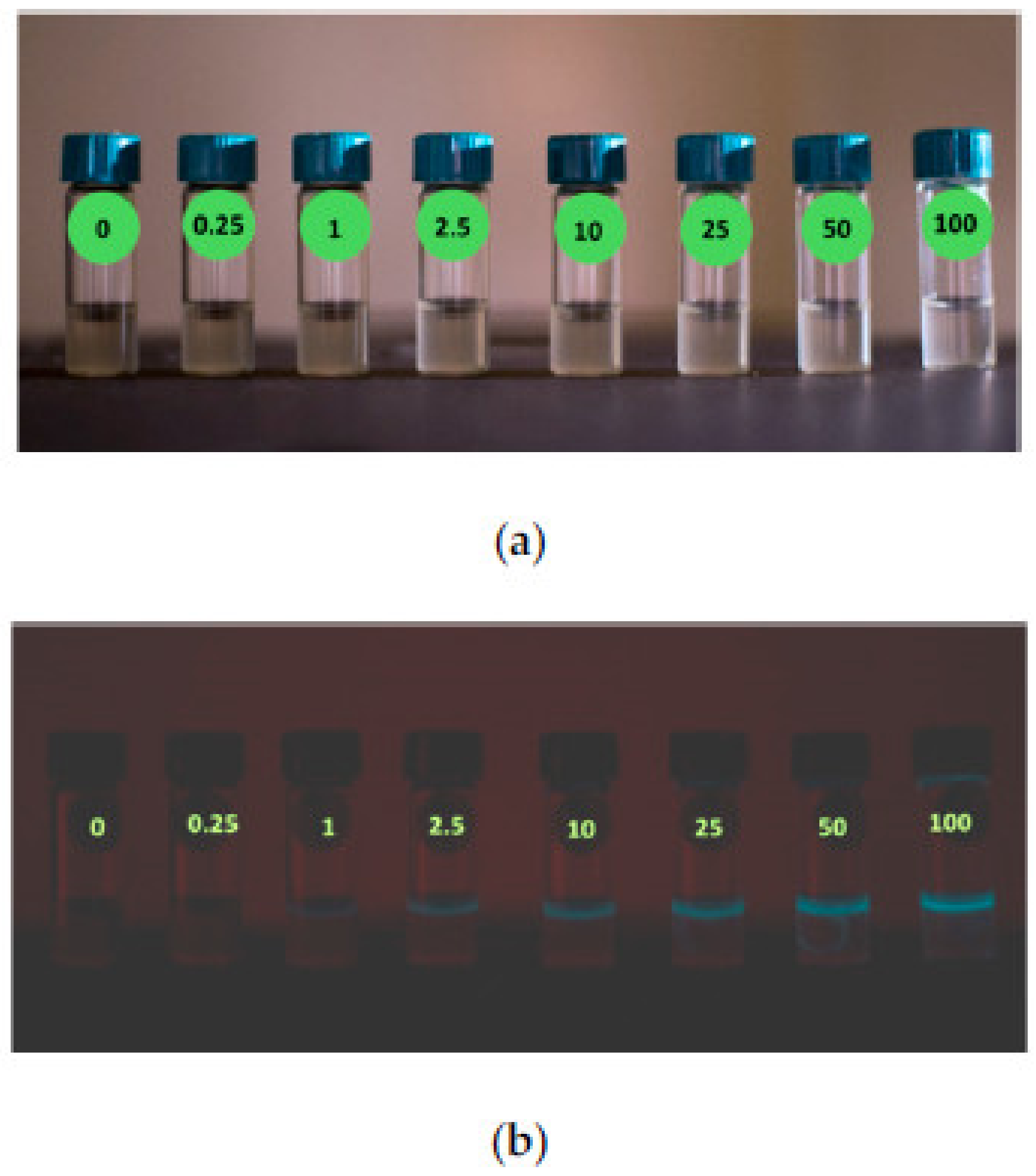

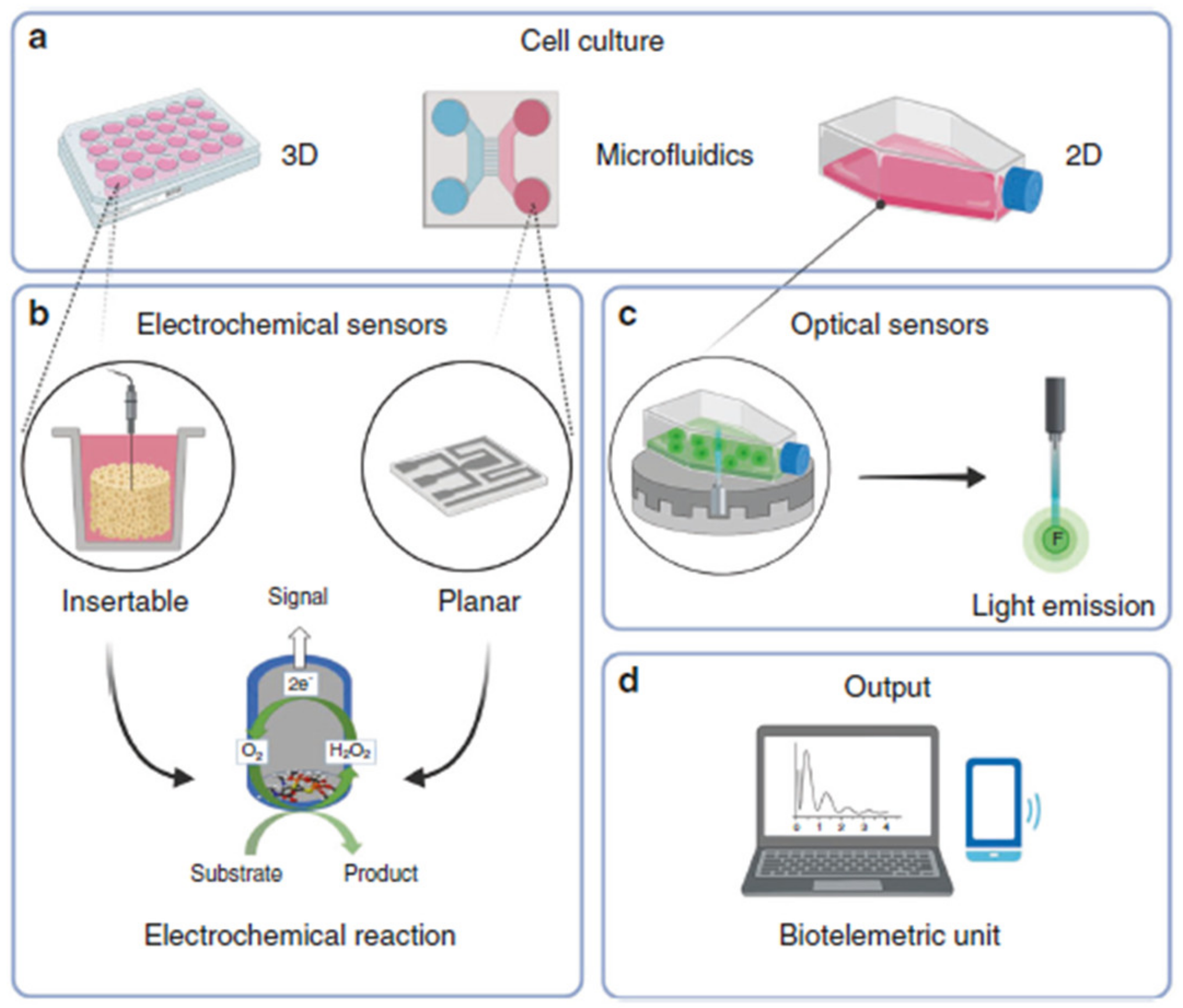
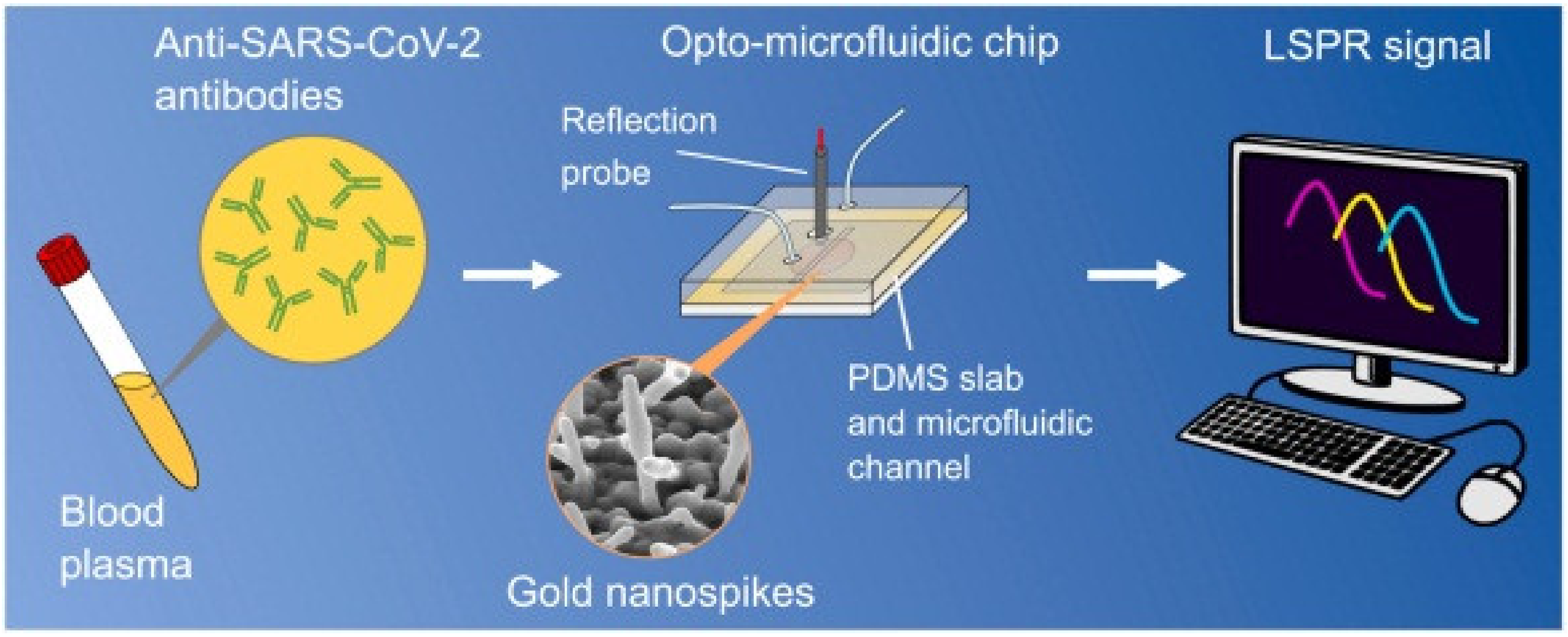
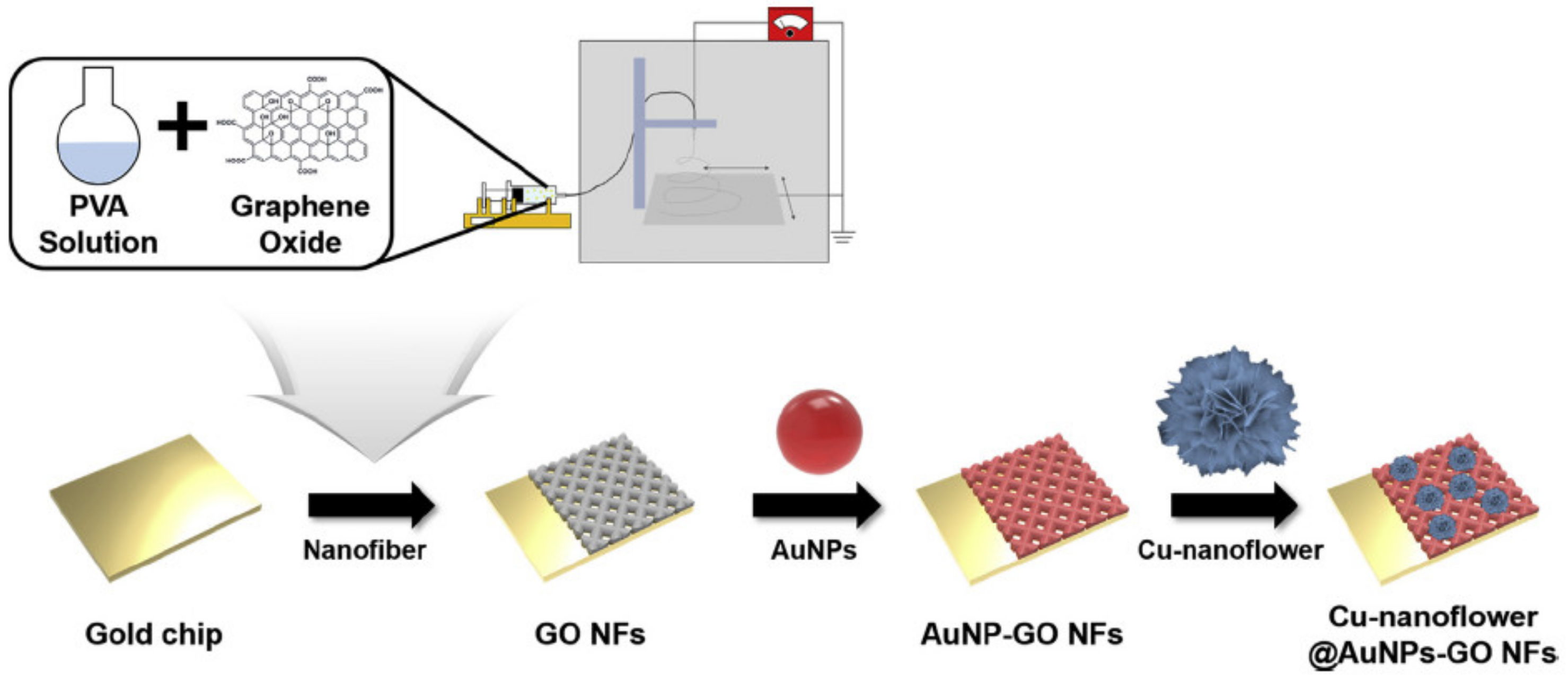
| Material | Target Analyte | Methodology | LOD | Detection Range | Ref. |
|---|---|---|---|---|---|
| Graphene | ZIKA NS1 | FEB technology | 0.45 nM | - | [74] |
| Graphene | Living cells | Refractive index changes | 1.2 × 108 mV/RIU | - | [81] |
| Graphene | SARS-COV 2 | FET biosensing | 1 fg/mL | - | [82] |
| Graphene | COVID 19 | Receptor binding domains | 0.2 pM | - | [83] |
| rGO | E7 protein of HPV | Electrolyte gated FET | 100 pg/mL | - | [84] |
| GNP | Protein biomarker | Micro-cantilever technology | 21 pg/mL | - | [91] |
| GNP | Gene allergic to peanut, sesame, and soybean | Colorimetric | 0.5 nm | - | [89] |
| GNP | SARS-COV2 | Dual sensing- Colorimetric Electrochemical | 48 ng/mL 1 pg/mL | - | [90] |
| Graphene-GNP | Hepatitis-B | π-π stacking of ssDNA | 50 pg/mL | - | [92] |
| CNT-PAN | Microbacterium Tuberculosis | Electrochemical biosensing | 0.33 fm | - | [95] |
| CNT | Lactase | Fabric-based wearable gloves | 1.4 nm | - | [96] |
| CNT/PLA | Thermal injuries | Microneedle array matrix | 180 µm | - | [97] |
| CQD | Acrylamide | Fluorescence | 2.4 × 10−8 M | - | [99] |
| ZnO QDs | Cysteine | Fluorescence | 0.642 µm | 0.1 µm–600 µm | [100] |
| GQD-GNP | Lung cancer | Fluorescence | 0.09 pg mL−1 | 0.11 pg mL−1 to 1005 ng mL−1 | [101] |
| PB-CdSe QD-rGO QD | Glucose and H2O2 | Wearable sensor | 37.26 µA/mM.cm2 (Glucose) 53.8 µA/mM.cm2 (H2O2) | - | [102] |
| Saccharomyces cerevisiae | Cu(II) | Colorimetric and Olfactory output | 0.322 ppm and 21.0 ± 1.48 µg/mL | - | [105] |
| Escherichia coli | Hg2+ | SiPM optical detector | 0.25 µg/L | 0.25 to 25 µg/L | [106] |
| Co3O4 | Glucose | Electrocatalytic | 10 µM | 10–600 µM | [117] |
| TiO2/CdS QDs | Asulam | Electrochemical | 4.1 pg/mL | 0.02–2.0 ng mL−1 | [119] |
| ZnOnanorods | Phosphate | FET biosensing | ~0.51 µM | 0.11 µM–7.2 mM | [120] |
| NiO@PANINS | Glucose | Amperometry | 0.06 µM | - | [121] |
| Nanomaterial | Applications | Target Analyte | Methodology | LOD | Detection Range | Ref. |
|---|---|---|---|---|---|---|
| Si nanowire | Cancer detection | 8-OHdG | Electrochemical | 1 ng/mL | - | [175] |
| GO | Cancer detection | PEAK1 | Electrochemical | 10 pg/mL | - | [176] |
| TiO2/Au/graphene | Cancer detection | MCF7 | Surface Plasmon Resonance | 292.86 deg/RIU | - | [142] |
| Au/HCP PS | Cancer detection | 2-NAT | Surface enhanced Raman scattering | 100 fM | 10−8–10−3 M | [143] |
| SPRi-Gold chip | Rheumatoid arthritis | miR-15a | Surface Plasmon Resonance | 0.56 fM | 5 fM–0.5 nM | [145] |
| Poly hydrogel film | Osteoarthritis | Proteases | Electrochemical | 2 nM | 2–2000 nM | [146] |
| Silver nanoparticle | Kidney tissue | MCF-7 | Aptamer conjugation | - | - | [177] |
| Graphene/gold | Liver metastasis | PHD-1 | Electrochemical | 4 μM | - | [178] |
| nMgO | Periodontal tissue regeneration | - | GTR | - | - | [179] |
| Zeolite | Neuro transmitter | Dopamine | Amperometry | - | - | [150] |
| Nanoporous gold | Fungus detection | Ascorbic acid | Electrochemical | 2 μmol L−1 | - | [151] |
| Carbon | Brain tissue | Oxygen | Biotelemetry | ≤5 M | - | [152] |
| - | Cancer detection | microRNA | microfluidic | 84 aM | 1 fM–1 nM | [153] |
| Gold nanospike | COVID-19 | Cov 2 spike | microfluidic | 0.5 pM | - | [154] |
| - | Influenza A | H 5 H 9 | microfluidic | 0.016 HAU (H5) 0.25 HAU (H9) | - | [155] |
| Ni-Phthalocyanine | Cancer detection | CA125 | Optical sensing | 1.0 × 10−4 U mL−1 | - | [163] |
| Co9S8-Porphyrin | H2O2 detection | - | Colorimetry | 6.803 μM | 7–100 μM | [166] |
| NiO | Glucose detection | Saliva | Electrochemical | ~84 nM | ~5 µM to 0.825 mM | [168] |
| CuO nanoleaves | Glucose detection | - | Electrochemical | 12 nM | 0.005–5.89 mM | [169] |
| Au/Cu/GO | Glucose detection | - | Electrocatalysis | 0.018 μM | 0.001–0.1 mM | [170] |
| MnCaO2 | Glucose detection | Human serum | Colorimetry | 6.12 × 10−6 M | (0–82.3) × 10−6 M | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2023, 13, 40. https://doi.org/10.3390/bios13010040
Ramesh M, Janani R, Deepa C, Rajeshkumar L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors. 2023; 13(1):40. https://doi.org/10.3390/bios13010040
Chicago/Turabian StyleRamesh, Manickam, Ravichandran Janani, Chinnaiyan Deepa, and Lakshminarasimhan Rajeshkumar. 2023. "Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications" Biosensors 13, no. 1: 40. https://doi.org/10.3390/bios13010040
APA StyleRamesh, M., Janani, R., Deepa, C., & Rajeshkumar, L. (2023). Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors, 13(1), 40. https://doi.org/10.3390/bios13010040








