Near-Field Communication Tag for Colorimetric Glutathione Determination with a Paper-Based Microfluidic Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments and Software
2.3. Preparation of µPAD
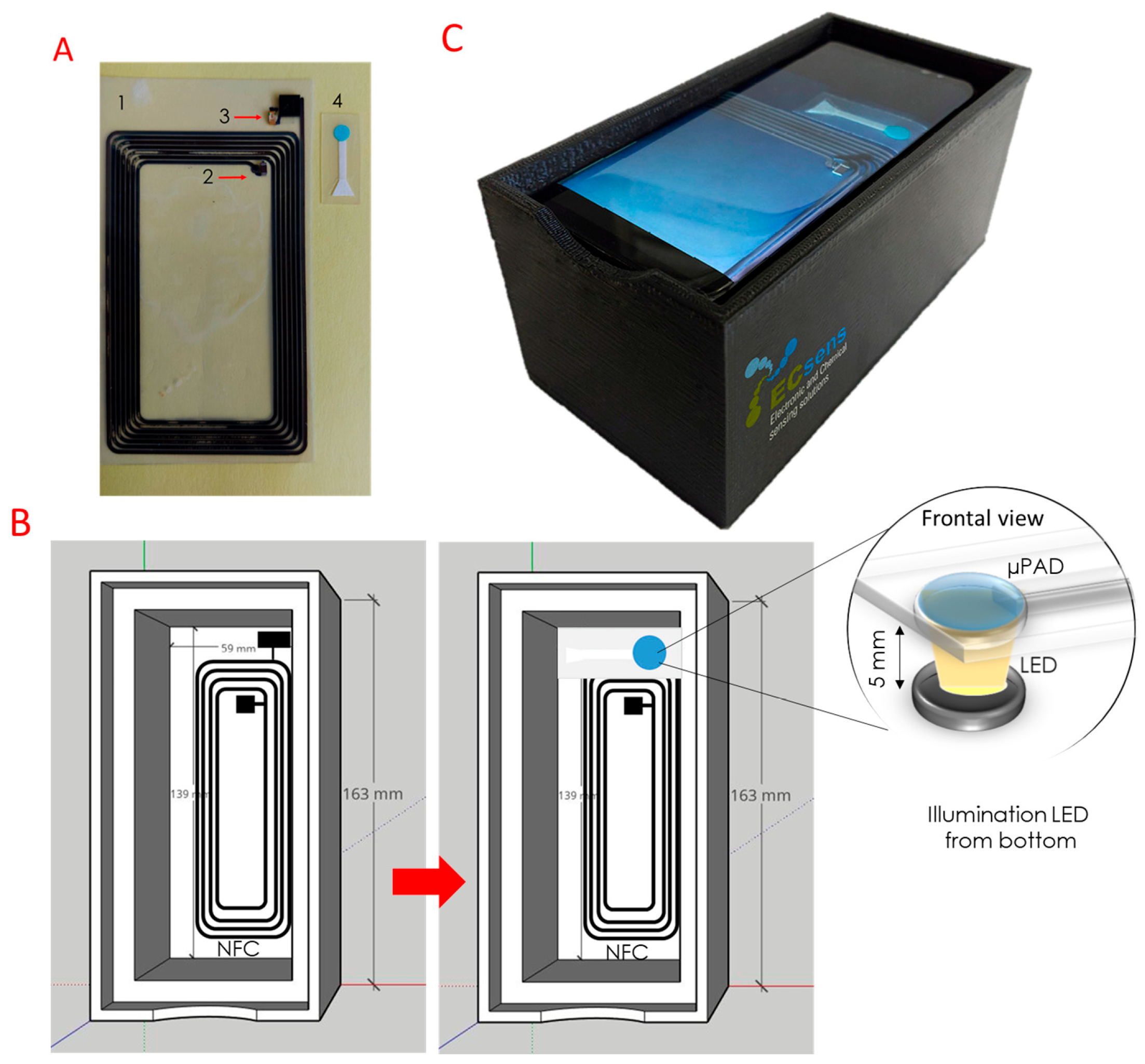
2.4. Tag Design and Fabrication
2.5. Measurement Protocol
2.6. Detection of Glutathione in Real Samples
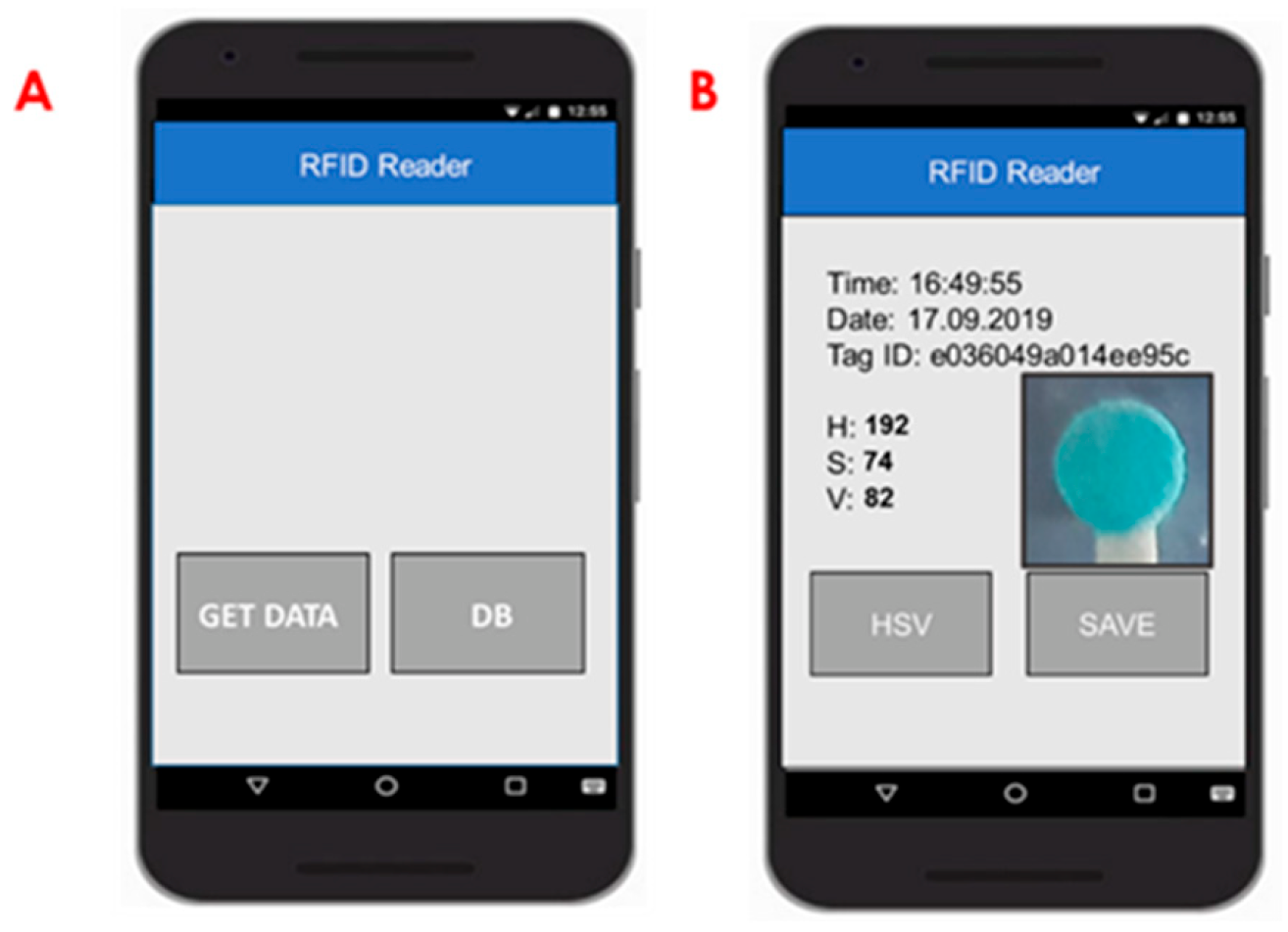
2.7. Mobile Application
3. Results
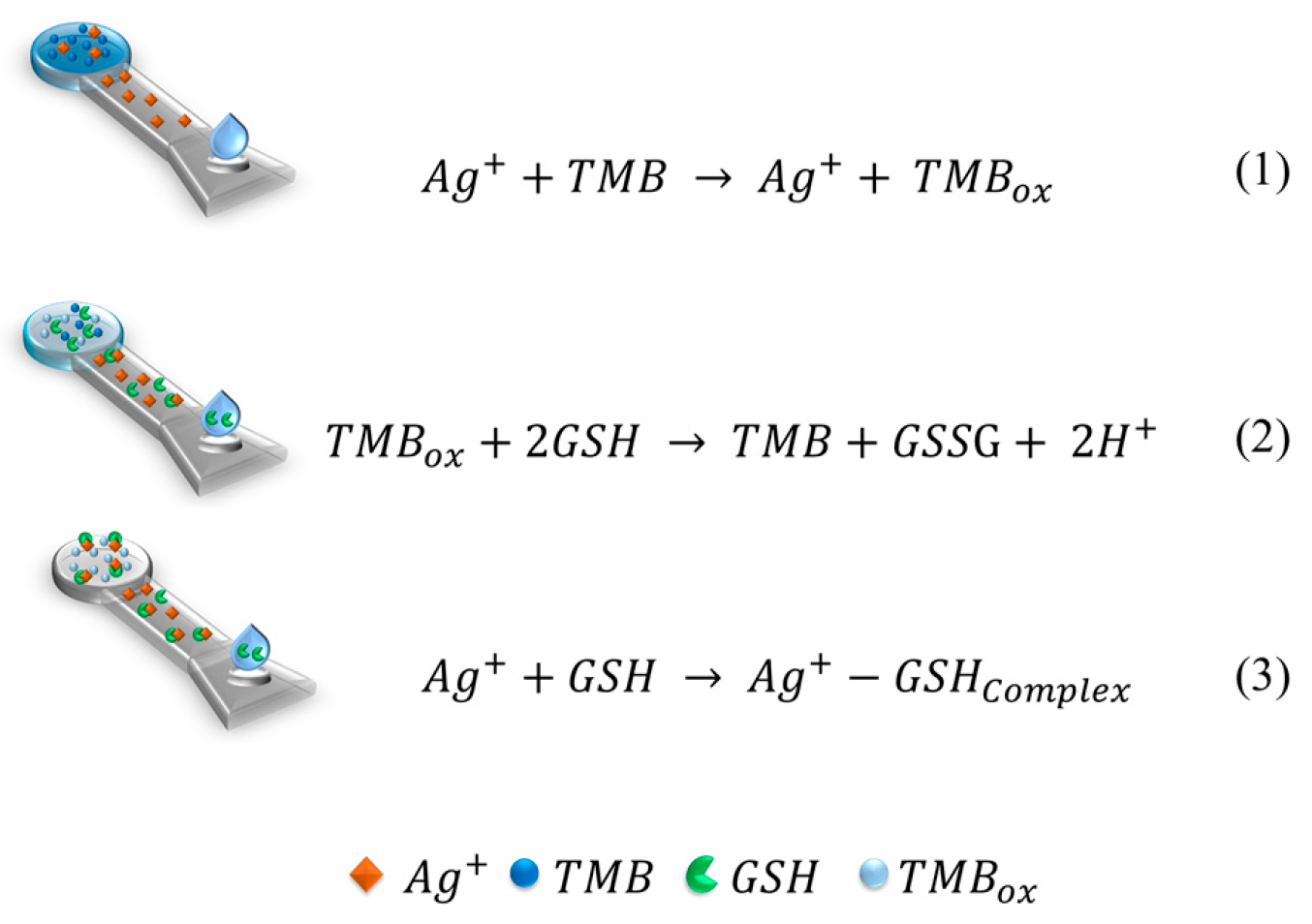
3.1. Mechanism of GSH Detection
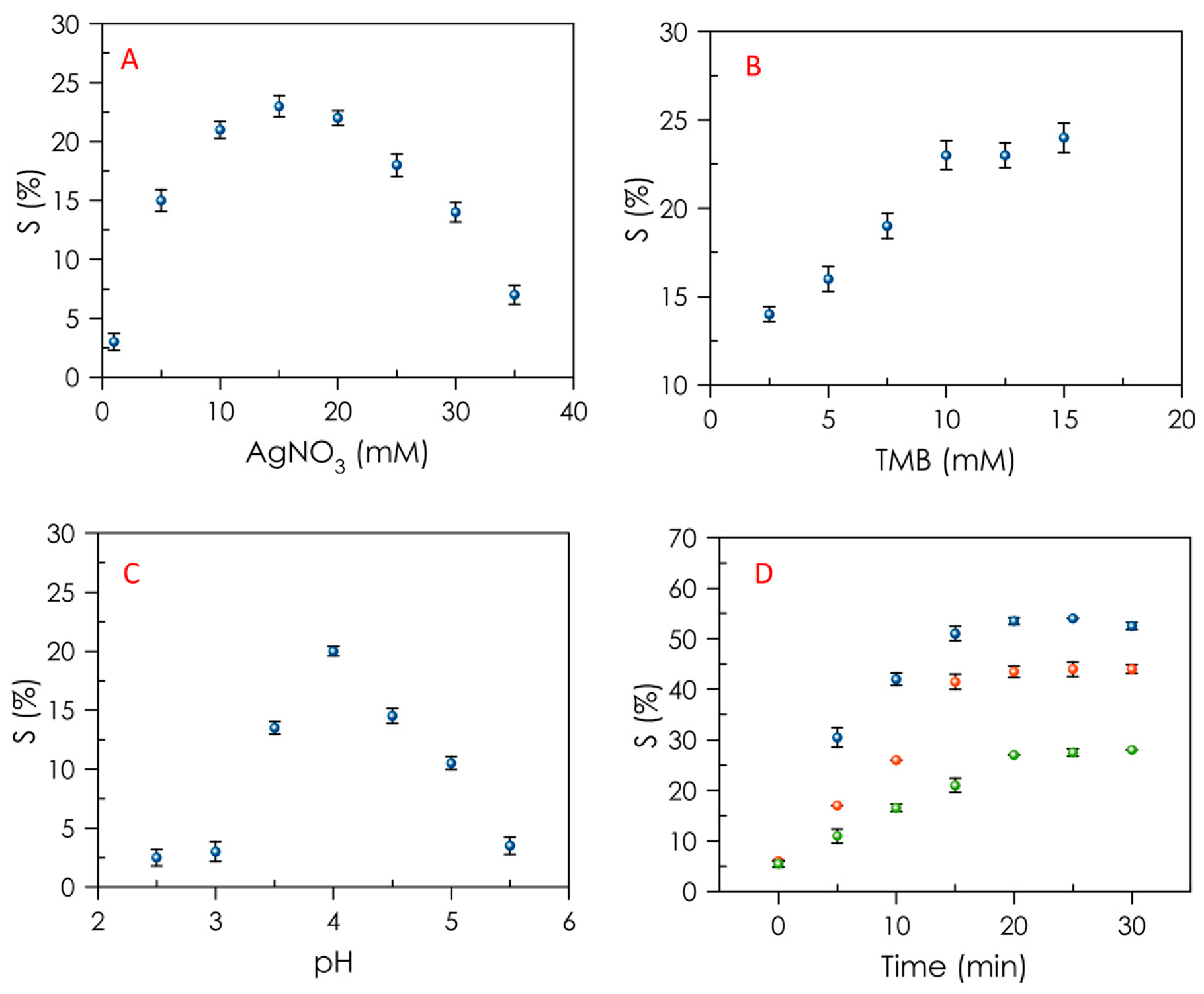
3.2. Optimization of GSH Determination Conditions
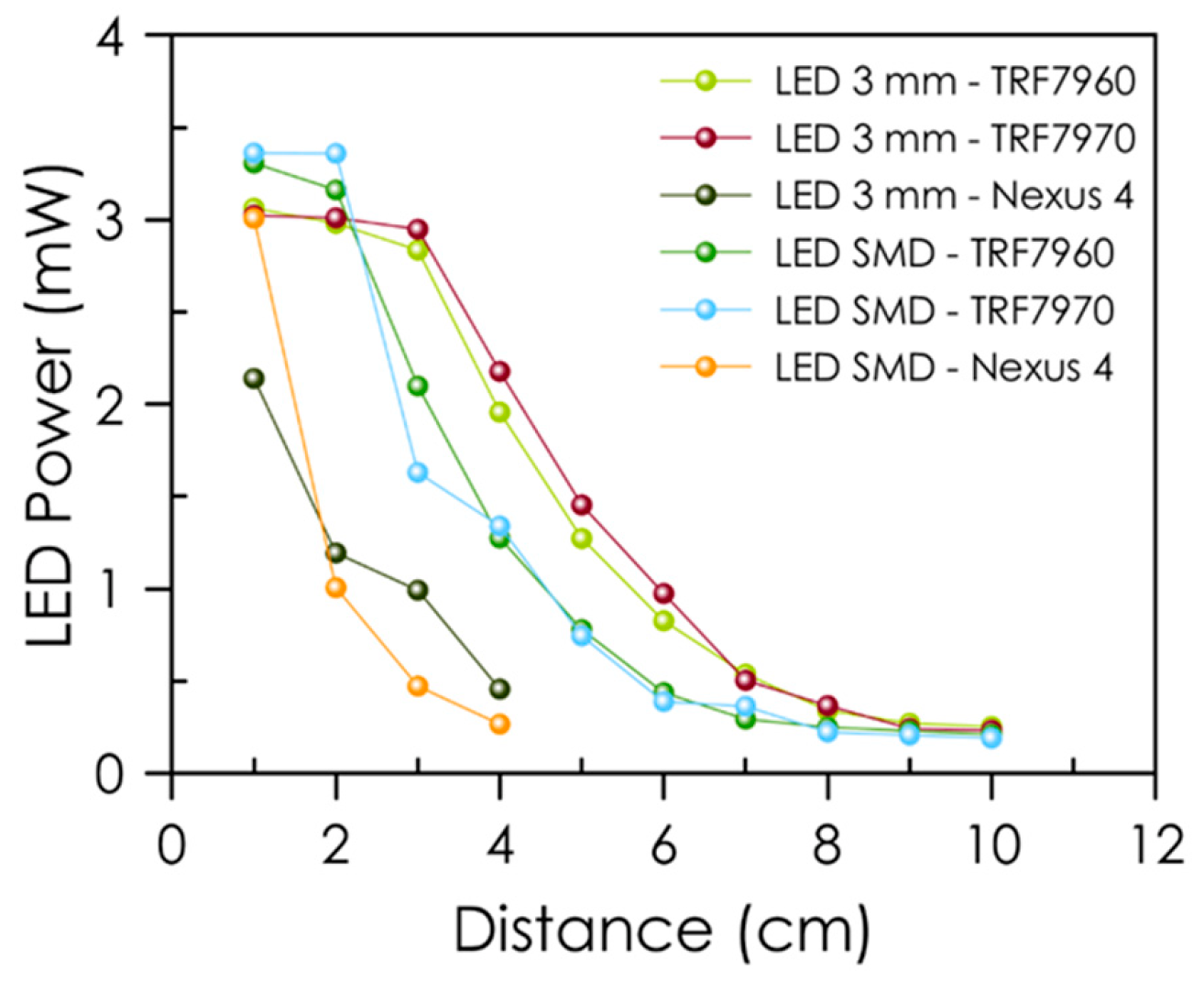
3.3. Characterization of the Tag Acquisition System
3.4. Analytical Characterization
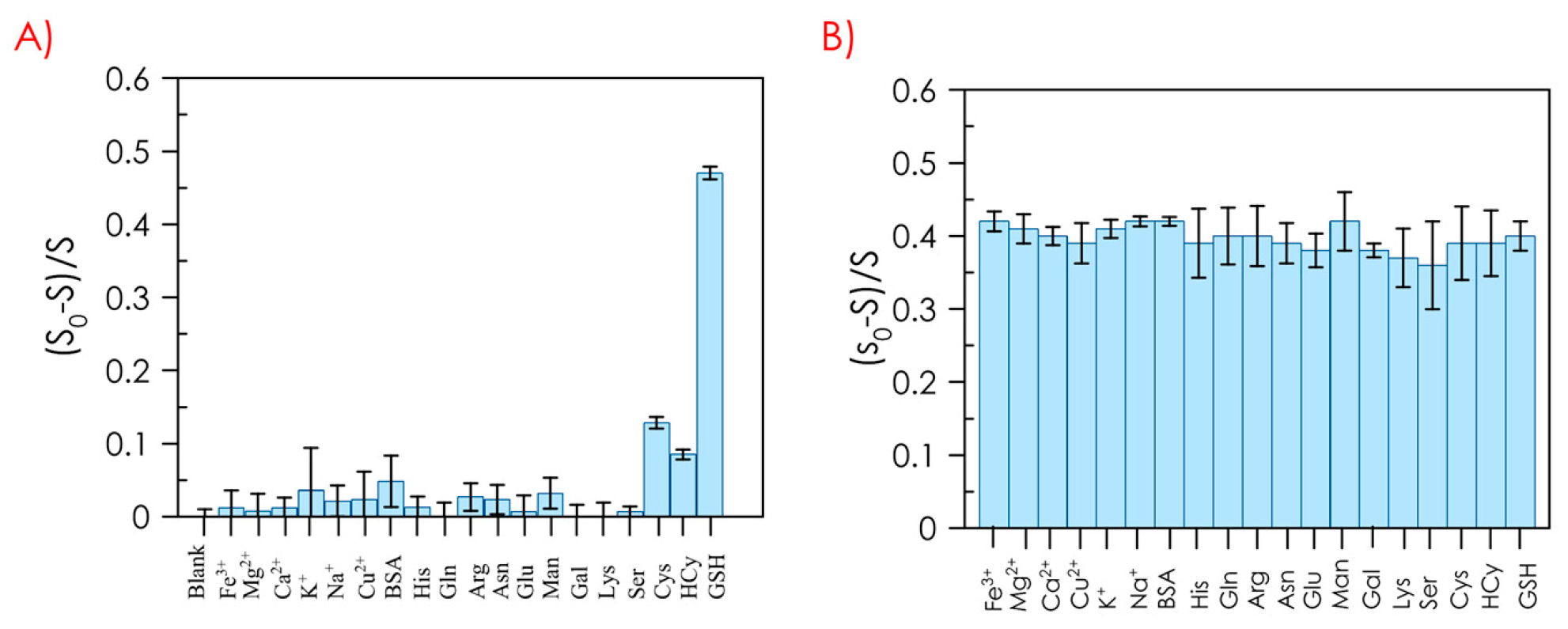
4. Interferences
5. Real Samples Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tseng, C.-C.; Kung, C.-T.; Chen, R.-F.; Tsai, M.-H.; Chao, H.-R.; Wang, Y.-N.; Fu, L.-M. Recent advances in microfluidic paper-based assay devices for diagnosis of human diseases using saliva, tears and sweat samples. Sens. Actuators B Chem. 2021, 342, 130078. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. A New Paper-Based Microfluidic Device for Improved Detection of Nitrate in Water. Sensors 2020, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- Sikora, T.; Morawska, K.; Lisowski, W.; Rytel, P.; Dylong, A. Application of Optical Methods for Determination of Concentration of Doxorubicin in Blood and Plasma. Pharmaceuticals 2022, 15, 112. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, Y.-J.; Ahn, Y.-J.; Kim, M.; Lee, G.-J. In Situ Detection of Hydrogen Sulfide in 3D-Cultured, Live Prostate Cancer Cells Using a Paper-Integrated Analytical Device. Chemosensors 2022, 10, 27. [Google Scholar] [CrossRef]
- Kudo, H.; Yamada, K.; Watanabe, D.; Suzuki, K.; Citterio, D. Paper-Based Analytical Device for Zinc Ion Quantification in Water Samples with Power-Free Analyte Concentration. Micromachines 2017, 8, 127. [Google Scholar] [CrossRef]
- Li, F.; You, M.; Li, S.; Hu, J.; Liu, C.; Gong, Y.; Yang, H.; Xu, F. Paper-based point-of-care immunoassays: Recent advances and emerging trends. Biotechnol. Adv. 2019, 39, 107442. [Google Scholar] [CrossRef]
- Yamada, K.; Shibata, H.; Suzuki, K.; Citterio, D. Toward practical application of paper-based microfluidics for medical diagnostics: State-of-the-art and challenges. Lab Chip 2017, 17, 1206–1249. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Tang, H.; Su, J.; He, M.; Chen, G.; Guan, L.; Tian, J. Portable paper sensors for the detection of heavy metals based on light transmission-improved quantification of colorimetric assays. Analyst 2019, 144, 6382–6390. [Google Scholar] [CrossRef]
- Gershenfeld, N.; Krikorian, R.; Cohen, D. The Internet of Things. Sci. Am. 2004, 291, 76–81. [Google Scholar] [CrossRef]
- Ward, M.; van Kranenburg, R. RFID: Frequency, Standards, Adoption and Innovation; JISC TechWatch: Liecester, UK, 2006; Available online: https://core.ac.uk/download/pdf/17299996.pdf (accessed on 1 May 2006).
- Landt, J. The history of RFID. IEEE Potentials 2005, 24, 8–11. [Google Scholar] [CrossRef]
- Domdouzis, K.; Kumar, B.; Anumba, C. Radio-Frequency Identification (RFID) applications: A brief introduction. Adv. Eng. Inform. 2007, 21, 350–355. [Google Scholar] [CrossRef]
- Peris-Lopez, P.; Hernandez-Castro, J.C.; Estevez-Tapiador, J.M.; Ribagorda, A. RFID Systems: A Survey on Security Threats and Proposed Solutions. In Personal Wireless Communications; Springer: Berlin/Heidelberg, Germany, 2006; pp. 159–170. [Google Scholar] [CrossRef]
- Kassal, P.; Steinberg, I.M.; Steinberg, M.D. Wireless smart tag with potentiometric input for ultra low-power chemical sensing. Sens. Actuators B Chem. 2013, 184, 254–259. [Google Scholar] [CrossRef]
- Cecil, S.; Bammer, M.; Schmid, G.; Lamedschwandner, K.; Oberleitner, A. Smart NFC-sensors for healthcare applications and further development trends. E I Elektrotechnik Inf. 2013, 130, 191–200. [Google Scholar] [CrossRef]
- Escobedo, P.; Erenas, M.M.; Martínez-Olmos, A.; Carvajal, M.A.; Gonzalez-Chocano, S.; Capitán-Vallvey, L.F.; Palma, A.J. General-purpose passive wireless point–of–care platform based on smartphone. Biosens. Bioelectron. 2019, 141, 111360. [Google Scholar] [CrossRef]
- Escobedo, P.; Bhattacharjee, M.; Nikbakhtnasrabadi, F.; Dahiya, R. Flexible Strain Sensor with NFC Tag for Food Packaging. In Proceedings of the 2020 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 16–19 August 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Escobedo, P.; Nikbakhtnasrabadi, F.; Dahiya, R. Printed Flexible Temperature Sensor with NFC Interface. In Proceedings of the 2020 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 16–19 August 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Lazaro, A.; Boada, M.; Villarino, R.; Girbau, D. Color Measurement and Analysis of Fruit with a Battery-Less NFC Sensor. Sensors 2019, 19, 1741. [Google Scholar] [CrossRef]
- Tavallali, H.; Deilamy-Rad, G.; Parhami, A.; Zebarjadi, R.; Najafi-Nejad, A.; Mosallanejad, N. A novel design of multiple ligands for ultrasensitive colorimetric chemosensor of glutathione in plasma sample. Anal. Biochem. 2021, 637, 114475. [Google Scholar] [CrossRef]
- Mitton, K.P.; Trevithick, J.R. [55] High-performance liquid chromatography-electrochemical detection of antioxidants in vertebrate lens: Glutathione, tocopherol, and ascorbate. Methods Enzymol. 1994, 233, 523–539. [Google Scholar] [CrossRef]
- Mika, A.; Skorkowski, E.; Stepnowski, P. The Use of Different MS Techniques to Determine Glutathione Levels in Marine Tissues. Food Anal. Methods 2012, 6, 789–802. [Google Scholar] [CrossRef]
- Alshatteri, A.H.; Omer, K.M. Dual-nanocluster of copper and silver as a ratiometric-based smartphone-assisted visual detection of biothiols. Microchem. J. 2023, 187, 108385. [Google Scholar] [CrossRef]
- Yuan, X.; Bai, F.; Ye, H.; Zhao, H.; Zhao, L.; Xiong, Z. Smartphone-assisted ratiometric fluorescence sensing platform and logical device based on polydopamine nanoparticles and carbonized polymer dots for visual and point-of-care testing of glutathione. Anal. Chim. Acta 2021, 1188, 339165. [Google Scholar] [CrossRef]
- Lai, Y.; Li, M.; Liao, X.; Zou, L. Smartphone-Assisted Colorimetric Detection of Glutathione and Glutathione Reductase Activity in Human Serum and Mouse Liver Using Hemin/G-Quadruplex DNAzyme. Molecules 2021, 26, 5016. [Google Scholar] [CrossRef] [PubMed]
- Shariati, S.; Khayatian, G. The colorimetric and microfluidic paper-based detection of cysteine and homocysteine using 1,5-diphenylcarbazide-capped silver nanoparticles. RSC Adv. 2021, 11, 3295–3303. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Wang, H.; Du, Y.; Yang, F.; Yang, L.; Jiang, C. Portable Smartphone Platform Integrated with a Nanoprobe-Based Fluorescent Paper Strip: Visual Monitoring of Glutathione in Human Serum for Health Prognosis. ACS Sustain. Chem. Eng. 2020, 8, 8175–8183. [Google Scholar] [CrossRef]
- Ni, P.; Sun, Y.; Dai, H.; Hu, J.; Jiang, S.; Wang, Y.; Li, Z. Highly sensitive and selective colorimetric detection of glutathione based on Ag [I] ion–3,3′,5,5′-tetramethylbenzidine (TMB). Biosens. Bioelectron. 2015, 63, 47–52. [Google Scholar] [CrossRef]
- Ortiz-Gomez, I.; Muñoz, M.O.; Marín-Sánchez, A.; de Orbe-Payá, I.; Hernandez-Mateo, F.; Capitan-Vallvey, L.F.; Santoyo-Gonzalez, F.; Salinas-Castillo, A. A vinyl sulfone clicked carbon dot-engineered microfluidic paper-based analytical device for fluorometric determination of biothiols. Microchim. Acta 2020, 187, 421. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Sun, X. Highly sensitive and selective colorimetric detection of Ag(I) ion using 3,3′,5,5′,-tetramethylbenzidine (TMB) as an indicator. Sens. Actuators B Chem. 2012, 165, 44–47. [Google Scholar] [CrossRef]
- Zhang, N.; Qu, F.; Luo, H.Q.; Li, N.B. Sensitive and selective detection of biothiols based on target-induced agglomeration of silvernanoclusters. Biosens. Bioelectron. 2013, 42, 214–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Chen, K.; Yang, Q.; Hu, N.; Suo, Y.; Wang, J. Highly sensitive and selective colorimetric detection of glutathione via enhanced Fenton-like reaction of magnetic metal organic framework. Sens. Actuators B Chem. 2018, 262, 95–101. [Google Scholar] [CrossRef]
- Lin, M.; Guo, Y.; Liang, Z.; Zhao, X.; Chen, J.; Wang, Y. Simple and fast determination of biothiols using Fe3+-3, 3′, 5, 5′-tetramethylbenzidine as a colorimetric probe. Microchem. J. 2019, 147, 319–323. [Google Scholar] [CrossRef]
- Cai, H.-H.; Wang, H.; Wang, J.; Wei, W.; Yang, P.-H.; Cai, J. Naked eye detection of glutathione in living cells using rhodamine B-functionalized gold nanoparticles coupled with FRET. Dye. Pigment. 2012, 92, 778–782. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhang, Y.; Zhang, L.; Su, Y.; Lv, Y. Colorimetric detection of glutathione in human blood serum based on the reduction of oxidized TMB. New J. Chem. 2013, 37, 2174–2178. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Z.; Ren, C.; Liu, G.; Chen, X. A novel colorimetric determination of reduced glutathione in A549 cells based on Fe3O4 magnetic nanoparticles as peroxidase mimetics. Analyst 2011, 137, 485–489. [Google Scholar] [CrossRef]
- Kappi, F.A.; Tsogas, G.Z.; Routsi, A.-M.; Christodouleas, D.C.; Giokas, D.L. Paper-based devices for biothiols sensing using the photochemical reduction of silver halides. Anal. Chim. Acta 2018, 1036, 89–96. [Google Scholar] [CrossRef]
- Markina, M.; Stozhko, N.; Krylov, V.; Vidrevich, M.; Brainina, K. Nanoparticle-based paper sensor for thiols evaluation in human skin. Talanta 2017, 165, 563–569. [Google Scholar] [CrossRef]
- Han, B.; Wang, E. Oligonucleotide-stabilized fluorescent silver nanoclusters for sensitive detection of biothiols in biological fluids. Biosens. Bioelectron. 2011, 26, 2585–2589. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Yang, J.; Hu, Y.; Yang, R.; Li, Z.; Qu, L.; Lin, Y. High performance fluorescence biosensing of cysteine in human serum with superior specificity based on carbon dots and cobalt-derived recognition. Sens. Actuators B Chem. 2018, 280, 62–68. [Google Scholar] [CrossRef]
- Khani, H.; Abbasi, S.; Yaraki, M.T.; Gholivand, M.B. Colorimetric detection and determination of glutathione based on superoxide radical-assisted etching approach. Microchem. J. 2021, 173, 107006. [Google Scholar] [CrossRef]
- Kapoor, A.; Rajput, J.K. Open chain conjugated azomethine derived optical biosensor for selective and ultrasensitive colorimetric detection of biomarker glutathione in human blood serum. Dye. Pigment. 2022, 203, 110336. [Google Scholar] [CrossRef]



| Analytical Parameters | Smartphone |
|---|---|
| Measurement range (µM) | 3.0–250.0 |
| Slope (µM) | 0.2 |
| Intercept (a.u.) | 74.9 |
| LOD (µM) | 1.0 |
| LOQ (µM) | 3.0 |
| Precision (%) 200 µM | 2.3 |
| Materials | Linear Range (µM) | LOD (µM) | Reaction Time (min) | Method | References |
|---|---|---|---|---|---|
| Fe+3-TMB | 2.0–24.0 | 1.04 | 10 | Solution | [33] |
| Rhodamine B-gold NPs | 12–1384 | 1.0 | 60 | Solution | [34] |
| Ag+-TMB | 0.05–8.0 | 0.05 | 10 | Solution | [30] |
| MnO2 NPs-TMB | 0.26–26 | 0.1 | 15 | Solution | [35] |
| Fe3O4-ABTS-H2O2 | 3.0–30 | Not given | 20 | Solution | [36] |
| Ag:AgCl | 10–100 | 10.0 | 5 | Paper | [37] |
| Au NPs | 8–75 | 6.9 | 40 | Paper | [38] |
| Ag+-TMB | 3.0–250.0 | 1.0 | 20 | Paper | This work |
| Concentration of GSH (µM) | |||
|---|---|---|---|
| Sample | Amount Added | Amount Found | Recovery (%) |
| Serum 1 | 50 | 54.9 | 100.9 |
| 200 | 209.1 | 104.5 | |
| 250 | 254.9 | 101.9 | |
| Serum 2 | 50 | 50.8 | 101.5 |
| 200 | 196.6 | 98.3 | |
| 250 | 254.9 | 101.9 | |
| Serum 3 | 50 | 51.2 | 102.4 |
| 200 | 197.4 | 98.7 | |
| 250 | 254.9 | 101.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Gómez, I.; Rivadeneyra, A.; Salmerón, J.F.; Orbe-Payá, I.d.; Morales, D.P.; Capitán-Vallvey, L.F.; Salinas-Castillo, A. Near-Field Communication Tag for Colorimetric Glutathione Determination with a Paper-Based Microfluidic Device. Biosensors 2023, 13, 267. https://doi.org/10.3390/bios13020267
Ortiz-Gómez I, Rivadeneyra A, Salmerón JF, Orbe-Payá Id, Morales DP, Capitán-Vallvey LF, Salinas-Castillo A. Near-Field Communication Tag for Colorimetric Glutathione Determination with a Paper-Based Microfluidic Device. Biosensors. 2023; 13(2):267. https://doi.org/10.3390/bios13020267
Chicago/Turabian StyleOrtiz-Gómez, Inmaculada, Almudena Rivadeneyra, José F. Salmerón, Ignacio de Orbe-Payá, Diego P. Morales, Luis Fermín Capitán-Vallvey, and Alfonso Salinas-Castillo. 2023. "Near-Field Communication Tag for Colorimetric Glutathione Determination with a Paper-Based Microfluidic Device" Biosensors 13, no. 2: 267. https://doi.org/10.3390/bios13020267
APA StyleOrtiz-Gómez, I., Rivadeneyra, A., Salmerón, J. F., Orbe-Payá, I. d., Morales, D. P., Capitán-Vallvey, L. F., & Salinas-Castillo, A. (2023). Near-Field Communication Tag for Colorimetric Glutathione Determination with a Paper-Based Microfluidic Device. Biosensors, 13(2), 267. https://doi.org/10.3390/bios13020267









