Photoluminescence Sensing of Soluble Lead in Children’s Crayons Using Perovskite Nanocrystal In Situ Growth on an Aluminum Hydroxide Layer
Abstract
:1. Introduction
2. Materials and Methods
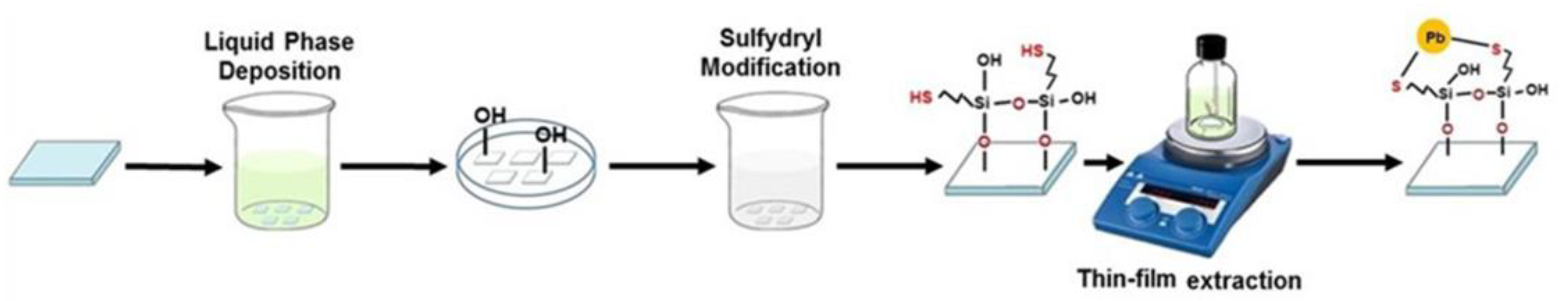
2.1. Preparation of Aluminum Hydroxide Thin Layer and Pb2+ Extraction
2.2. In Situ Growth of MAPbBr3 Perovskite on the Al(OH)3-SH Layer Extracted Pb2+
2.3. Sample Preparation
3. Results and Discussion
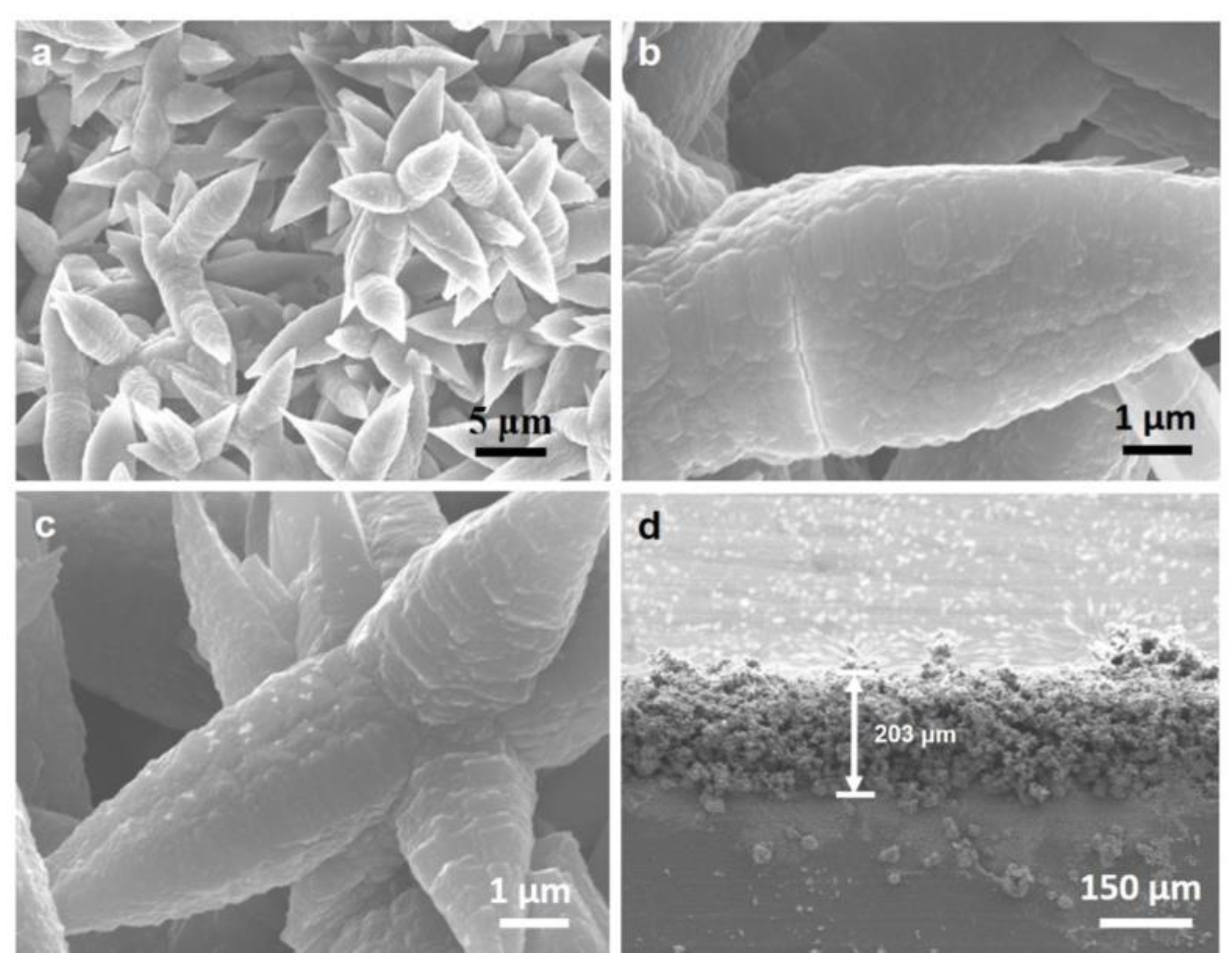
3.1. Surface Characterization of Al(OH)3 Layer
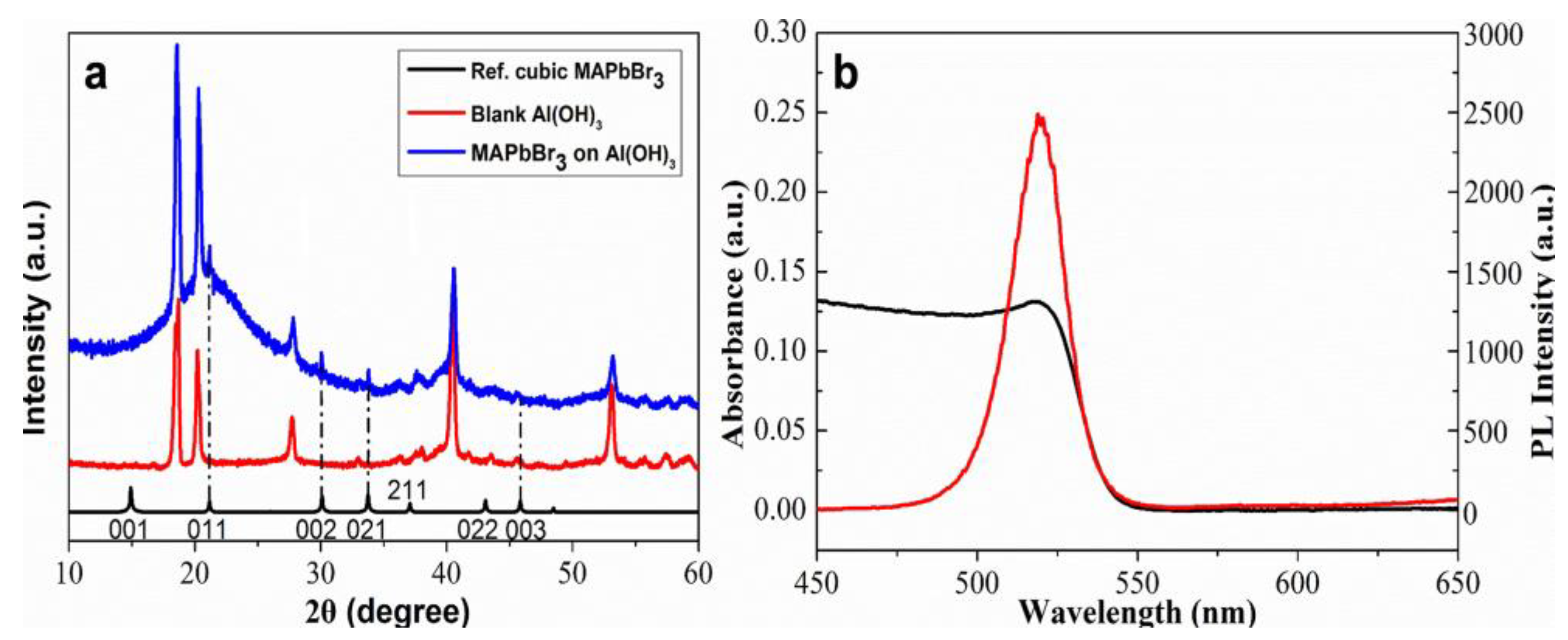
3.2. In Situ Growth of MAPbBr3 Perovskite on Al(OH)3 Layer
3.3. Fluorescence Sensing of Pb2+ Using Al(OH)3-SH
3.4. Stability and Selectivity Investigation for the Sensing Layer
3.5. Sensing Application for Pb2+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flegal, A.R.; Smith, D.R. Current needs for increased accuracy and precision in measurements of low levels of lead in blood. Environ. Res. 1992, 58, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Košak, A.; Bauman, M.; Padežnik-Gomilšek, J.; Lobnik, A. Lead (II) complexation with 3-mercaptopropyl-groups in the surface layer of silica nanoparticles: Sorption, kinetics and EXAFS/XANES study. J. Mol. Liq. 2017, 229, 371–379. [Google Scholar] [CrossRef]
- Muntean, E.; Nicoleta, M.; Creta, C.; Marcel, D.U.D.A. Occurrence of lead and cadmium in some baby foods and cereal products. ProEnvironment Promediu 2013, 6, 587–590. [Google Scholar]
- Zhang, C.; Wang, Y.; Cheng, X.; Xia, H.; Liang, P. Determination of Cadmium and Lead in Human Teeth Samples Using Dispersive Liquid-liquid Microextraction and Graphite Furnace Atomic Absorption Spectrometry. J. Chin. Chem. Soc. 2011, 58, 919–924. [Google Scholar] [CrossRef]
- Abadin, H.; Ashizawa, A.; Stevens, Y.; Llados, F.; Diamond, G.; Sage, G.; Citra, M.; Quinones, A.; Bosch, S.J.; Swarts, S.G. Toxicological Profıle for Lead; United States Agency for Toxic Substances and Disease Registry, Department of Health and Human Services: Atlanta, GA, USA, 2007. [Google Scholar]
- Coco, F.L.; Monotti, P.; Cozzi, F.; Adami, G. Determination of cadmium and lead in fruit juices by stripping chronopotentiometry and comparison of two sample pretreatment procedures. Food Control. 2006, 17, 966–970. [Google Scholar] [CrossRef]
- Lidsky, T.I.; Schneider, J.S. Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain 2003, 126 Pt 1, 5–19. [Google Scholar] [CrossRef]
- GB 21027-2020; Request in Common Use of Security for Student’s Articles. Standards Press of China: Beijing, China, 2020.
- Cai, Y.; Ren, B.; Peng, C.; Zhang, C.; Wei, X. Highly Sensitive and Selective Fluorescence “Turn-On” Detection of Pb (II) Based on Fe3O4@Au–FITC Nanocomposite. Molecules 2021, 26, 3180. [Google Scholar] [CrossRef]
- Zhang, C.; Lai, Z.; Liu, X.; Ye, M.; Zhang, L.; Zhang, L.; Chen, X. Voltammetric determination of Pb2+ in water using Mn-doped MoS2/MWCNTs/Nafion electrode coupled with an electrochemical flow analysis device. Electroanalysis 2022, 34, 1638. [Google Scholar] [CrossRef]
- Đogo-Mračević, S.; Ražić, S.; Trišić, J.; Mitrović, N.; Đukić-Ćosić, D. Toxic elements in children’s crayons and colored pencils: Bioaccessibility assessment. J. Serb. Chem. Soc. 2022, 87, 723–734. [Google Scholar] [CrossRef]
- Wilcockson, J.B.; Gobas, F.A. Thin-film solid-phase extraction to measure fugacities of organic chemicals with low volatility in biological samples. Environ. Sci. Technol. 2001, 35, 1425–1431. [Google Scholar] [CrossRef]
- Bruheim, I.; Liu, X.; Pawliszyn, J. Thin-film microextraction. Anal Chem. 2003, 75, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Olcer, Y.A.; Tascon, M.; Eroglu, A.E.; Boyacı, E. Thin film microextraction: Towards faster and more sensitive microextraction. TrAC-Trends Anal Chem. 2019, 113, 93–101. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Gionfriddo, E.; Poole, J.; Pawliszyn, J. Ultrafast Screening and Quantitation of Pesticides in Food and Environmental Matrices by Solid-Phase Microextraction-Transmission Mode (SPME-TM) and Direct Analysis in Real Time (DART). Anal Chem. 2017, 89, 7240–7248. [Google Scholar] [CrossRef] [PubMed]
- Piri-Moghadam, H.; Gionfriddo, E.; Rodriguez-Lafuente, A.; Grandy, J.J.; Lord, H.L.; Obal, T.; Pawliszyn, J. Inter-laboratory validation of a thin film microextraction technique for determination of pesticides in surface water samples. Anal Chim. Acta 2017, 964, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Garces, N.; Bojko, B.; Pawliszyn, J. High throughput quantification of prohibited substances in plasma using thin film solid phase microextraction. J. Chromatogr. A 2014, 1374, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Dong, J.; Wang, Y.; Chen, X. A review of developments and applications of thin-film microextraction coupled to surface-enhanced Raman scattering. Electrophoresis 2019, 40, 2041–2049. [Google Scholar] [CrossRef]
- Nwosu, F.O.; Ajala, O.J.; Owoyemi, R.M.; Raheem, B.G. Preparation and characterization of adsorbents derived from bentonite and kaolin clays. Appl. Water Sci. 2018, 8, 195. [Google Scholar] [CrossRef]
- Gong, W.X.; Qu, J.H.; Liu, R.P.; Lan, H.C. Effect of aluminum fluoride complexation on fluoride removal by coagulation. Colloid Surf. A 2012, 395, 88–93. [Google Scholar] [CrossRef]
- Ju, J.; Liu, R.; He, Z.; Liu, H.; Zhang, X.; Qu, J. Utilization of aluminum hydroxide waste generated in fluoride adsorption and coagulation processes for adsorptive removal of cadmium ion. Front. Environ. Sci. Eng. 2015, 10, 467–476. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Zhang, L.; Li, F.; Lin, F.; Wang, Y.; Chen, X. Highly selective fluorescence turn-on determination of Pb(II) in Water by in-situ enrichment of Pb(II) and MAPbBr3 perovskite growth in sulfydryl functionalized mesoporous alumina film. Sens. Actuators B-Chem. 2021, 326, 128975. [Google Scholar] [CrossRef]
- Lin, G.; Chen, Q. The Methodology of Lead Detection and Lead Survey in Stationery for Painting and Writing. Master’s Thesis, Southern Medical University, Guangzhou, China, 2002. [Google Scholar]
- Jaffe, A.; Lin, Y.; Beavers, C.M.; Voss, J.; Mao, W.L.; Karunadasa, H.I. High-Pressure Single-Crystal Structures of 3D Lead-Halide Hybrid Perovskites and Pressure Effects on their Electronic and Optical Properties. ACS Cent. Sci. 2016, 2, 201–209. [Google Scholar] [CrossRef]
- Oranskaia, A.; Yin, J.; Bakr, O.M.; Brédas, J.L.; Mohammed, O.F. Halogen Migration in Hybrid Perovskites: The Organic Cation Matters. J. Phys. Chem. Lett. 2018, 9, 5474–5480. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, D.; Ren, W.; Guo, X.; Wu, S.; Zhu, Y.; Rogach, A.L.; Chhowalla, M.; Jen, A.K.Y. Water-resistant perovskite nanodots enable robust two-photon lasing in aqueous environment. Nat. Commun. 2020, 11, 1192. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Liu, X.; Chen, G.; Wang, Y.; Bao, J.; Xu, X.; Liu, X.; Zhang, Q.; Yu, K.; et al. Heterostructural CsPbX(3)-PbS (X = Cl, Br, I) Quantum Dots with Tunable Vis-NIR Dual Emission. J. Am. Chem. Soc. 2020, 142, 4464–4471. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pritzl, G. Migration of some toxic metals from crayons and water colors. Bull. Environ. Contam. Toxicol. 1996, 56, 527–533. [Google Scholar] [CrossRef] [PubMed]

| Sample | Before Spiking (mg/kg) | Spiking Level (mg/kg) | Found (mg/kg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Sample 1 | 53.5 | 25 | 70.8 ± 1.6 | 90.3 | 1.7 |
| 50 | 101.4 ± 3.1 | 98.0 | 3.4 | ||
| 100 | 162.6 ± 2.3 | 105.9 | 5.7 | ||
| Sample 2 | 100.7 | 50 | 142.7 ± 1.5 | 94.7 | 2.1 |
| 100 | 207.4 ± 2.1 | 103.1 | 2.2 | ||
| 150 | 275.3 ± 4.4 | 109.8 | 3.8 |
| Sample | Sensing Method Detected (mg/kg) |
|---|---|
| Sample 1 | aN.D. |
| Sample 2 | 97.7 ± 7.6 |
| Sample 3 | aN.D. |
| Sample 4 | aN.D. |
| Sample 5 | aN.D. |
| Sample 6 | aN.D. |
| Sample 7 | aN.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, S.; Jin, J.; Luo, H.; Wang, Y.; Chen, X. Photoluminescence Sensing of Soluble Lead in Children’s Crayons Using Perovskite Nanocrystal In Situ Growth on an Aluminum Hydroxide Layer. Biosensors 2023, 13, 213. https://doi.org/10.3390/bios13020213
Zhang C, Wang S, Jin J, Luo H, Wang Y, Chen X. Photoluminescence Sensing of Soluble Lead in Children’s Crayons Using Perovskite Nanocrystal In Situ Growth on an Aluminum Hydroxide Layer. Biosensors. 2023; 13(2):213. https://doi.org/10.3390/bios13020213
Chicago/Turabian StyleZhang, Chen, Shuya Wang, Jingwen Jin, Hezhou Luo, Yiru Wang, and Xi Chen. 2023. "Photoluminescence Sensing of Soluble Lead in Children’s Crayons Using Perovskite Nanocrystal In Situ Growth on an Aluminum Hydroxide Layer" Biosensors 13, no. 2: 213. https://doi.org/10.3390/bios13020213
APA StyleZhang, C., Wang, S., Jin, J., Luo, H., Wang, Y., & Chen, X. (2023). Photoluminescence Sensing of Soluble Lead in Children’s Crayons Using Perovskite Nanocrystal In Situ Growth on an Aluminum Hydroxide Layer. Biosensors, 13(2), 213. https://doi.org/10.3390/bios13020213






