Amine Functionalized Gadolinium Oxide Nanoparticles-Based Electrochemical Immunosensor for Cholera
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gd2O3 NPs
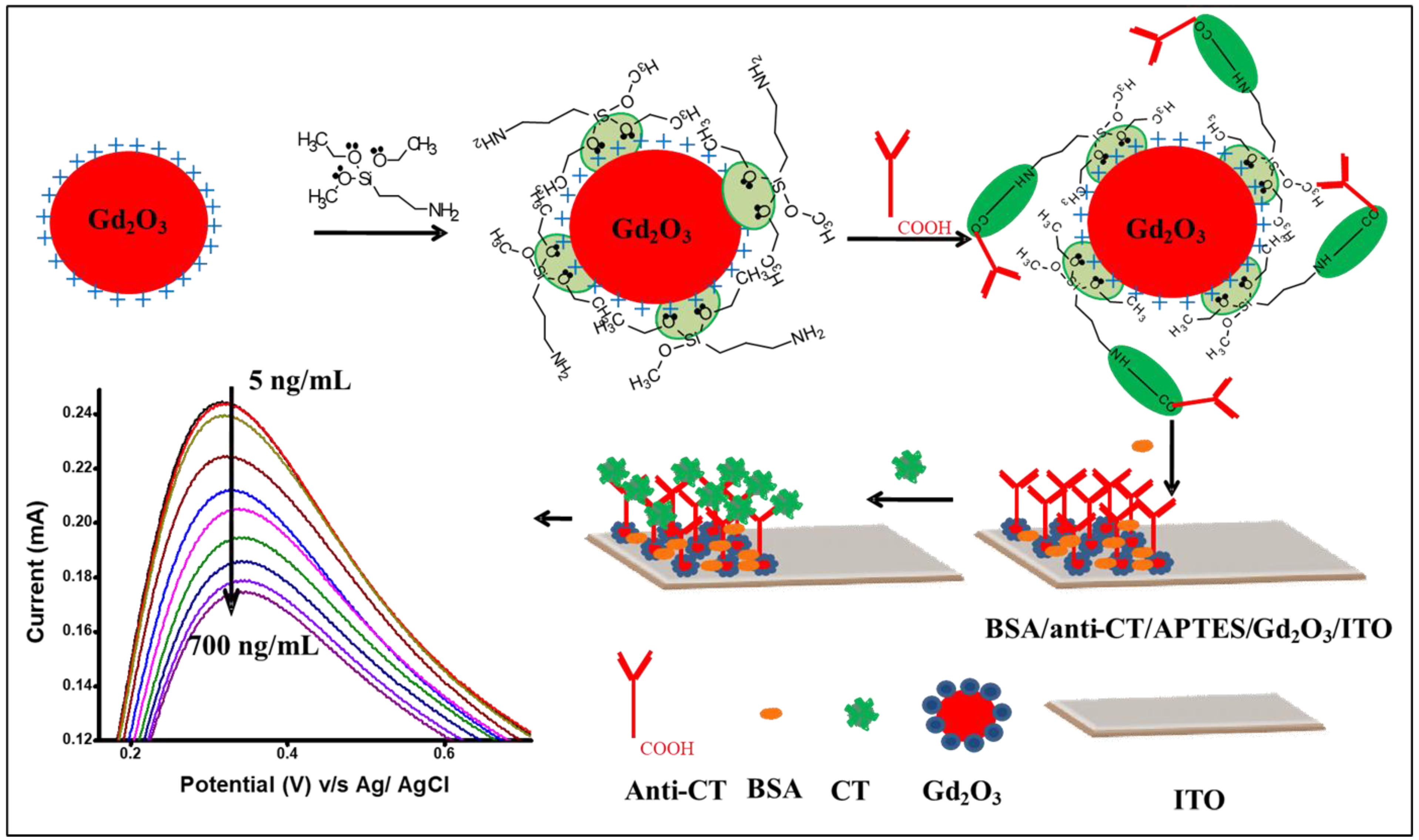
2.3. Functionalization of Gd2O3 NPs with APTES
2.4. Electrophoretic Deposition of APTES-Gd2O3 NPs onto ITO-Coated Glass Substrate
2.5. Immobilization of Anti-CT onto APTES-Gd2O3/ITO Electrode
2.6. Characterizations
3. Results and Discussion
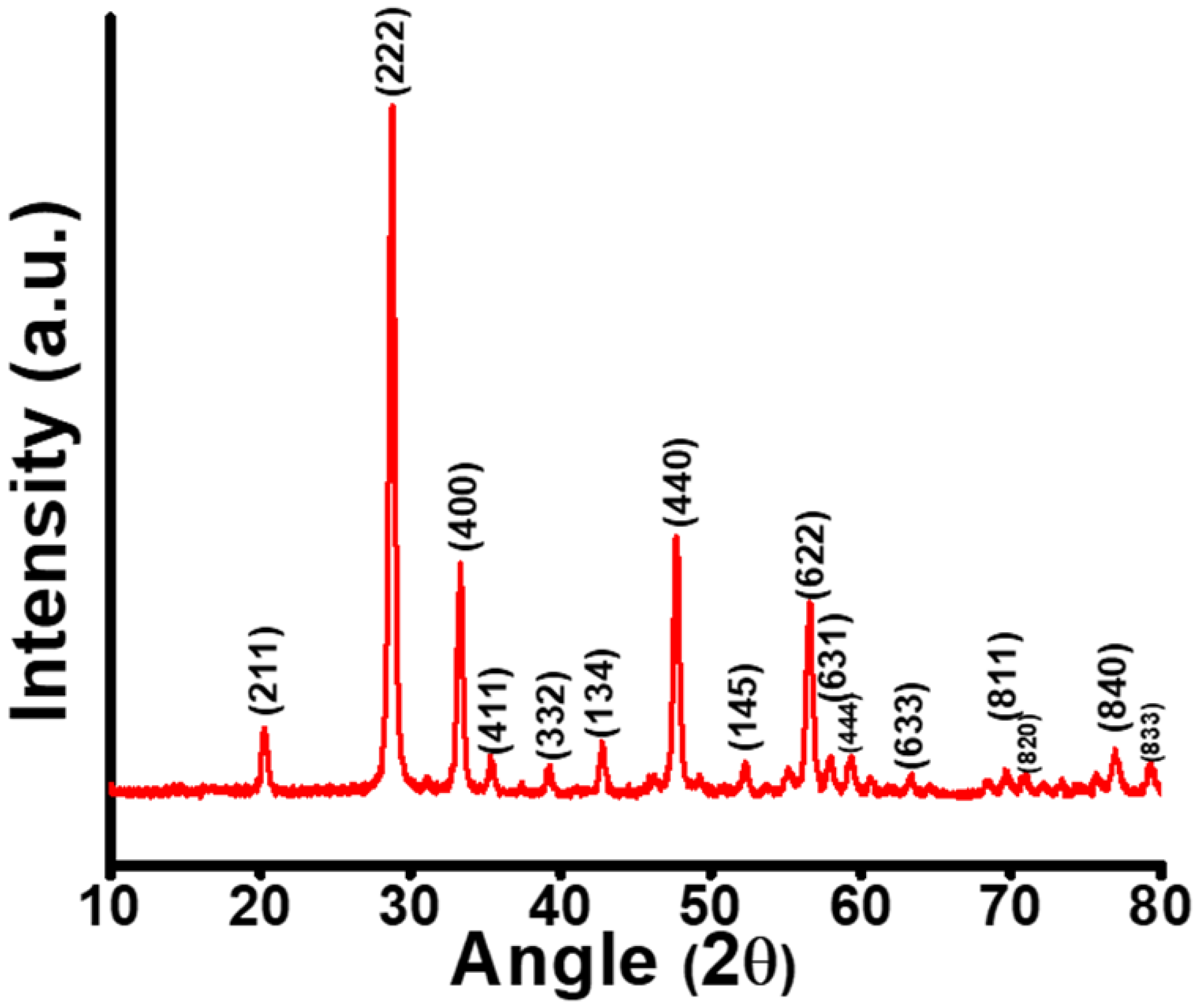
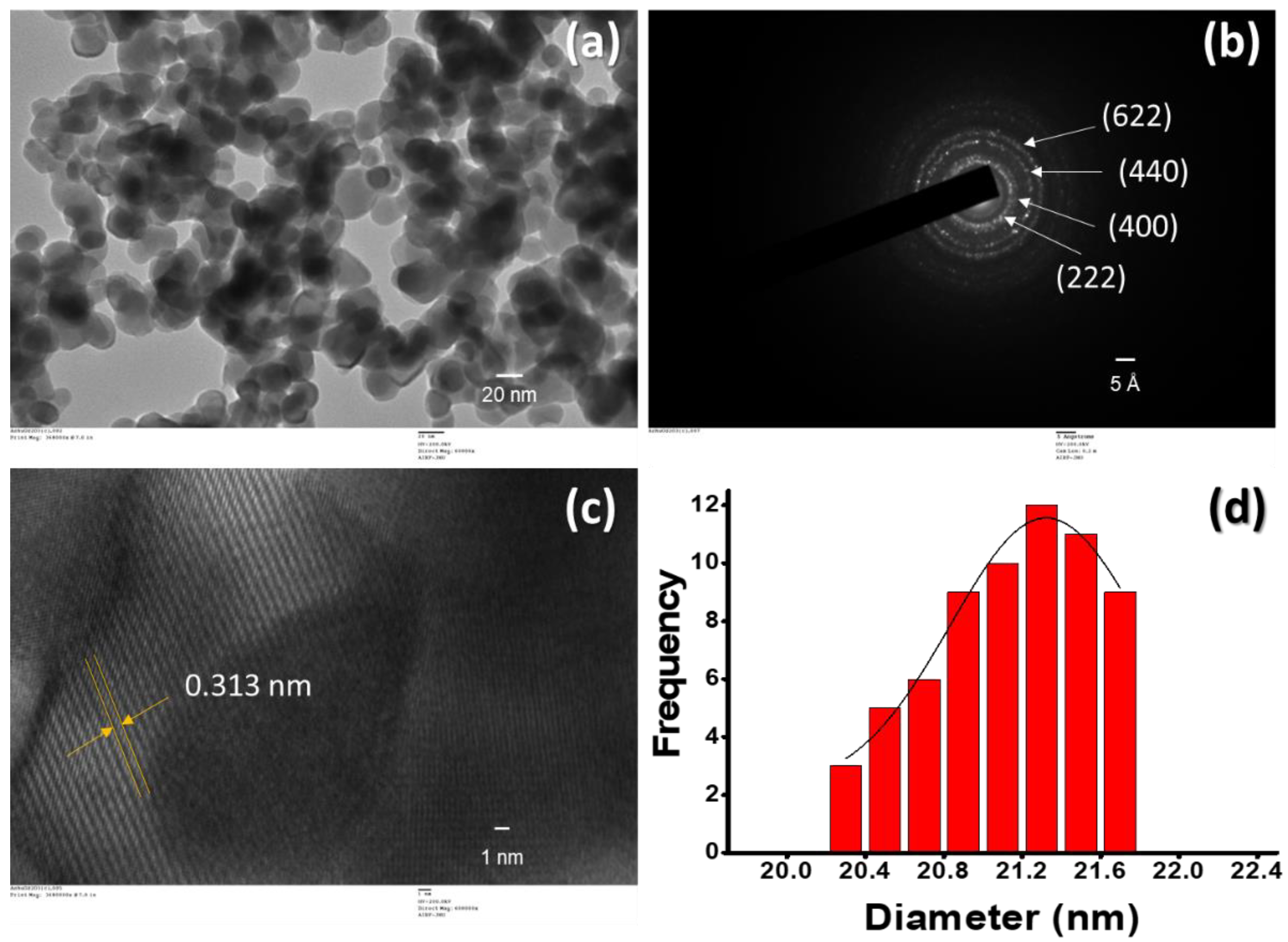
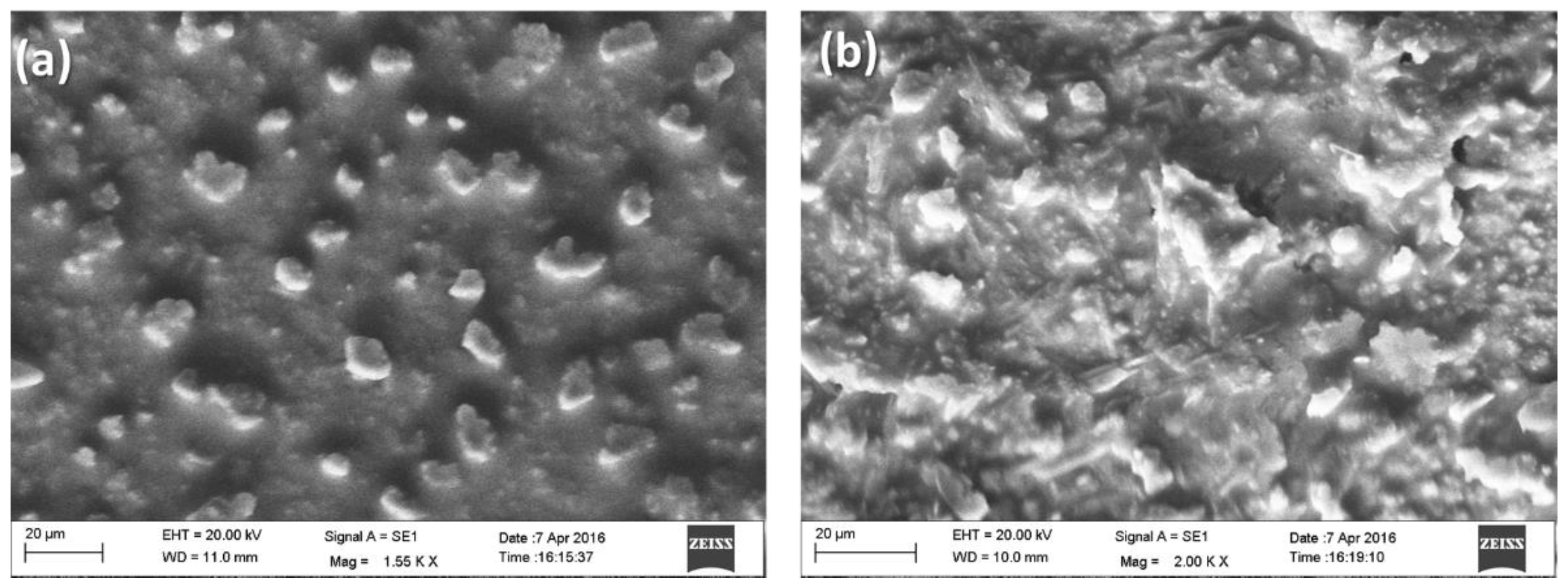
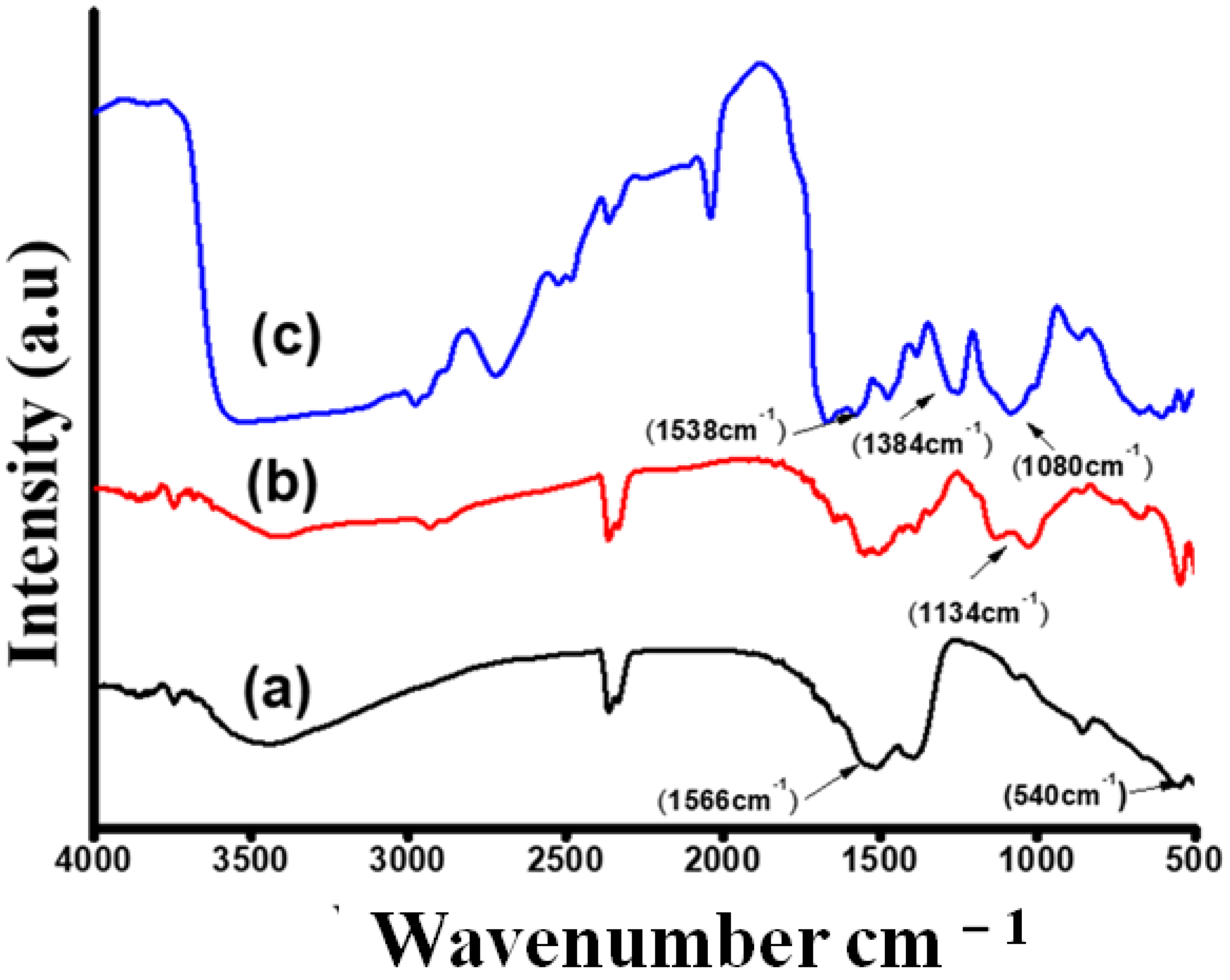
3.1. Structural and Morphological Study
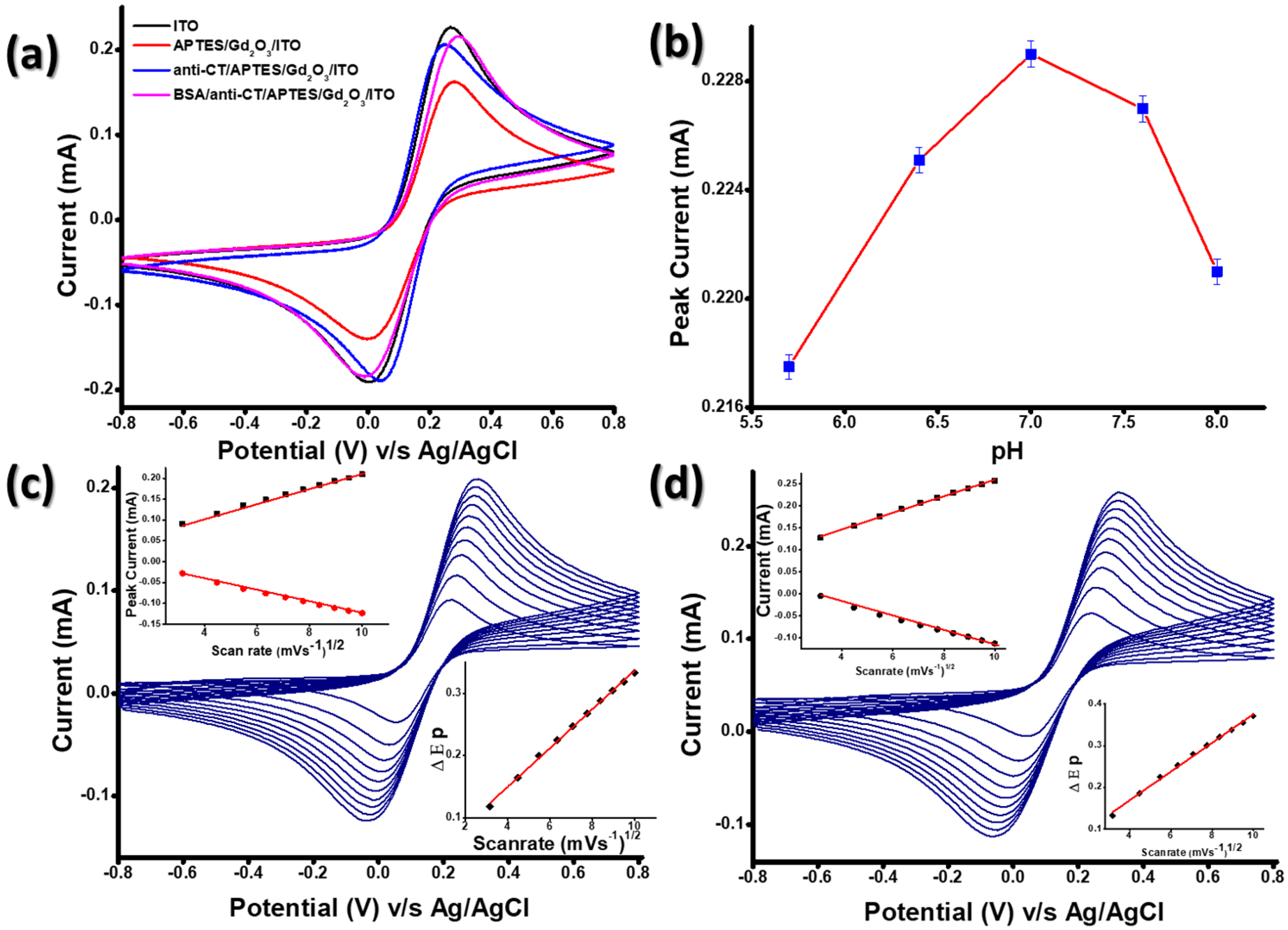
3.2. Electrochemical Characterizations
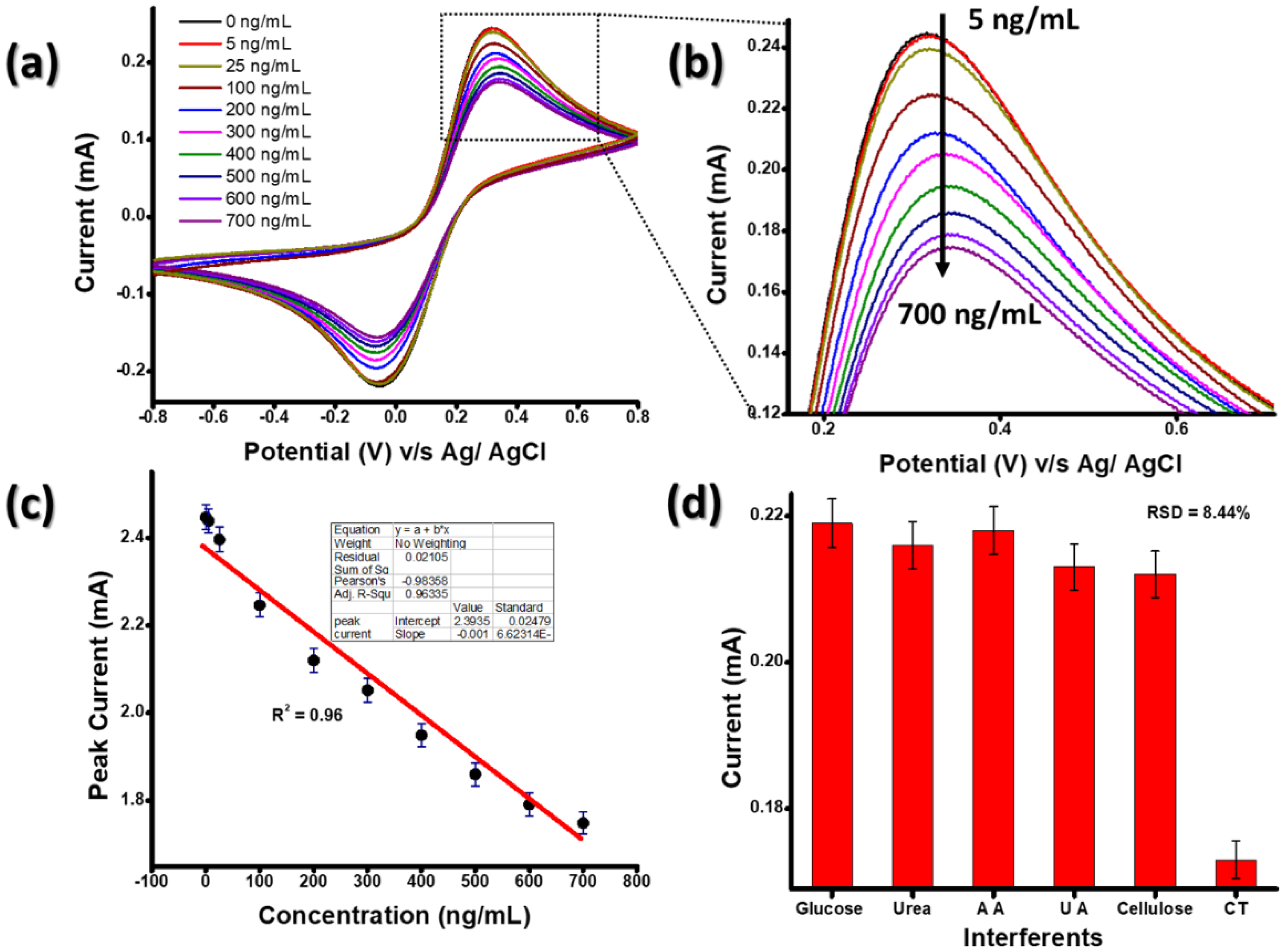
3.3. Electrochemical Response Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for Biosensing Applications: A Review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Purohit, B.; Mahato, K.; Roy, S.; Srivastava, A.; Chandra, P. Design and Development of Ultrafast Sinapic Acid Sensor Based on Electrochemically Nanotuned Gold Nanoparticles and Solvothermally Reduced Graphene Oxide. Electroanalysis 2020, 32, 59–69. [Google Scholar] [CrossRef]
- Kumar, A.; Purohit, B.; Mahato, K.; Mandal, R.; Srivastava, A.; Chandra, P. Gold-Iron Bimetallic Nanoparticles Impregnated Reduced Graphene Oxide Based Nanosensor for Label-Free Detection of Biomarker Related to Non-Alcoholic Fatty Liver Disease. Electroanalysis 2019, 31, 2417–2428. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Pandey, L.M.; Chandra, P. Nanoengineered Material Based Biosensing Electrodes for Enzymatic Biofuel Cells Applications. Mater. Sci. Energy Technol. 2018, 1, 38–48. [Google Scholar] [CrossRef]
- Kumar, A.; Purohit, B.; Maurya, P.K.; Pandey, L.M.; Chandra, P. Engineered Nanomaterial Assisted Signal-Amplification Strategies for Enhancing Analytical Performance of Electrochemical Biosensors. Electroanalysis 2019, 31, 1615–1629. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-Based Contrast Agent Toxicity: A Review of Known and Proposed Mechanisms. BioMetals 2016, 29, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.; Yang, P.; Wang, D.; Li, C.; Niu, N.; Li, X. Monodisperse Gd2O3:Ln (Ln = Eu3+, Tb3+, Dy3+, Sm3+, Yb3+/Er3+, Yb3+/Tm3+, and Yb3+/Ho3+) nanocrystals with tunable size and multicolor luminescent properties. CrystEngComm 2011, 13, 5480–5487. [Google Scholar] [CrossRef]
- Singh, M.P.; Thakur, C.S.; Shalini, K.; Banerjee, S.; Bhat, N.; Shivashankar, S.A. Structural, Optical, and Electrical Characterization of Gadolinium Oxide Films Deposited by Low-Pressure Metalorganic Chemical Vapor Deposition Structural, Optical, and Electrical Characterization of Gadolinium Oxide Films Deposited by Low-Pressure metalorganic chemical vapor deposition. J. Appl. Phys. 2015, 96, 5631–5637. [Google Scholar] [CrossRef]
- Tamrakar, R.K.; Bisen, D.P.; Brahme, N. Comparison of photoluminescence properties of Gd2O3 phosphor synthesized by combustion and solid state reaction method. J. Radiat. Res. Appl. Sci. 2014, 7, 550–559. [Google Scholar] [CrossRef]
- Hazarika, S.; Paul, N.; Mohanta, D. Rapid Hydrothermal Route to Synthesize Cubic-Phase Gadolinium Oxide Nanorods. Bull. Mater. Sci. 2014, 37, 789–796. [Google Scholar] [CrossRef]
- Vahdatkhah, P.; Hosseini, H.R.M.; Khodaei, A.; Montazerabadi, A.R.; Irajirad, R.; Oghabian, M.A.; Delavari, H. Rapid Microwave-Assisted Synthesis of PVP-Coated Ultrasmall Gadolinium Oxide Nanoparticles for Magnetic Resonance Imaging. Chem. Phys. 2015, 453–454, 35–41. [Google Scholar] [CrossRef]
- Gayathri, T.; Sundaram, N.M.; Kumar, R.A. Gadolinium Oxide Nanoparticles for Magnetic Resonance Imaging and Cancer Theranostics Gadolinium Oxide Nanoparticles for Magnetic Resonance Imaging and Cancer Theranostics. J. Bionanosci. 2016, 9, 409–423. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry; John Wiley and Sons, Inc.: Hoboken, USA, 1999; Volume 6. [Google Scholar]
- Bentivoglio, M.; Pacini, P. Filippo Pacini: A Determined Observer. Brain Res. Bull. 1995, 38, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Howard-Jones, N. Robert Koch and the Cholera Vibrio: A Centenary. Br. Med. J. Clin. Res. Ed. 1984, 288, 379. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Waldor, M.K. Filamentous Phages Linked to Virulence of Vibrio Cholerae. Curr. Opin. Microbiol. 2003, 6, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, A.; Kaushik, A.; Bhansali, S. Electrochemical Immunosensor for Label Free Epidermal Growth Factor Receptor (EGFR) Detection. Biosens. Bioelectron. 2013, 39, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Srivastava, S.; Ali, A.; Srivastava, R.K.; Srivastava, A.; Malhotra, B.D. RSC Advances for Label-Free Biosensor. RSC Adv. 2014, 4, 60386–60396. [Google Scholar] [CrossRef]
- Sharma, A.; Baral, D.; Rawat, K.; Solanki, P.R.; Bohidar, H.B. Biocompatible Capped Iron Oxide Nanoparticles for Vibrio Cholerae Detection. Nanotechnology 2015, 26, 175302. [Google Scholar] [CrossRef]
- Bagbi, Y.; Sharma, A.; Bohidar, H.B.; Solanki, P.R. Immunosensor Based on Nanocomposite of Nanostructured Zirconium Oxide and Gelatin-A. Int. J. Biol. Macromol. 2016, 82, 480–487. [Google Scholar] [CrossRef]
- Samuel, J.; Raccurt, O.; Mancini, C.; Dujardin, C.; Amans, D.; Ledoux, G.; Poncelet, O.; Tillement, O. Homogeneous Dispersion of Gadolinium Oxide Nanoparticles into a Non-Aqueous-Based Polymer by Two Surface Treatments. J. Nanopart. Res. 2011, 13, 2417–2428. [Google Scholar] [CrossRef]
- Hemmer, E.; Yamano, T.; Kishimoto, H.; Venkatachalam, N.; Hyodo, H.; Soga, K. Cytotoxic Aspects of Gadolinium Oxide Nanostructures for Up-Conversion and NIR Bioimaging. Acta Biomater. 2013, 9, 4734–4743. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Tiwari, S.; Srivastava, S.; Srivastava, M.; Yadav, B.K.; Kumar, S.; Tran, T.T.; Dewan, A.K.; Mulchandani, A.; et al. Biofunctionalized Nanostructured Zirconia for Biomedical Application: A Smart Approach for Oral Cancer Detection. Adv. Sci. 2015, 2, 1500048. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.; Hazarika, S.; Saha, A.; Mohanta, D. Optical Emission, Vibrational Feature, and Shear- Thinning Aspect of Tb3+-Doped Gd2O3 Nanoparticle-Based Novel Ferrofluids Irradiated by Gamma Photons. J. Appl. Phys. 2013, 114, 134903. [Google Scholar] [CrossRef]
- Hazarika, S.; Mohanta, D. Production and Optoelectronic Response of Tb3 Activated Gadolinium Oxide Nanocrystalline Phosphors. Eur. Phys. J. Appl. Phys. 2013, 62, 30401. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Zhang, D.; Yang, S. Structural, Luminescence and Magnetic Properties of Yb3+-Er3+Codoped Gd2O3 Hierarchical Architectures. CrystEngComm 2015, 17, 1106–1114. [Google Scholar] [CrossRef]
- da Silva, A.C.N.; Deda, D.K.; da Róz, A.L.; Prado, R.A.; Carvalho, C.C.; Viviani, V.; Leite, F.L. Nanobiosensors Based on Chemically Modified AFM Probes: A Useful Tool for Metsulfuron-Methyl Detection. Sensors 2013, 13, 1477–1489. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Tiwari, S.; Augustine, S.; Srivastava, S.; Yadav, B.K.; Malhotra, B.D. Highly Sensitive Protein Functionalized Nanostructured Hafnium Oxide Based Biosensing Platform for Non-Invasive Oral Cancer Detection. Sens. Actuators B Chem. 2016, 235, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y. Graphene Based Immunosensors. In Immunosensors; Royal Society of Chemistry: London, UK, 2019; pp. 156–185. [Google Scholar]
- Turner, A.; Karube, I.; Wilson, G.S. Biosensors: Fundamentals and Applications; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Teker, K.; Wickstrom, E.; Panchapakesan, B. Biomolecular Tuning of Electronic Transport Properties of Carbon Nanotubes via Antibody Functionalization. IEEE Sens. J. 2006, 6, 1422–1428. [Google Scholar] [CrossRef]
- Ali, A.; Ansari, A.A.; Kaushik, A.; Solanki, P.R.; Barik, A.; Pandey, M.K.; Malhotra, B.D. Nanostructured Zinc Oxide Film for Urea Sensor. Mater. Lett. 2009, 63, 2473–2475. [Google Scholar] [CrossRef]
- Tiwari, S.; Gupta, P.K.; Bagbi, Y.; Sarkar, T.; Solanki, P.R. L-Cysteine Capped Lanthanum Hydroxide Nanostructures for Non-Invasive Detection of Oral Cancer Biomarker. Biosens. Bioelectron. 2017, 89, 1042–1052. [Google Scholar] [CrossRef]

| Electrode/Immobilization | Transducer | Biomolecules | Detection Range | Low Detection Range | Sensitivity | References |
|---|---|---|---|---|---|---|
| Au Electrode | DNA biosensors | ss-DNA | 100–500 ng mL−1 | 100 ng mL−1 | 0.027 μA/ng cm−2 | [12] |
| RGO-TiO2/ITO | Electrochemical impedance spectroscopy | Antibodies | 10–450 ng mL−1 | 0.15 ng mL−1 | 21.8 × 10−3μF/ng L−1cm−2 | [13] |
| BSA/Ab/CA-Fe3O4/ITO | Electrochemical Impedance spectroscopy | Antibodies | 12.5–500 ng mL−1 | 0.32 ng mL−1 | 0.03 Ω/ng mL−1 cm−2 | [14] |
| BSA/Ab/GelA-ZrO2/ITO | Electrochemical impedance spectroscopy | Antibodies | 50–400 ng mL−1 | 0.74 ng mL−1 | 0.03 Ω/ng mL−1cm−2 | [15] |
| BSA/Ab/OA-Fe3O4/ITO | Electrochemical impedance spectroscopy | Antibodies | 12.5–500 ng mL−1 | 0.5 ng mL−1 | 0.1 Ω/ng mL−1cm−2 | [28] |
| NiO/ITO nanowires | Electrochemical impedance spectroscopy | Antibodies | 37–350 ng mL−1 | 0.553 ng mL−1 | 11.12 Ω/ng mL−1 cm−2 | [29] |
| Nanocoaxial array | Differential Pulse Voltammetry | ELISA | 10 ng mL−1–1 μg mL−1 | 2 ng mL−1 | ------- | [30] |
| IDUA electrodes | Microfluidic assay | Antibodies | 0–100 ng mL−1 | 31.7ng mL−1 | -------- | [31] |
| Graphene Nano sheets with incorporated lipid films | Potentiometric | GM1 | 10 × 10−9M to 10 × 10−6M | 1 nM | ∼60 mV/decade | [32] |
| BSA/anti-CT/APTES/Gd2O3/ ITO | Cyclic Voltammetry | Antibodies | 5–700 ng mL−1 | 1.48 ng mL−1 | 8.37 mA ng−1mL cm−2 | Present Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Sarkar, T.; Solanki, P.R. Amine Functionalized Gadolinium Oxide Nanoparticles-Based Electrochemical Immunosensor for Cholera. Biosensors 2023, 13, 177. https://doi.org/10.3390/bios13020177
Kumar A, Sarkar T, Solanki PR. Amine Functionalized Gadolinium Oxide Nanoparticles-Based Electrochemical Immunosensor for Cholera. Biosensors. 2023; 13(2):177. https://doi.org/10.3390/bios13020177
Chicago/Turabian StyleKumar, Ashutosh, Tamal Sarkar, and Pratima R. Solanki. 2023. "Amine Functionalized Gadolinium Oxide Nanoparticles-Based Electrochemical Immunosensor for Cholera" Biosensors 13, no. 2: 177. https://doi.org/10.3390/bios13020177
APA StyleKumar, A., Sarkar, T., & Solanki, P. R. (2023). Amine Functionalized Gadolinium Oxide Nanoparticles-Based Electrochemical Immunosensor for Cholera. Biosensors, 13(2), 177. https://doi.org/10.3390/bios13020177






