Microfluidics for COVID-19: From Current Work to Future Perspective
Abstract
1. Introduction
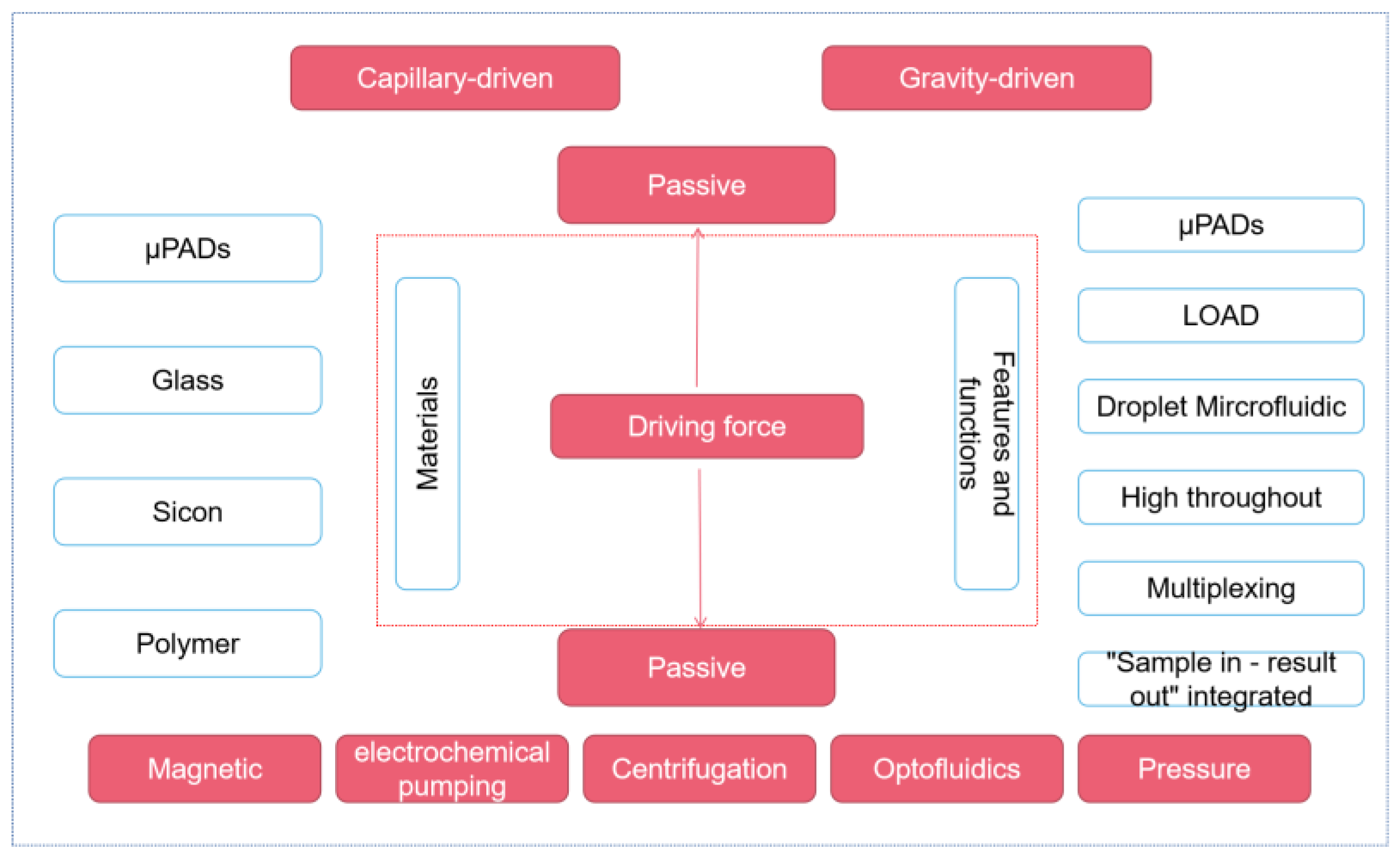
2. Microfluidic Platform
2.1. Passive Microfluidic Platform—μPADs
2.2. Active Microfluidic Platforms
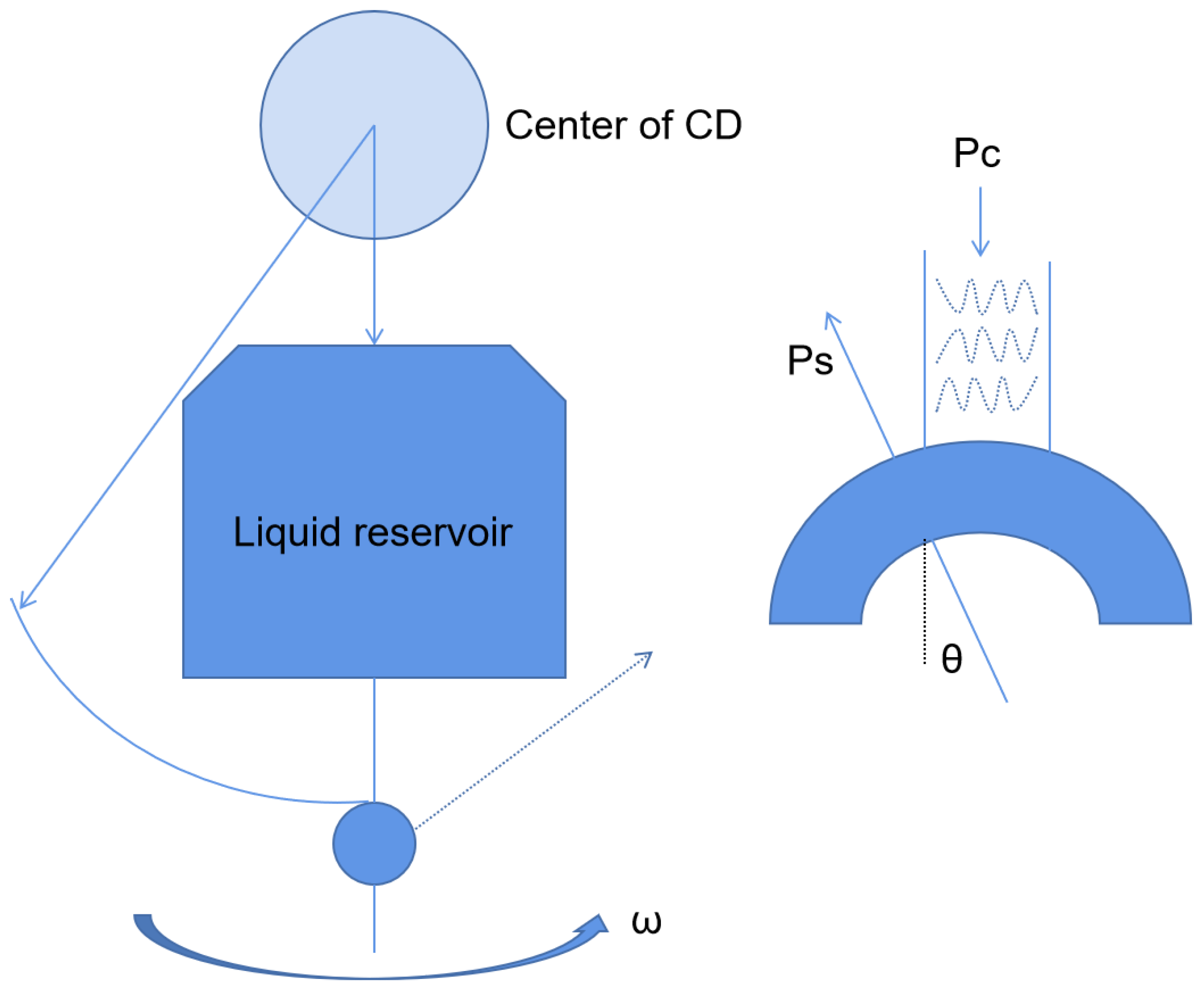
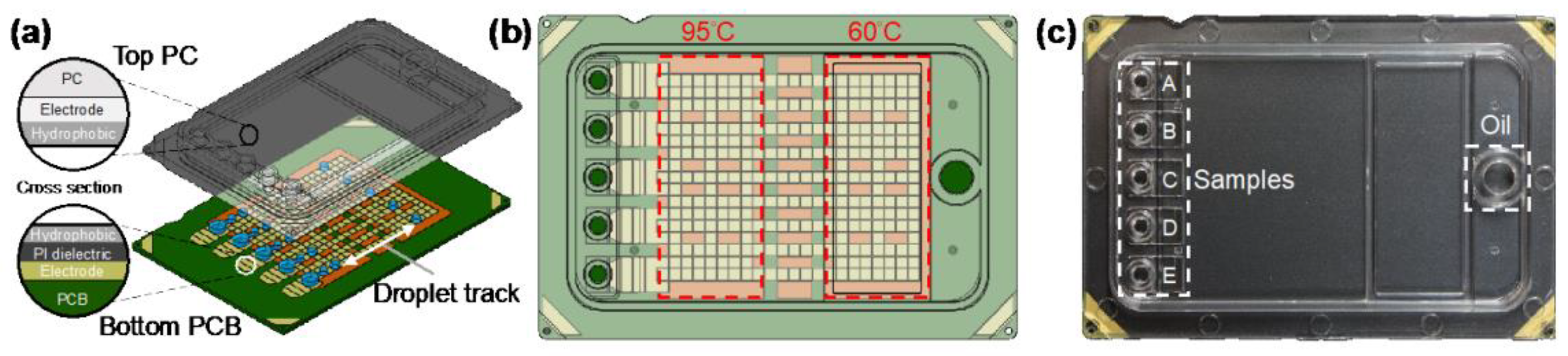
2.2.1. Centrifugal Microfluidic Platform
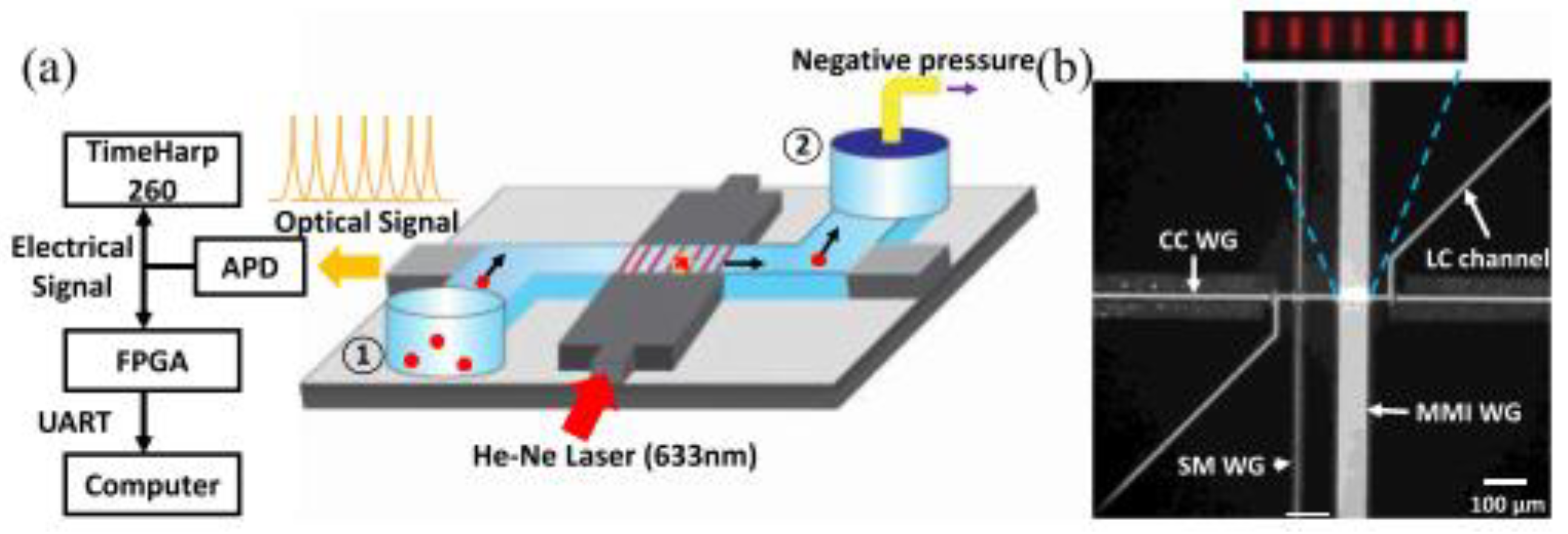
2.2.2. Optical Fluid Microfluidic Platform
2.2.3. Digital Microfluidics Platform
3. Detection Method for COVID-19 Diagnosis Based on Microfluidics
3.1. Nucleic Acid Detection Method
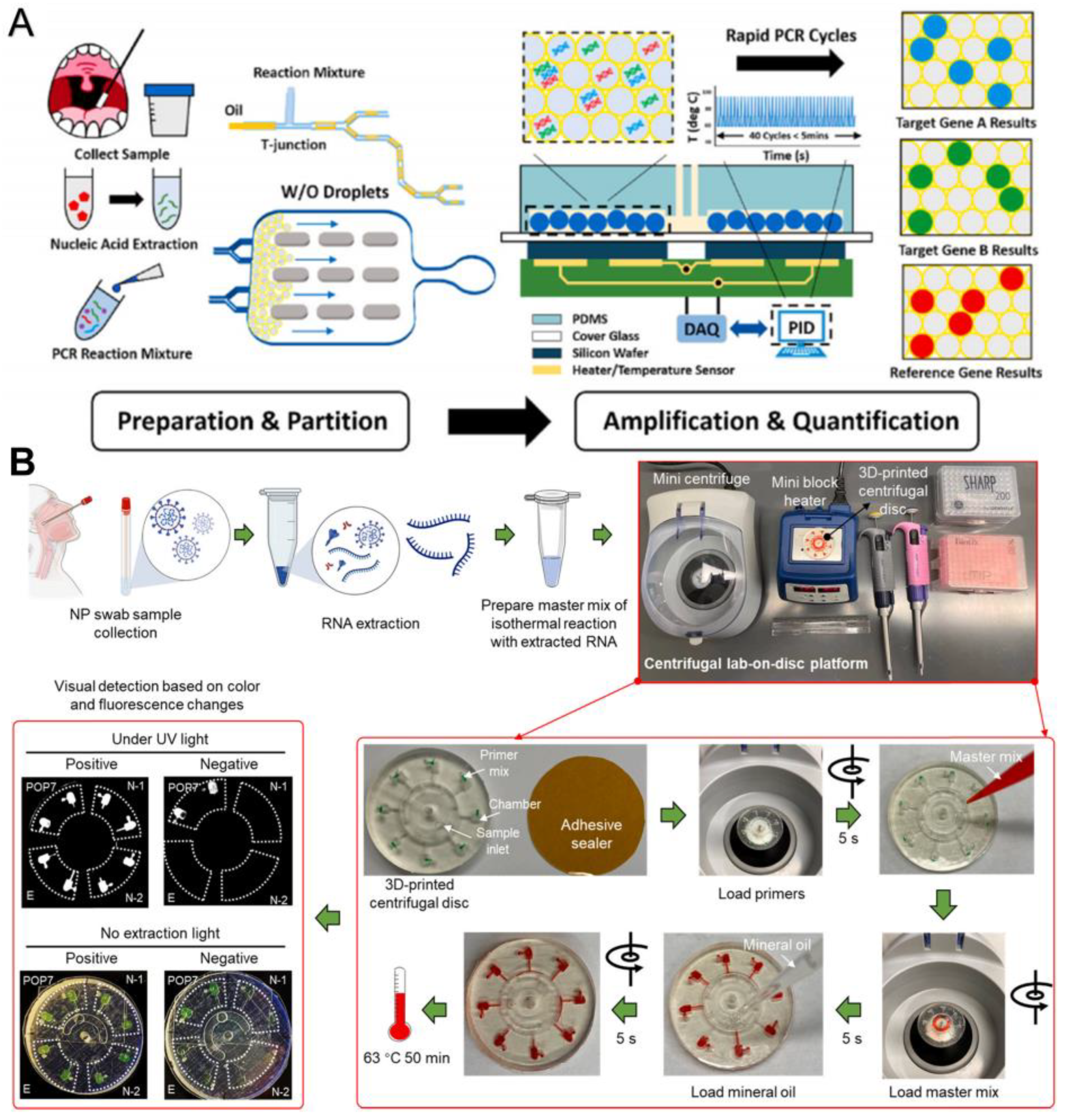
3.1.1. Based on PCR
Combining with Microfluidics to Simplify the Steps and Reduce the Detection Time
Combining with Microfluidics to Improve the Automation, Accuracy, and Sensitivity
3.1.2. Based on Isothermal Amplification Technology
Combining with Microfluidic to Improve the Accuracy
Combining with Microfluidics to Improve the Sensitivity
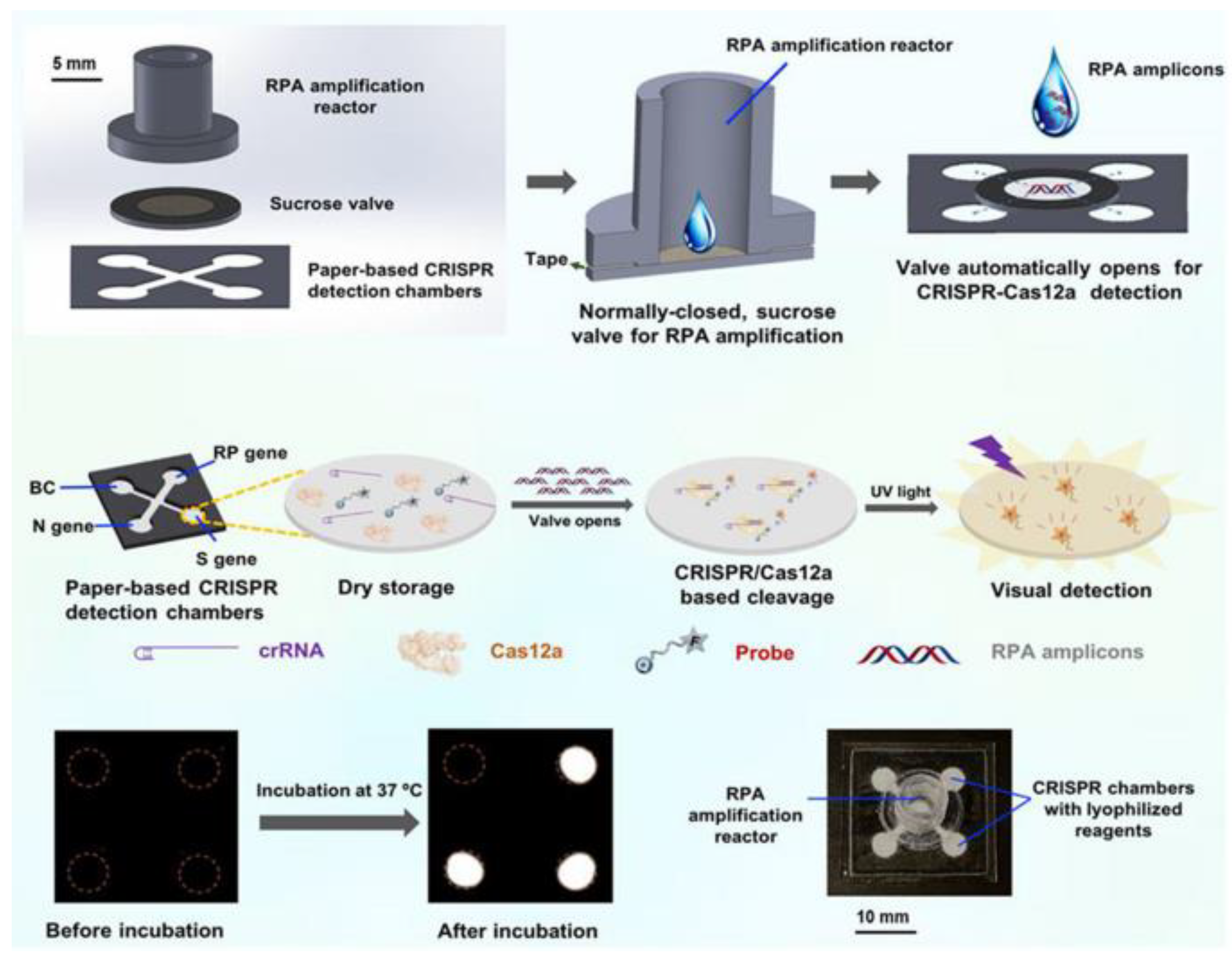
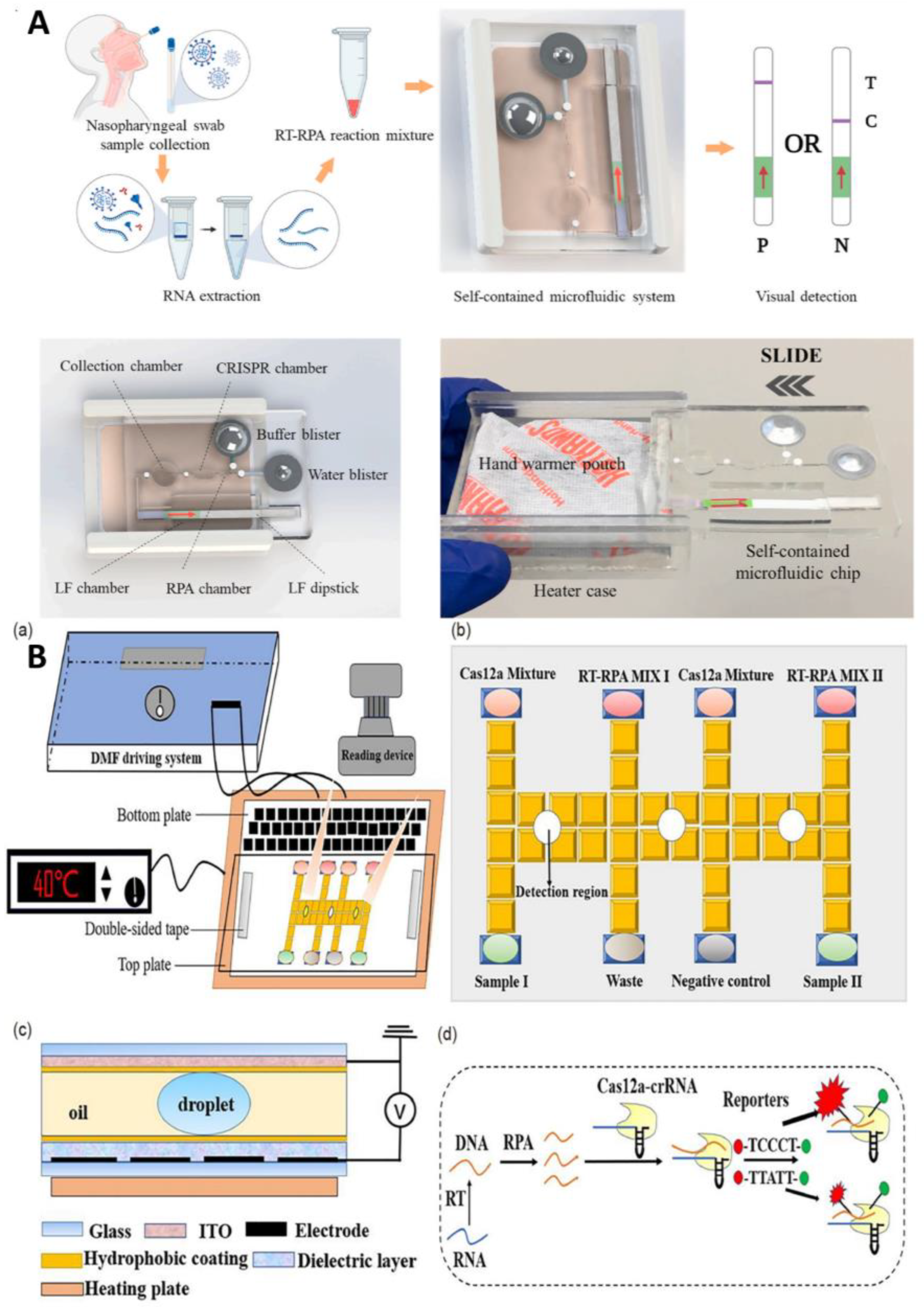
3.1.3. Based on CRISPR
Combining with Microfluidics to Improve the Degree of Automation and Reduce Cost
Combining with Microfluidics to Improve the Sensitivity and Throughput
3.1.4. Other Nucleic Acid Detection Methods
3.2. Immunoassay
3.2.1. Labeled Immunoassay
Fluorescence Immunoassay
Combining with Microfluidics to Improve the Sensitivity and Throughput
Combining with Microfluidics to Simplified the Step
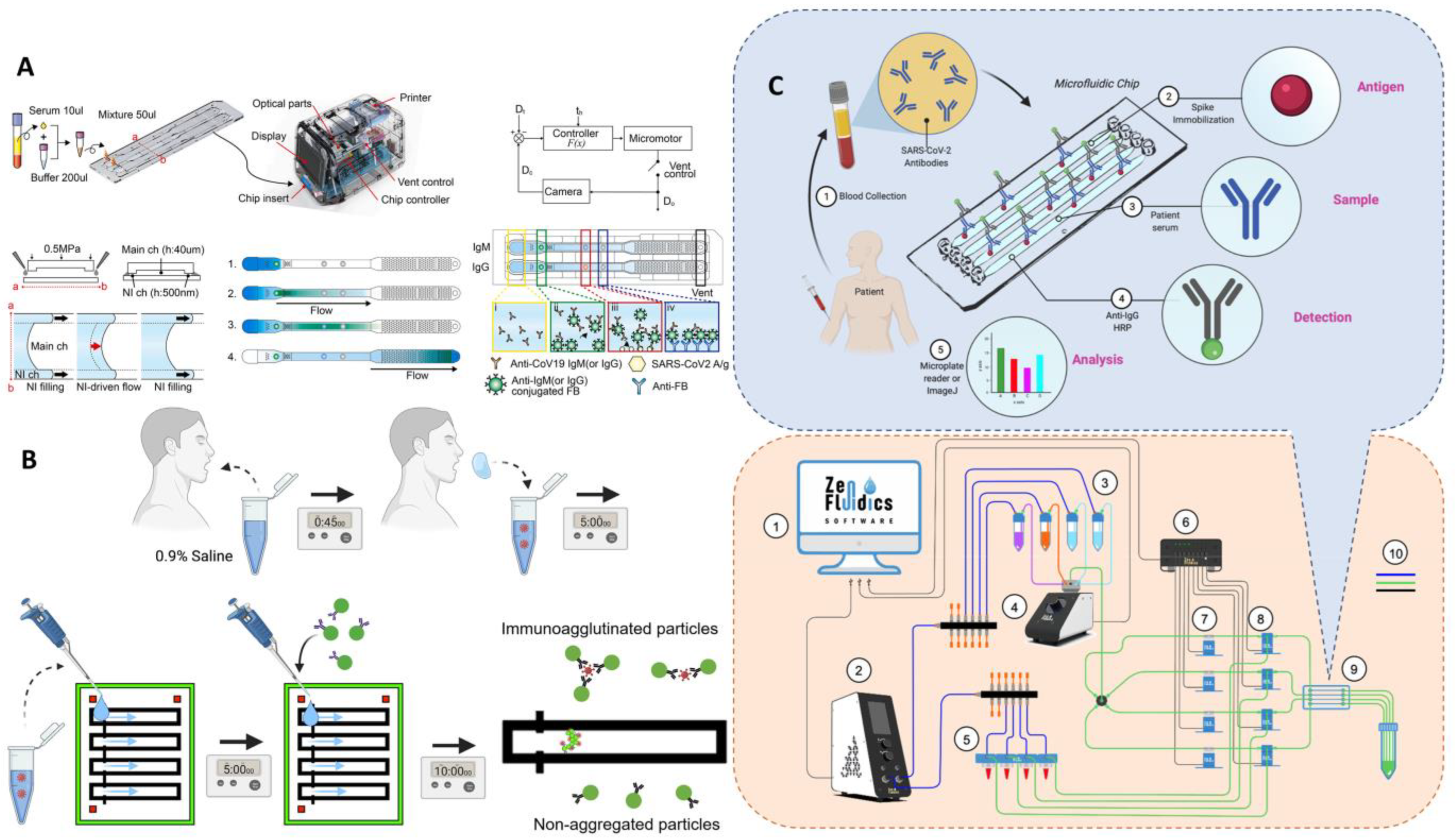
ELISA
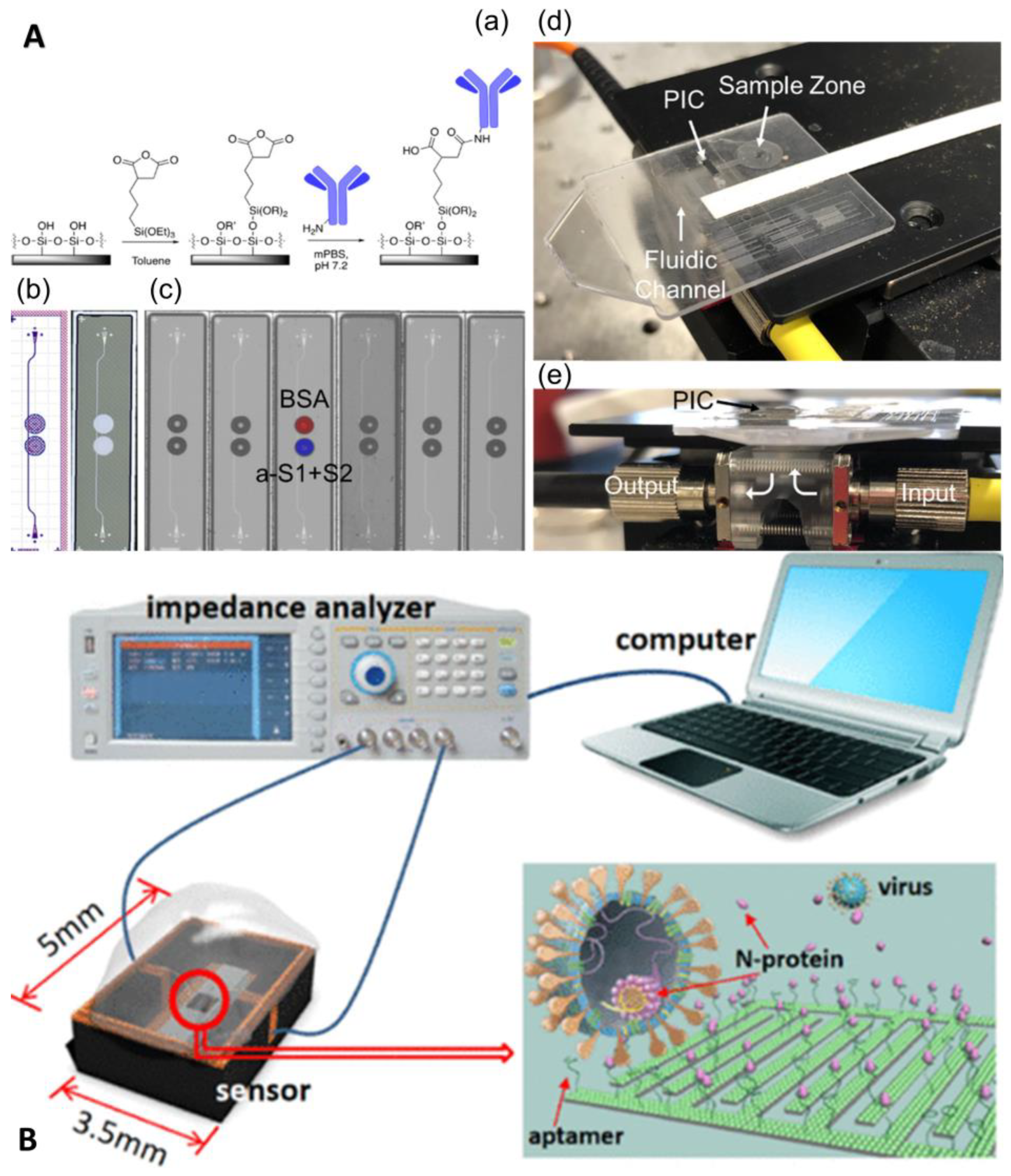
3.2.2. Unlabeled Immunoassay
4. Commercially Available Microfluidic Platform
5. Limitations and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.C.; Chen, C.S.; Chan, Y.J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020, 395, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 31 October 2022).
- Backer, J.A.; Klinkenberg, D.; Wallinga, J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020, 25, 2000062. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C. Transposed Data in a Table. JAMA—J. Am. Med. Assoc. 2021, 325, 1113. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Y.; Xiao, T.; Lu, J.; Peng, H.; Sterling, S.M.; Walsh, R.M., Jr.; Rits-Volloch, S.; Zhu, H.; Woosley, A.N.; et al. Structural impact on SARS-CoV-2 spike protein by D614G substitution. Science 2021, 372, 525–530. [Google Scholar] [CrossRef]
- Weigl, J. Challenges in infectious disease control and the current pandemic by skewed distributions. Pravent. Gesundh. 2020, 15, 97–101. [Google Scholar] [CrossRef]
- Nie, K.X.; Wang, C.; Li, X.W. Success of Big Infectious Disease Reimbursement Policy in China. Inq. J. Health Care Organ. Provis. Financ. 2020, 57, 46958020907788. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA—J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Deng, S.Q.; Peng, H.J. Characteristics of and Public Health Responses to the Coronavirus Disease 2019 Outbreak in China. J. Clin. Med. 2020, 9, 575. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef] [PubMed]
- Jarrom, D.; Elston, L.; Washington, J.; Prettyjohns, M.; Cann, K.; Myles, S.; Groves, P. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: A rapid systematic review. BMJ Evid. Based Med. 2022, 27, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kruttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.W.; Imohl, M.; Kleines, M. Comparison of the SARS-CoV-2 Rapid antigen test to the real star Sars-CoV-2 RT PCR kit. J. Virol. Methods 2021, 288, 114024. [Google Scholar] [CrossRef] [PubMed]
- Kruttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.; Imohl, M.; Kleines, M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J. Clin. Virol. 2020, 128, 104394. [Google Scholar] [CrossRef]
- Shrivastava, S.; Trung, T.Q.; Lee, N.E. Recent progress, challenges, and prospects of fully integrated mobile and wearable point-of-care testing systems for self-testing. Chem. Soc. Rev. 2020, 49, 1812–1866. [Google Scholar] [CrossRef]
- Wang, M.; Gong, Q.; Liu, W.; Tan, S.; Xiao, J.; Chen, C. Applications of capillary electrophoresis in the fields of environmental, pharmaceutical, clinical, and food analysis (2019–2021). J. Sep. Sci. 2022, 45, 1918–1941. [Google Scholar] [CrossRef]
- Liang, W.; Lin, H.; Chen, J.; Chen, C. Utilization of nanoparticles in microfluidic systems for optical detection. Microsyst. Technol. 2016, 22, 2363–2370. [Google Scholar] [CrossRef]
- Peng, S.; Hong, T.; Liang, W.; Liu, W.; Chen, C. A multichannel microchip containing 16 chambers packed with antibody-functionalized beads for immunofluorescence assay. Anal. Bioanal. Chem. 2019, 411, 1579–1589. [Google Scholar] [CrossRef]
- Li, F.; You, M.; Li, S.; Hu, J.; Liu, C.; Gong, Y.; Yang, H.; Xu, F. Paper-based point-of-care immunoassays: Recent advances and emerging trends. Biotechnol. Adv. 2020, 39, 107442. [Google Scholar] [CrossRef]
- Li, X.; Qin, Z.; Fu, H.; Li, T.; Peng, R.; Li, Z.; Rini, J.M.; Liu, X. Enhancing the performance of paper-based electrochemical impedance spectroscopy nanobiosensors: An experimental approach. Biosens. Bioelectron. 2021, 177, 112672. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Ding, X.; Li, Z.; Sfeir, M.M.; Ballesteros, E.; Liu, C. Autonomous lab-on-paper for multiplexed, CRISPR-based diagnostics of SARS-CoV-2. Lab Chip 2021, 21, 2730–2737. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Weber, P.; Lutz, S.; Focke, M.; Zengerle, R.; von Stetten, F. Aliquoting on the centrifugal microfluidic platform based on centrifugo-pneumatic valves. Microfluid. Nanofluidics 2011, 10, 1279–1288. [Google Scholar] [CrossRef]
- Gorkin, R.; Park, J.; Siegrist, J.; Amasia, M.; Lee, B.S.; Park, J.-M.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.-K. Centrifugal microfluidics for biomedical applications. Lab Chip 2010, 10, 1758–1773. [Google Scholar] [CrossRef]
- Malic, L.; Brassard, D.; Da Fonte, D.; Nassif, C.; Mounier, M.; Ponton, A.; Geissler, M.; Shiu, M.; Morton, K.J.; Veres, T. Automated sample-to-answer centrifugal microfluidic system for rapid molecular diagnostics of SARS-CoV-2. Lab Chip 2022, 22, 3157–3171. [Google Scholar] [CrossRef]
- Dignan, L.M.; Woolf, M.S.; Tomley, C.J.; Nauman, A.Q.; Landers, J.P. Multiplexed Centrifugal Microfluidic System for Dynamic Solid-Phase Purification of Polynucleic Acids Direct from Buccal Swabs. Anal. Chem. 2021, 93, 7300–7309. [Google Scholar] [CrossRef]
- Sampad, M.J.N.; Amin, M.N.; Hawkins, A.R.; Schmidt, H. FPGA Integrated Optofluidic Biosensor for Real-Time Single Biomarker Analysis. IEEE Photonics J. 2022, 14, 3701806. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.-L.; Liao, H.-Y.; Liu, H.M.; Lu, Y.-W.; Yeh, P.-K.; Chang, J.Y.; Fan, S.-K. Digital Microfluidic qPCR Cartridge for SARS-CoV-2 Detection. Micromachines 2022, 13, 196. [Google Scholar] [CrossRef]
- Perlman, S. Another Decade, Another Coronavirus. N. Engl. J. Med. 2020, 382, 760–762. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Dong, X.; Liu, L.; Tu, Y.; Zhang, J.; Miao, G.; Zhang, L.; Ge, S.; Xia, N.; Yu, D.; Qiu, X. Rapid PCR powered by microfluidics: A quick review under the background of COVID-19 pandemic. Trac-Trends Anal. Chem. 2021, 143, 116377. [Google Scholar] [CrossRef] [PubMed]
- Moschou, D.; Vourdas, N.; Kokkoris, G.; Papadakis, G.; Parthenios, J.; Chatzandroulis, S.; Tserepi, A. All-plastic, low-power, disposable, continuous-flow PCR chip with integrated microheaters for rapid DNA amplification. Sens. Actuator B Chem. 2014, 199, 470–478. [Google Scholar] [CrossRef]
- Prakash, R.; Pabbaraju, K.; Wong, S.; Wong, A.; Tellier, R.; Kaler, K.V.I.S. Multiplex, Quantitative, Reverse Transcription PCR Detection of Influenza Viruses Using Droplet Microfluidic Technology. Micromachines 2015, 6, 63–79. [Google Scholar] [CrossRef]
- Hsieh, H.-Y.; Chang, R.; Huang, Y.-Y.; Juan, P.-H.; Tahara, H.; Lee, K.-Y.; Vo, D.N.K.; Tsai, M.-H.; Wei, P.-K.; Sheen, H.-J.; et al. Continuous polymerase chain reaction microfluidics integrated with a gold-capped nanoslit sensing chip for Epstein-Barr virus detection. Biosens. Bioelectron. 2022, 195, 113672. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kidd, M.; Nordquist, A.R.; Smith, S.D.; Hurth, C.; Modlin, I.M.; Zenhausern, F. A Sensitive, Portable Microfluidic Device for SARS-CoV-2 Detection from Self-Collected Saliva. Infect. Dis. Rep. 2021, 13, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Lee, Y.; Yu, E.S.; Na, H.; Kang, M.; Huh, H.J.; Jeong, K.H. Ultrafast and Real-Time Nanoplasmonic On-Chip Polymerase Chain Reaction for Rapid and Quantitative Molecular Diagnostics. ACS Nano 2021, 15, 10194–10202. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Wang, Y.; Yang, N.; Shu, B.; Zhang, G.; Liu, D. A fully automated microfluidic PCR-array system for rapid detection of multiple respiratory tract infection pathogens. Anal. Bioanal. Chem. 2021, 413, 1787–1798. [Google Scholar] [CrossRef]
- Yin, H.; Wu, Z.; Shi, N.; Qi, Y.; Jian, X.; Zhou, L.; Tong, Y.; Cheng, Z.; Zhao, J.; Mao, H. Ultrafast multiplexed detection of SARS-CoV-2 RNA using a rapid droplet digital PCR system. Biosens. Bioelectron. 2021, 188, 113282. [Google Scholar] [CrossRef]
- Ding, X.; Li, Z.; Liu, C. Monolithic, 3D-printed lab-on-disc platform for multiplexed molecular detection of SARS-CoV-2. Sens. Actuator B Chem. 2022, 351, 130998. [Google Scholar] [CrossRef]
- Donia, A.; Furqan Shahid, M.; Hassan, S.U.; Shahid, R.; Ahmad, A.; Javed, A.; Nawaz, M.; Yaqub, T.; Bokhari, H. Integration of RT-LAMP and Microfluidic Technology for Detection of SARS-CoV-2 in Wastewater as an Advanced Point-of-Care Platform. Food Environ. Virol. 2022, 14, 364–373. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, Z.Y. Detection of Exosomal MicroRNAs Using Rolling Circle Amplification on Microfluidic Chip. Basic Clin. Pharmacol. Toxicol. 2020, 127, 30. [Google Scholar]
- Wozniakowski, G.; Fraczyk, M.; Mazur, N. Comparison of loop-mediated isothermal amplification (LAMP) and cross-priming amplification (CPA) for detection of African swine fever virus. Pol. J. Vet. Sci. 2018, 21, 827–830. [Google Scholar] [PubMed]
- Shi, C.; Liu, Q.; Zhou, M.L.; Zhao, H.J.; Yang, T.; Ma, C.P. Nicking endonuclease-mediated isothermal exponential amplification for double-stranded DNA detection. Sens. Actuator B Chem. 2016, 222, 221–225. [Google Scholar] [CrossRef]
- Wu, Q.; Suo, C.; Brown, T.; Wang, T.; Teichmann, S.A.; Bassett, A.R. INSIGHT: A population-scale COVID-19 testing strategy combining point-of-care diagnosis with centralized high-throughput sequencing. Sci. Adv. 2021, 7, eabe5054. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Y.; Lau, Y.L. Detection of Plasmodium knowlesi using recombinase polymerase amplification (RPA) combined with SYBR Green I. Acta Trop. 2020, 208, 105511. [Google Scholar] [CrossRef]
- Gill, P.; Ghaemi, A. Nucleic acid isothermal amplification technologies: A review. Nucleosides Nucleotides Nucleic Acids 2008, 27, 224–243. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Babu, B.; Ochoa-Corona, F.M.; Paret, M.L. Recombinase polymerase amplification applied to plant virus detection and potential implications. Anal. Biochem. 2018, 546, 72–77. [Google Scholar] [CrossRef]
- Goo, N.I.; Kim, D.E. Rolling circle amplification as isothermal gene amplification in molecular diagnostics. BioChip J. 2016, 10, 262–271. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, J.; Ye, Y.; Zhang, J.; Zhang, Y.; Sun, X. Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit. Rev. Food Sci. Nutr. 2020, 60, 201–224. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Das, S.; Ahmed, R.; Mori, Y.; Notomi, T.; Kevadiya, B.D.; Thakor, A.S. Loop-Mediated Isothermal Amplification (LAMP): A Rapid, Sensitive, Specific, and Cost-Effective Point-of-Care Test for Coronaviruses in the Context of COVID-19 Pandemic. Biology 2020, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Jhou, Y.-R.; Wang, C.-H.; Tsai, H.-P.; Shan, Y.-S.; Lee, G.-B. An integrated microfluidic platform featuring real-time reverse transcription loop-mediated isothermal amplification for detection of COVID-19. Sens. Actuator B Chem. 2022, 358, 131447. [Google Scholar] [CrossRef] [PubMed]
- Hau Van, N.; Vu Minh, P.; Seo, T.S. Total integrated centrifugal genetic analyzer for point-of-care Covid-19 testing with automatic and high-throughput capability. Sens. Actuator B Chem. 2022, 353, 131088. [Google Scholar]
- Soares, R.R.G.; Akhtar, A.S.; Pinto, I.F.; Lapins, N.; Barrett, D.; Sandh, G.; Yin, X.; Pelechano, V.; Russom, A. Sample-to-answer COVID-19 nucleic acid testing using a low-cost centrifugal microfluidic platform with bead-based signal enhancement and smartphone read-out. Lab Chip 2021, 21, 2932–2944. [Google Scholar] [CrossRef]
- Liu, D.; Shen, H.; Zhang, Y.; Shen, D.; Zhu, M.; Song, Y.; Zhu, Z.; Yang, C. A microfluidic-integrated lateral flow recombinase polymerase amplification (MI-IF-RPA) assay for rapid COVID-19 detection. Lab Chip 2021, 21, 2019–2026. [Google Scholar] [CrossRef]
- Lyu, W.; Zhang, J.; Yu, Y.; Xu, L.; Shen, F. Slip formation of a high-density droplet array for nucleic acid quantification by digital LAMP with a random-access system. Lab Chip 2021, 21, 3086–3093. [Google Scholar] [CrossRef]
- Kim, H.S.; Abbas, N.; Shin, S. A rapid diagnosis of SARS-CoV-2 using DNA hydrogel formation on microfluidic pores. Biosens. Bioelectron. 2021, 177, 113005. [Google Scholar] [CrossRef]
- Bai, Y.M.; Ji, J.C.; Ji, F.D.; Wu, S.; Tian, Y.; Jin, B.R.; Li, Z.D. Recombinase polymerase amplification integrated with microfluidics for nucleic acid testing at point of care. Talanta 2022, 240, 123209. [Google Scholar] [CrossRef]
- Kong, M.; Li, Z.; Wu, J.; Hu, J.; Sheng, Y.; Wu, D.; Lin, Y.; Li, M.; Wang, X.; Wang, S. A wearable microfluidic device for rapid detection of HIV-1 DNA using recombinase polymerase amplification. Talanta 2019, 205, 120155. [Google Scholar] [CrossRef]
- Yang, B.; Kong, J.; Fang, X. Bandage-like wearable flexible microfluidic recombinase polymerase amplification sensor for the rapid visual detection of nucleic acids. Talanta 2019, 204, 685–692. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhao, Z.; Lian, K.; Yin, L.; Wang, J.; Man, S.; Liu, G.; Ma, L. SERS-based CRISPR/Cas assay on microfluidic paper analytical devices for supersensitive detection of pathogenic bacteria in foods. Biosens. Bioelectron. 2022, 207, 114167. [Google Scholar] [CrossRef] [PubMed]
- Li, P.T.; Zeng, X.W.; Xue, H.J.; Ye, X.; Yang, B.; Kong, J.L.; Yin, L.P.; Fang, X.E. CRISPR-microfluidic array for single-copy DNA mini barcoding and rapid field species identification. Sens. Actuator B Chem. 2022, 359, 131567. [Google Scholar] [CrossRef]
- Li, Z.; Ding, X.; Yin, K.; Avery, L.; Ballesteros, E.; Liu, C. Instrument-free, CRISPR-based diagnostics of SARS-CoV-2 using self-contained microfluidic system. Biosens. Bioelectron. 2022, 199, 113865. [Google Scholar] [CrossRef]
- Ramachandran, A.; Huyke, D.A.; Sharma, E.; Sahoo, M.K.; Huang, C.; Banaei, N.; Pinsky, B.A.; Santiago, J.G. Electric field-driven microfluidics for rapid CRISPR-based diagnostics and its application to detection of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 29518–29525. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lin, K.F.; Zhao, Z.H.; Wang, Y.; Hong, X.X.; Guo, J.G.; Ruan, Q.Y.; Lu, L.Y.; Li, X.; Zhang, R.; et al. An automated nucleic acid detection platform using digital microfluidics with an optimized Cas12a system. Sci. China Chem. 2022, 65, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, C.M.; Myhrvold, C.; Thakku, S.G.; Freije, C.A.; Metsky, H.C.; Yang, D.K.; Ye, S.H.; Boehm, C.K.; Kosoko-Thoroddsen, T.-S.F.; Kehe, J.; et al. Massively multiplexed nucleic acid detection with Cas13. Nature 2020, 582, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, J.; Liu, D.; Hu, B.; Luo, G.; Huang, Z. A novel microfluidic RNA chip for direct, single-nucleotide specific, rapid and partially-degraded RNA detection. Talanta 2022, 239, 122974. [Google Scholar] [CrossRef]
- Chu, Y.; Qiu, J.; Wang, Y.; Wang, M.; Zhang, Y.; Han, L. Rapid and High-Throughput SARS-CoV-2 RNA Detection without RNA Extraction and Amplification by Using a Microfluidic Biochip. Chem. Eur. J. 2022, 28, e202104054. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Chen, Y.; Ho, N.R.Y.; Sundah, N.R.; Natalia, A.; Liu, Y.; Miow, Q.H.; Wang, Y.; Tambyah, P.A.; et al. Accessible detection of SARS-CoV-2 through molecular nanostructures and automated microfluidics. Biosens. Bioelectron. 2021, 194, 113629. [Google Scholar] [CrossRef]
- Maiolini, E.; Ferri, E.; Pitasi, A.L.; Montoya, A.; Di Giovanni, M.; Errani, E.; Girotti, S. Bisphenol A determination in baby bottles by chemiluminescence enzyme-linked immunosorbent assay, lateral flow immunoassay and liquid chromatography tandem mass spectrometry. Analyst 2014, 139, 318–324. [Google Scholar] [CrossRef]
- Lee, J.H.; Bae, P.K.; Kim, H.; Song, Y.J.; Yi, S.Y.; Kwon, J.; Seo, J.-S.; Lee, J.-M.; Jo, H.-S.; Park, S.M.; et al. A rapid quantitative on-site coronavirus disease 19 serological test. Biosens. Bioelectron. 2021, 191, 113406. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Moncayo, R.; Cedillo-Alcantar, D.F.; Guevara-Pantoja, P.E.; Chavez-Pineda, O.G.; Hernandez-Ortiz, J.A.; Amador-Hernandez, J.U.; Rojas-Velasco, G.; Sanchez-Munoz, F.; Manzur-Sandoval, D.; Patino-Lopez, L.D.; et al. A high-throughput multiplexed microfluidic device for COVID-19 serology assays. Lab Chip 2021, 21, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wen, D.; Wu, J.; Liu, L.; Wu, W.; Fang, X.; Kong, J. Microfluidic Immunoassays for Sensitive and Simultaneous Detection of IgG/IgM/Antigen of SARS-CoV-2 within 15 min. Anal. Chem. 2020, 92, 9454–9458. [Google Scholar] [CrossRef] [PubMed]
- Breshears, L.E.; Nguyen, B.T.; Akarapipad, P.; Sosnowski, K.; Kaarj, K.; Quirk, G.; Uhrlaub, J.L.; Nikolich-Zugich, J.; Worobey, M.; Yoon, J.Y. Sensitive, smartphone-based SARS-CoV-2 detection from clinical saline gargle samples. PNAS Nexus 2022, 1, pgac028. [Google Scholar] [CrossRef]
- Swank, Z.; Michielin, G.; Yip, H.M.; Cohen, P.; Andrey, D.O.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; Meyer, B.; Maerkl, S.J. A high-throughput microfluidic nanoimmunoassay for detecting anti-SARS-CoV-2 antibodies in serum or ultralow-volume blood samples. Proc. Natl. Acad. Sci. USA 2021, 118, e2025289118. [Google Scholar] [CrossRef]
- Kim, S.; Akarapipad, P.; Nguyen, B.T.; Breshears, L.E.; Sosnowski, K.; Baker, J.; Uhrlaub, J.L.; Nikolich-Zugich, J.; Yoon, J.Y. Direct capture and smartphone quantification of airborne SARS-CoV-2 on a paper microfluidic chip. Biosens. Bioelectron. 2022, 200, 113912. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, E.; Garcia-Ramirez, R.; Diaz-Armas, G.G.; Esparza, M.; Aguilar-Avelar, C.; Flores-Contreras, E.A.; Rodriguez-Sanchez, I.P.; Delgado-Balderas, J.R.; Soto-Garcia, B.; Araiz-Hernandez, D.; et al. Automated ELISA On-Chip for the Detection of Anti-SARS-CoV-2 Antibodies. Sensors 2021, 21, 6785. [Google Scholar] [CrossRef]
- Gong, F.; Wei, H.-x.; Qi, J.; Ma, H.; Liu, L.; Weng, J.; Zheng, X.; Li, Q.; Zhao, D.; Fang, H.; et al. Pulling-Force Spinning Top for Serum Separation Combined with Paper-Based Microfluidic Devices in COVID-19 ELISA Diagnosis. ACS Sens. 2021, 6, 2709–2719. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, Y.; Fu, Q.; Lin, M.; He, J.; He, S.; Yang, M.; Chen, S.; Zhou, J. Reciprocating-flowing on-a-chip enables ultra-fast immunobinding for multiplexed rapid ELISA detection of SARS-CoV-2 antibody. Biosens. Bioelectron. 2021, 176, 112920. [Google Scholar] [CrossRef]
- Funari, R.; Fukuyama, H.; Shen, A.Q. Nanoplasmonic multiplex biosensing for COVID-19 vaccines. Biosens. Bioelectron. 2022, 208, 114193. [Google Scholar] [CrossRef]
- Cognetti, J.S.; Miller, B.L. Monitoring Serum Spike Protein with Disposable Photonic Biosensors Following SARS-CoV-2 Vaccination. Sensors 2021, 21, 5857. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, J.; Song, D.; Li, C.; Zhu, A.; Long, F. Rapid, label-free, and sensitive point-of-care testing of anti-SARS-CoV-2 IgM/IgG using all-fiber Fresnel reflection microfluidic biosensor. Microchim. Acta 2021, 188, 261. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Hu, Z.; Yang, Z.; Zhang, J.; Wu, J.J.; Cheng, C.; Wang, C.; Zheng, L. Capacitive Aptasensor Coupled with Microfluidic Enrichment for Real-Time Detection of Trace SARS-CoV-2 Nucleocapsid Protein. Anal. Chem. 2022, 94, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, C.; Chu, Y.; Han, Y.; Gao, Y.; Wang, Y.; Wang, C.; Liu, H.; Han, L.; Zhang, Y. Graphene oxide-graphene Van der Waals heterostructure transistor biosensor for SARS-CoV-2 protein detection. Talanta 2022, 240, 123197. [Google Scholar] [CrossRef] [PubMed]
- ID NOW COVID-19 Testing|Abbott U.S. Available online: https://www.abbott.com/IDNOW.html (accessed on 5 June 2022).
- Foaming Test—COVID-19 Screening Test Kit|PharmaNona. Available online: https://foamingtest.com/en (accessed on 5 June 2022).
- Credo Diagnostics Biomedical VitaPCR SARS-CoV-2 Assay Garners CE Mark. Credo Diagnostics Biomedical Pte. Ltd. Available online: https://www.credodxbiomed.com/en/news/100-credo-diagnostics-biomedical-vitapcr-sars-cov-2-assay-garners-ce-mark (accessed on 5 June 2022).
- POC Respiratory Panel Test|Respiratory EZ Panel|BioFire Diagnostics. Available online: https://www.biofiredx.com/products/the-filmarray-panels/filmarray-respiratory-panel-ez (accessed on 5 June 2022).
- Cepheid|Cepheid|Xpert®, Xpress SARS-CoV-2—FDA Emergency Use Authorization. Available online: https://www.cepheid.com/en/coronavirus (accessed on 11 February 2022).
- 1copy COVID-19 qPCR Multi Kit—Instructions for Use. Available online: https://www.fda.gov/media/137935/download (accessed on 5 June 2022).
- TEST COVID-19—Biosynex. Available online: https://www.biosynex.com/en/pharmacie-para-test-covid-19/ (accessed on 5 June 2022).
- AQ-TOP COVID-19 Rapid Detection Kit PLUS—Instructions for Use. Available online: https://www.fda.gov/media/142800/download (accessed on 5 June 2022).
- COVID-19 Mutation Multiplex RT-PCR Detection Kit (Lyophilized). Available online: https://www.chkbiotech.com/covid-19-mutation-multiplex-rt-pcr-detection-kit-lyophilized-product/ (accessed on 5 June 2022).
- Zyobio_INSERTO.pdf. Available online: www.bvs.hn/COVID-19/Plataforma/Zyobio_INSERTO.pdf (accessed on 5 June 2022).
- Lucira COVID-19 All-In-One Test Kit + PDF Report (Good For Travel)—Plus PDF Results (Good for Travel). Available online: https://www.meenta.io/product/lucira-covid-19-all-in-one-test/ (accessed on 11 February 2022).
- Respiratory Virus Nucleic Acid Detection Kit (Isothermal Amplification Chip Meth—FIND). Available online: https://www.finddx.org/product/respiratory-virus-nucleic-acid-detection-kit-isothermal-amplification-chip-meth/ (accessed on 11 February 2022).
- Qorvo Biotechnologies Omnia SARS-CoV-2 Antigen Test Detects Delta and Other Circulating Variants in Two Studies—Qorvo. Available online: https://www.qorvo.com/newsroom/news/2021/qorvo-biotechnologies-omnia-sars-cov-2-antigen-test-detects-delta-and-other-circulating-variants (accessed on 11 February 2022).
- The LumiraDx SARS-CoV-2 Ag Test Is a Rapid Microfluidic Immunoassay Detecting SARS-CoV-2 Antigen. Available online: https://www.lumiradx.com/uk-en/test-menu/antigen-test (accessed on 10 February 2022).
- SAMPINUTETM. Available online: https://www.celltrion.com/en-us/kit/sampinute (accessed on 10 February 2022).


| Microfluidic Platform | Improvement | Type of Technique | Target Gene | LOD | Processing of Time | Characteristics | Reference |
|---|---|---|---|---|---|---|---|
| μPADs | High integration | CRISPR-RPA | N, S | 100 copies/rnx | <60 min | Paper-based sugar valve | [23] |
| Centrifugal microfluidic platform | High integration | LAMP | N | NR | 8 min | Automatic control of laser airborne microvalve instead of passive valve | [26] |
| Centrifugal microfluidic platform | Step simplification and High integration | LAMP | N, E | 0.5 copies/uL | 60 min | Rapid test in whole blood | [27] |
| Optical flow control platform | High sensitivity | NR | NR | NR | <10 min | FPGA optical flow control platform | [28] |
| Digital droplet microfluidic platform | High sensitivity | qPCR | N | NR | NR | Dielectric electrowetting | [29] |
| Sample-in–result-out integrated microfluidic platform | Step simplification and High integration | qPCR | N | 9 copies/rnx | 10 min | Use a saliva sample directly | [36] |
| Array-based microfluidic chip | Reduce detection time | PF-PCR | E | 259/rnx | 326 s | Pressure driven, vacuum assisted plasma nanocolumn array | [37] |
| Sample-in–result-out integrated microfluidic platform Multiplexing platform | High integration | qPCR | ORF1ab | 1 copies/uL | <90 min | Pressure driven, parallel channel multiplexing | [38] |
| Digital droplet microfluidic platform | High accuracy | dPCR | N, ORF1ab | 5 copies/rnx | <15 min | In situ array heater | [39] |
| Centrifugal microfluidic platform | High throughput and visualization | qPCR | N, E | 5 copies/rnx | 70 min | Centrifugal force drive, 3D printing technology | [40] |
| Sample-in–result-out integrated microfluidic platform | High accuracy and integration | LAMP | N, E, and ORF1ab | 5 × 103 copies/rnx | <90 min | Pneumatic film based micropump | [53] |
| Centrifugal microfluidic platform | High accuracy and integration | LAMP | N, S, and ORF1ab | 2 copies/µL | <90 min | Centrifugal force drive, smartphone modularization | [54] |
| Centrifugal microfluidic platform | High accuracy and integration | LAMP | ORF1ab | 100 copies/rnx | <60 min | Capillary action driven, multifunctional agarose bead strategy | [55] |
| Sample-in–result-out integrated microfluidic platform | High accuracy and integration | RPA | N | 30 copies/rnx | 30 min | Capillary driven, integrated lateral flow test bar, visible | [56] |
| Digital droplet microfluidic platform | High sensitivity | dLAMP | NR | 185 copies/mL | NR | Droplet array sliding chip | [57] |
| A microfluidic platform based on capillary interaction | High sensitivity | RCA | ORF1ab | 0.7 aM/rxn | 5–15 min | DNA hydrogel | [58] |
| Sample-in–result-out integrated microfluidic platform | High degree of automation and integration | LFA-CRISPR-RPA | N | 10 copies/rnx | 45 min | warm bag as heat source | [67] |
| Electric field mediated microfluidic platform | High degree of automation | CRISPR | N, E | 10 copies/uL | 30–40 min | Electric field mediated microfluidic iso-electrophoresis for nucleic acid extraction | [68] |
| Digital droplet microfluidic platform | High sensitivity | CRISPR | N, ORF1ab | 5.2 copies/rnx | 30 min | Digital microfluidic | [69] |
| Array-based microfluidic chip | High throughput | CRISPR | N | aM | NR | Microporous array | [70] |
| Optical flow control platform | High sensitivity | Molecular hybridization | N, ORF1ab | 1.0 pM | 20 min | Probe DNA-RNA-DNA | [71] |
| Electric chemistry microfluidic | High accuracy | Molecular hybridization | N, E, and ORF1ab | 600 copies/ mL | 40 min | graphene and poly-lysine materials | [72] |
| Electric chemistry microfluidic | High sensitivity | Molecular hybridization | S | 7 copies/uL | 20 min | Integrated reconfigurable enzyme-DNA nanostructures | [73] |
| Microfluidic Platform | Improvement | Method | Protein | LOD | Time | Test Method | Characteristic | Reference |
|---|---|---|---|---|---|---|---|---|
| μPADs | Step simplification | Electrochemistry | NR | NR | 30 min | Electrochemical | ZnO nanowires were synthesized directly | [22] |
| Digital droplet microfluidic platform | High sensitivity | Indirect immunofluorescence | Anti-N | NR | <5 min | Fluorescence | Nanogap filling and digital fluid control | [72] |
| Array-based microfluidic chip | High sensitivity and throughput | Indirect immunofluorescence | Anti-N, S and RBD | 1.6 ng/mL | 2.6 h | Fluorescence | Pressure pumping, microarray | [73] |
| Sample in- result out integrated microfluidic platform | High sensitivity | Indirect immunofluorescence | VP | NR | 15 min | Fluorescence | Smartphone | [74] |
| μPADs | Improved sampling mode | Direct immunofluorescence | N | 10 ag/uL | 20 min | Fluorescence | Capillary action, mouthwash | [75] |
| Array-based microfluidic chip | Improved sampling mode | Indirect immunofluorescence | Anti-S | 327 pg/unit | NR | Fluorescence | Microarray robot | [76] |
| μPADs | Improved sampling mode | Direct immunofluorescence | Anti-N | 200 pg/mL | 30 min | Fluorescence | Air sample collection | [77] |
| Array-based microfluidic chip | High degree of automation | ELISA | Anti-S | NR | <2.5 h | Colorimetric | Smartphone | [78] |
| μPADs | High degree of automation | ELISA | Anti-RBD | NR | 4–5 h | Fluorescence | Foldable paper-based pair | [79] |
| Sample in- result out integrated microfluidic platform | Reduce detection time | ELISA | N | 4.14 pg/mL | 5 min | Fluorescence | Reverse phase flow immunocoupling technique | [80] |
| Optical flow control platform | Mass production and high sensitivity | Label-free optical immunoassay | Anti-RBD | 0.08 ng/mL | <30 min | Reflected light | Peak shift of local surface plasmon resonance (LSPR) wavelength in gold nanorods | [81] |
| Optical flow control platform | Mass production and high sensitivity | Label-free optical immunoassay | Anti-RBD | NR | 3 min | Absorbance | Photonic ring harmonic oscillator | [82] |
| Optical flow control platform | Reduce detection time and high sensitivity | Label-free optical immunoassay | Anti-S | 0.82 ng/mL 0.45 ng/mL | 7 min | Reflected light | All-fiber Fresnel reflection | [83] |
| Micromotor array chip | High sensitivity | Label-free electrochemical immunoassay | N | fg/mL | 15 s | Electrochemical | Solid-liquid interface capacitance | [84] |
| Electric chemistry microfluidic | High sensitivity | Label-free electrochemical immunoassay | S | 8 fg/mL | 20 min | Electrochemical | Graphene oxide | [85] |
| Product | Manufacturer Name |
|---|---|
| Simplified steps | Sample collection method; capillary driven; 3D printing; paper-based microfluidic |
| Reduced time | Small volume and large surface area array; heating cycle in-situ heater array; digital droplet efficient mixed; centrifugal microfluidic |
| High throughput | Array; centrifugal microfluidic |
| Multiplexing | Centrifugal microfluidic; multiple closed reaction chambers |
| Improved accuracy | Strategy of sealing multifunctional agarose beads; closed reaction chambers |
| Improved sensitivity | Digital droplet; DNA hydrogel; graphene and poly 1-lysine material; E-construct enzyme-DNA nanostructure; photonic ring oscillator |
| Improved integration and automation | Valve; setting air cavity; nano-array; centrifugal microfluidic; pneumatic film-based micropump; warm handbag as heat source; smartphone modularization |
| Product | Manufacturer Name | City and Country | Type of Platform | Target | Limit of Detection | Processing Time (Minutes) | Reference |
|---|---|---|---|---|---|---|---|
| ID NOW™ COVID-19 | Abbott Diagnostics Scarborough, Inc. | Illinois, USA | NEAR | RdRp gene | 125 genome equivalents per mL | 15 | [86] |
| Foaming Test | Pharma Nona | Udine, Italy | POC/Near POC | N Gene | NR | 1 | [87] |
| Vita PCR™ SARS-CoV-2 Gen2 Assay | Credo Diagnostics Biomedical Pte. Ltd. | New Taipei, Singapore | RT-PCR | N Gene | 30 copies/reaction | 25 | [88] |
| The Bio-Fire® Respiratory 2.1-EZ (RP2.1-EZ) Panel (EUA) | Bio-Fire Diagnostics, LLC | Utah, USA | RT-PCR | S, E Gene | 500 copies/L | 45 | [89] |
| Xpert Xpress SARS-CoV-2 test | Cepheid | California, USA | RT-PCR | N, E gene | NR | 45 | [90] |
| 1copy COVID-19 qPCR Kit | 1drop Inc | Seongnam, Republic of Korea | RT-PCR | E and RdRp gene | 200 copies/L | 22 | [91] |
| Biosynex COVID-19 Ag+ BSS Rapid Test | BIOSYNEX S.A., Switzerland | llkirch-graffenstaden, France | RT-PCR | N-protein | 750 TCID50/mL | 10 | [92] |
| AQ-TOP COVID-9 Rapid Detection Kit PLUS | SEASUN BIOMATERIALS | Seoul, Republic of Korea | RT-PCR | Orf1ab | 1 copy/uL in single reaction | 30 | [93] |
| Novel Coronavirus (2019-nCoV) RT-PCR Detection Kit (Lyophilized) | Shanghai Chuangkun Bitech Inc. | Shanghai, China | RT-PCR | S | 500 copies/uL | 70 | [94] |
| SARS-CoV-2 IgM/IgG Antibody Assay Kit | Zybio Inc. | Chongqing, China | Colloidal Gold method | NR | NR | 15 | [95] |
| Lucira COVID-19 All-In-One Test Kit | Lucira Health, Inc. | Delaware, United States | RT-LAMP | N Gene | 1100 TCID50/mL | 30 | [96] |
| Respiratory Virus Nucleic Acid Detection kit | CapitalBio Technology | Beijing, China | Isothermal amplification | N | NR | 90 | [97] |
| Omnia SARS-CoV-2 | Qorvo Biotechnologies | Minnesota, USA | Antigen immunoassay | Protein | NR | ~20 | [98] |
| LumiraDx SARS-CoV-2 Ag test | LumiraDx | London, United Kingdom | Antigen immunoassay | N-protein | 32 TCID50/mL | 12 | [99] |
| Sampinute COVID-19 | Celltrion | Incheon, Republic of Korea | Antigen immunoassay | N, S-protein | NR | 30–45 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhou, X.; Wang, Q.; Liu, W.; Chen, C. Microfluidics for COVID-19: From Current Work to Future Perspective. Biosensors 2023, 13, 163. https://doi.org/10.3390/bios13020163
Li Q, Zhou X, Wang Q, Liu W, Chen C. Microfluidics for COVID-19: From Current Work to Future Perspective. Biosensors. 2023; 13(2):163. https://doi.org/10.3390/bios13020163
Chicago/Turabian StyleLi, Qi, Xingchen Zhou, Qian Wang, Wenfang Liu, and Chuanpin Chen. 2023. "Microfluidics for COVID-19: From Current Work to Future Perspective" Biosensors 13, no. 2: 163. https://doi.org/10.3390/bios13020163
APA StyleLi, Q., Zhou, X., Wang, Q., Liu, W., & Chen, C. (2023). Microfluidics for COVID-19: From Current Work to Future Perspective. Biosensors, 13(2), 163. https://doi.org/10.3390/bios13020163





