A Novel Aptamer Biosensor Based on a Localized Surface Plasmon Resonance Sensing Chip for High-Sensitivity and Rapid Enrofloxacin Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
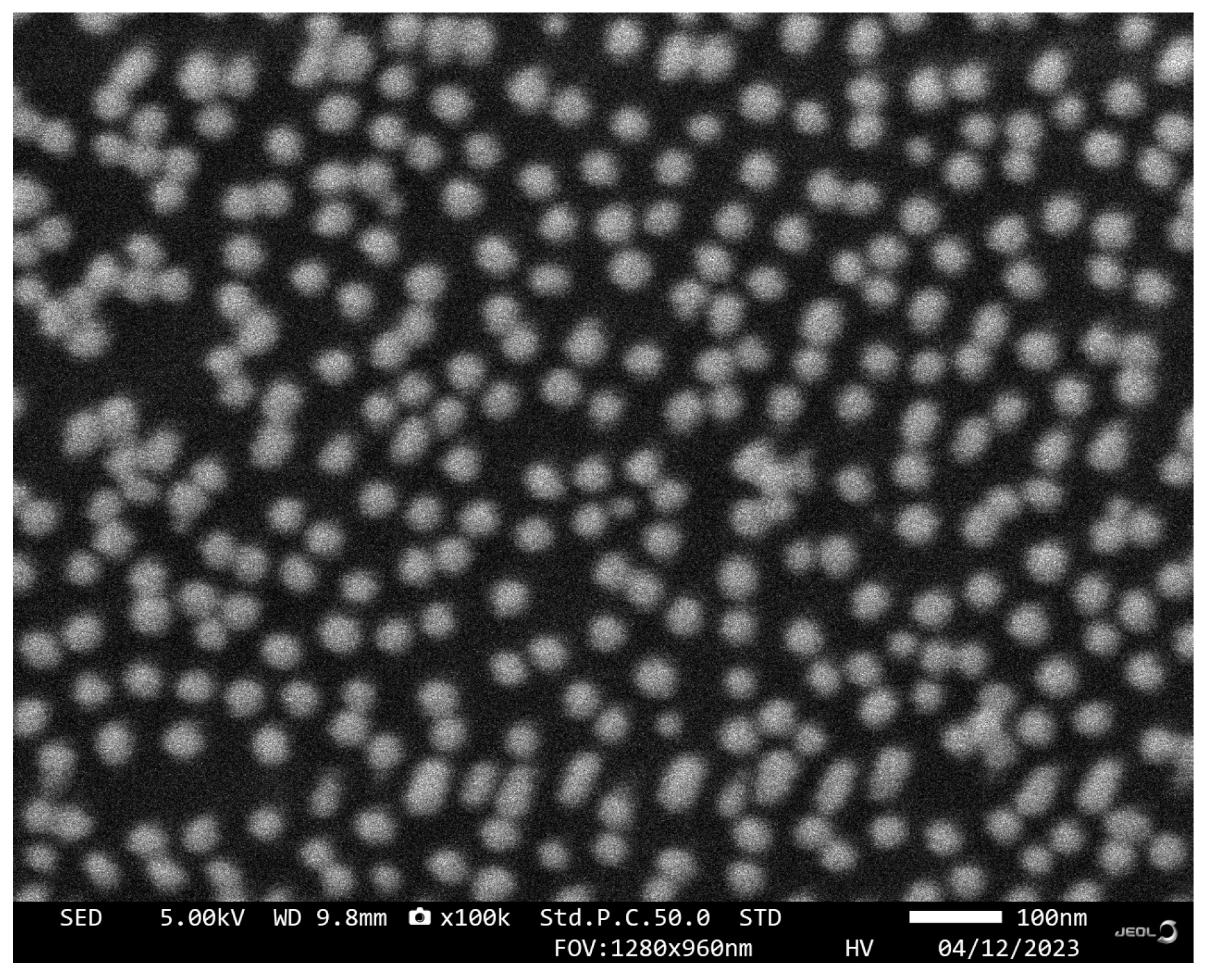
2.2. Preparation of AuNPs
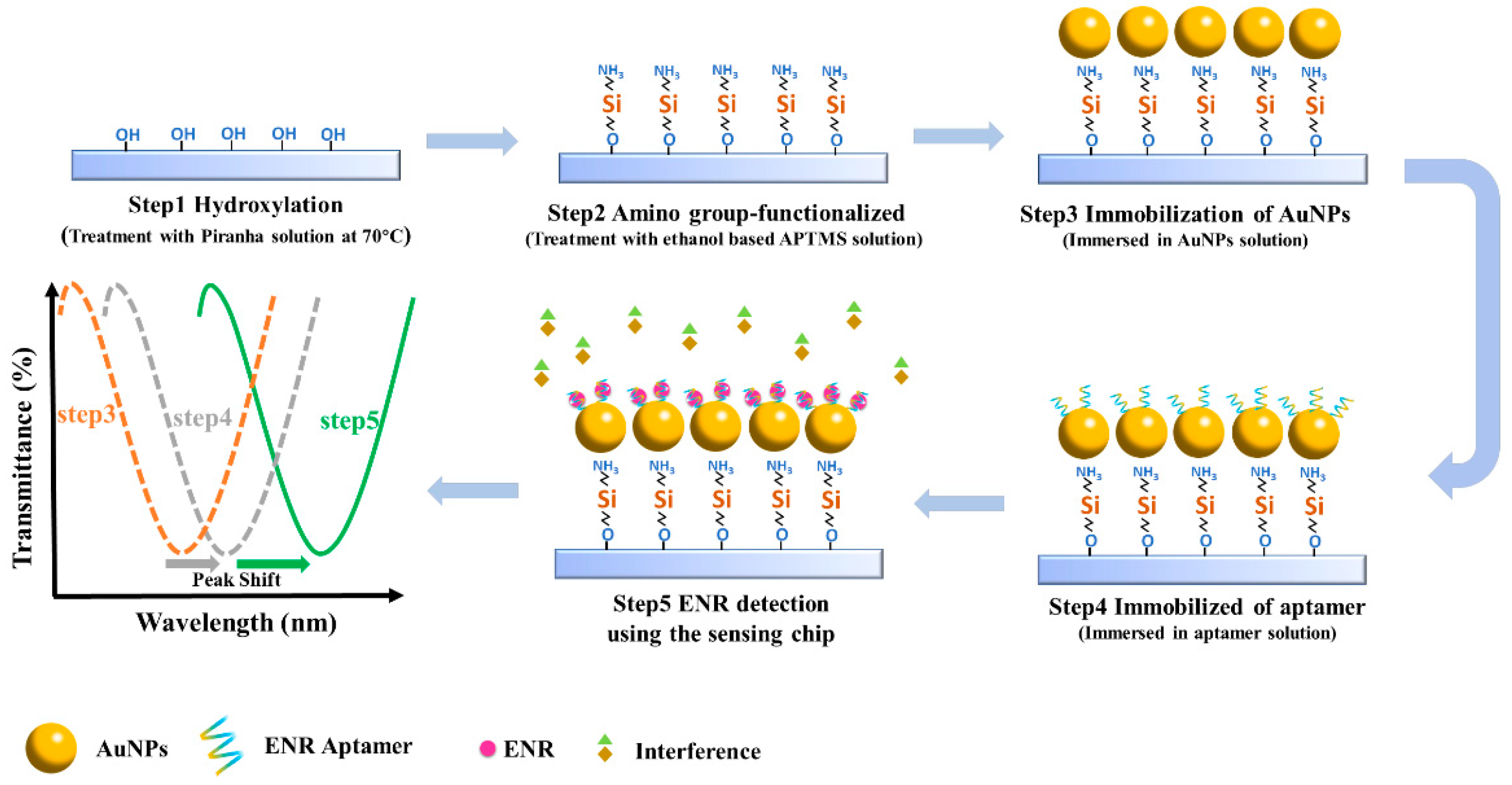
2.3. Preparation of the LSPR Aptamer Sensing Chip
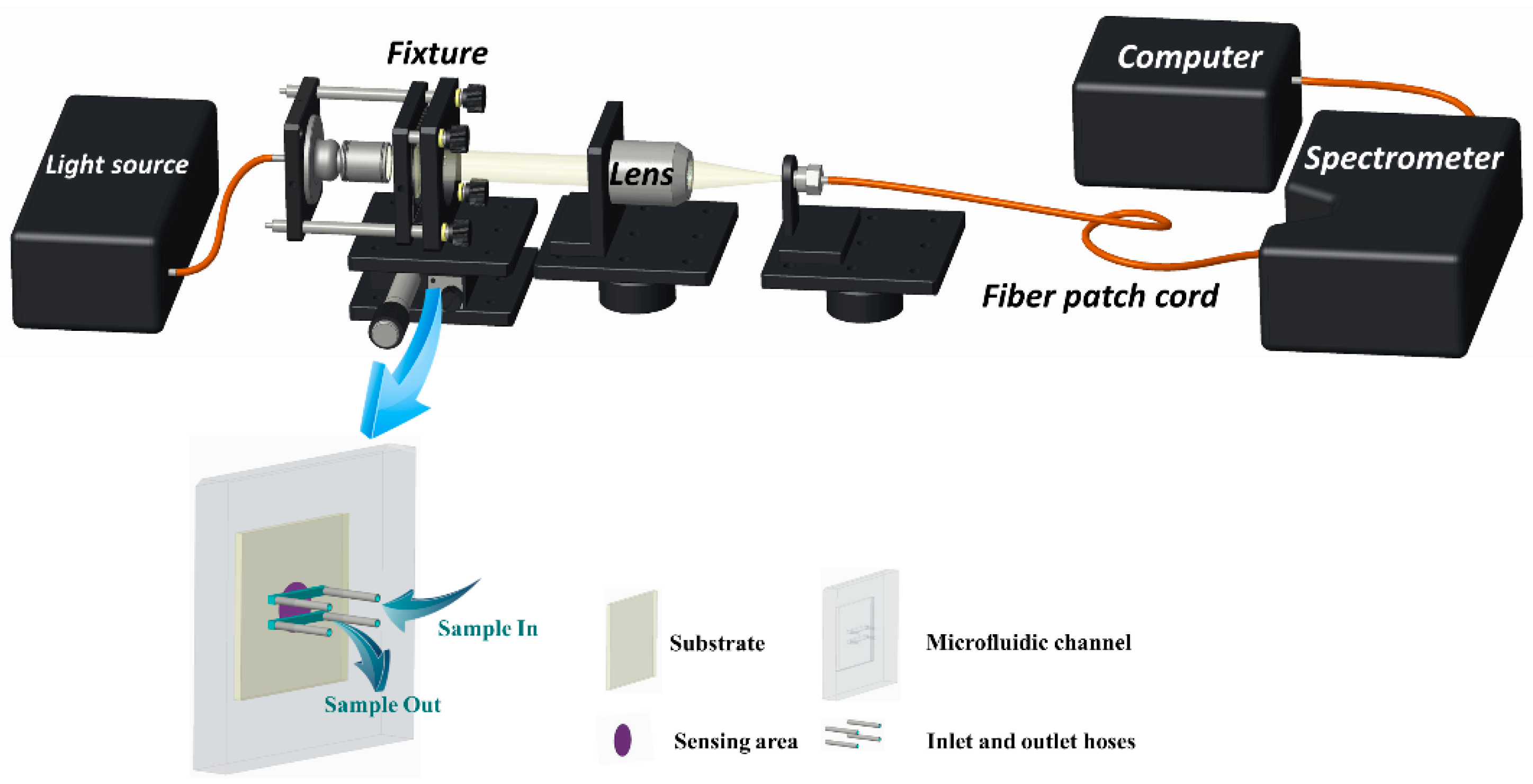
2.4. Construction of the LSPR Sensing Platform
2.5. ENR Detection
2.6. Characterization
3. Results and Discussion
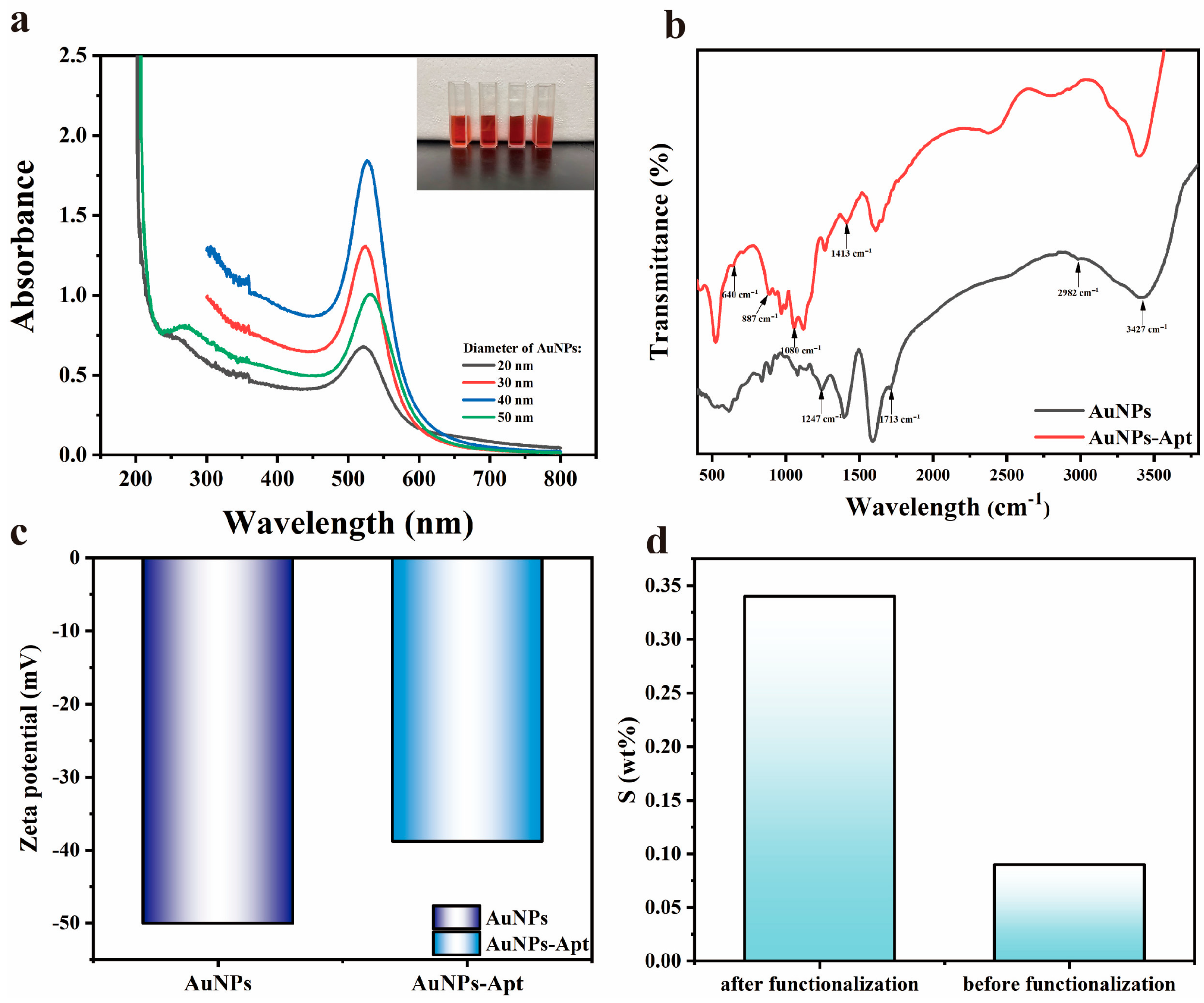
3.1. Characterization of the AuNPs-Apt and LSPR Sensing Chip
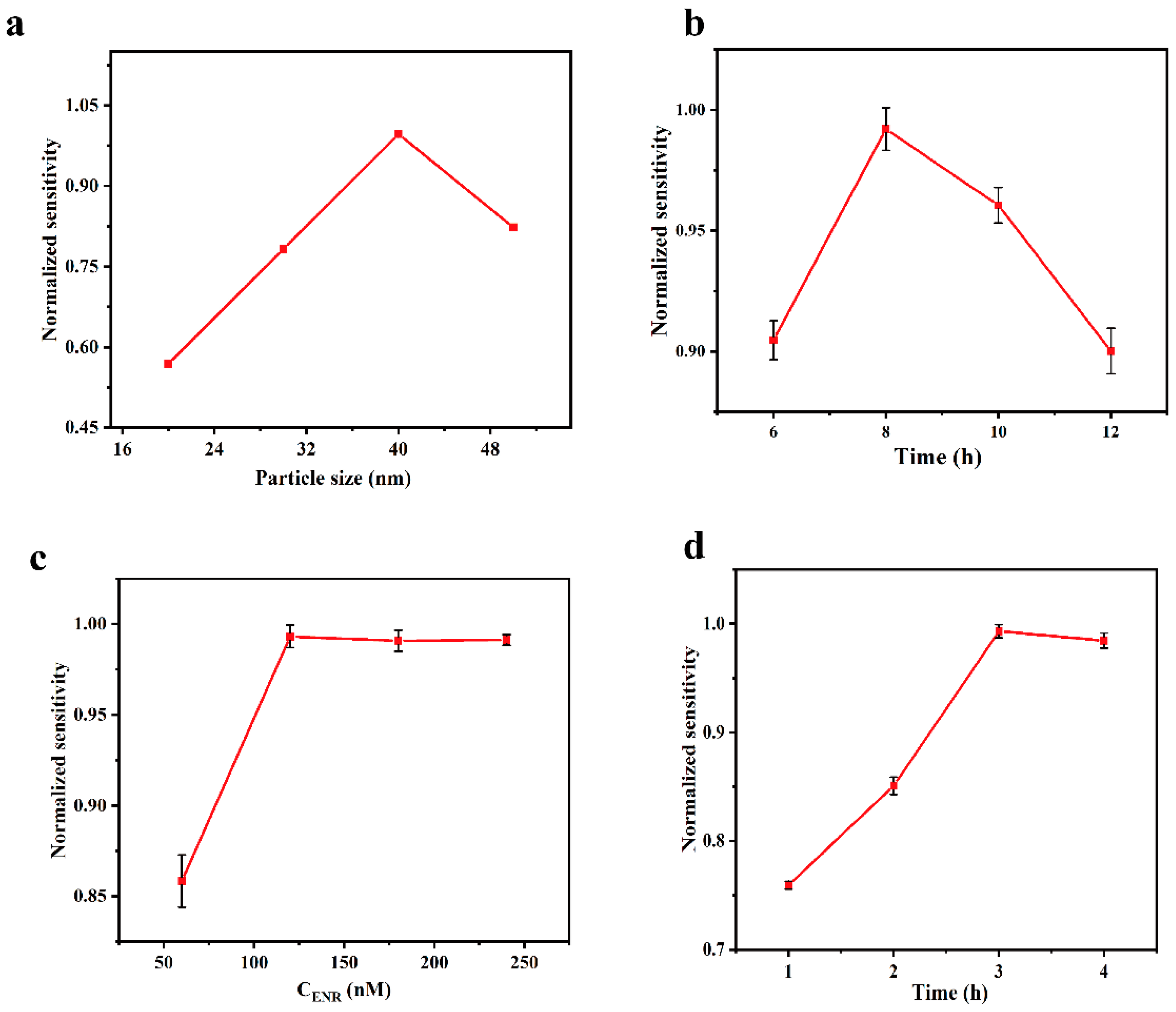
3.2. Optimization of the Experimental Conditions
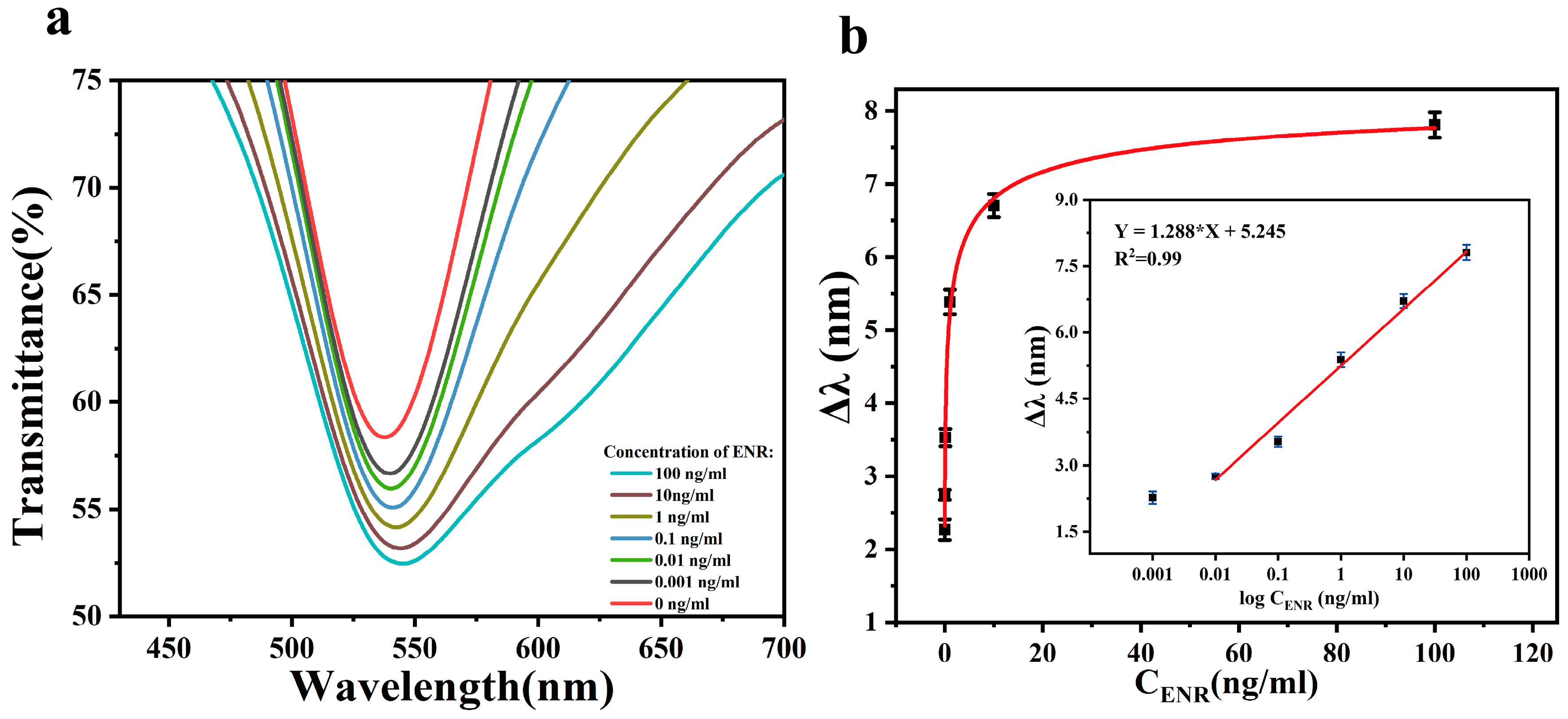
3.3. ENR Analysis Using Standard Solutions
3.4. Interference Immunity of the LSPR Sensing Chip
3.5. Comprehensive Performance of the LSPR Sensing Chip
3.6. Analysis of ENR in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwon, D.; Lee, H.; Yoo, H.; Hwang, J.; Lee, D.; Jeon, S. Facile method for enrofloxacin detection in milk using a personal glucose meter. Sens. Actuators B Chem. 2018, 254, 935–939. [Google Scholar] [CrossRef]
- Zhang, G.; Lai, X.; He, W.; Su, L.; Zhang, G.; Lai, W.; Deng, S. High-flux smartphone-integrated lateral flow assay based on chrysanthemum-like Au@polydopamine for sensitive detection of enrofloxacin in milk. Lab Chip 2023, 23, 3207–3216. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Dong, W.; Wei, K.; Li, J.; Sun, J.; Wang, S.; Mao, X. A colorimetric aptasensor fabricated with group-specific split aptamers and complex nanozyme for enrofloxacin and ciprofloxacin determination. J. Hazard. Mater. 2023, 458, 131995. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, B.; Gu, X.; Li, M.; Qi, K.; Yan, Y. Detection of enrofloxacin residues in dairy products based on their fluorescence quenching effect on AgInS2 QDs. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 301, 122985. [Google Scholar] [CrossRef]
- Yuting, L.; Jing, Z.; Donghui, L. Preparation of cadmium sulfide nanoparticles and their application for improving the properties of the electrochemical sensor for the determination of enrofloxacin in real samples. Chirality 2019, 31, 174–184. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Kuang, L.; Li, Y.; Chen, L.; Lin, C.; Wang, L.; Song, Y. Benzotrithiophene-based covalent organic frameworks for real-time visual onsite assays of enrofloxacin. Biosens. Bioelectron. 2022, 214, 114527. [Google Scholar] [CrossRef]
- Jin, X.; Liu, S.; Zhang, Z.; Liu, T.; Li, N.; Liang, Y.; Zheng, J.; Peng, N. Enrofloxacin-induced transfer of multiple-antibiotic resistance genes and emergence of novel resistant bacteria in red swamp crayfish guts and pond sediments. J. Hazard. Mater. 2023, 443, 130261. [Google Scholar] [CrossRef]
- Hardie, K.R. Antimicrobial resistance: The good, the bad, and the ugly. Emerg. Top. Life Sci. 2020, 4, 129–136. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Zhang, M.; You, F.; Hao, N.; Ding, C.; Wang, K. Simultaneous detection of enrofloxacin and ciprofloxacin in milk using a bias potentials controlling-based photoelectrochemical aptasensor. J. Hazard. Mater. 2021, 416, 125988. [Google Scholar] [CrossRef]
- Tumini, M.; Nagel, O.; Molina, M.P.; Althaus, R. Microbiological assay with Bacillus licheniformis for the easy detection of quinolones in milk. Int. Dairy, J. 2017, 64, 9–13. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Wang, Z.; Sun, Y.; Liu, Q.; Dong, W.; Hao, A. Fluorescent carbon dots based sensing system for detection of enrofloxacin in water solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Nie, L.; Han, L.; Wen, W.; Wang, J.; Wang, S. Glutathione-Capped CdTe Quantum Dots Based Sensors for Detection of H2O2 and Enrofloxacin in Foods Samples. Foods 2023, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, L.; Dong, G.-Y. Two dual-responsive luminescent coordination polymers for highly effective sensing of enrofloxacin and Al3+ ions. Inorganica Chim. Acta 2022, 540, 121064. [Google Scholar] [CrossRef]
- Wu, S.; Mao, J.; Zhang, Y.; Wang, S.; Huo, M.; Guo, H. Sensitive electrochemical detection of enrofloxacin in eggs based on carboxylated multi-walled carbon nanotubes-reduced graphene oxide nanocomposites: Molecularlyimprintedrecognition versus direct electrocatalytic oxidation. Food Chem. 2023, 413, 135579. [Google Scholar] [CrossRef] [PubMed]
- Dönmez, F.; Yardım, Y.; Şentürk, Z. Electroanalytical determination of enrofloxacin based on the enhancement effect of the anionic surfactant at anodically pretreated boron-doped diamond electrode. Diam. Relat. Mater. 2018, 84, 95–102. [Google Scholar] [CrossRef]
- Cháfer-Pericás, C.; Maquieira, Á.; Puchades, R. Fast screening methods to detect antibiotic residues in food samples. TrAC Trends Anal. Chem. 2010, 29, 1038–1049. [Google Scholar] [CrossRef]
- Chen, J.; Ding, L.; Zhao, J.; Jiang, X.; Ma, F.; Li, H.; Zhang, Y. A L-glutamine binding protein modified MNM structured optical fiber biosensor based on surface plasmon resonance sensing for detection of L-glutamine metabolism in vitro embryo culture. Biosens. Bioelectron. 2023, 237, 115537. [Google Scholar] [CrossRef]
- Yu, S.; Ding, L.; Lin, H.; Wu, W.; Huang, J. A novel optical fiber glucose biosensor based on carbon quantum dots-glucose oxidase/cellulose acetate complex sensitive film. Biosens. Bioelectron. 2019, 146, 111760. [Google Scholar] [CrossRef]
- Butt, M.A.; Kaźmierczak, A.; Kazanskiy, N.L.; Khonina, S.N. Metal-Insulator-Metal Waveguide-Based Racetrack Integrated Circular Cavity for Refractive Index Sensing Application. Electronics 2021, 10, 1419. [Google Scholar] [CrossRef]
- Chou Chau, Y.-F.; Chou Chao, C.-T.; Huang, H.J.; Anwar, U.; Lim, C.M.; Voo, N.Y.; Mahadi, A.H.; Kumara, N.T.R.N.; Chiang, H.-P. Plasmonic perfect absorber based on metal nanorod arrays connected with veins. Results Phys. 2019, 15, 102567. [Google Scholar] [CrossRef]
- Kravets, V.G.; Wu, F.; Yu, T.; Grigorenko, A.N. Metal-Dielectric-Graphene Hybrid Heterostructures with Enhanced Surface Plasmon Resonance Sensitivity Based on Amplitude and Phase Measurements. Plasmonics 2022, 17, 973–987. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Alapan, Y.; ElKabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; De Luca, A.; Strangi, G. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 2016, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Chen, P.C.; Mwakwari, S.C.; Oyelere, A.K. Gold nanoparticles: From nanomedicine to nanosensing. Nanotechnol. Sci. Appl. 2008, 1, 45–65. [Google Scholar] [PubMed]
- Ramalingam, V. Multifunctionality of gold nanoparticles: Plausible and convincing properties. Adv. Colloid Interface Sci. 2019, 271, 101989. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yeung, J.; Zhou, J.; Retout, M.; Yim, W.; Fajtová, P.; Gosselin, B.; Jabin, I.; Bruylants, G.; Mattoussi, H.; et al. Empirical Optimization of Peptide Sequence and Nanoparticle Colloidal Stability: The Impact of Surface Ligands and Implications for Colorimetric Sensing. ACS Appl. Mater. Interfaces 2023, 15, 20483–20494. [Google Scholar] [CrossRef]
- Yang, T.; Luo, Z.; Tian, Y.; Qian, C.; Duan, Y. Design strategies of AuNPs-based nucleic acid colorimetric biosensors. TrAC Trends Anal. Chem. 2020, 124, 115795. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Kumar, S.; Singh, R.; Zhang, B.; Bai, C.; Pu, X. Development of Glucose Sensor Using Gold Nanoparticles and Glucose-Oxidase Functionalized Tapered Fiber Structure. Plasmonics 2020, 15, 841–848. [Google Scholar] [CrossRef]
- Špringer, T.; Ermini, M.L.; Špačková, B.; Jabloňků, J.; Homola, J. Enhancing Sensitivity of Surface Plasmon Resonance Biosensors by Functionalized Gold Nanoparticles: Size Matters. Anal. Chem. 2014, 86, 10350–10356. [Google Scholar] [CrossRef]
- Melikishvili, S.; Piovarci, I.; Hianik, T. Advances in Colorimetric Assay Based on AuNPs Modified by Proteins and Nucleic Acid Aptamers. Chemosensors 2021, 9, 281. [Google Scholar] [CrossRef]
- Guo, H.; Sun, Y.; Ma, P.; Khan, I.M.; Duan, N.; Wang, Z. Sensitive detection of patulin based on DNase I-assisted fluorescent aptasensor by using AuNCs-modified truncated aptamer. Food Control 2022, 131, 108430. [Google Scholar] [CrossRef]
- Ma, P.; Ye, H.; Guo, H.; Ma, X.; Yue, L.; Wang, Z. Aptamer truncation strategy assisted by molecular docking and sensitive detection of T-2 toxin using SYBR Green I as a signal amplifier. Food Chem. 2022, 381, 132171. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Canoura, J.; Guntupalli, B.; Lou, X.; Xiao, Y. A cooperative-binding split aptamer assay for rapid, specific and ultra-sensitive fluorescence detection of cocaine in saliva. Chem. Sci. 2017, 8, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Geleta, G.S. A colorimetric aptasensor based on gold nanoparticles for detection of microbial toxins: An alternative approach to conventional methods. Anal. Bioanal. Chem. 2022, 414, 7103–7122. [Google Scholar] [CrossRef]
- Kaur, H.; Shorie, M. Nanomaterial based aptasensors for clinical and environmental diagnostic applications. Nanoscale Adv. 2019, 1, 2123–2138. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Q.U.A.; Luo, Z.; Ali, R.; Khan, M.I.; Li, F.; Qiu, B. Advances in Gold Nanoparticles-Based Colorimetric Aptasensors for the Detection of Antibiotics: An Overview of the Past Decade. Nanomaterials 2021, 11, 840. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xiang, W.; Deng, Z.; Shi, K.; Wang, H.; Shi, H. Cocaine detection using aptamer and molybdenum disulfide-gold nanoparticle-based sensors. Nanomedicine 2020, 15, 325–335. [Google Scholar] [CrossRef]
- Yang, S.; Teng, Y.; Cao, Q.; Bai, C.; Fang, Z.; Xu, W. Electrochemical Sensor Based on Molecularly Imprinted Polymer-Aptamer Hybrid Receptor for Voltammetric Detection of Thrombin. J. Electrochem. Soc. 2019, 166, B23. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, M.; Liu, Y.; Wan, S.; Cui, C.; Zhang, L.; Tan, W. Aptamer/AuNP Biosensor for Colorimetric Profiling of Exosomal Proteins. Angew. Chem. Int. Ed. 2017, 56, 11916–11920. [Google Scholar] [CrossRef]
- Wei, P.; Wang, S.; Wang, W.; Niu, Z.; Rodas-Gonzalez, A.; Li, K.; Li, L.; Yang, Q. CoNi bimetallic metal–organic framework and gold nanoparticles-based aptamer electrochemical sensor for enrofloxacin detection. Appl. Surf. Sci. 2022, 604, 154369. [Google Scholar] [CrossRef]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 nm: Size Focusing versus Ostwald Ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV–Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ding, L.; Gong, P.; Huang, J. A colorimetric detection of microRNA-148a in gastric cancer by gold nanoparticle-RNA conjugates. Nanotechnology 2019, 31, 095501. [Google Scholar] [CrossRef] [PubMed]
- Foss, C.A., Jr.; Hornyak, G.L.; Stockert, J.A.; Martin, C.R. Template-Synthesized Nanoscopic Gold Particles: Optical Spectra and the Effects of Particle Size and Shape. J. Phys. Chem. 1994, 98, 2963–2971. [Google Scholar] [CrossRef]
- Ye, J.; Van Dorpe, P.; Van Roy, W.; Borghs, G.; Maes, G. Fabrication, Characterization, and Optical Properties of Gold Nanobowl Submonolayer Structures. Langmuir 2009, 25, 1822–1827. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, B.; Bao, Q.; Yang, T.; Wei, T.; Wang, J.; Mao, C.; Zhang, C.; Yang, M. Aptamer-modified sensitive nanobiosensors for the specific detection of antibiotics. J. Mater. Chem. B 2020, 8, 8607–8613. [Google Scholar] [CrossRef]
- Alhammadi, M.; Yoo, J.; Sonwal, S.; Park, S.Y.; Umapathi, R.; Oh, M.-H.; Huh, Y.S. A highly sensitive lateral flow immunoassay for the rapid and on-site detection of enrofloxacin in milk. Front. Nutr. 2022, 9, 1036826. [Google Scholar] [CrossRef]
- Cao, L.; Lin, H.; Mirsky, V.M. Surface plasmon resonance biosensor for enrofloxacin based on deoxyribonucleic acid. Anal. Chim. Acta 2007, 589, 1–5. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Liao, M.; Kidd, M.T.; Li, Y. Rapid detection of enrofloxacin using a localized surface plasmon resonance sensor based on polydopamine molecular imprinted recognition polymer. J. Food Meas. Charact. 2021, 15, 3376–3386. [Google Scholar] [CrossRef]

| Detection Method | Probe Used | Liner Range | LOD | Assay Time | Reference |
|---|---|---|---|---|---|
| Fluorescent biosensor | N-CDs | 1~15 μg/mL | 0.009 μg/mL | 4 min | [12] |
| Upconversion nanoparticle | CSUNPs | 0.976~62.5 ng/mL | 0.47 ng/mL | 2 h | [47] |
| Lateral flow immunoassay | AuNPs-Ab | 20~50 ng/mL | 20 ng/mL | 10 min | [48] |
| SPR | Au | 3~20 ng/mL | 3 μg/mL | — | [49] |
| LSPR | PDA-MIP | 25~1000 ng/mL | 61.1 ng/mL | 20 min | [50] |
| Electrochemistry | CoNi-MOF-AuNPs | 10−6~100 ng/mL | 3.3 × 10−3 pg/mL | 100 min | [41] |
| Proposed method | AuNPs-Apt | 0.01~100 ng/mL | 0.001 ng/mL | 20 min | This work |
| Interferent | Amount of Interferents Added (ng/mL) | Addition Amount of ENR C0 (ng/mL) | Detection Amount of ENR C (ng/mL) | Interference Ratio (%) |
|---|---|---|---|---|
| Ciprofloxacin | 100 | 10 | 10.23 | 2.3 |
| Pefloxacin | 100 | 10 | 9.76 | −2.4 |
| Norfloxacin | 100 | 10 | 9.72 | −2.8 |
| Ofloxacin | 100 | 10 | 10.26 | 2.6 |
| Na+ | 100 | 10 | 10.15 | 1.5 |
| K+ | 100 | 10 | 10.19 | 1.9 |
| Sample | Added Concentration (ng/mL) | Detected Concentration (ng/mL) | Recovery (%) (n = 3) | RSD (%) |
|---|---|---|---|---|
| 1 | 0.1 | 0.09194 | 91.94 | 3.26 |
| 2 | 1 | 1.0838 | 108.38 | 4.45 |
| 3 | 10 | 10.4757 | 104.75 | 3.53 |
| 4 | 100 | 98.0527 | 98.05 | 3.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Ding, L.; Zhang, Y.; Jiang, X. A Novel Aptamer Biosensor Based on a Localized Surface Plasmon Resonance Sensing Chip for High-Sensitivity and Rapid Enrofloxacin Detection. Biosensors 2023, 13, 1027. https://doi.org/10.3390/bios13121027
Wang P, Ding L, Zhang Y, Jiang X. A Novel Aptamer Biosensor Based on a Localized Surface Plasmon Resonance Sensing Chip for High-Sensitivity and Rapid Enrofloxacin Detection. Biosensors. 2023; 13(12):1027. https://doi.org/10.3390/bios13121027
Chicago/Turabian StyleWang, Pan, Liyun Ding, Yumei Zhang, and Xingdong Jiang. 2023. "A Novel Aptamer Biosensor Based on a Localized Surface Plasmon Resonance Sensing Chip for High-Sensitivity and Rapid Enrofloxacin Detection" Biosensors 13, no. 12: 1027. https://doi.org/10.3390/bios13121027
APA StyleWang, P., Ding, L., Zhang, Y., & Jiang, X. (2023). A Novel Aptamer Biosensor Based on a Localized Surface Plasmon Resonance Sensing Chip for High-Sensitivity and Rapid Enrofloxacin Detection. Biosensors, 13(12), 1027. https://doi.org/10.3390/bios13121027




