Biosensors for Odor Detection: A Review
Abstract
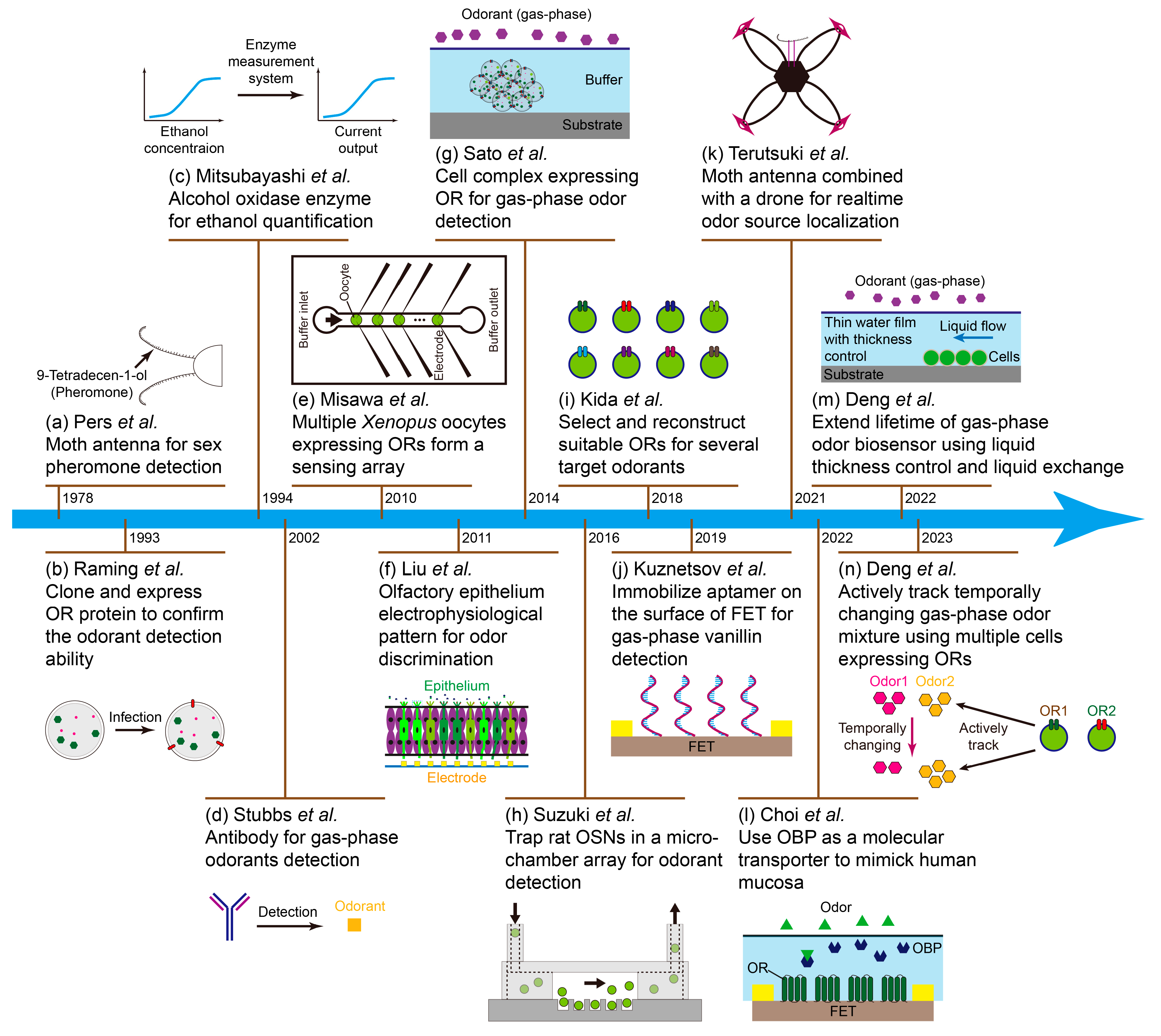
1. Introduction
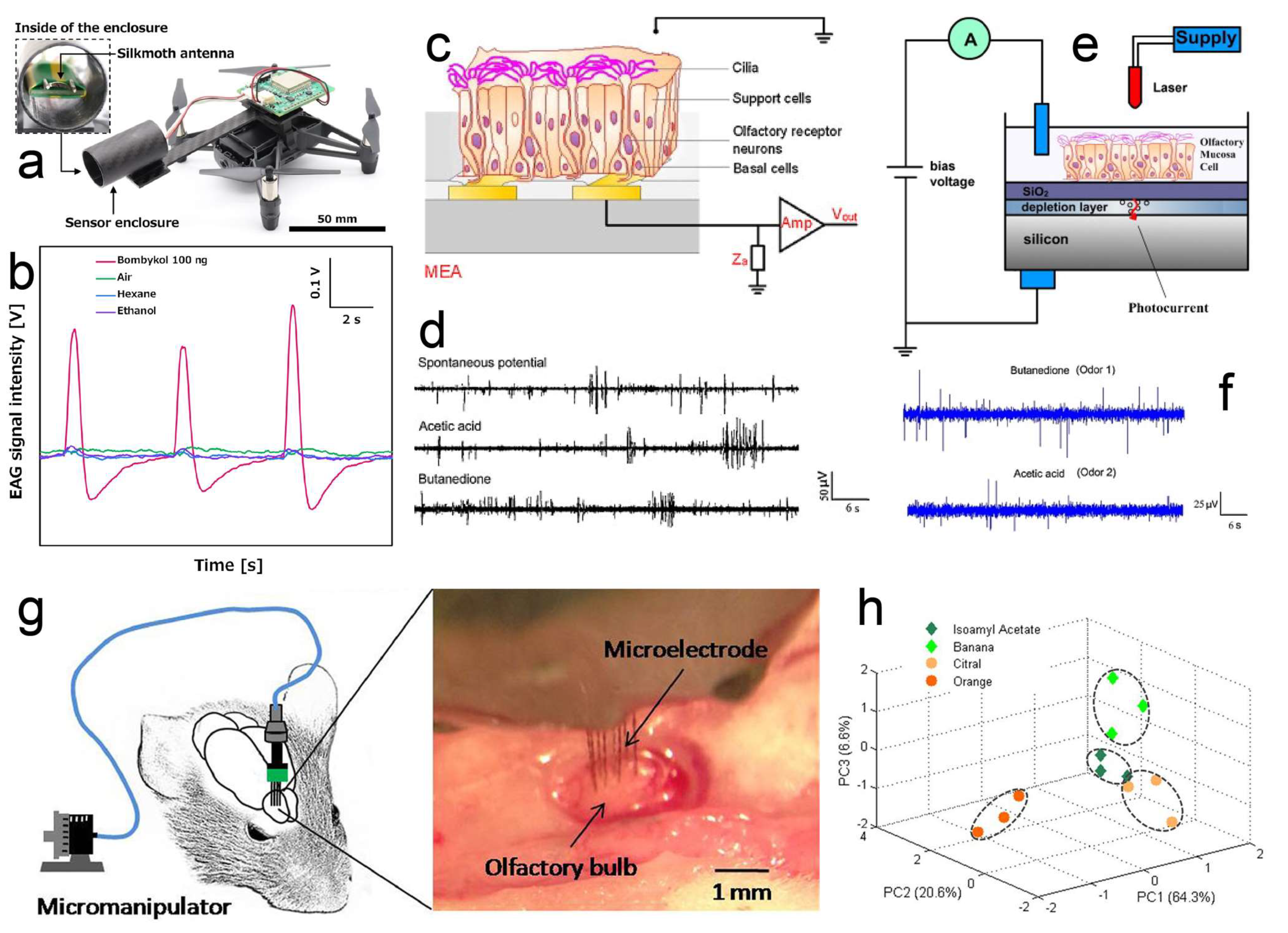
2. Organ/Tissue-Based Odor Biosensors
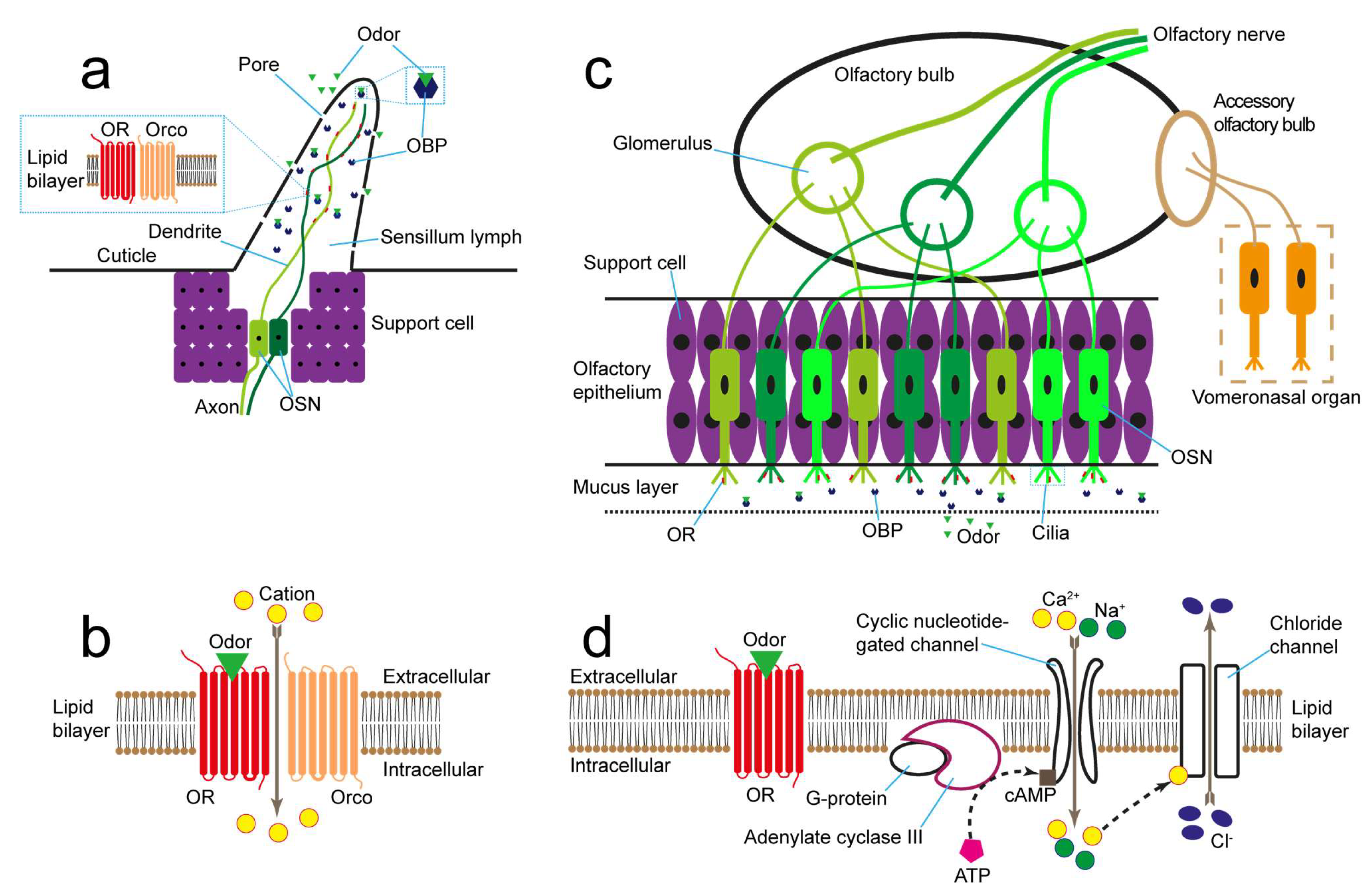
2.1. Antenna-Based Odor Biosensor
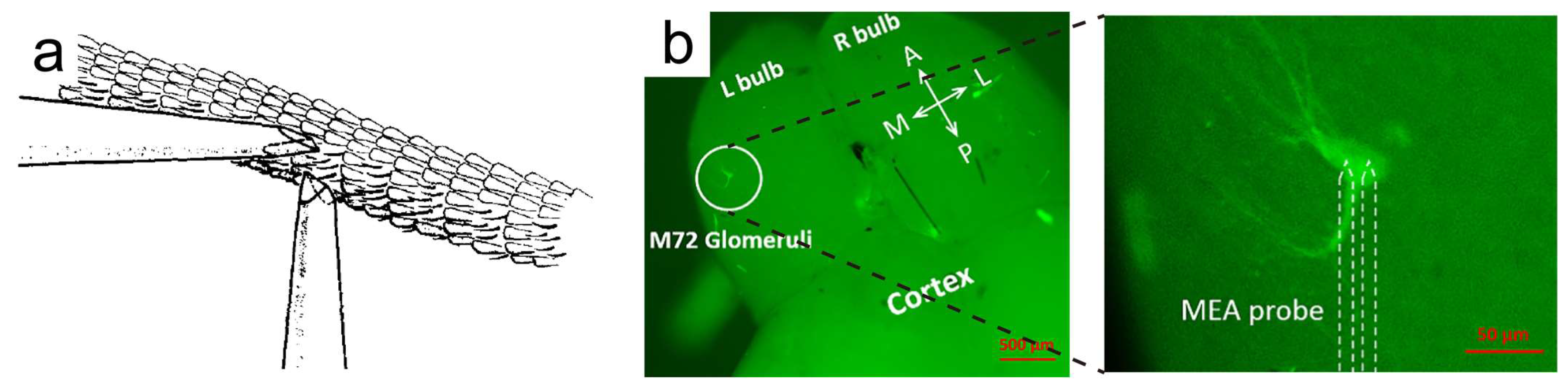
2.2. Olfactory-Epithelium- and Olfactory-Bulb-Based Odor Biosensors
3. Cell-Based Odor Biosensor
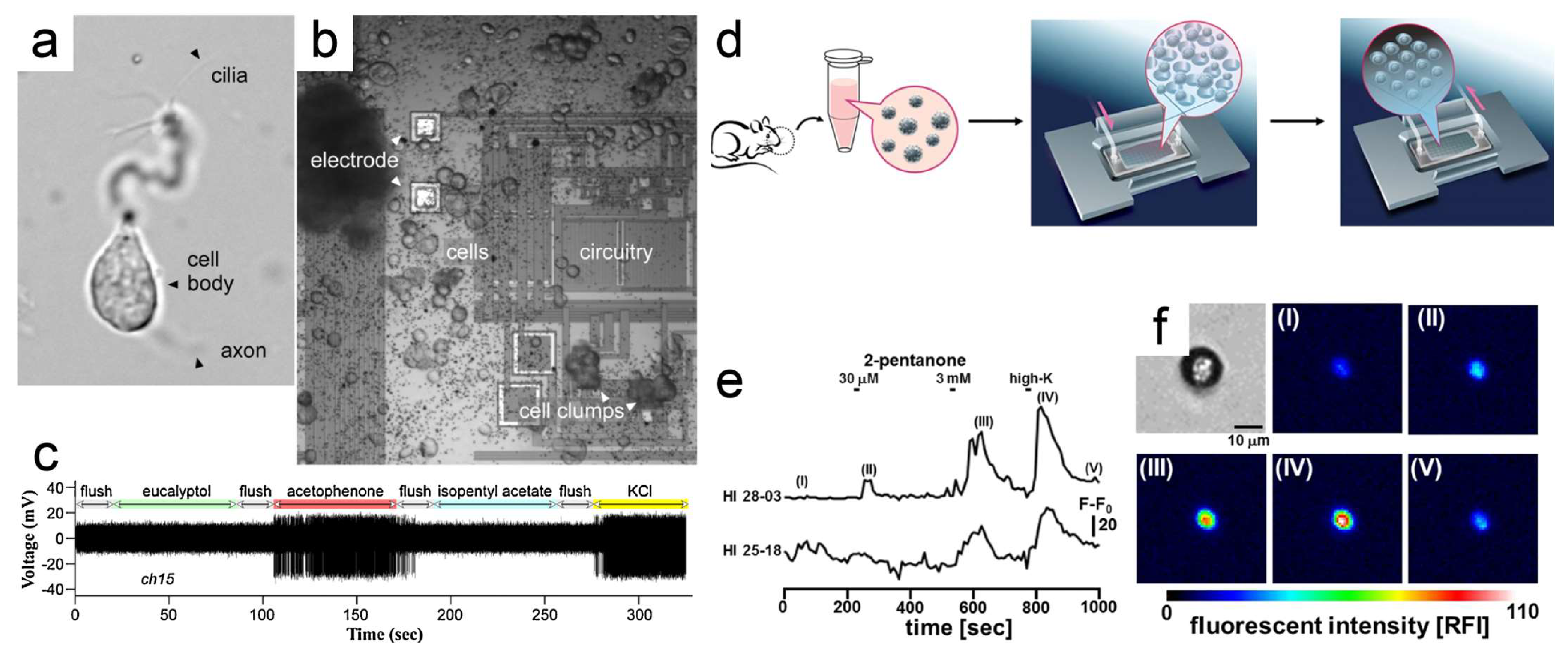
3.1. Olfactory Sensory-Neuron-Based Odor Biosensors
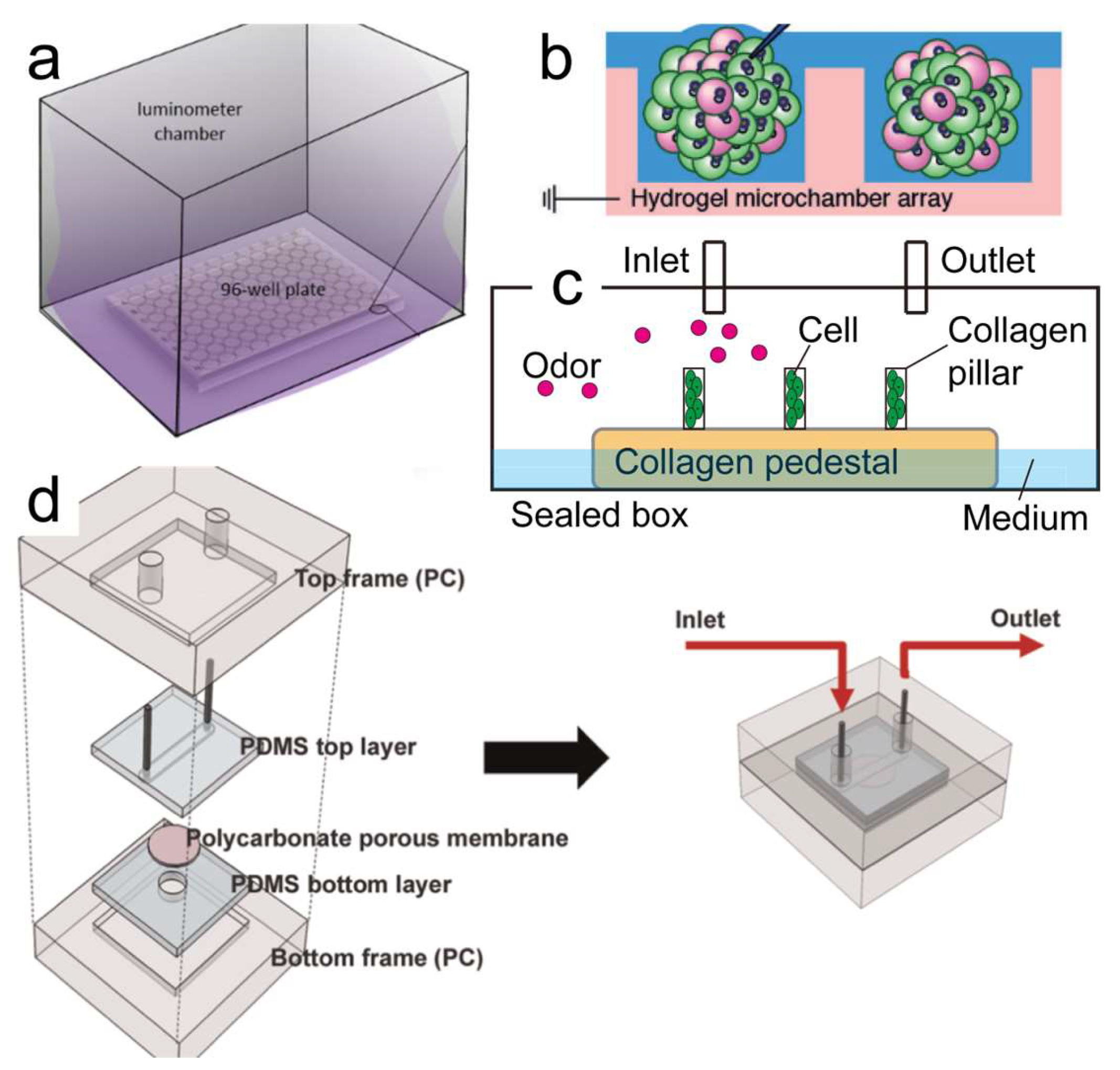
3.2. Cell’s Expressing Olfactory-Receptor-Based Odor Biosensor
| OR Type | Expressed on | Odorant | Sensing Method | Odorant Concentration | Importance | Ref. |
|---|---|---|---|---|---|---|
| Human OR 5 | E. coli | Lilial | Fluorescence | 0.2–1 mM | Glutathione S-transferase can improve the OR expression level | [99] |
| Mouse OR-EG | HEK293 | Eugenol | Fluorescence | 0.01–3 mM | Reconstituted mouse OR in HEK293 cell has a similar detection function to the original one | [100] |
| Rat I7 | Yeast | Octyl aldehyde | Fluorescence | 10–50 μM | Screen the proper OR that is sensitive to a specific odorant | [101] |
| Drosophila melanogaster Or85b | Xenopus oocytes | 2-Heptanoe | Electrode | 10–1000 nM | Build a highly sensitive portable odor biosensor | [43] |
| Caenorhabditis elegans ODR-10 | HEK293 | Diacetyl | LAPS | 10–100 nM | Label-free functional assays of olfactory receptor | [102] |
| Rat OR I7 | HEK293 | Octanal | SPR | 0.1–100 mM | Measure molecular interactions in realtime without any labeling | [103] |
| Caenorhabditis elegans ODR-10 | MCF-7 | Diacetyl | SAW | 10−10–10−4 mM | Build a highly sensitive odor biosensor | [104] |
| Rat OR I7 | HEK293 | Octanal | QCM | 10−8–100 mM | Find a linear relationship between response and the odorant concentration logarithmic value | [105] |
| Silk moth BmOR3 | Sf21 | Bombykal | FET | 1–10 μM | Explore the suitable surface for the cell expressing OR | [106] |
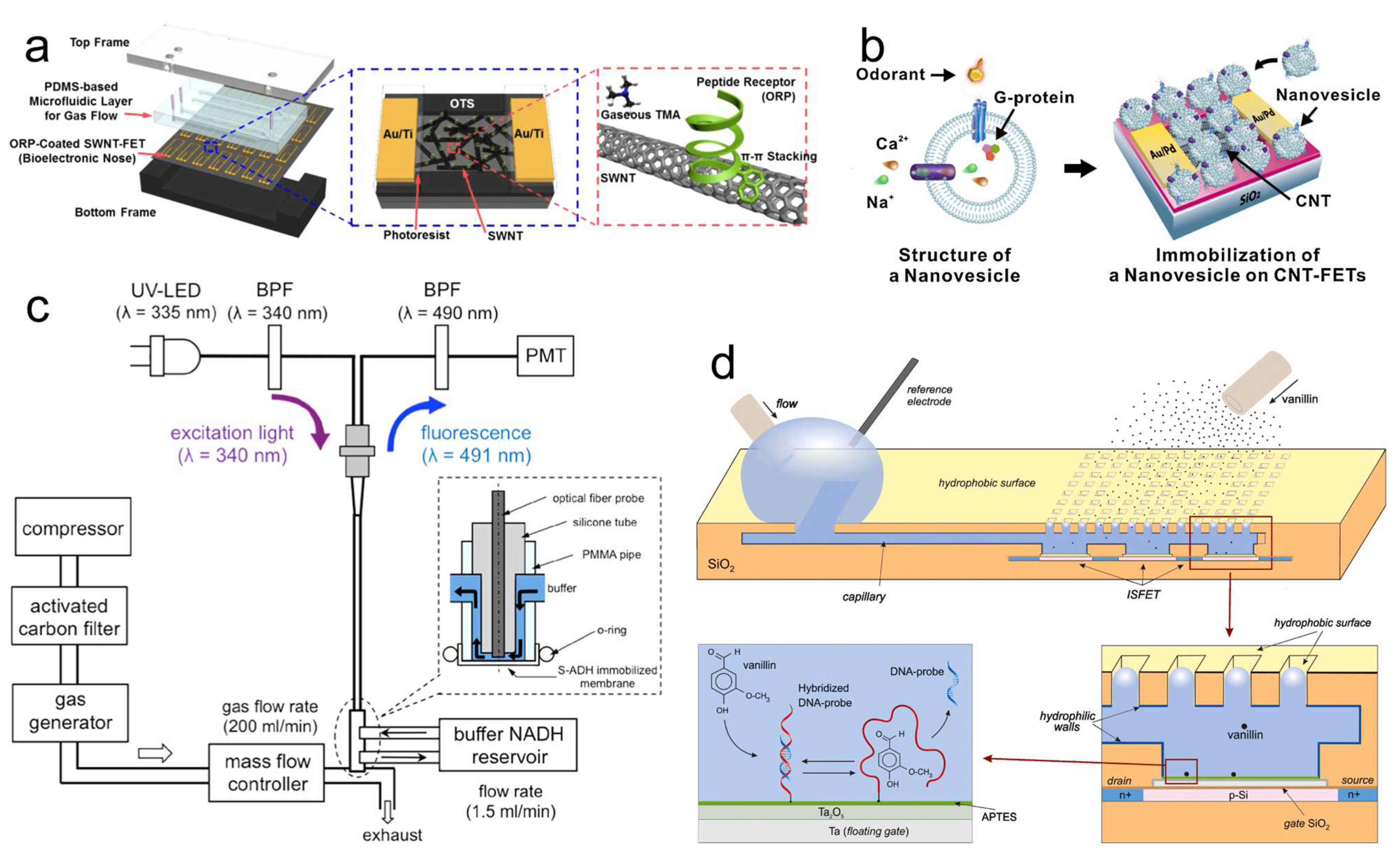
4. Protein-Based Odor Biosensor
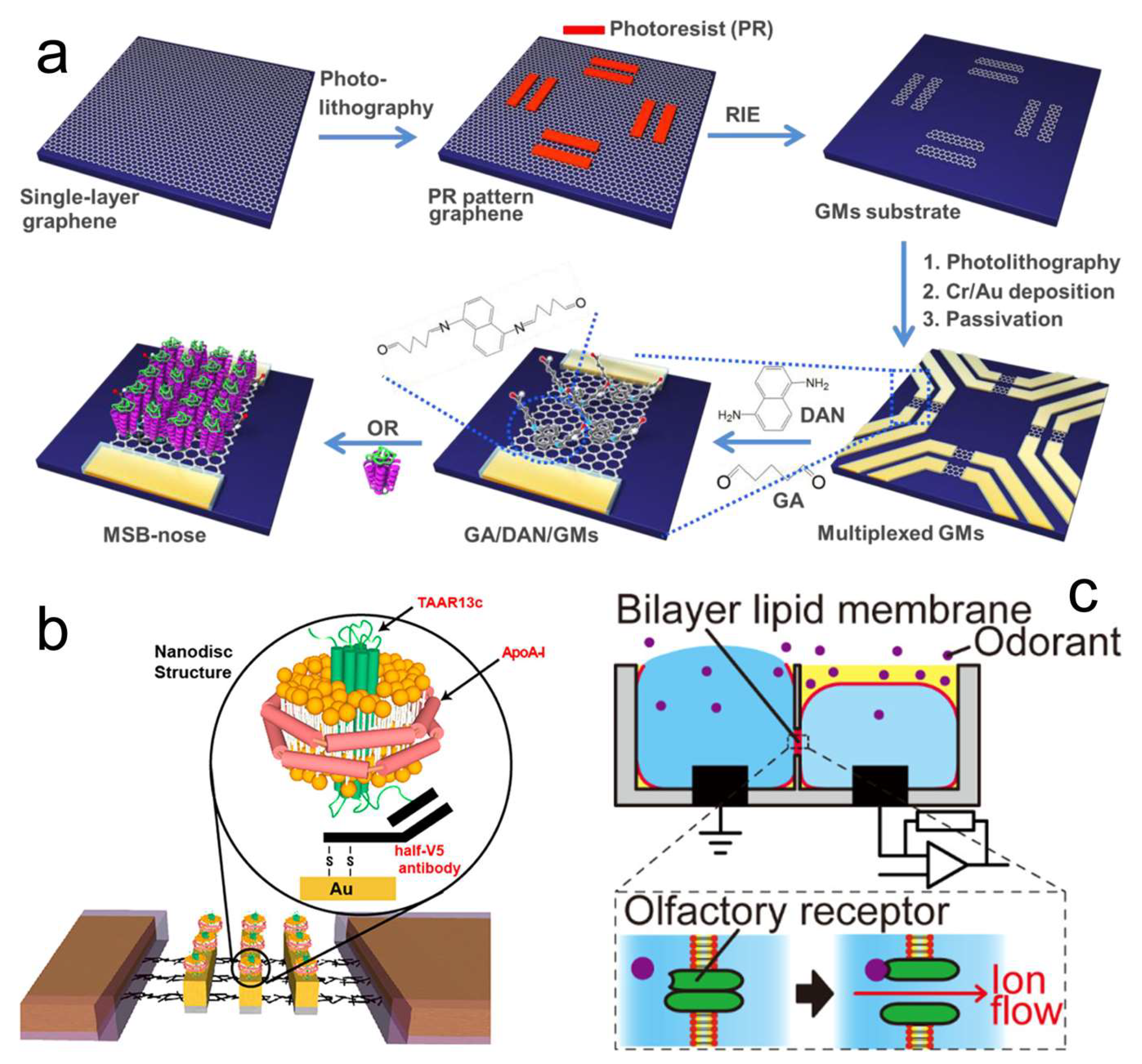
4.1. OR Protein-Based Odor Biosensors
4.2. OBP-Based Odor Biosensors
| Type | Sensing Material | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Organ/Tissue | Antenna | Low cost Good sensitivity | Low selectivity Low reproducibility Short lifetime | [39,69] |
| Olfactory epithelium/bulb | Low cost Multi-channel data | Complex operation Short lifetime | [72,153] | |
| Cell | OSN | Easy to form a large sensor array Good sensitivity and selectivity | Hard to obtain the desired OSN type Unable to subculture | [46,83] |
| Cell expressing OR | Low cost Easy for use Good sensitivity and selectivity Stable characteristic | Hard to obtain a favorable cell line Large individual difference | [47,154] | |
| Protein | OR protein | High sensitivity and selectivity Easy to combine with transducers | Hard to purify High cost | [119,125] |
| OBP | Easy to purify Good sensitivity Easy to combine with transducers | High cost Low selectivity | [147,150] |
5. Other Biological Materials for Odor Biosensors
6. Conclusions
7. Future Perspectives
Funding
Conflicts of Interest
References
- Danchuk, A.I.; Komova, N.S.; Mobarez, S.N.; Doronin, S.Y.; Burmistrova, N.A.; Markin, A.V.; Duerkop, A. Optical sensors for determination of biogenic amines in food. Anal. Bioanal. Chem. 2020, 412, 4023–4036. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Phan, M.A.T.; Arcot, J.; Chandrawati, R. Anthocyanin-based sensors derived from food waste as an active use-by date indicator for milk. Food Chem. 2020, 326, 127017. [Google Scholar] [CrossRef]
- Saraiva, L.R.; Kondoh, K.; Ye, X.; Yoon, K.H.; Hernandez, M.; Buck, L.B. Combinatorial effects of odorants on mouse behavior. Proc. Natl. Acad. Sci. USA 2016, 113, E3300–E3306. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, A.; Badonnel, K.; Persuy, M.A.; Durieux, D.; Bombail, V.; Favreau-Peigné, A.; Baly, C. Effect of environmental exposure to a maternally-learned odorant on anxiety-like behaviors at weaning in mice. Anim. Cogn. 2020, 23, 881–891. [Google Scholar] [CrossRef] [PubMed]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Lahondère, C.; Vinauger, C.; Okubo, R.P.; Wolff, G.H.; Chan, J.K.; Akbari, O.S.; Riffell, J.A. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. USA 2020, 117, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef]
- Pelletier, J.; Xu, P.; Yoon, K.S.; Clark, J.M.; Leal, W.S. Odorant receptor-based discovery of natural repellents of human lice. Insect Biochem. Mol. Biol. 2015, 66, 103–109. [Google Scholar] [CrossRef]
- Dweck, H.K.M.; Ebrahim, S.A.M.; Thoma, M.; Mohamed, A.A.M.; Keesey, I.W.; Trona, F.; Lavista-Llanos, S.; Svatoš, A.; Sachse, S.; Knaden, M.; et al. Pheromones mediating copulation and attraction in Drosophila. Proc. Natl. Acad. Sci. USA 2015, 112, E2829–E2835. [Google Scholar] [CrossRef]
- Sarolidou, G.; Tognetti, A.; Lasselin, J.; Regenbogen, C.; Lundström, J.N.; Kimball, B.A.; Garke, M.; Lekander, M.; Axelsson, J.; Olsson, M.J. Olfactory Communication of Sickness Cues in Respiratory Infection. Front. Psychol. 2020, 11, 1004. [Google Scholar] [CrossRef]
- Tognetti, A.; Williams, M.N.; Lybert, N.; Lekander, M.; Axelsson, J.; Olsson, M.J. Humans can detect axillary odor cues of an acute respiratory infection in others. Evol. Med. Public Health 2023, 11, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Debeda, H.; Dulau, L.; Dondon, P.; Menil, F.; Lucat, C.; Massok, P. Development of a reliable methane detector. Sens. Actuators B Chem. 1997, 44, 248–256. [Google Scholar] [CrossRef]
- Darmastuti, Z.; Bur, C.; Möller, P.; Rahlin, R.; Lindqvist, N.; Andersson, M.; Schütze, A.; Spetz, A.L. SiC-FET based SO2 sensor for power plant emission applications. Sens. Actuators B Chem. 2014, 194, 511–520. [Google Scholar] [CrossRef]
- Liu, S.F.; Lin, S.; Swager, T.M. An Organocobalt-Carbon Nanotube Chemiresistive Carbon Monoxide Detector. ACS Sens. 2016, 1, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-A.; Li, M.; Knipp, R.J.; Nantz, M.H.; Bousamra, M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014, 3, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.; Zhang, W.; Zhang, Z.; et al. Breath Analysis Based on Surface-Enhanced Raman Scattering Sensors Distinguishes Early and Advanced Gastric Cancer Patients from Healthy Persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef] [PubMed]
- Dent, A.G.; Sutedja, T.G.; Zimmerman, P.V. Exhaled breath analysis for lung cancer. J. Thorac. Dis. 2013, 5 (Suppl. S5), S540. [Google Scholar]
- Kumar, M.A.; Jayavel, R.; Mahalingam, S.; Kim, J.; Atchudan, R. Detection of Interleukin-6 Protein Using Graphene Field-Effect Transistor. Biosensors 2023, 13, 834. [Google Scholar] [CrossRef]
- Kwak, H.T.; Kim, H.; Yoo, H.; Choi, M.; Oh, K.; Kim, Y.; Kong, B.D.; Baek, C.K. Hydrogen Fluoride Gas Sensor by Silicon Nanosheet Field-Effect Transistor. IEEE Sens. J. 2023, 23, 16545–16552. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Van Nguyen, K.; Lee, J.H.; Lee, W.H. Optimization of Gas-Sensing Properties in Poly(triarylamine) Field-Effect Transistors by Device and Interface Engineering. Polymers 2023, 15, 3463. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Ruan, C.; Xu, S.; Yu, C. MoS2 based dual mine gas disaster sensor that operates at room temperature. Sens. Actuators A Phys. 2023, 359, 114517. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Lazarov, Y.; Grakov, T.; Fedchenko, Y.I.; Vergov, L.G.; Staykov, S. Metal–Phenolic Film Coated Quartz Crystal Microbalance as a Selective Sensor for Methanol Detection in Alcoholic Beverages. Micromachines 2023, 14, 1274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Che, J.; Cao, Q.; Shen, S.; Tang, Y. Highly Sensitive Surface Acoustic Wave H2S Gas Sensor Using Electron-beam-evaporated CuO as Sensitive Layer. Sens. Mater. 2023, 35, 2293–2304. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, L.; Wang, X. Surface acoustic wave sensor for formaldehyde gas detection using the multi-source spray-deposited graphene/PMMA composite film. Front. Mater. 2023, 9, 1025903. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Chen, Q.; Li, H.; Gao, Z. Surface plasmon resonance induced methane gas sensor in hollow core anti-resonant fiber. Opt. Fiber Technol. 2023, 78, 103293. [Google Scholar] [CrossRef]
- Sassi, I.A.; El Hadj Rhouma, M.B.; Daher, M.G. Highly sensitive refractive index gas sensor using two-dimensional silicon carbide grating based on surface plasmon resonance. Opt. Quantum Electron. 2023, 55, 402. [Google Scholar] [CrossRef]
- Ito, Y.; Morimoto, K.; Tsunoda, Y. Light-addressable potentiometric (LAP) gas sensor. Sens. Actuators B. Chem. 1993, 13, 348–350. [Google Scholar] [CrossRef]
- Kaaliveetil, S.; Lee, Y.; Li, Z.; Cheng, Y.; Menon, N.H.; Dongare, S.; Gurkan, B.; Basuray, S. Ionic Liquid-Packed Microfluidic Device with Non-Planar Microelectrode as a Miniaturized Electrochemical Gas Sensor. J. Electrochem. Soc. 2023, 170, 087508. [Google Scholar] [CrossRef]
- Anjos, T.G.; Hahn, C.E.W. The development of a membrane-covered microelectrode array gas sensor for oxygen and carbon dioxide measurement. Sens. Actuators B Chem. 2008, 135, 224–229. [Google Scholar] [CrossRef]
- Farooq, A.; Al-Jowder, R.; Narayanaswamy, R.; Azzawi, M.; Roche, P.J.R.; Whitehead, D.E. Gas detection using quenching fluorescence of dye-immobilised silica nanoparticles. Sens. Actuators B Chem. 2013, 183, 230–238. [Google Scholar] [CrossRef]
- Wang, B.; Deng, Z.; Fu, Y.; Kerman, S.; Xu, W.; Li, H.; Liu, H.; He, Q.; Chen, C.; Cheng, J. On-Chip Fluorescent Sensor for Chemical Vapor Detection. Adv. Mater. Technol. 2023, 8, 2300609. [Google Scholar] [CrossRef]
- Micholt, E.; Jans, D.; Callewaert, G.; Bartic, C.; Lammertyn, J.; Nicolaï, B. Extracellular recordings from rat olfactory epithelium slices using micro electrode arrays. Sens. Actuators B Chem. 2013, 184, 40–47. [Google Scholar] [CrossRef]
- Wu, C.S.; Chen, P.H.; Yuan, Q.; Wang, P. Response enhancement of olfactory sensory neurons-based biosensors for odorant detection. J. Zhejiang Univ. Sci. B 2009, 10, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Kim, S.G.; Ahn, H.; Yoo, J.; Jang, J.; Park, T.H. Ni-rGO Sensor Combined with Human Olfactory Receptor-Embedded Nanodiscs for Detecting Gas-Phase DMMP as a Simulant of Nerve Agents. ACS Sens. 2023, 8, 3095–3103. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Liu, S.; Wang, Z.; Wei, J.; Qin, G. High-performance olfactory receptor-derived peptide sensor for trimethylamine detection on the pyramid substrate structure. Sens. Actuators A Phys. 2023, 358, 114452. [Google Scholar] [CrossRef]
- Kuroda, S.; Nakaya-Kishi, Y.; Tatematsu, K.; Hinuma, S. Human Olfactory Receptor Sensor for Odor Reconstitution. Sensors 2023, 23, 6164. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Qiu, F.; Wang, C.; Qian, Y.; Zhang, Y.; Guo, Q.; Wang, Q.; Wang, S.; Yang, M.; Yu, J. Possibility for detecting 14 typical odorants occurring in drinking water by employing human odor-binding protein OBP2a. Environ. Sci. Eur. 2023, 35, 1–12. [Google Scholar] [CrossRef]
- Nakano-Baker, O.; Fong, H.; Shukla, S.; Lee, R.V.; Cai, L.; Godin, D.; Hennig, T.; Rath, S.; Novosselov, I.; Dogan, S.; et al. Data-driven design of a multiplexed, peptide-sensitized transistor to detect breath VOC markers of COVID-19. Biosens. Bioelectron. 2023, 229, 115237. [Google Scholar] [CrossRef]
- Van Der Pers, J.N.C.; Den Otter, C.J. Single cell responses from olfactory receptors of small ermine moths to sex-attractants. J. Insect Physiol. 1978, 24, 337–343. [Google Scholar] [CrossRef]
- Raming, K.; Krieger, J.; Strotmann, J.; Boekhoff, I.; Kubick, S.; Baumstark, C.; Breer, H. Cloning and expression of odorant receptors. Nature 1993, 361, 353–356. [Google Scholar] [CrossRef]
- Mitsubayashi, K.; Yokoyama, K.; Takeuchi, T.; Karube, I. Gas-Phase Biosensor for Ethanol. Anal. Chem. 1994, 66, 3297–3302. [Google Scholar] [CrossRef]
- Stubbs, D.D.; Hunt, W.D.; Lee, S.H.; Doyle, D.F. Gas phase activity of anti-FITC antibodies immobilized on a surface acoustic wave resonator device. Biosens. Bioelectron. 2002, 17, 471–477. [Google Scholar] [CrossRef]
- Misawa, N.; Mitsuno, H.; Kanzaki, R.; Takeuchi, S. Highly sensitive and selective odorant sensor using living cells expressing insect olfactory receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 15340–15344. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, N.; Ye, W.; Cai, H.; Zhang, F.; Wang, P. Extracellular recording of spatiotemporal patterning in response to odors in the olfactory epithelium by microelectrode arrays. Biosens. Bioelectron. 2011, 27, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takeuchi, S. Chemical vapor detection using a reconstituted insect olfactory receptor complex. Angew. Chem. Int. Ed. 2014, 53, 11798–11802. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yoshimoto, N.; Shimono, K.; Kuroda, S. Deciphering the Receptor Repertoire Encoding Specific Odorants by Time-Lapse Single-Cell Array Cytometry. Sci. Rep. 2016, 6, 19934. [Google Scholar] [CrossRef] [PubMed]
- Kida, H.; Fukutani, Y.; Mainland, J.D.; de March, C.A.; Vihani, A.; Li, Y.R.; Chi, Q.; Toyama, A.; Liu, L.; Kameda, M.; et al. Vapor detection and discrimination with a panel of odorant receptors. Nat. Commun. 2018, 9, 4556. [Google Scholar] [CrossRef]
- Kuznetsov, A.E.; Komarova, N.V.; Kuznetsov, E.V.; Andrianova, M.S.; Grudtsov, V.P.; Rybachek, E.N.; Puchnin, K.V.; Ryazantsev, D.V.; Saurov, A.N. Integration of a field effect transistor-based aptasensor under a hydrophobic membrane for bioelectronic nose applications. Biosens. Bioelectron. 2019, 129, 29–35. [Google Scholar] [CrossRef]
- Terutsuki, D.; Uchida, T.; Fukui, C.; Sukekawa, Y.; Okamoto, Y.; Kanzaki, R. Real-time odor concentration and direction recognition for efficient odor source localization using a small bio-hybrid drone. Sens. Actuators B Chem. 2021, 339, 129770. [Google Scholar] [CrossRef]
- Choi, D.; Lee, S.J.; Baek, D.; Kim, S.; Shin, J.; Choi, Y.; Cho, Y.; Bang, S.; Park, J.Y.; Lee, S.H.; et al. Bioelectrical Nose Platform Using Odorant-Binding Protein as a Molecular Transporter Mimicking Human Mucosa for Direct Gas Sensing. ACS Sens. 2022, 7, 3399–3408. [Google Scholar] [CrossRef]
- Deng, H.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Extending lifetime of gas-phase odor biosensor using liquid thickness control and liquid exchange. Biosens. Bioelectron. 2022, 199, 113887. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Sukekawa, Y.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Active Tracking of Temporally Changing Gas-Phase Odor Mixture Using an Array of Cells Expressing Olfactory Receptors. Anal. Chem. 2023, 95, 11558–11565. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Qin, S.; Casademunt, H.; Wu, M.; Hung, W.; Cain, G.; Tan, N.Z.; Valenzuela, R.; Lesanpezeshki, L.; Venkatachalam, V.; et al. Functional imaging and quantification of multineuronal olfactory responses in C. elegans. Sci. Adv. 2023, 9, eade1249. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.K.; Schwarz, E.M.; Muirhead, C.S.; Robidoux, A.N.; Narayan, A.; Doma, M.K.; Sternberg, P.W.; Srinivasan, J. Transcriptomic profiling of sex-specific olfactory neurons reveals subset-specific receptor expression in Caenorhabditis elegans. Genetics 2023, 223, iyad026. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Ko, H.J.; Park, T.H. Piezoelectric biosensor using olfactory receptor protein expressed in Escherichia coli. Biosens. Bioelectron. 2006, 21, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Kaissling, K.E. Chemo-electrical transduction in insect olfactory receptors. Annu. Rev. Neurosci. 1986, 9, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Jacquin-Joly, E.; Merlin, C. Insect olfactory receptors: Contributions of molecular biology to chemical ecology. J. Chem. Ecol. 2004, 30, 2359–2397. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Sekine, S.; Inagaki, S.; Misaki, K.; Badel, L.; Moriya, H.; Sami, M.M.; Itakura, Y.; Chihara, T.; Kazama, H. Nanopore Formation in the Cuticle of an Insect Olfactory Sensillum. Curr. Biol. 2019, 29, 1512–1520.e6. [Google Scholar] [CrossRef]
- Stengl, M.; Funk, N.W. The role of the coreceptor Orco in insect olfactory transduction. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2013, 199, 897–909. [Google Scholar] [CrossRef]
- Mukunda, L.; Lavista-Llanos, S.; Hansson, B.S.; Wicher, D. Dimerisation of the Drosophila odorant coreceptor Orco. Front. Cell. Neurosci. 2014, 8, 261. [Google Scholar] [CrossRef]
- Hayden, S.; Teeling, E.C. The Molecular Biology of Vertebrate Olfaction. Anat. Rec. 2014, 297, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Mitsubayashi, K.; Toma, K.; Iitani, K.; Arakawa, T. Gas-phase biosensors: A review. Sens. Actuators B Chem. 2022, 367, 132053. [Google Scholar] [CrossRef]
- El Kazzy, M.; Weerakkody, J.S.; Hurot, C.; Mathey, R.; Buhot, A.; Scaramozzino, N.; Hou, Y. An overview of artificial olfaction systems with a focus on surface plasmon resonance for the analysis of volatile organic compounds. Biosensors 2021, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Oda, H.; Osaki, T.; Takeuchi, S. Biohybrid sensor for odor detection. Lab Chip 2021, 21, 2643–2657. [Google Scholar] [CrossRef] [PubMed]
- Hurot, C.; Scaramozzino, N.; Buhot, A.; Hou, Y. Bio-inspired strategies for improving the selectivity and sensitivity of artificial noses: A review. Sensors 2020, 20, 1803. [Google Scholar] [CrossRef]
- Brito, N.F.; Oliveira, D.S.; Santos, T.C.; Moreira, M.F.; Melo, A.C.A. Current and potential biotechnological applications of odorant-binding proteins. Appl. Microbiol. Biotechnol. 2020, 104, 8631–8648. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Lee, Y.; Heath, J.; Kim, M. Applications of animal biosensors: A review. IEEE Sens. J. 2014, 15, 637–645. [Google Scholar]
- Sakurai, T.; Mitsuno, H.; Haupt, S.S.; Uchino, K.; Yokohari, F.; Nishioka, T.; Kobayashi, I.; Sezutsu, H.; Tamura, T.; Kanzaki, R. A single sex pheromone receptor determines chemical response specificity of sexual behavior in the silkmoth Bombyx mori. PLoS Genet. 2011, 7, e1002115. [Google Scholar] [CrossRef]
- Pawson, S.M.; Kerr, J.L.; O’Connor, B.C.; Lucas, P.; Martinez, D.; Allison, J.D.; Strand, T.M. Light-Weight Portable Electroantennography Device as a Future Field-Based Tool for Applied Chemical Ecology. J. Chem. Ecol. 2020, 46, 557–566. [Google Scholar] [CrossRef]
- Schütz, S.; Weißbecker, B.; Hummel, H.E. Biosensor for volatiles released by damaged plants. Biosens. Bioelectron. 1996, 11, 427–433. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, W.; Yu, H.; Hu, N.; Du, L.; Wang, P.; Yang, M. Olfactory mucosa tissue-based biosensor: A bioelectronic nose with receptor cells in intact olfactory epithelium. Sens. Actuators B Chem. 2010, 146, 527–533. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, W.; Xiao, L.; Du, L.; Hu, N.; Wang, P. Extracellular potentials recording in intact olfactory epithelium by microelectrode array for a bioelectronic nose. Biosens. Bioelectron. 2010, 25, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, F.; Zhang, D.; Hu, N.; Hsia, K.J.; Wang, P. Extracellular potentials recording in intact taste epithelium by microelectrode array for a bioelectronic nose. Biosens. Bioelectron. 2013, 43, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Davison, I.G.; Katz, L.C. Sparse and selective odor coding by mitral/tufted neurons in the main olfactory bulb. J. Neurosci. 2007, 27, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Hu, N.; Dong, Q.; Liu, Q.; Wang, P. A high sensitive in vivo biosensing detection for odors by multiunit in rat olfactory bulb. Sens. Actuators B Chem. 2013, 186, 308–314. [Google Scholar] [CrossRef]
- Zhuang, L.; Hu, N.; Tian, F.; Dong, Q.; Hu, L.; Li, R.; Wang, P. A high-sensitive detection method for carvone odor by implanted electrodes in rat olfactory bulb. Chin. Sci. Bull. 2014, 59, 29–37. [Google Scholar] [CrossRef]
- Zhuang, L.; Guo, T.; Cao, D.; Ling, L.; Su, K.; Hu, N.; Wang, P. Detection and classification of natural odors with an in vivo bioelectronic nose. Biosens. Bioelectron. 2015, 67, 694–699. [Google Scholar] [CrossRef]
- Gao, K.; Li, S.; Zhuang, L.; Qin, Z.; Zhang, B.; Huang, L.; Wang, P. In vivo bioelectronic nose using transgenic mice for specific odor detection. Biosens. Bioelectron. 2018, 102, 150–156. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, S.; Tian, Y.; Chen, Y.; Chen, W.; Wang, P.; Du, L.; Wu, C. In Vivo Bioelectronic Nose Based on a Bioengineered Rat Realizes the Detection and Classification of Multiodorants. ACS Chem. Neurosci. 2022, 13, 1727–1737. [Google Scholar] [CrossRef]
- Dasgupta, D.; Warner, T.P.A.; Erskine, A.; Schaefer, A.T. Coupling of Mouse Olfactory Bulb Projection Neurons to Fluctuating Odor Pulses. J. Neurosci. 2022, 42, 4278–4296. [Google Scholar] [CrossRef]
- Liu, P.; Cao, T.; Xu, J.; Mao, X.; Wang, D.; Li, A. Plasticity of Sniffing Pattern and Neural Activity in the Olfactory Bulb of Behaving Mice During Odor Sampling, Anticipation, and Reward. Neurosci. Bull. 2020, 36, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, X.A.; Cooksey, G.A.; Votaw, S.V.; Horowitz, L.F.; Folch, A. Large-scale investigation of the olfactory receptor space using a microfluidic microwell array. Lab Chip 2010, 10, 1120–1127. [Google Scholar] [CrossRef]
- Datta-Chaudhuri, T.; Araneda, R.C.; Abshire, P.; Smela, E. Olfaction on a chip. Sens. Actuators B Chem. 2016, 235, 74–78. [Google Scholar] [CrossRef]
- Gao, K.; Gao, F.; Li, J.; He, C.; Liu, M.; Zhu, Q.; Qian, Z.; Ma, T.; Wang, P. Biomimetic integrated olfactory sensory and olfactory bulb systems in vitro based on a chip. Biosens. Bioelectron. 2021, 171, 112739. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Reed, R.R. Golf: An olfactory neuron specific-G protein involved in odorant signal transduction. Science 1989, 244, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Pace, U.; Hanski, E.; Salomon, Y.; Lancet, D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature 1985, 316, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Dahmen, N.; Wang, H.-L.; Margolis, F.L. Expression of Olfactory Receptors in Xenopus Oocytes. J. Neurochem. 1992, 58, 1176–1179. [Google Scholar] [CrossRef]
- Deng, H.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Study of Liquid Film Thickness for Gas Phase Odor Biosensor. IEEE Sens. J. 2022, 22, 16785–16793. [Google Scholar] [CrossRef]
- Deng, H.; Mitsuno, H.; Kanzaki, R.; Nomoto, S.; Nakamoto, T. Gas-phase Odorant Fast Quantification by Odor Biosensor Based on Reference Response Model. IEEE Sens. J. 2023, 23, 24169–24178. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, S.B.; Ko, H.J.; Kim, S.J.; Park, T.H. Cell-based olfactory biosensor using microfabricated planar electrode. Biosens. Bioelectron. 2009, 24, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chou, J.H.; Bradley, J.; Bargmann, C.I.; Zinn, K. The Caenorhabditis elegans seven-transmembrane protein ODR-10 functions as an odorant receptor in mammalian cells. Proc. Natl. Acad. Sci. USA 1997, 94, 12162–12167. [Google Scholar] [CrossRef] [PubMed]
- Krautwurst, D.; Yau, K.W.; Reed, R.R. Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell 1998, 95, 917–926. [Google Scholar] [CrossRef]
- Gimelbrant, A.A.; Stoss, T.D.; Landers, T.M.; McClintock, T.S. Truncation releases olfactory receptors from the endoplasmic reticulum of heterologous cells. J. Neurochem. 1999, 72, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- De March, C.A.; Fukutani, Y.; Vihani, A.; Kida, H.; Matsunami, H. Real-time in vitro monitoring of odorant receptor activation by an odorant in the vapor phase. J. Vis. Exp. 2019, 146, e59446. [Google Scholar]
- Deng, H.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Gas Phase Odorant Detection by Insect Olfactory Receptor. IEEE Sens. J. 2021, 21, 21184–21191. [Google Scholar] [CrossRef]
- Hirata, Y.; Morimoto, Y.; Takeuchi, S. Cell-laden micropillars detect gaseous odorants on a liquid-air interface. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Belfast, UK, 21–25 January 2018; IEEE: Piscataway, NJ, USA, 2018; Volume 2018, pp. 304–307. [Google Scholar]
- Lee, S.H.; Oh, E.H.; Park, T.H. Cell-based microfluidic platform for mimicking human olfactory system. Biosens. Bioelectron. 2015, 74, 554–561. [Google Scholar] [CrossRef]
- Kiefer, H.; Krieger, J.; Olszewski, J.D.; Von Heijne, G.; Prestwich, G.D.; Breer, H. Expression of an olfactory receptor in Escherichia coli: Purification, reconstitution, and ligand binding. Biochemistry 1996, 35, 16077–16084. [Google Scholar] [CrossRef]
- Kajiya, K.; Inaki, K.; Tanaka, M.; Haga, T.; Kataoka, H.; Touhara, K. Molecular bases of odor discrimination: Reconstitution of olfactory receptors that recognize overlapping sets of odorants. J. Neurosci. 2001, 21, 6018–6025. [Google Scholar] [CrossRef]
- Radhika, V.; Proikas-Cezanne, T.; Jayaraman, M.; Onesime, D.; Ha, J.H.; Dhanasekaran, D.N. Chemical sensing of DNT by engineered olfactory yeast strain. Nat. Chem. Biol. 2007, 3, 325–330. [Google Scholar] [CrossRef]
- Du, L.; Zou, L.; Zhao, L.; Huang, L.; Wang, P.; Wu, C. Label-free functional assays of chemical receptors using a bioengineered cell-based biosensor with localized extracellular acidification measurement. Biosens. Bioelectron. 2014, 54, 623–627. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, H.J.; Park, T.H. Real-time monitoring of odorant-induced cellular reactions using surface plasmon resonance. Biosens. Bioelectron. 2009, 25, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Du, L.; Wang, D.; Wang, L.; Zhao, L.; Wang, P. A novel surface acoustic wave-based biosensor for highly sensitive functional assays of olfactory receptors. Biochem. Biophys. Res. Commun. 2011, 407, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Park, T.H. Piezoelectric olfactory biosensor: Ligand specificity and dose-dependence of an olfactory receptor expressed in a heterologous cell system. Biosens. Bioelectron. 2005, 20, 1327–1332. [Google Scholar] [CrossRef]
- Terutsuki, D.; Mitsuno, H.; Sakurai, T.; Okamoto, Y.; Tixier-Mita, A.; Toshiyoshi, H.; Mita, Y.; Kanzaki, R. Increasing cell–device adherence using cultured insect cells for receptor-based biosensors. R. Soc. Open Sci. 2018, 5, 172366. [Google Scholar] [CrossRef] [PubMed]
- Termtanasombat, M.; Mitsuno, H.; Misawa, N.; Yamahira, S.; Sakurai, T.; Yamaguchi, S.; Nagamune, T.; Kanzaki, R. Cell-Based Odorant Sensor Array for Odor Discrimination Based on Insect Odorant Receptors. J. Chem. Ecol. 2016, 42, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Sukekawa, Y.; Mitsuno, H.; Kanzaki, R.; Nakamoto, T. Odor discrimination using cell-based odor biosensor system with fluorescent image processing. IEEE Sens. J. 2019, 19, 7192–7200. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Jones, P.L.; Pask, G.M.; Rinker, D.C.; Zwiebel, L.J. Functional agonism of insect odorant receptor ion channels. Proc. Natl. Acad. Sci. USA 2011, 108, 8821–8825. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, O.S.; Song, H.S.; Park, S.J.; Sung, J.H.; Jang, J.; Park, T.H. Mimicking the human smell sensing mechanism with an artificial nose platform. Biomaterials 2012, 33, 1722–1729. [Google Scholar] [CrossRef]
- Du, L.; Wu, C.; Peng, H.; Zou, L.; Zhao, L.; Huang, L.; Wang, P. Piezoelectric olfactory receptor biosensor prepared by aptamer-assisted immobilization. Sens. Actuators B Chem. 2013, 187, 481–487. [Google Scholar] [CrossRef]
- Kwon, O.S.; Song, H.S.; Park, S.J.; Lee, S.H.; An, J.H.; Park, J.W.; Yang, H.; Yoon, H.; Bae, J.; Park, T.H.; et al. An Ultrasensitive, Selective, Multiplexed Superbioelectronic Nose That Mimics the Human Sense of Smell. Nano Lett. 2015, 15, 6559–6567. [Google Scholar] [CrossRef] [PubMed]
- Garenne, D.; Haines, M.C.; Romantseva, E.F.; Freemont, P.; Strychalski, E.A.; Noireaux, V. Cell-free gene expression. Nat. Rev. Methods Primers 2021, 1, 49. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Du, L.; Zhang, X.; Zhang, F.; Chen, W.; Cai, W.; Wu, C.; Wang, P. Functional expression of olfactory receptors using cell-free expression system for biomimetic sensors towards odorant detection. Biosens. Bioelectron. 2019, 130, 382–388. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Man, Y.; Chen, Q.; Pei, D.; Wu, W. Cell-free expression, purification and characterization of Drosophila melanogaster odorant receptor OR42a and its co-receptor. Protein Expr. Purif. 2019, 159, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ritz, S.; Hulko, M.; Zerfaß, C.; May, S.; Hospach, I.; Krasteva, N.; Nelles, G.; Sinner, E.K. Cell-free expression of a mammalian olfactory receptor and unidirectional insertion into small unilamellar vesicles (SUVs). Biochimie 2013, 95, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, L.; Graveland-Bikker, J.; Steuerwald, D.; Vanberghem, M.; Herlihy, K.; Zhang, S. Efficient cell-free production of olfactory receptors: Detergent optimization, structure, and ligand binding analyses. Proc. Natl. Acad. Sci. USA 2008, 105, 15726–15731. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, D.; Kim, J.; Moon, D.; Song, H.S.; Lee, M.; Hong, S.; Park, T.H. Nanodisc-Based Bioelectronic Nose Using Olfactory Receptor Produced in Escherichia coli for the Assessment of the Death-Associated Odor Cadaverine. ACS Nano 2017, 11, 11847–11855. [Google Scholar] [CrossRef]
- Lee, M.; Yang, H.; Kim, D.; Yang, M.; Park, T.H.; Hong, S. Human-like smelling of a rose scent using an olfactory receptor nanodisc-based bioelectronic nose. Sci. Rep. 2018, 8, 13945. [Google Scholar] [CrossRef]
- Murugathas, T.; Zheng, H.Y.; Colbert, D.; Kralicek, A.V.; Carraher, C.; Plank, N.O.V. Biosensing with Insect Odorant Receptor Nanodiscs and Carbon Nanotube Field-Effect Transistors. ACS Appl. Mater. Interfaces 2019, 11, 9530–9538. [Google Scholar] [CrossRef]
- Oh, J.; Yang, H.; Jeong, G.E.; Moon, D.; Kwon, O.S.; Phyo, S.; Lee, J.; Song, H.S.; Park, T.H.; Jang, J. Ultrasensitive, Selective, and Highly Stable Bioelectronic Nose That Detects the Liquid and Gaseous Cadaverine. Anal. Chem. 2019, 91, 12181–12190. [Google Scholar] [CrossRef]
- Fujii, S.; Nobukawa, A.; Osaki, T.; Morimoto, Y.; Kamiya, K.; Misawa, N.; Takeuchi, S. Pesticide vapor sensing using an aptamer, nanopore, and agarose gel on a chip. Lab Chip 2017, 17, 2421–2425. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Fujii, S.; Kamiya, K.; Osaki, T.; Takaku, T.; Takahashi, Y.; Takeuchi, S. Construction of a Biohybrid Odorant Sensor Using Biological Olfactory Receptors Embedded into Bilayer Lipid Membrane on a Chip. ACS Sens. 2019, 4, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sugiura, H.; Mimura, H.; Kamiya, K.; Osaki, T.; Takeuchi, S. Highly sensitive VOC detectors using insect olfactory receptors reconstituted into lipid bilayers. Sci. Adv. 2021, 7, eabd2013. [Google Scholar] [CrossRef] [PubMed]
- Khadka, R.; Carraher, C.; Hamiaux, C.; Travas-Sejdic, J.; Kralicek, A. Synergistic improvement in the performance of insect odorant receptor based biosensors in the presence of Orco. Biosens. Bioelectron. 2020, 153, 112040. [Google Scholar] [CrossRef] [PubMed]
- Khadka, R.; Aydemir, N.; Carraher, C.; Hamiaux, C.; Colbert, D.; Cheema, J.; Malmström, J.; Kralicek, A.; Travas-Sejdic, J. An ultrasensitive electrochemical impedance-based biosensor using insect odorant receptors to detect odorants. Biosens. Bioelectron. 2019, 126, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.R.; Shim, J.; Park, J.H.; Kim, Y.S.; Kim, M.J. Discovery of orphan olfactory receptor 6m1 as a new anticancer target in mcf-7 cells by a combination of surface plasmon resonance-based and cell-based systems. Sensors 2021, 21, 3468. [Google Scholar] [CrossRef]
- Hou, Y.; Jaffrezic-Renault, N.; Martelet, C.; Zhang, A.; Minic-Vidic, J.; Gorojankina, T.; Persuy, M.A.; Pajot-Augy, E.; Salesse, R.; Akimov, V. A novel detection strategy for odorant molecules based on controlled bioengineering of rat olfactory receptor I7. Biosens. Bioelectron. 2007, 22, 1550–1555. [Google Scholar] [CrossRef]
- Khadka, R.; Aydemir, N.; Carraher, C.; Hamiaux, C.; Baek, P.; Cheema, J.; Kralicek, A.; Travas-Sejdic, J. Investigating Electrochemical Stability and Reliability of Gold Electrode-electrolyte Systems to Develop Bioelectronic Nose Using Insect Olfactory Receptor. Electroanalysis 2019, 31, 726–738. [Google Scholar] [CrossRef]
- Matarazzo, V.; Zsurger, N.; Guillemot, J.C.; Clot-Faybesse, O.; Botto, J.M.; Dal Farra, C.; Crowe, M.; Demaille, J.; Vincent, J.P.; Mazella, J. Porcine odorant-binding protein selectively binds to a human olfactory receptor. Chem. Senses 2002, 27, 691–701. [Google Scholar] [CrossRef]
- Pevsner, J.; Hou, V.; Snowman, A.M.; Snyder, S.H. Odorant-binding protein. Characterization of ligand binding. J. Biol. Chem. 1990, 265, 6118–6125. [Google Scholar] [CrossRef]
- Steinbrecht, M.A. Odorant-Binding Proteins: Expression and Function. Ann. N. Y. Acad. Sci. 1998, 855, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wojtasek, H.; Leal, W.S. Conformational change in the pheromone-binding protein from Bombyx mori induced by pH and by interaction with membranes. J. Biol. Chem. 1999, 274, 30950–30956. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.; Spinelli, S.; Ramoni, R.; Grolli, S.; Pelosi, P.; Cambillau, C.; Tegoni, M. Complexes of porcine odorant binding protein with odorant molecules belonging to different chemical classes. J. Mol. Biol. 2000, 300, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Tegoni, M.; Pelosi, P.; Vincent, F.; Spinelli, S.; Campanacci, V.; Grolli, S.; Ramoni, R.; Cambillau, C. Mammalian odorant binding proteins. Biochim. Et Biophys. Acta-Protein Struct. Mol. Enzymol. 2000, 1482, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Campanacci, V.; Krieger, J.; Bette, S.; Sturgis, J.N.; Lartigue, A.; Cambillau, C.; Breer, H.; Tegoni, M. Revisiting the Specificity of Mamestra brassicae and Antheraea polyphemus Pheromone-binding Proteins with a Fluorescence Binding Assay. J. Biol. Chem. 2001, 276, 20078–20084. [Google Scholar] [CrossRef] [PubMed]
- Sankarganesh, D.; Kirkwood, R.N.; Meillour, P.N.-L.; Angayarkanni, J.; Achiraman, S.; Archunan, G. Pheromones, binding proteins, and olfactory systems in the pig (Sus scrofa): An updated review. Front. Vet. Sci. 2022, 9, 989409. [Google Scholar] [CrossRef]
- Li, M.Y.; Jiang, X.Y.; Qi, Y.Z.; Huang, Y.J.; Li, S.G.; Liu, S. Identification and expression profiles of 14 odorant-binding protein genes from Pieris rapae (lepidoptera: Pieridae). J. Insect Sci. 2020, 20, 2. [Google Scholar] [CrossRef]
- Capo, A.; Cozzolino, S.; Cavallari, A.; Bruno, U.; Calabrese, A.; Pennacchio, A.; Camarca, A.; Staiano, M.; D’auria, S.; Varriale, A. The Porcine Odorant-Binding Protein as a Probe for an Impedenziometric-Based Detection of Benzene in the Environment. Int. J. Mol. Sci. 2022, 23, 4039. [Google Scholar] [CrossRef]
- Kaupp, U.B. Olfactory signalling in vertebrates and insects: Differences and commonalities. Nat. Rev. Neurosci. 2010, 11, 188–200. [Google Scholar] [CrossRef]
- Forstner, M.; Breer, H.; Krieger, J. A receptor and binding protein interplay in the detection of a distinct pheromone component in the silkmoth Antheraea polyphemus. Int. J. Biol. Sci. 2009, 5, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Panigrahi, S.; Mallik, S. Odorant binding protein based biomimetic sensors for detection of alcohols associated with Salmonella contamination in packaged beef. Biosens. Bioelectron. 2011, 26, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Cannatà, D.; Di Pietrantonio, F.; Varriale, A.; D’Auria, S.; Staiano, M.; Verona, E.; Benetti, M. Detection of odorant molecules via surface acoustic wave biosensor array based on odorant-binding proteins. Biosens. Bioelectron. 2012, 41, 328–334. [Google Scholar]
- Lu, Y.; Li, H.; Zhuang, S.; Zhang, D.; Zhang, Q.; Zhou, J.; Dong, S.; Liu, Q.; Wang, P. Olfactory biosensor using odorant-binding proteins from honeybee: Ligands of floral odors and pheromones detection by electrochemical impedance. Sens. Actuators B Chem. 2014, 193, 420–427. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Huang, Y.; Yao, Y.; Zhang, D.; Luo, S.; Lu, Y.; Liu, Q. Impedance spectroscopy analysis of human odorant binding proteins immobilized on nanopore arrays for biochemical detection. Biosens. Bioelectron. 2015, 79, 251–257. [Google Scholar]
- Larisika, M.; Kotlowski, C.; Steininger, C.; Mastrogiacomo, R.; Pelosi, P.; Schütz, S.; Peteu, S.F.; Kleber, C.; Reiner-Rozman, C.; Nowak, C.; et al. Electronic Olfactory Sensor Based on A. mellifera Odorant-Binding Protein 14 on a Reduced Graphene Oxide Field-Effect Transistor. Angew. Chem. Int. Ed. 2015, 54, 13245–13248. [Google Scholar] [CrossRef] [PubMed]
- Di Pietrantonio, F.; Benetti, M.; Cannatà, D.; Verona, E.; Palla-Papavlu, A.; Fernández-Pradas, J.M.; Serra, P.; Staiano, M.; Varriale, A.; D’Auria, S. A surface acoustic wave bio-electronic nose for detection of volatile odorant molecules. Biosens. Bioelectron. 2015, 67, 516–523. [Google Scholar] [CrossRef]
- Gao, A.; Wang, Y.; Zhang, D.; He, Y.; Zhang, L.; Liu, Y.; Wang, Y.; Song, H.; Li, T. Highly sensitive and selective detection of human-derived volatile organic compounds based on odorant binding proteins functionalized silicon nanowire array. Sens. Actuators B Chem. 2020, 309, 127762. [Google Scholar] [CrossRef]
- Scorsone, E.; Manai, R.; Cali, K.; Ricatti, M.J.; Farno, S.; Persaud, K.; Mucignat, C. Biosensor array based on ligand binding proteins for narcotics and explosives detection. Sens. Actuators B Chemical. 2021, 334, 129587. [Google Scholar] [CrossRef]
- Ko, H.J.; Park, T.H. Enhancement of odorant detection sensitivity by the expression of odorant-binding protein. Biosens. Bioelectron. 2008, 23, 1017–1023. [Google Scholar] [CrossRef]
- Fukutani, M.Y.Y.; Hori, A.; Tsukada, S.; Sato, R.; Ishii, J.; Kondo, A.; Matsunami, H. Improving the odorant sensitivity of olfactory receptor-expressing yeast with accessory proteins. Anal. Biochem. J. 2015, 35, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhuang, L.; Qin, Z.; Zhang, B.; Hu, N.; Wang, P. Multi-odor discrimination by a novel bio-hybrid sensing preserving rat’s intact smell perception in vivo. Sens. Actuators B Chem. 2016, 225, 34–41. [Google Scholar] [CrossRef]
- Mitsuno, H.; Sakurai, T.; Namiki, S.; Mitsuhashi, H.; Kanzaki, R. Novel cell-based odorant sensor elements based on insect odorant receptors. Biosens. Bioelectron. 2015, 65, 287–294. [Google Scholar] [CrossRef]
- Son, M.; Kim, D.; Kang, J.; Lim, J.H.; Lee, S.H.; Ko, H.J.; Hong, S.; Park, T.H. Bioelectronic nose using odorant binding protein-derived peptide and carbon nanotube field-effect transistor for the assessment of salmonella contamination in food. Anal. Chem. 2016, 88, 11283–11287. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Szulczyński, B.; Wojciechowski, M.; Kamysz, W.; Gębicki, J. A highly selective biosensor based on peptide directly derived from the harmOBP7 aldehyde binding site. Sensors 2019, 19, 4284. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Park, J.; Ahn, J.H.; Jin, H.J.; Hong, S.; Park, T.H. A peptide receptor-based bioelectronic nose for the real-time determination of seafood quality. Biosens. Bioelectron. 2013, 39, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lim, J.H.; Park, J.; Hong, S.; Park, T.H. Bioelectronic nose combined with a microfluidic system for the detection of gaseous trimethylamine. Biosens. Bioelectron. 2015, 71, 179–185. [Google Scholar] [CrossRef]
- Homma, C.; Tsukiiwa, M.; Noguchi, H.; Tanaka, M.; Okochi, M.; Tomizawa, H.; Sugizaki, Y.; Isobayashi, A.; Hayamizu, Y. Designable peptides on graphene field-effect transistors for selective detection of odor molecules. Biosens. Bioelectron. 2023, 224, 115047. [Google Scholar] [CrossRef]
- Jin, H.J.; Lee, S.H.; Kim, T.H.; Park, J.; Song, H.S.; Park, T.H.; Hong, S. Nanovesicle-based bioelectronic nose platform mimicking human olfactory signal transduction. Biosens. Bioelectron. 2012, 35, 335–341. [Google Scholar] [CrossRef]
- Son, M.; Cho, D.G.; Lim, J.H.; Park, J.; Hong, S.; Ko, H.J.; Park, T.H. Real-time monitoring of geosmin and 2-methylisoborneol, representative odor compounds in water pollution using bioelectronic nose with human-like performance. Biosens. Bioelectron. 2015, 74, 199–206. [Google Scholar] [CrossRef]
- Ye, M.; Chien, P.J.; Toma, K.; Arakawa, T.; Mitsubayashi, K. An acetone bio-sniffer (gas phase biosensor) enabling assessment of lipid metabolism from exhaled breath. Biosens. Bioelectron. 2015, 73, 208–213. [Google Scholar] [CrossRef]
- Son, M.; Kim, D.; Ko, H.J.; Hong, S.; Park, T.H. A portable and multiplexed bioelectronic sensor using human olfactory and taste receptors. Biosens. Bioelectron. 2017, 87, 901–907. [Google Scholar] [CrossRef]
- Son, M.; Park, T.H. The bioelectronic nose and tongue using olfactory and taste receptors: Analytical tools for food quality and safety assessment. Biotechnol. Adv. 2018, 36, 371–379. [Google Scholar] [CrossRef]
- Münch, D.; Galizia, C.G. DoOR 2.0—Comprehensive Mapping of Drosophila melanogaster Odorant Responses. Sci. Rep. 2016, 6, 21841. [Google Scholar] [CrossRef] [PubMed]
- Ambhorkar, P.; Wang, Z.; Ko, H.; Lee, S.; Koo, K.I.; Kim, K.; Cho, D.I.D. Nanowire-based biosensors: From growth to applications. Micromachines 2018, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.F.; Smith, A.F.; Liu, X.; Woodard, T.L.; Fu, T.; Emrick, T.; Jiménez, J.M.; Lovley, D.R.; Yao, J. Bioelectronic protein nanowire sensors for ammonia detection. Nano Res. 2020, 13, 1479–1484. [Google Scholar] [CrossRef]
- Selimoğlu, F.; Gür, B.; Ayhan, M.E.; Gür, F.; Kalita, G.; Tanemura, M.; Alma, M.H. Silver nanoparticle doped graphene-based impedimetric biosensor towards sensitive detection of procalcitonin. Mater. Chem. Phys. 2023, 297, 127339. [Google Scholar] [CrossRef]
- Tshobeni, Z.Z.; January, J.L.; Ngema, N.P.P.; Jijana, A.N.; Iwuoha, E.I.; Mulaudzi, T.; Douman, S.F.; Ajayi, R.F. Thioglycolic acid-capped gold nanoparticle/cytochrome P450-2E1 electrochemical biosensor for isoniazid. Sens. Bio-Sens. Res. 2023, 41, 100583. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, F.; Fu, J.; Jia, N.; Huang, X.; Sun, C.; Liu, C.; Huan, H.; Wang, Y. Rapid detection of monkeypox virus by multiple cross displacement amplification combined with nanoparticle-based biosensor platform. J. Med. Virol. 2023, 95, e28479. [Google Scholar] [CrossRef]
- Edwards, C.; Duanghathaipornsuk, S.; Goltz, M.; Kanel, S.; Kim, D.S. Peptide nanotube encapsulated enzyme biosensor for vapor phase detection of malathion, an organophosphorus compound. Sensors 2019, 19, 3856. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, P.; Zhou, X.; Liu, D.; Garcia, B.B.; Cao, G. Carbon monoxide annealed TiO2 nanotube array electrodes for efficient biosensor applications. J. Mater. Chem. 2009, 19, 948–953. [Google Scholar] [CrossRef]
- Terutsuki, D.; Mitsuno, H.; Sato, K.; Sakurai, T.; Mase, N.; Kanzaki, R. Highly effective volatile organic compound dissolving strategy based on mist atomization for odorant biosensors. Anal. Chim. Acta 2020, 1139, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Warden, A.C.; Trowell, S.C.; Gel, M. A Miniature Gas Sampling Interface with Open Microfluidic Channels: Characterization of Gas-to-Liquid Extraction Efficiency of Volatile Organic Compounds. Micromachines 2019, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Kida, H.; Tsukada, S.; Tagawa, Y.; Sato, R.; Kameda, M. Effective collection of volatile organic compounds in water using rimming flow with odorant-binding proteins. Mech. Eng. J. 2015, 2, 15-00244. [Google Scholar] [CrossRef][Green Version]
- Yokoshiki, Y.; Nagayoshi, K.; Mitsuno, H.; Niki, S.; Tokuda, T.; Nakamoto, T. Improving Performance of Odor Biosensor System Using Olfactory Receptor. Sens. Mater. 2022, 34, 1617–1626. [Google Scholar] [CrossRef]
- Park, T.H.; Lee, S.H.; Oh, E.H.; Lee, S.H.; Ko, H.J. Cell-based high-throughput odorant screening system through visualization on a microwell array. Biosens. Bioelectron. 2013, 53, 18–25. [Google Scholar]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans can discriminate more than 1 trillion olfactory stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- Yu, T.; Xianyu, Y. Array-Based Biosensors for Bacteria Detection: From the Perspective of Recognition. Small 2021, 17, 2006230. [Google Scholar] [CrossRef]
- Arano-Martinez, J.A.; Martínez-González, C.L.; Salazar, M.I.; Torres-Torres, C. A Framework for Biosensors Assisted by Multiphoton Effects and Machine Learning. Biosensors 2022, 12, 710. [Google Scholar] [CrossRef]
- Ma, J.; Guan, Y.; Xing, F.; Eltzov, E.; Wang, Y.; Li, X.; Tai, B. Accurate and non-destructive monitoring of mold contamination in foodstuffs based on whole-cell biosensor array coupling with machine-learning prediction models. J. Hazard. Mater. 2023, 449, 131030. [Google Scholar] [CrossRef]
- Cui, J.; Han, X.; Shi, G.; Li, K.; Yuan, W.; Zhou, W.; Li, Z.; Wang, M. Hierarchical structure SERS biosensor: A machine learning-driven ultra-sensitive platform for trace detection of amygdalin. Opt. Mater. 2023, 143, 114170. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Nakamoto, T. Biosensors for Odor Detection: A Review. Biosensors 2023, 13, 1000. https://doi.org/10.3390/bios13121000
Deng H, Nakamoto T. Biosensors for Odor Detection: A Review. Biosensors. 2023; 13(12):1000. https://doi.org/10.3390/bios13121000
Chicago/Turabian StyleDeng, Hongchao, and Takamichi Nakamoto. 2023. "Biosensors for Odor Detection: A Review" Biosensors 13, no. 12: 1000. https://doi.org/10.3390/bios13121000
APA StyleDeng, H., & Nakamoto, T. (2023). Biosensors for Odor Detection: A Review. Biosensors, 13(12), 1000. https://doi.org/10.3390/bios13121000





