Capacitance Contribution of NIH/3T3 Cells Existing on and between Electrodes of an Impedance Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Impedance Biosensor Fabrication Process
2.2. NIH/3T3 Cell Growth and Drug Reaction Process
2.3. NIH/3T3 Cell Culture
2.4. Impedance Biosensor Design: Reference, Wide, and Narrow Patterns
2.5. Electrical Measurement Methods
3. Results and Discussion
3.1. Capacitance Monitoring of NIH/3T3 Cell Using 0.3 mm Reference Pattern
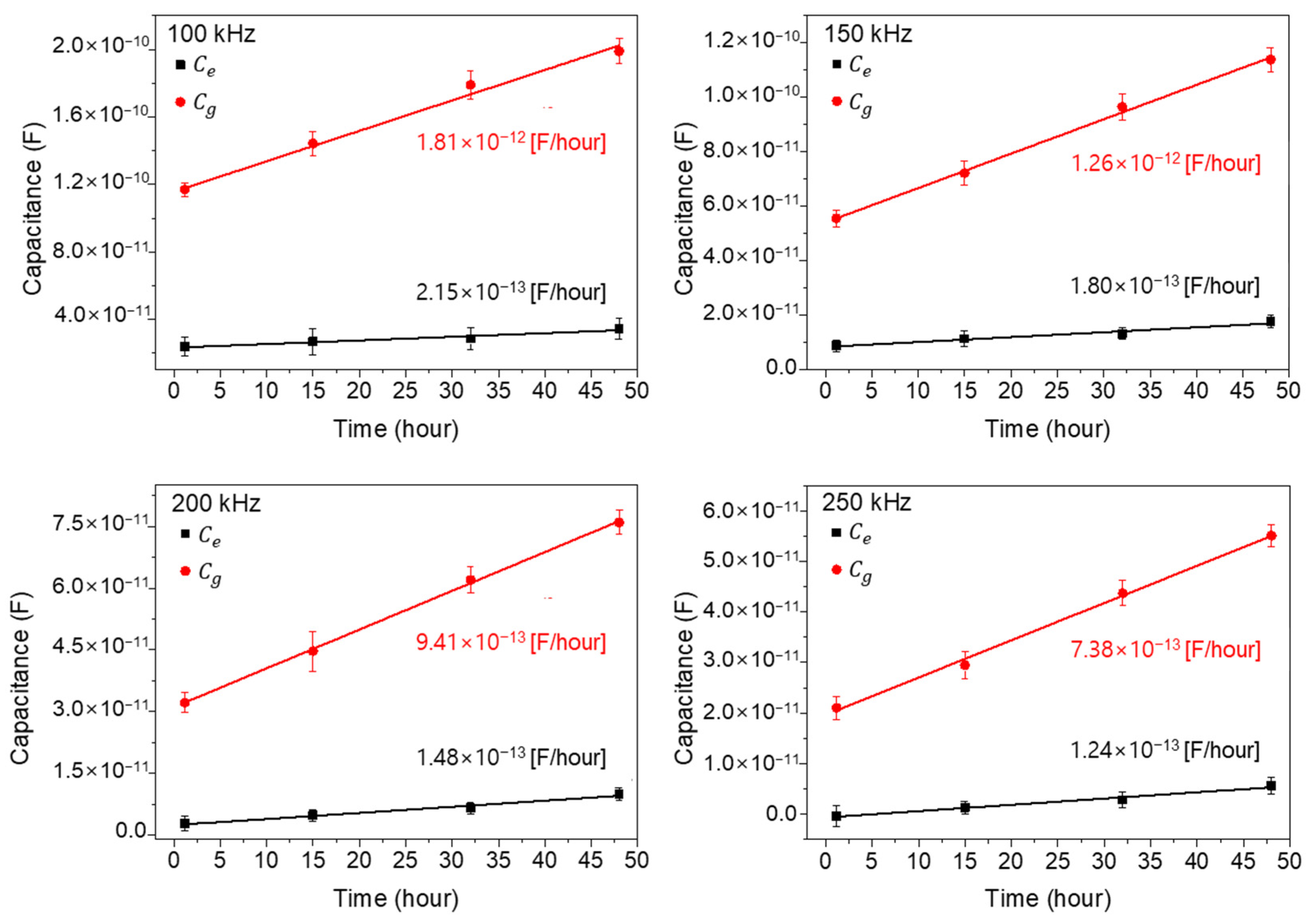
3.2. Capacitance Measurements as a Function of Time for Wide and Narrow Impedance Patterns
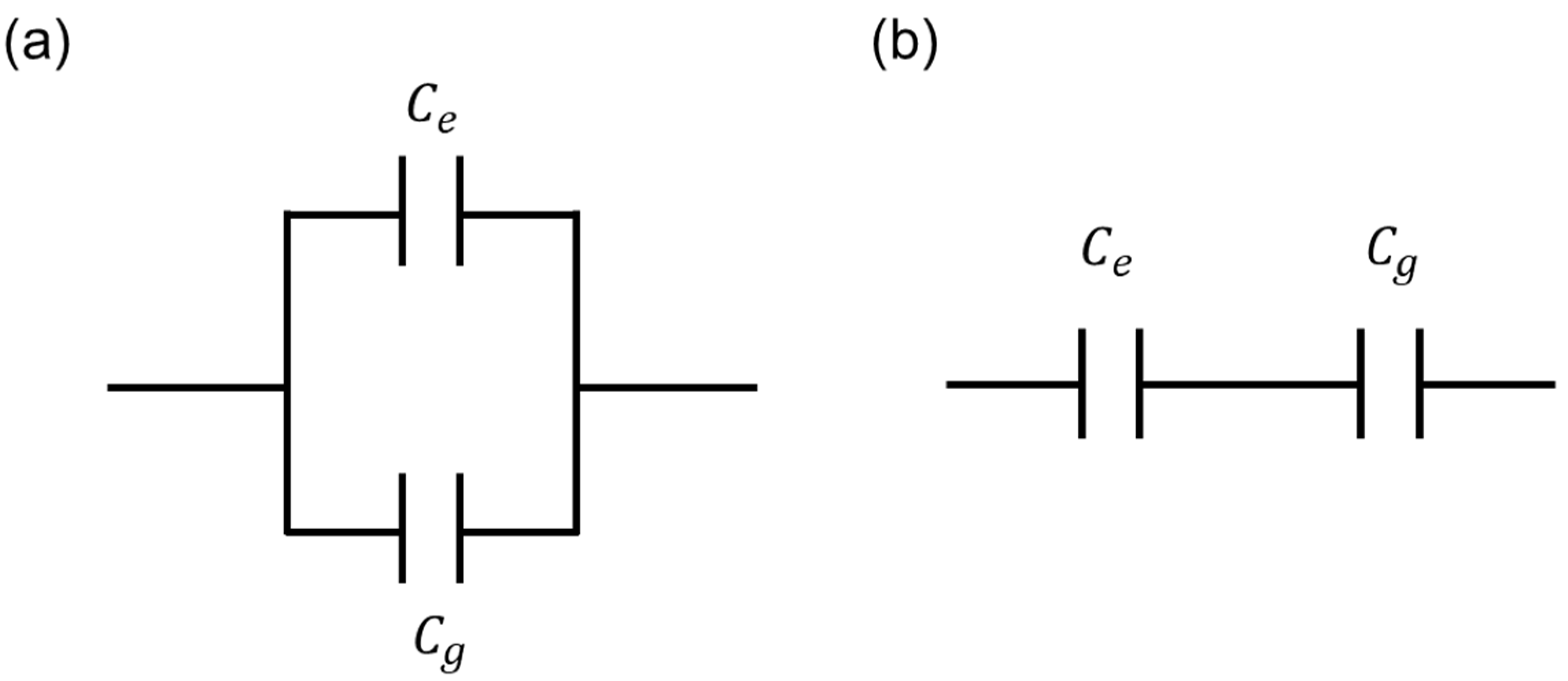
3.3. Analysis of Capacitance Contribution of the Cells Existing on and between Electrodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Merkoçi, A.; Li, C.Z.; Lechuga, L.M.; Ozcan, A. COVID-19 biosensing technologies. Biosens. Bioelectron. 2021, 178, 113046. [Google Scholar] [CrossRef]
- Amano, Y.; Cheng, Q.J.A. Detection of influenza virus: Traditional approaches and development of biosensors. Anal. Bioanal. Chem. 2005, 381, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, J.; Du, X. Electrochemical Biosensors for Detection of Foodborne Pathogens. Micromachines 2019, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar]
- Kim, Y.; Gonzales, J.; Zheng, Y. Optical Biosensing: Sensitivity-Enhancing Strategies in Optical Biosensing. Small 2021, 17, 2170016. [Google Scholar] [CrossRef]
- Pan, S.; Arnold, M. Selectivity enhancement for glutamate with a Nafion/glutamate oxidase biosensor. Talanta 1996, 43, 1157–1162. [Google Scholar] [CrossRef]
- Pradhan, A.; Lahare, P.; Sinha, P.; Singh, N.; Gupta, B.; Kuca, K.; Ghosh, K.K.; Krejcar, O. Biosensors as Nano-Analytical Tools for COVID-19 Detection. Sensors 2021, 21, 7823. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano Biosensors: Properties, applications and electrochemical techniques. J. Mater. Res. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Strong, M.; Richards, J.; Torres, M.; Beck, C.; La Belle, T. Faradaic electrochemical impedance spectroscopy for enhanced analyte detection in diagnostics. Biosens. Bioelectron. 2021, 177, 112949–112961. [Google Scholar] [CrossRef]
- Laborde, C.; Pittino, F.; Verhoeven, H.A.; Lemay, S.G.; Selmi, L.; Jongsma, M.A.; Widdershoven, F.P. Real-time imaging of microparticles and living cells with CMOS nanocapacitor arrays. Nat. Nanotechnol. 2015, 10, 791–795. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Micromotion of mammalian cells measured electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef] [PubMed]
- Heine, V.; Kremers, T.; Menzel, N.; Schnakenberg, U.; Elling, L. Electrochemical Impedance Spectroscopy Biosensor Enabling Kinetic Monitoring of Fucosyltransferase Activity. ACS Sens. 2021, 6, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Sacci, R.L.; Seland, F.; Harrington, D.A. Dynamic electrochemical impedance spectroscopy, for electrocatalytic reactions. Electrochim. Acta 2014, 131, 13–19. [Google Scholar] [CrossRef]
- Giaever, L.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef]
- Jiang, D.; Wei, X.; Zhu, Y. Evaluating the efficacy and cardiotoxicity of EGRF-TKI AC0010 with a novel multifunctional biosensor. Microsyst. Nanoeng. 2023, 9, 57. [Google Scholar] [CrossRef]
- Engel, N.; Dau, M.; Engel, V.; Franz, D.; Klemmstein, F.; Thanisch, C.; Kolb, J.F.; Frank, M.; Springer, A.; Köhling, R.; et al. Combining Electrostimulation with Impedance Sensing to Promote and Track Osteogenesis within a Titanium Implant. Biomedicines 2023, 11, 697. [Google Scholar] [CrossRef]
- Yagati, A.k.; Chavan, S.G.; Baek, C.; Lee, D.; Lee, M.; Min, J. RGO-PANI composite Au microelectrodes for sensitive ECIS analysis of human gastric(MKN-1) cancer cells. Bioelectrochemistry 2022, 150, 108347. [Google Scholar] [CrossRef]
- Hsiao, Y.S.; Quiñones, E.D.; Yen, S.H.; Yu, S.C.; Fang, J.T.; Chen, P.; Juang, R.S. PEDOT:PSS-Based Bioelectrodes for Multifunctional Drug Release and Electric Cell-Substrate Impedance Sensing. ACS Appl. Mater. Interfaces 2023, 15, 21953–21964. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, X.; Guo, H.; Shang, P. The Influence of Electrode Design on Detecting the Effects of Ferric Ammonium Citrate (FAC) on Pre-Osteoblast through Electrical Cell-Substrate Impedance Sensing (ECIS). Biosensors 2023, 13, 322. [Google Scholar] [CrossRef]
- Chen, M.; Patra, P.K.; Warner, S.B.; Bhowmick, S. Role of Fiber Diameter in Adhesion and Proliferation of NIH 3T3 Fibroblast on Electrospun Polycaprolactone Scaffolds. Tissue Eng. 2007, 13, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Bashur, C.A.; Dahlgren, L.A.; Goldstein, A.S. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d,l-lactic-co-glycolic acid) meshes. Biomaterials 2006, 27, 5681–5688. [Google Scholar] [CrossRef] [PubMed]
- Asphahani, F.; Thein, M.; Wang, K.; Wood, D.; Wong, S.S.; Xu, J.; Zhang, M. Real-time characterization of cytotoxicity using single0cell impedance monitoring. Analyst 2012, 137, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Jeong, J.; Kim, Y.; Kang, D.; Shin, S.; Lee, J.; Jeon, S.H.; Jang, M. Growth and Drug Interaction Monitoring of NIH 3T3 Cells by Image Analysis and Capacitive Biosensor. Micromachines 2021, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.R. Impedance Spectroscopy. Ann. Biomed. Eng. 1992, 20, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Robilliard, L.; Kho, D.; Johnson, R.; Anchan, A.; O’Carroll, S.; Graham, E. The Importance of Multifrequency Impedance Sensing of Endothelial Barrier Formation Using ECIS Technology for the Generation of a Strong and Durable Paracellular Barrier. Biosensors 2018, 8, 64. [Google Scholar] [CrossRef]
- Yoo, T.; Lim, K.; Sultan, M.T.; Lee, J.S.; Park, J.; Ju, H.W.; Park, C.; Jang, M. The real-time monitoring of drug reaction in HeLa cancer cell using temperature/impedance integrated biosensors. Sens. Actuators B Chem. 2019, 291, 17–24. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kang, D.; Kim, S.; Hong, E.; Jang, M. Capacitance Contribution of NIH/3T3 Cells Existing on and between Electrodes of an Impedance Biosensor. Biosensors 2023, 13, 970. https://doi.org/10.3390/bios13110970
Kim Y, Kang D, Kim S, Hong E, Jang M. Capacitance Contribution of NIH/3T3 Cells Existing on and between Electrodes of an Impedance Biosensor. Biosensors. 2023; 13(11):970. https://doi.org/10.3390/bios13110970
Chicago/Turabian StyleKim, Yeeun, Dahyun Kang, Seokgyu Kim, Eunchae Hong, and Moongyu Jang. 2023. "Capacitance Contribution of NIH/3T3 Cells Existing on and between Electrodes of an Impedance Biosensor" Biosensors 13, no. 11: 970. https://doi.org/10.3390/bios13110970
APA StyleKim, Y., Kang, D., Kim, S., Hong, E., & Jang, M. (2023). Capacitance Contribution of NIH/3T3 Cells Existing on and between Electrodes of an Impedance Biosensor. Biosensors, 13(11), 970. https://doi.org/10.3390/bios13110970