A Novel Molecularly Imprinted Sensor Based on CuO Nanoparticles with Peroxidase-like Activity for the Selective Determination of Astragaloside-IV
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
2.3. Synthesis of CuO NPs
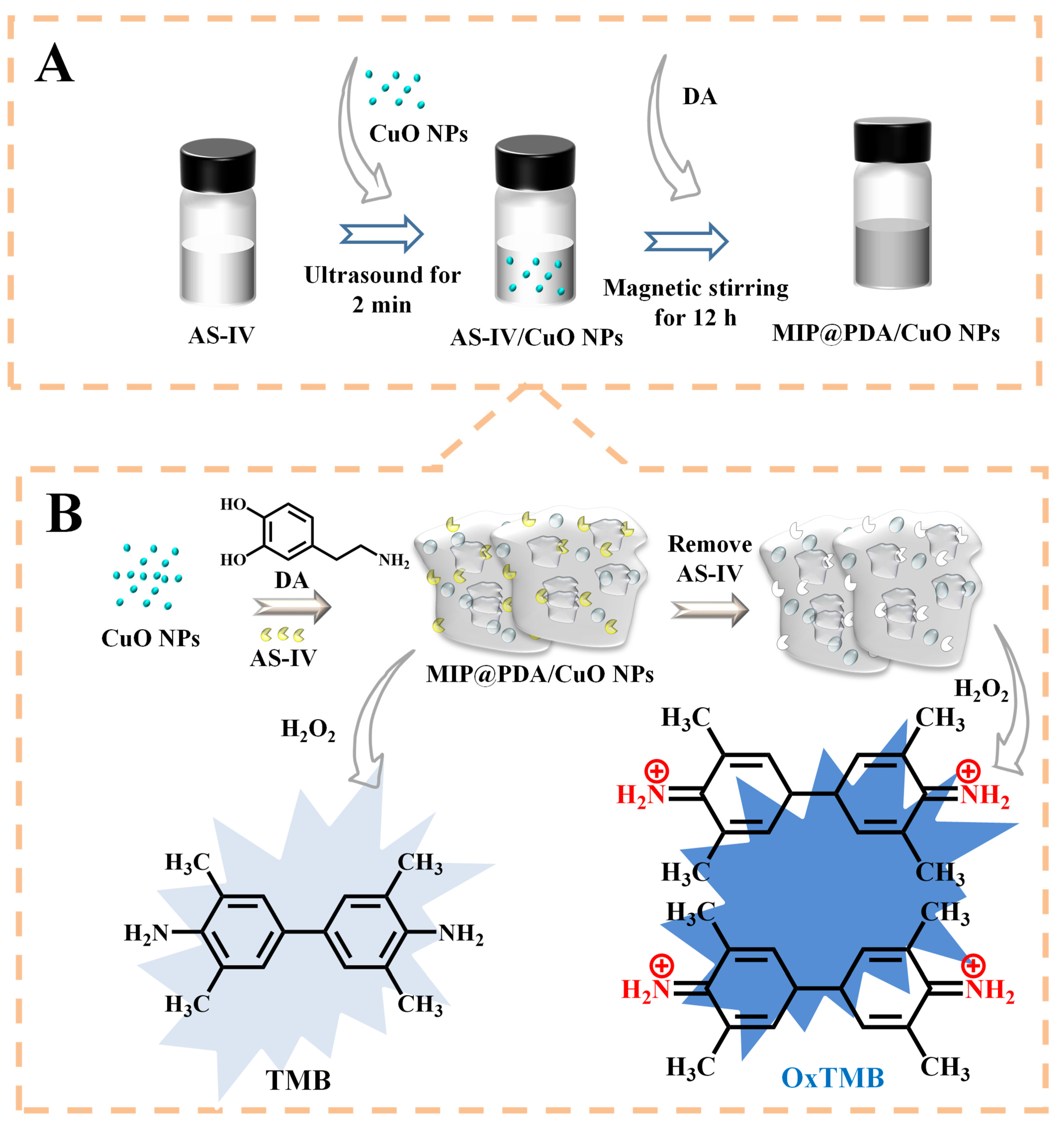
2.4. Synthesis of MIP@PDA/CuO NPs
2.5. Preparation of Huangqi Granules and Ganweikang Tablets Solution
2.6. Peroxidase-like Catalytic Activity of MIP@PDA/CuO NPs and Detection of AS-IV
2.7. HPLC-ELSD Detection of AS-IV
3. Results and Discussion
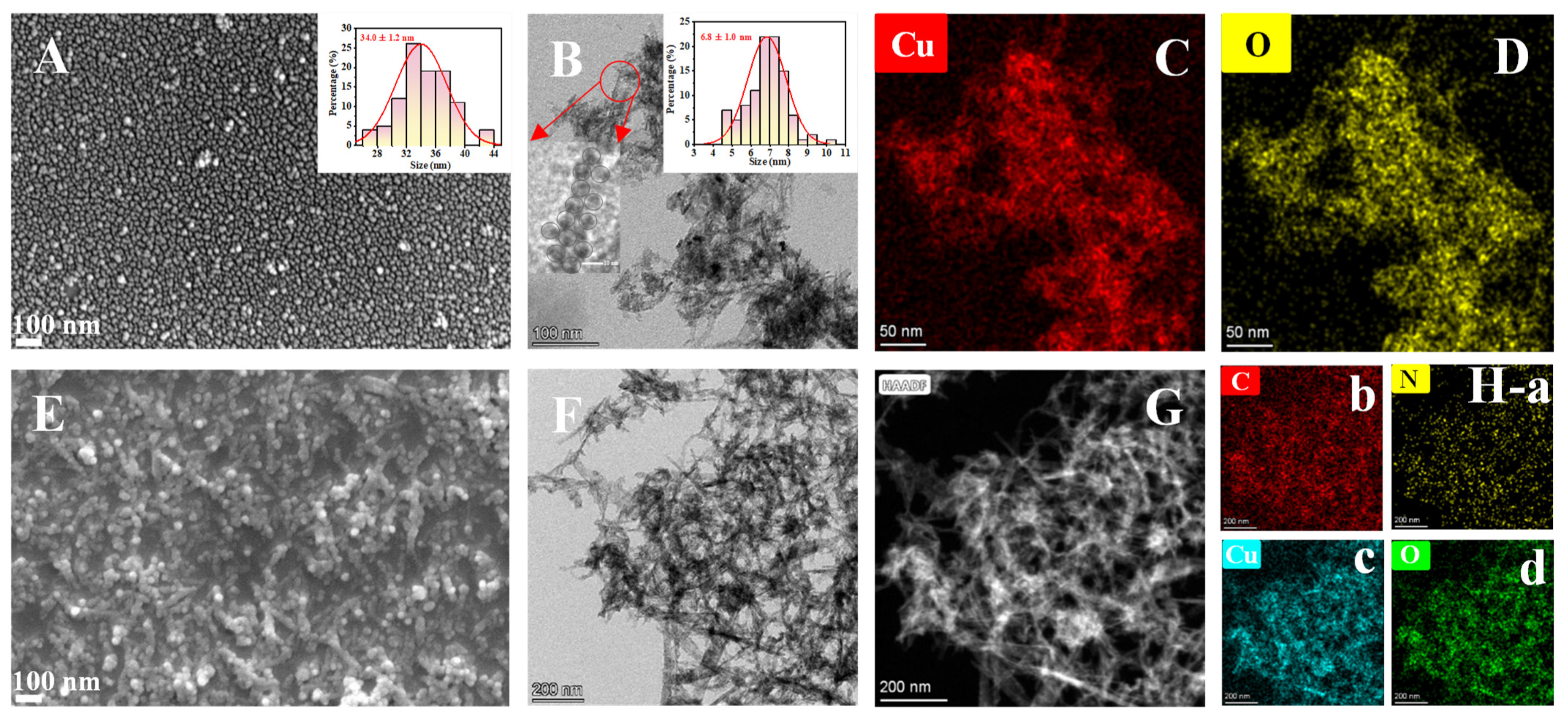
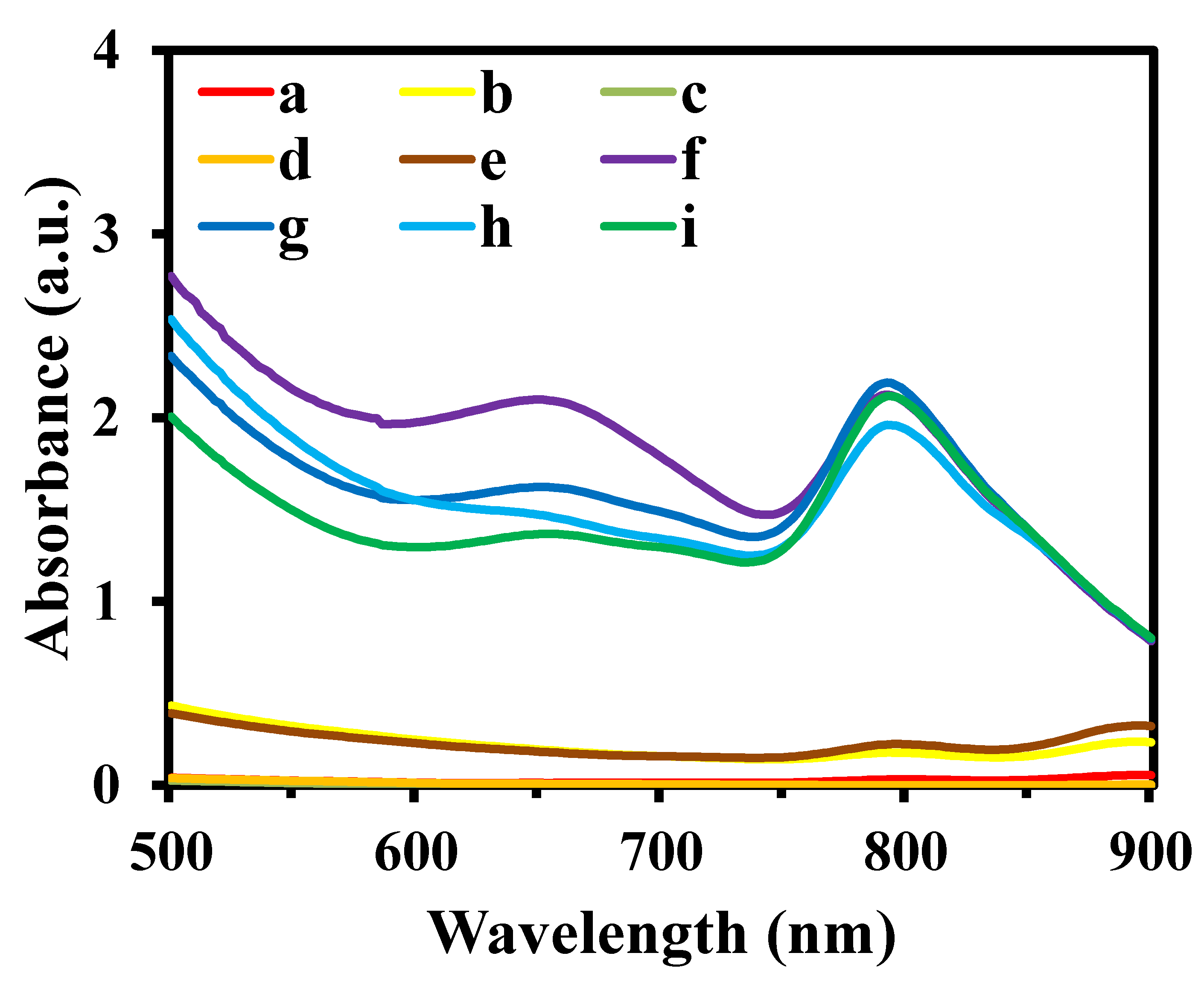
3.1. Characterization of CuO NPs and MIP@PDA/CuO NPs Nanocomposites and Feasibility of the Established Method for the Detection of AS-IV
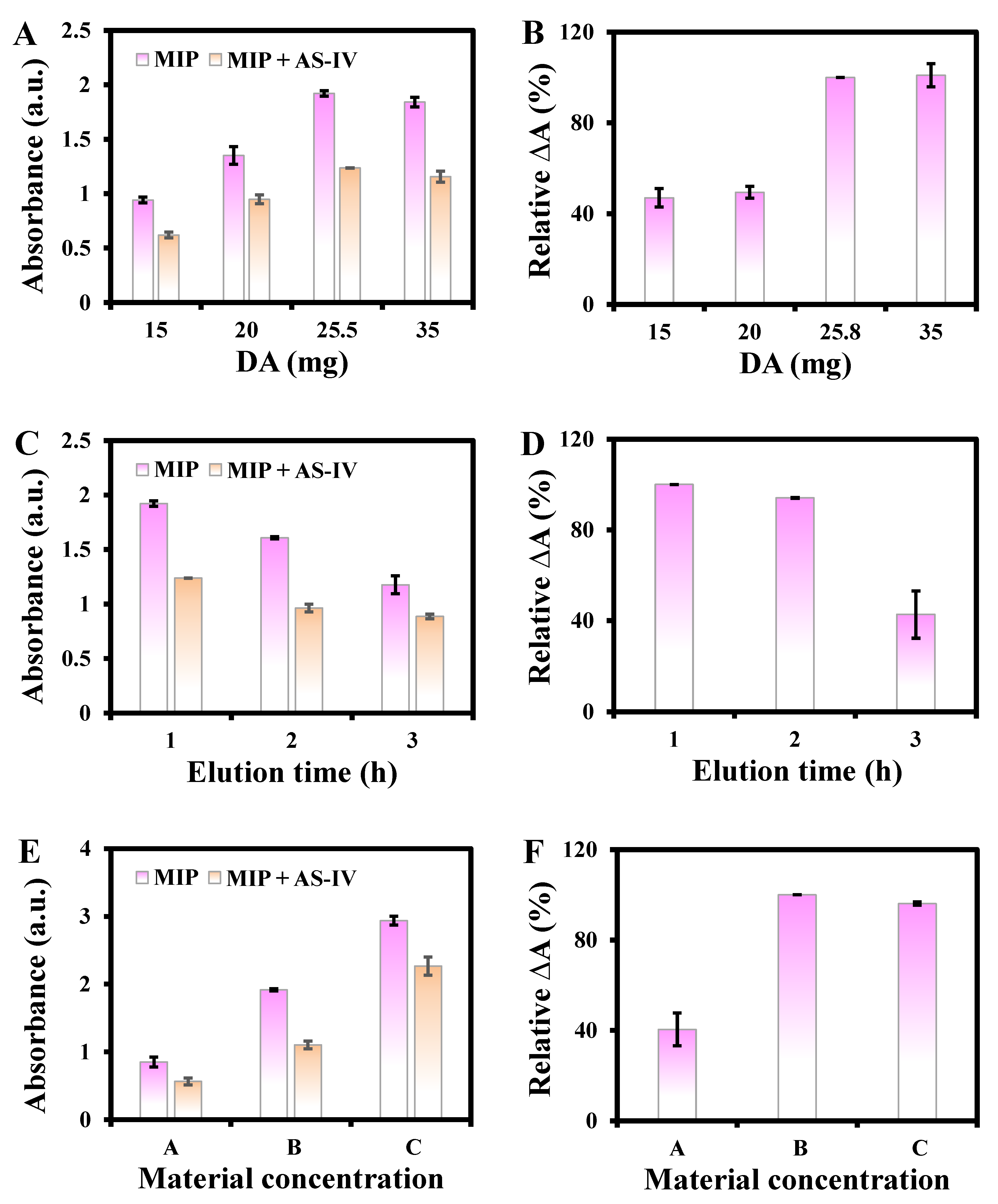
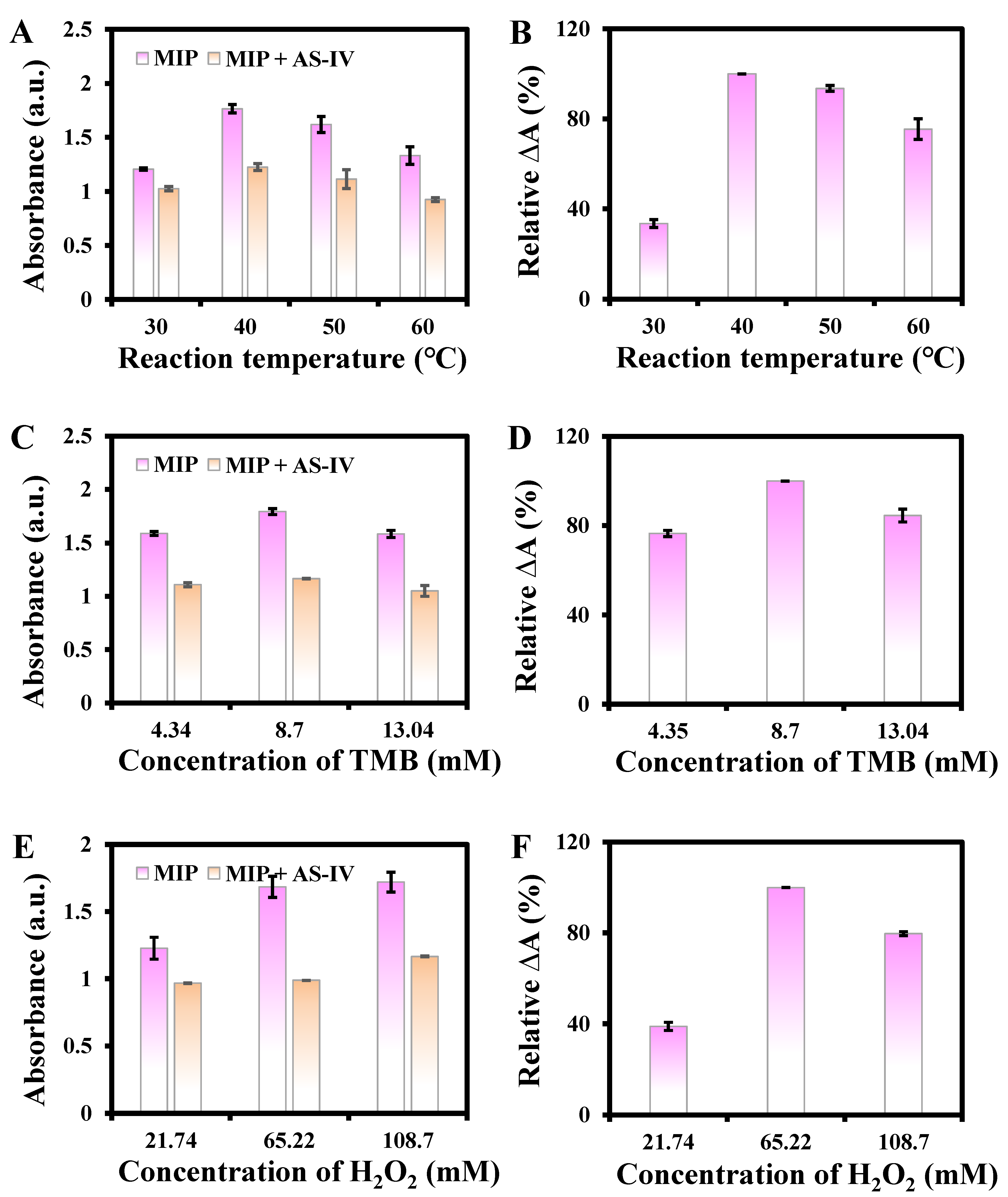
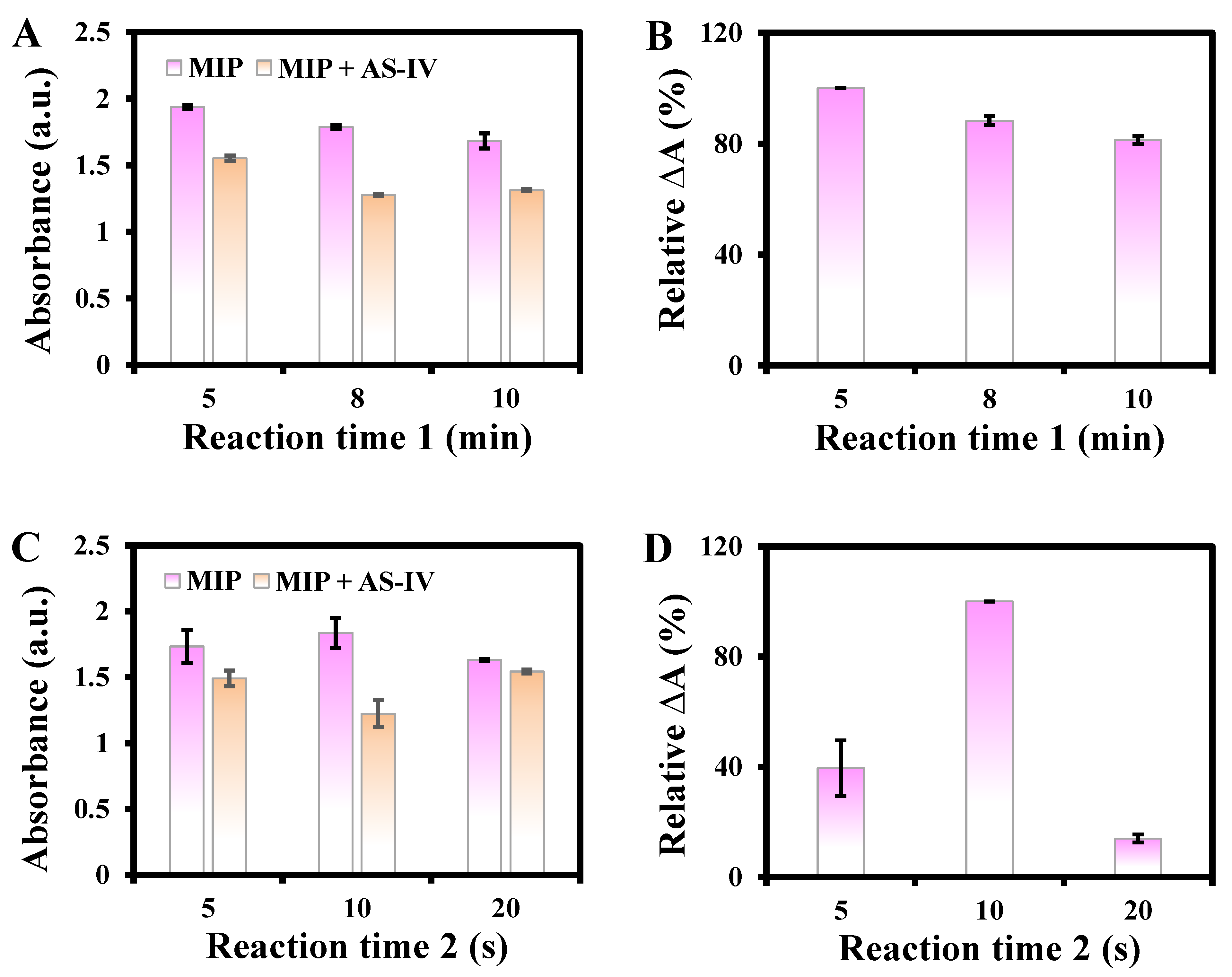
3.2. Study of Reaction Variables
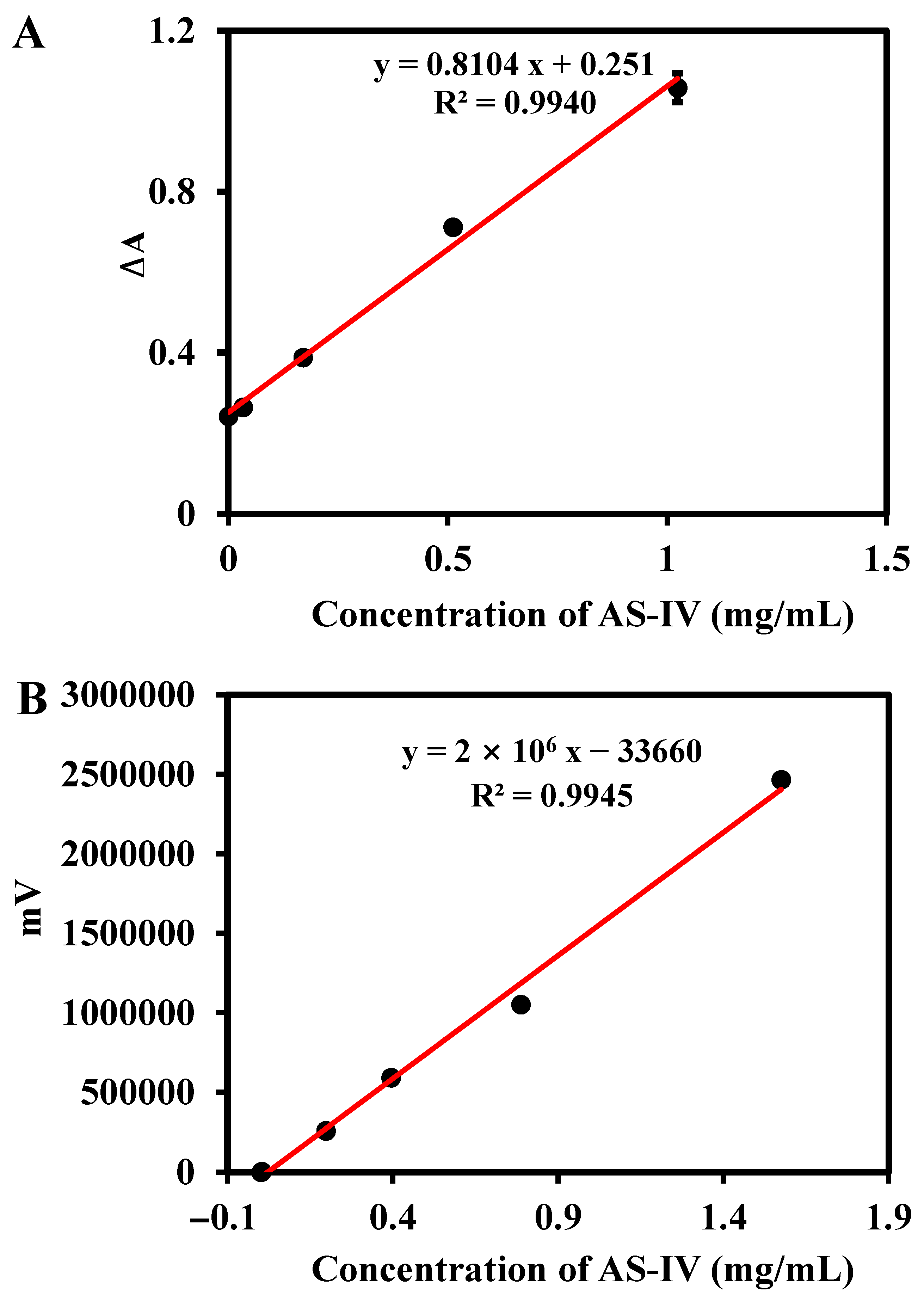
3.3. Detection of AS-IV Based on MIP@PDA/CuO NPs
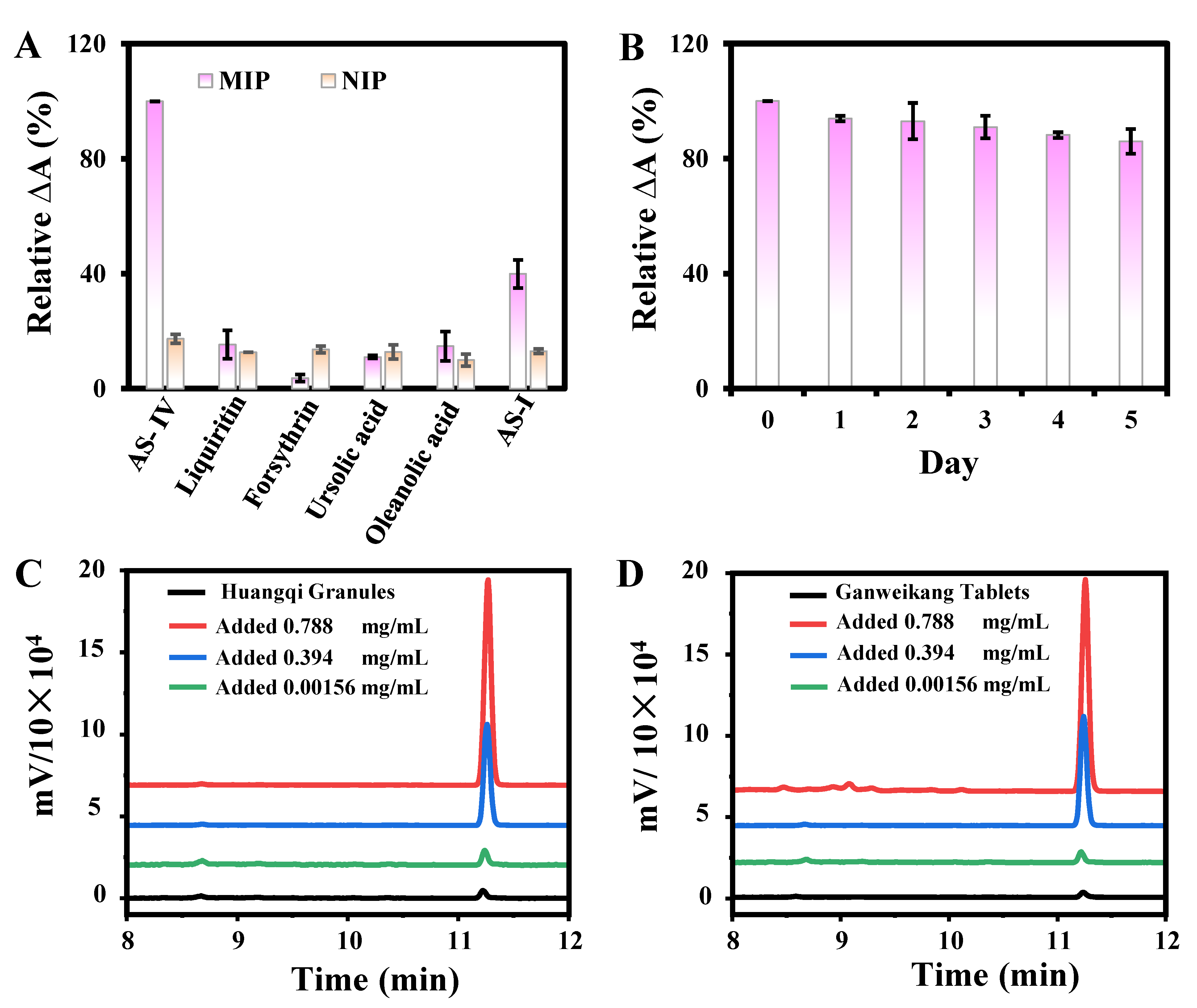
3.4. AS-IV Detection in Huangqi Granules and Ganweikang Tablets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Z.Z.; Lou, R.X.; Pan, W.; Li, N.; Tang, B. Nanoenzymes in disease diagnosis and therapy. Chem. Commun. 2020, 56, 15513–15524. [Google Scholar] [CrossRef]
- Tan, J.; Wu, S.Y.; Cai, Q.Q.; Wang, Y.; Zhang, P. Reversible regulation of enzyme-like activity of molybdenum disulfide quantum dots for colorimetric pharmaceutical analysis. J. Pharm. Anal. 2022, 12, 113–121. [Google Scholar] [CrossRef] [PubMed]
- He, X.X.; Zhang, F.Y.; Liu, J.F.; Fang, G.Z.; Wang, S. Homogenous graphene oxide-peptide nanofiber hybrid hydrogel as biomimetic polysaccharide hydrolase. Nanoscale 2017, 9, 18066–18074. [Google Scholar] [CrossRef]
- Hu, X.N.; Liu, J.B.; Hou, S.; Wen, T.; Liu, W.Q.; Zhang, K.; He, W.W.; Ji, Y.L.; Ren, H.X.; Wang, Q.; et al. Research progress of nanoparticles as enzyme mimetics. Sci. China Phys. Mech. Astron. 2011, 54, 1749–1756. [Google Scholar] [CrossRef]
- Du, J.Y.; Qi, S.Q.; Chen, J.; Yang, Y.; Fan, T.T.; Zhang, P.; Zhuo, S.J.; Zhu, C.Q. Fabrication of highly active phosphatase-like fluorescent cerium-doped carbon dots for in situ monitoring the hydrolysis of phosphate diesters. RSC Adv. 2020, 10, 41551–41559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Lu, N.N.; Wang, C.; Zhang, R.S.; Chen, W.J.; Zhang, Z.Q.; Xing, D.M. Biomimetic cascade nanoreactor with triple-enzyme mimetic activities for colorimetric detection of acid phosphatase. Chem. Eng. J. 2022, 437, 135267. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.Y.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci.-Nano. 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Yang, H.Y.; He, Q.Y.; Pan, J.K.; Lin, M.X.; Lao, Z.T.; Li, Q.L.; Cui, X.P.; Zhao, S.Q. PtCu nanocages with superior tetra-enzyme mimics for colorimetric sensing and fluorescent sensing dehydroepiandrosterone. Sens. Actuator B-Chem. 2022, 351, 130905. [Google Scholar] [CrossRef]
- Liu, C.Y.; Cai, Y.Y.; Wang, J.; Liu, X.; Ren, H.; Yan, L.; Zhang, Y.J.; Yang, S.Q.; Guo, J.; Liu, A.H. Facile preparation of homogeneous copper nanoclusters exhibiting excellent tetraenzyme mimetic activities for colorimetric glutathione sensing and fluorimetric ascorbic acid sensing. ACS Appl. Mater. Interfaces 2020, 12, 42521–42530. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Sheth, S.; Zhou, H.F.; Song, Q.J. Highly selective detection of L-phenylalanine by molecularly imprinted polymers coated Au nanoparticles via surface-enhanced Raman scattering. Talanta 2020, 211, 120745. [Google Scholar] [CrossRef]
- Dong, C.Y.; Shi, H.X.; Han, Y.R.; Yang, Y.Y.; Wang, R.X.; Men, J.Y. Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J. 2021, 145, 110231. [Google Scholar] [CrossRef]
- Zeng, L.S.; Cui, H.R.; Chao, J.L.; Huang, K.; Wang, X.; Zhou, Y.K.; Jing, T. Colorimetric determination of tetrabromobisphenol A based on enzyme-mimicking activity and molecular recognition of metal-organic framework-based molecularly imprinted polymers. Microchim. Acta 2020, 187, 142. [Google Scholar] [CrossRef] [PubMed]
- Sawetwong, P.; Chairam, S.; Jarujamrus, P.; Amatatongchai, M. Enhanced selectivity and sensitivity for colorimetric determination of glyphosate using Mn-ZnS quantum dot embedded molecularly imprinted polymers combined with a 3D-microfluidic paper-based analytical device. Talanta 2021, 225, 122077. [Google Scholar] [CrossRef]
- Guo, L.L.; Zheng, H.J.; Zhang, C.J.; Qu, L.B.; Yu, L.L. A novel molecularly imprinted sensor based on PtCu bimetallic nanoparticle deposited on PSS functionalized graphene with peroxidase-like activity for selective determination of puerarin. Talanta 2020, 210, 120621. [Google Scholar] [CrossRef] [PubMed]
- He, L.Y.; Lu, Y.X.; Gao, X.Y.; Song, P.S.; Huang, Z.X.; Liu, S.; Liu, Y.Y. Self-cascade system based on cupric oxide nanoparticles as dual-functional enzyme mimics for ultrasensitive detection of silver ions. ACS Sustain. Chem. Eng. 2018, 6, 12132–12139. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Weil, T.; Ng, D.Y.W.; Ball, V. Polydopamine at biological interfaces. Adv. Colloid Interface Sci. 2022, 305, 102689. [Google Scholar] [CrossRef]
- Chai, X.Y.; Gu, Y.Q.; Lv, L.; Chen, C.; Feng, F.; Cao, Y.; Liu, Y.; Zhu, Z.Y.; Hong, Z.Y.; Chai, Y.F.; et al. Screening of immune cell activators from Astragali Radix using a comprehensive two-dimensional NK-92MI cell membrane chromatography/C18 column/time-of-flight mass spectrometry system. J. Pharm. Anal. 2022, 12, 725–732. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Chen, H.W.; Li, J. Astragaloside IV: An effective drug for the treatment of cardiovascular diseases. Drug Des. Devel. Ther. 2020, 14, 3731–3746. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 11th ed.; China Medical Science Press: Beijing, China, 2020; p. 315.
- Wang, K.; Liu, J.; Wang, X.; Liu, X.Y.; Hu, J.S.; Li, E.S.; Zhao, Y.S.; Zhao, R.S.; Yang, S.H. Ratiometric fluorescent detection system based on dual-driving catalysis of CuO nanozyme with a classical univariate calibration for the determination of ascorbic acid in serum and fruits. Microchem. J. 2022, 172, 106921. [Google Scholar] [CrossRef]
- He, D.; Fu, C.; Xue, Z.G. Optimization study of direct morphology observation by cold field emission SEM without gold coating. Micron 2018, 109, 53–57. [Google Scholar] [CrossRef]
- Novotná, Z.; Rimpelová, S.; Juřík, P.; Veselý, M.; Kolská, Z.; Hubáček, T.; Borovec, J.; Švorčík, V. Tuning surface chemistry of polyetheretherketone by gold coating and plasma treatment. Nanoscale Res. Lett. 2017, 12, 424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Meng, M.; Jiang, Y.; Zhang, H.; Wang, F. A new approach to develop a standardized method for simultaneous analysis of astragaloside IV and formononetin in radix astragali by high-performance thin-layer chromatography. JPC-J. Planar Chromatogr. Mod. TLC 2015, 28, 268–273. [Google Scholar] [CrossRef]
- Jia, M.M.; Zhang, B.B.; Qi, Y.D.; Yang, J.; Yao, Z.H.; Qin, Z.F.; Zhang, X.J.; Yao, X.S. UHPLC coupled with mass spectrometry and chemometric analysis of Kang-Ai injection based on the chemical characterization, simultaneous quantification, and relative quantification of 47 herbal alkaloids and saponins. J. Sep. Sci. 2020, 43, 2539–2549. [Google Scholar] [CrossRef] [PubMed]

| UV-Vis | HPLC-ELSD | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Added (mg/mL) | Found (mg/mL) | Recovery (%) | RSD (n = 3, %) | Added (mg/mL) | Found (mg/mL) | Recovery (%) | RSD (n = 3, %) |
| Huangqi Granules | 0 | 0.000555 a | - | 1.0 | 0 | 0.0605 | - | 1.2 |
| 0.000341 | 0.000856 | 90.9 | 2.9 | 0.00156 | 0.0620 | 91.5 | 4.7 | |
| 0.512 | 0.494 | 93.8 | 3.0 | 0.394 | 0.374 | 80.9 | 5.9 | |
| 1.024 | 0.856 | 83.5 | 5.8 | 0.788 | 0.714 | 80.0 | 4.3 | |
| Ganweikang Tablets | 0 | 0.000352 b | - | 0.9 | 0 | 0.0244 | - | 1.5 |
| 0.000341 | 0.000745 | 113.1 | 2.2 | 0.00156 | 0.0261 | 109.0 | 1.3 | |
| 0.512 | 0.584 | 108.9 | 4.9 | 0.394 | 0.342 | 83.8 | 3.6 | |
| 1.024 | 1.059 | 102.6 | 0.8 | 0.788 | 0.715 | 89.0 | 5.0 | |
| Ganweikang Tablets | UV-Vis (μg/g) | HPLC-ELSD (μg/g) |
|---|---|---|
| 210202 | 122.93 ± 1.4 | 120.84 ± 0.8 |
| 210201 | 123.40 ± 1.0 | 121.20 ± 0.7 |
| 200102 | 122.15 ± 1.1 | 121.62 ± 1.2 |
| 200801 | 123.11 ± 0.9 | 120.26 ± 1.1 |
| 201201 | 122.68 ± 1.6 | 120.51 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.-Y.; Chen, L.-X.; Gao, J.; Chen, C.; Guan, J.; Cao, Z.; Hu, Y.; Yang, F.-Q. A Novel Molecularly Imprinted Sensor Based on CuO Nanoparticles with Peroxidase-like Activity for the Selective Determination of Astragaloside-IV. Biosensors 2023, 13, 959. https://doi.org/10.3390/bios13110959
Chen G-Y, Chen L-X, Gao J, Chen C, Guan J, Cao Z, Hu Y, Yang F-Q. A Novel Molecularly Imprinted Sensor Based on CuO Nanoparticles with Peroxidase-like Activity for the Selective Determination of Astragaloside-IV. Biosensors. 2023; 13(11):959. https://doi.org/10.3390/bios13110959
Chicago/Turabian StyleChen, Guo-Ying, Ling-Xiao Chen, Jin Gao, Chengyu Chen, Jianli Guan, Zhiming Cao, Yuanjia Hu, and Feng-Qing Yang. 2023. "A Novel Molecularly Imprinted Sensor Based on CuO Nanoparticles with Peroxidase-like Activity for the Selective Determination of Astragaloside-IV" Biosensors 13, no. 11: 959. https://doi.org/10.3390/bios13110959
APA StyleChen, G.-Y., Chen, L.-X., Gao, J., Chen, C., Guan, J., Cao, Z., Hu, Y., & Yang, F.-Q. (2023). A Novel Molecularly Imprinted Sensor Based on CuO Nanoparticles with Peroxidase-like Activity for the Selective Determination of Astragaloside-IV. Biosensors, 13(11), 959. https://doi.org/10.3390/bios13110959






