Application and Development of EEG Acquisition and Feedback Technology: A Review
Abstract
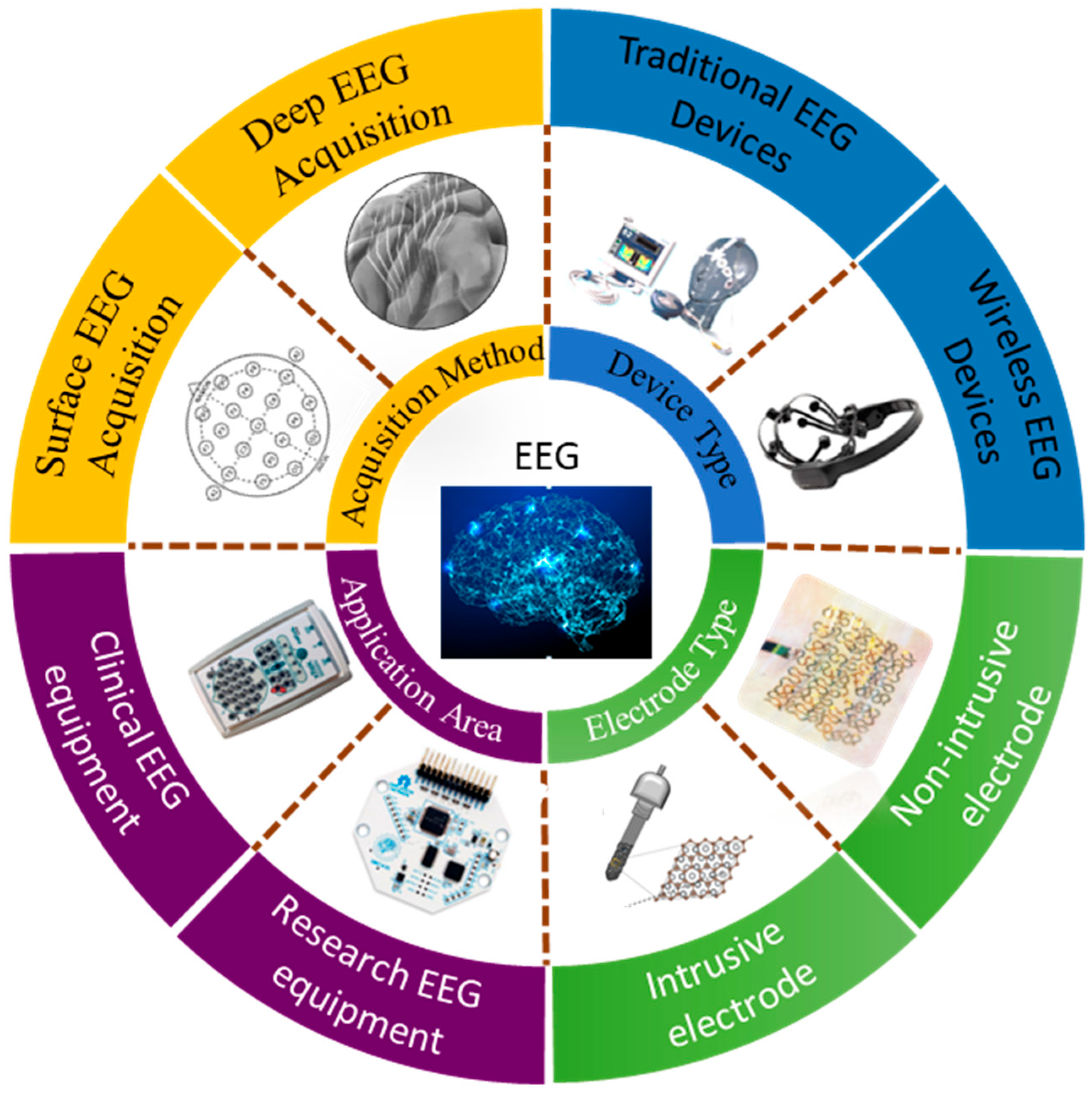
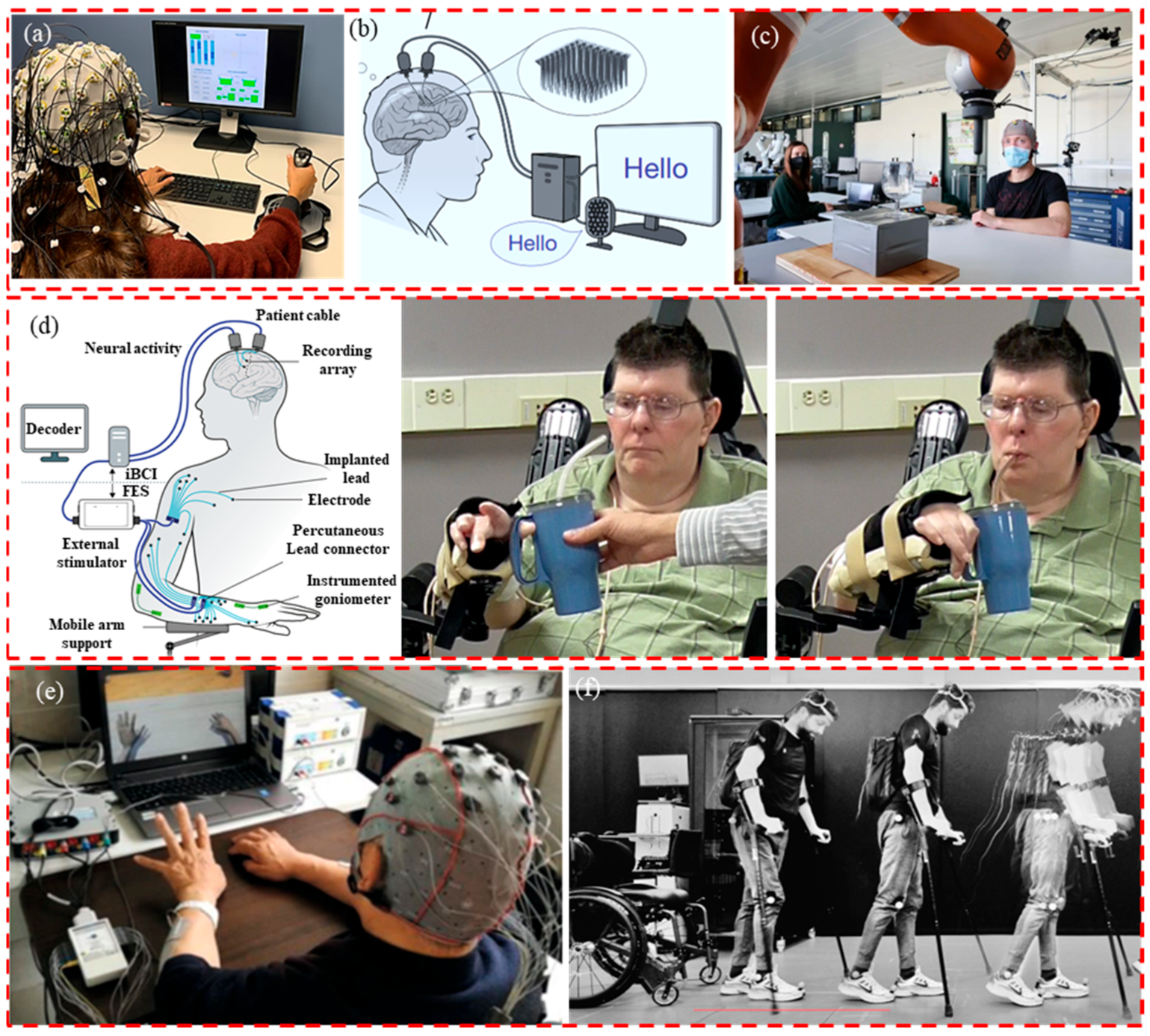
:1. Introduction
2. The Acquisition and Processing of EEG Acquisition and Feedback Devices
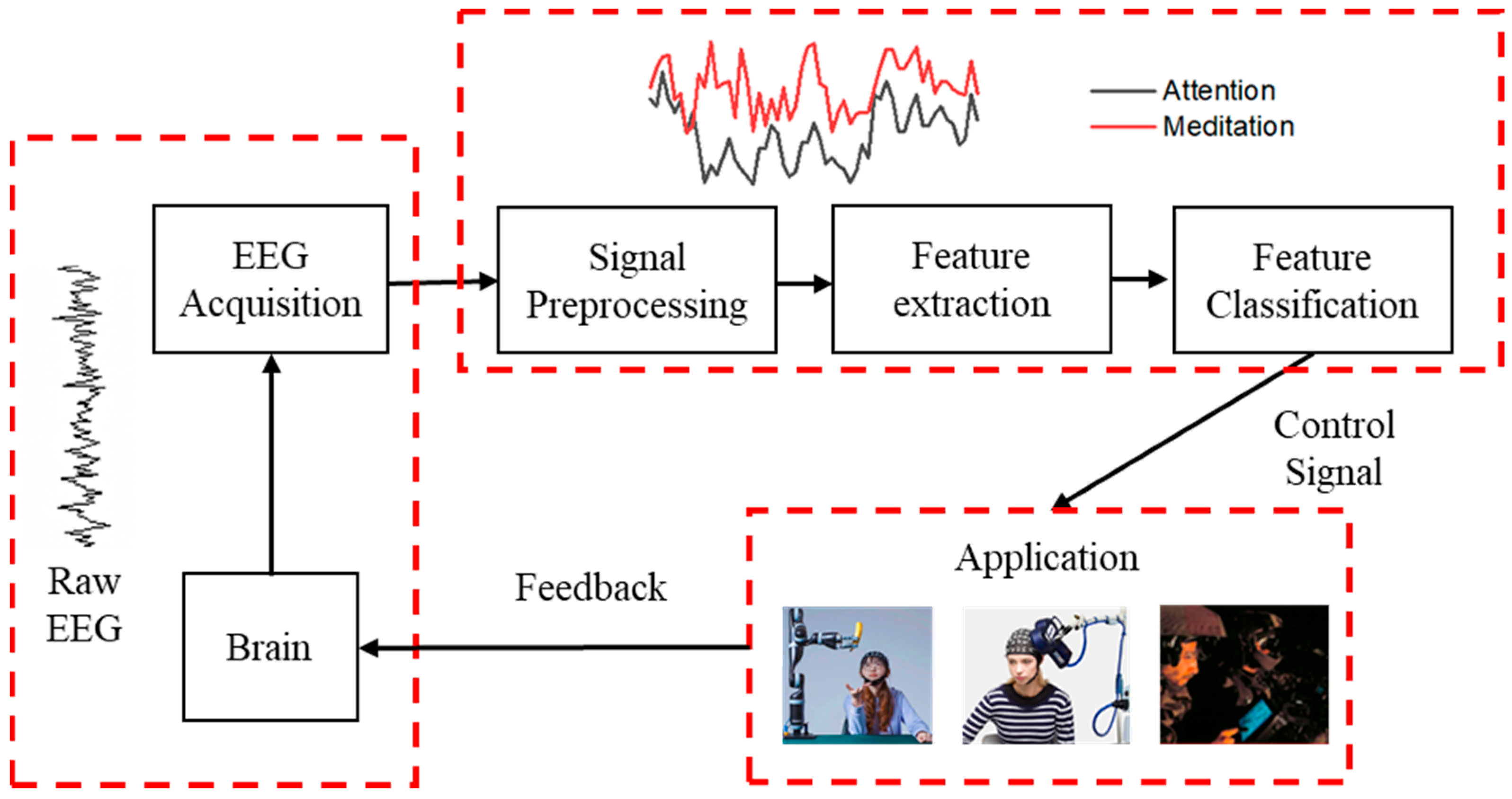
2.1. The Acquisition and Feedback Principle of EEG Acquisition Devices
2.2. Composition of EEG Signal Acquisition and Feedback Devices
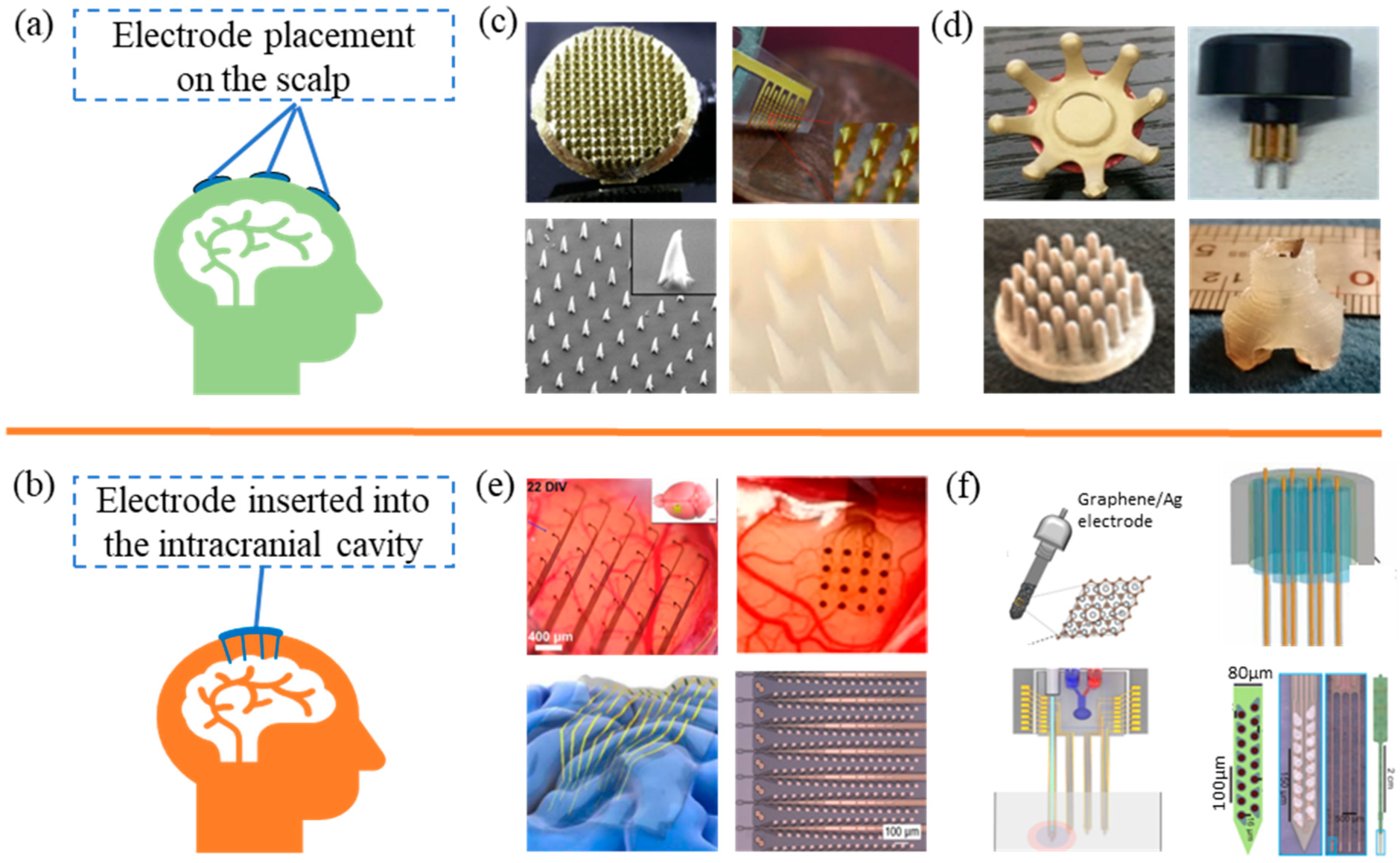
2.2.1. EEG Acquisition Electrode Type
2.2.2. Analog-to-Digital Conversion Circuit
2.2.3. Preprocessing Circuit
2.2.4. Processor Circuit
2.2.5. EEG Signal Control and Feedback
3. Application of EEG Acquisition and Feedback System
3.1. Emotional Recognition
3.2. Movement Assistance System
4. Data Analysis and Machine Learning in EEG Research
4.1. Common Data Analysis Methods
4.2. Application of Machine Learning in EEG Research
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, L.; Zhang, C.; Cui, Z. Experimental research on real-time acquisition and monitoring of wearable EEG based on TGAM module. Comput. Commun. 2020, 151, 76–85. [Google Scholar] [CrossRef]
- Xi, X.; Tao, Q.; Li, J.; Kong, W.; Zhao, Y.B.; Wang, H.; Wang, J. Emotion-movement relationship: A study using functional brain network and cortico-muscular coupling. J. Neurosci. Methods 2021, 362, 109320. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Deshpande, A.V. Fragmentary shape recognition: A BCI study. Comput.-Aided Des. 2016, 71, 51–64. [Google Scholar] [CrossRef]
- Zeynali, M.; Seyedarabi, H.; Afrouzian, R. Classification of EEG signals using Transformer based deep learning and ensemble models. Biomed. Signal Process. Control 2023, 86, 105130. [Google Scholar] [CrossRef]
- Acharya, J.N.; Hani, A.J.; Cheek, J.; Thirumala, P.; Tsuchida, T.N. American Clinical Neurophysiology Society Guideline 2: Guidelines for Standard Electrode Position Nomenclature. Neurodiagn. J. 2017, 56, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Du, M.; Wang, L.; Li, S.; Wang, G.; Yang, X.; Zhang, L.; Fang, Y.; Zheng, W.; Yang, G.; et al. Bacterial Cellulose as a Supersoft Neural Interfacing Substrate. ACS Appl. Mater. Interfaces 2018, 10, 33049–33059. [Google Scholar] [CrossRef]
- EEG Acquisition Devices. Available online: http://hanfeiyl.com/product-i12447.html (accessed on 29 August 2023).
- EEG Acquisition Devices. Available online: https://www.emotiv.com/epoc-x (accessed on 29 August 2023).
- Ban, S.; Lee, Y.J.; Kwon, S.; Kim, Y.-S.; Chang, J.W.; Kim, J.-H.; Yeo, W.-H. Soft Wireless Headband Bioelectronics and Electrooculography for Persistent Human–Machine Interfaces. ACS Appl. Electron. Mater. 2023, 5, 877–886. [Google Scholar] [CrossRef]
- Liu, S.; Liu, L.; Zhao, Y.; Wang, Y.; Wu, Y.; Zhang, X.-D.; Ming, D. A High-Performance Electrode Based on van der Waals Heterostructure for Neural Recording. Nano Lett. 2022, 22, 4400–4409. [Google Scholar] [CrossRef]
- Research-Grade EEG Gevices. Available online: https://openbci.com (accessed on 29 August 2023).
- Clinical EEG Devices. Available online: https://compumedicsneuroscan.com (accessed on 29 August 2023).
- Chiesi, M.; Guermandi, M.; Placati, S.; Scarselli, E.F.; Guerrieri, R. Creamino: A Cost-Effective, Open-Source EEG-Based BCI System. IEEE Trans. Biomed. Eng. 2019, 66, 900–909. [Google Scholar] [CrossRef]
- Kalra, J.; Mittal, P.; Mittal, N.; Arora, A.; Tewari, U.; Chharia, A.; Upadhyay, R.; Kumar, V.; Longo, L. How Visual Stimuli Evoked P300 is Transforming the Brain–Computer Interface Landscape: A PRISMA Compliant Systematic Review. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1429–1439. [Google Scholar] [CrossRef]
- Chaudhary, U.; Birbaumer, N.; Ramos-Murguialday, A. Brain–computer interfaces for communication and rehabilitation. Nat. Rev. Neurol. 2016, 12, 513–525. [Google Scholar] [CrossRef] [PubMed]
- EGI GES400 System. Available online: https://www.brainproducts.com (accessed on 29 August 2023).
- NeuSen W EEG Acquisition System. Available online: http://www.neuracle.cn/productinfo/148706.html (accessed on 29 August 2023).
- Zhang, W.; Sun, F.; Wu, H.; Tan, C.; Ma, Y. Asynchronous brain-computer interface shared control of robotic grasping. Tsinghua Sci. Technol. 2019, 24, 360–370. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Liu, H.; Wang, K.; Moses, K.; Feng, Z.; Li, P.; Huang, W. Flexible Electrodes for Brain-Computer Interface System. Adv. Mater. 2023, 7, 2211012. [Google Scholar] [CrossRef] [PubMed]
- Quitadamo, L.R.; Cavrini, F.; Sbernini, L.; Riillo, F.; Bianchi, L.; Seri, S.; Saggio, G. Support vector machines to detect physiological patterns for EEG and EMG-based human–computer interaction: A review. J. Neural Eng. 2017, 14, 011001. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Du, Y.; Pan, T.; Huang, C.; Zhang, Z.; Wong, K. A Deep Learning-Based Classification Method for Different Frequency EEG Data. Comput. Math. Methods Med. 2021, 2021, 1972662. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Li, D.; Chen, H.; Yu, P.; Yan, H.; Yue, M. An improved feature extraction algorithms of EEG signals based on motor imagery brain-computer interface. Alex. Eng. J. 2022, 61, 4807–4820. [Google Scholar] [CrossRef]
- Aggarwal, S.; Chugh, N. Review of Machine Learning Techniques for EEG Based Brain Computer Interface. Arch. Comput. Methods Eng. 2022, 29, 3001–3020. [Google Scholar] [CrossRef]
- Xia, X.; Liu, X.; Zheng, W.; Jia, X.; Wang, B.; Shi, Y.; Men, H. Recognition of odor and pleasantness based on olfactory EEG combined with functional brain network model. Int. J. Mach. Learn. Cybern. 2023, 14, 2761–2776. [Google Scholar] [CrossRef]
- Cheng, L.; Li, D.; Yu, G.; Zhang, Z.; Yu, S. Robotic arm control system based on brain-muscle mixed signals. Biomed. Signal Process. Control 2022, 77, 103754. [Google Scholar] [CrossRef]
- Peksa, J.; Mamchur, D. State-of-the-Art on Brain-Computer Interface Technology. Sensors 2023, 23, 6001. [Google Scholar] [CrossRef]
- Zafeiropoulos, G.C.; Drakakis, E.M. The neoEEG board: A 3 nV/Hz multi-channel wireless instrument for neonatal EEG monitoring. Measurement 2020, 154, 107442. [Google Scholar] [CrossRef]
- Lin, B.-S.; Lin, B.-S.; Yen, T.-H.; Hsu, C.-C.; Wang, Y.-C. Design of Wearable Headset with Steady State Visually Evoked Potential-Based Brain Computer Interface. Micromachines 2019, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tan, H.; Wang, X.; Teng, K. Research progress of the electrode for electroencephalogram acquisition. Sci. Technol. Eng. 2021, 21, 6097–6104. [Google Scholar]
- Dobkin, B.H. Brain-computer interface technology as a tool to augment plasticity and outcomes for neurological rehabilitation. J. Physiol. 2007, 579, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, D.; Jin, M.; Gao, H.; Wang, X.; Xia, L.; Li, D.; Sun, K.; Wang, H.; Dong, X.; et al. Hydrogel electrodes with conductive and substrate-adhesive layers for noninvasive long-term EEG acquisition. Microsyst. Nanoeng. 2023, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D printed dry electrode for ECG/EEG recording. Sens. Actuators A Phys. 2012, 174, 96–102. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, X.; Wang, W.; Li, Z. A microneedle electrode array on flexible substrate for long-term EEG monitoring. Sens. Actuators B Chem. 2017, 244, 750–758. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Bhartia, B.; Mukhopadhyay, K.; Sharma, A. Long term biopotential recording by body conformable photolithography fabricated low cost polymeric microneedle arrays. Sens. Actuators A Phys. 2015, 236, 164–172. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Lin, Y.-T.; Yu, J.; Chang, H.-T.; Lu, T.-Y.; Huang, T.-Y.; Preet, A.; Hsu, Y.-J.; Wang, L.; Lin, T.-E. MXene Nanosheet-Based Microneedles for Monitoring Muscle Contraction and Electrostimulation Treatment. ACS Appl. Nano Mater. 2021, 4, 7917–7924. [Google Scholar] [CrossRef]
- Xing, X.; Wang, Y.; Pei, W.; Guo, X.; Liu, Z.; Wang, F.; Ming, G.; Zhao, H.; Gui, Q.; Chen, H. A High-Speed SSVEP-Based BCI Using Dry EEG Electrodes. Sci. Rep. 2018, 8, 14708. [Google Scholar] [CrossRef]
- Hinrichs, H.; Scholz, M.; Baum, A.K.; Kam, J.W.Y.; Knight, R.T.; Heinze, H.-J. Comparison between a wireless dry electrode EEG system with a conventional wired wet electrode EEG system for clinical applications. Sci. Rep. 2020, 10, 5218. [Google Scholar] [CrossRef] [PubMed]
- Wunder, S.; Hunold, A.; Fiedler, P.; Schlegelmilch, F.; Schellhorn, K.; Haueisen, J. Novel bifunctional cap for simultaneous electroencephalography and transcranial electrical stimulation. Sci. Rep. 2018, 8, 7259. [Google Scholar] [CrossRef] [PubMed]
- Harati, A.; Jahanshahi, A. A reliable stretchable dry electrode for monitoring of EEG signals. Sens. Actuators A Phys. 2021, 326, 112727. [Google Scholar] [CrossRef]
- Lee, S.H.; Thunemann, M.; Lee, K.; Cleary, D.R.; Tonsfeldt, K.J.; Oh, H.; Azzazy, F.; Tchoe, Y.; Bourhis, A.M.; Hossain, L.; et al. Scalable Thousand Channel Penetrating Microneedle Arrays on Flex for Multimodal and Large Area Coverage BrainMachine Interfaces. Adv. Funct. Mater. 2022, 32, 2112045. [Google Scholar] [CrossRef] [PubMed]
- Ganji, M.; Paulk, A.C.; Yang, J.C.; Vahidi, N.W.; Lee, S.H.; Liu, R.; Hossain, L.; Arneodo, E.M.; Thunemann, M.; Shigyo, M.; et al. Selective Formation of Porous Pt Nanorods for Highly Electrochemically Efficient Neural Electrode Interfaces. Nano Lett. 2019, 19, 6244–6254. [Google Scholar] [CrossRef] [PubMed]
- Musk, E. An integrated brain-machine interface platform with thousands of channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef] [PubMed]
- Obaid, A.; Hanna, M.-E.; Wu, Y.-W.; Kollo, M.; Racz, R.; Angle, M.R.; Müller, J.; Brackbill, N.; Wray, W.; Franke, F. Massively parallel microwire arrays integrated with CMOS chips for neural recording. Sci. Adv. 2020, 6, eaay2789. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Son, Y.; Chae, U.; Kim, J.; Choi, N.; Lee, H.J.; Woo, J.; Cho, Y.; Yang, S.H.; Lee, C.J.; et al. Multifunctional multi-shank neural probe for investigating and modulating long-range neural circuits in vivo. Nat. Commun. 2019, 10, 3777. [Google Scholar] [CrossRef]
- Chung, J.E.; Joo, H.R.; Fan, J.L.; Liu, D.F.; Barnett, A.H.; Chen, S.; Geaghan-Breiner, C.; Karlsson, M.P.; Karlsson, M.; Lee, K.Y.; et al. High-Density, Long-Lasting, and Multi-region Electrophysiological Recordings Using Polymer Electrode Arrays. Neuron 2019, 101, 21–31.e25. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Cai, S.; Han, Z.; Liu, X.; Wang, F.; Cao, Y.; Wang, Z.; Li, H.; Chen, Y.; et al. Flexible Hybrid Electronics for Digital Healthcare. Adv. Mater. 2020, 32, e1902062. [Google Scholar] [CrossRef]
- Cheng, X.; Fan, Z.; Yao, S.; Jin, T.; Lv, Z.; Lan, Y.; Bo, R.; Chen, Y.; Zhang, F.; Shen, Z. Programming 3D curved mesosurfaces using microlattice designs. Science 2023, 379, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Jin, T.; Xu, S.; Bai, K.; He, Q.; Zhang, F.; Cheng, X.; Ji, Z.; Pang, W.; Shen, Z. Assembly of complex 3D structures and electronics on curved surfaces. Sci. Adv. 2022, 8, eabm6922. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Cheng, X.; Xu, S.; Lai, Y.; Zhang, Y. Deep learning aided inverse design of the buckling-guided assembly for 3D frame structures. J. Mech. Phys. Solids 2023, 179, 105398. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, F.; Jang, K.I.; Feng, X.; Rogers, J.A.; Zhang, Y.; Huang, Y.; Ma, Y. The equivalent medium of cellular substrate under large stretching, with applications to stretchable electronics. J. Mech. Phys. Solids 2018, 120, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Selberherr, S.; Vaz, F.A. A CMOS IC for portable EEG acquisition systems. IEEE Trans. Instrum. Meas. 1998, 47, 1191–1196. [Google Scholar] [CrossRef]
- Ng, D.W.-K.; Goh, S.Y. Indirect control of an autonomous wheelchair using SSVEP BCI. J. Robot. Mechatron. 2020, 32, 761–767. [Google Scholar] [CrossRef]
- Pengju, Z.; Dezhi, Z.; Shuailei, Z.; Kai, H. Digital EEG signal acquiring system based on FPGA. In Proceedings of the 2017 13th IEEE International Conference on Electronic Measurement & Instruments (ICEMI), Yangzhou, China, 20–22 October 2017; pp. 528–533. [Google Scholar]
- Tang, J.; Xu, M.; Han, J.; Liu, M.; Dai, T.; Chen, S.; Ming, D. Optimizing SSVEP-Based BCI System towards Practical High-Speed Spelling. Sensors 2020, 20, 4186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Voelker, M.; Hauer, J. A mixed-signal front-end ASIC for EEG acquisition system. In Proceedings of the 2012 19th IEEE International Conference on Electronics, Circuits, and Systems (ICECS 2012), Seville, Spain, 9–12 December 2012; pp. 649–652. [Google Scholar]
- Lee, C.-J.; Song, J.-I. A Chopper Stabilized Current-Feedback Instrumentation Amplifier for EEG Acquisition Applications. IEEE Access 2019, 7, 11565–11569. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Chen, C.; Sun, C.; Chen, W. A multichannel reconfigurable EEG acquisition system design with felt-based soft material electrodes. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018; pp. 1–6. [Google Scholar]
- Yi, X.; Hao, L.; Jiang, F.; Xu, L.; Song, S.; Li, G.; Lin, L. Synchronous acquisition of multi-channel signals by single-channel ADC based on square wave modulation. Rev. Sci. Instrum. 2017, 88, 085108. [Google Scholar] [CrossRef]
- McKee, J.J.; Evans, N.E.; Wallace, D. Sigma-delta analogue-to-digital converters for ECG signal acquisition. In Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherlands, 31 October–3 November 1996; pp. 19–20. [Google Scholar]
- Teo, T.H.; Gopalakrishnan, P.; Hwan, Y.; Qian, X.; Haridas, K.; Pang, C.; Je, M. A 700-μW single-chip IC for wireless continuous-time health monitoring in 0.18-μm CMOS. In Proceedings of the 2009 IEEE Asian Solid-State Circuits Conference, Taipei, Taiwan, 16–18 November 2009; pp. 361–364. [Google Scholar]
- ADS1299 Series Chip. Available online: https://www.ti.com.cn/sitesearch/zh-cn/docs/universalsearch.tsp?langPref=zh-CN&searchTerm=ADS1299&nr=2497#q=ADS1299&sort=relevancy&numberOfResults=25 (accessed on 29 August 2023).
- Jha, P.; Patra, P.; Naik, J.; Dutta, A.; Acharya, A.; Rajalakshmi, P.; Singh, S.G. A 2 μW biomedical frontend with ΣΔ ADC for self-powered U-healthcare devices in 0.18 μm CMOS technology. In Proceedings of the 2015 IEEE 13th International New Circuits and Systems Conference (NEWCAS), Grenoble, France, 7–10 June 2015; pp. 1–4. [Google Scholar]
- ADSD1299 Series Chip. Available online: https://www.sinoxtech.com/search/?keyword=ADSD1299&submit= (accessed on 29 August 2023).
- Sahu, A.K.; Sahu, A.K. A review on different filter design techniques and topologies for bio-potential signal acquisition systems. In Proceedings of the 2018 3rd International Conference on Communication and Electronics Systems (ICCES), Coimbatore, India, 15–16 October 2018; pp. 934–937. [Google Scholar]
- Yazicioglu, R.F.; Merken, P.; Puers, R.; Van Hoof, C. A 200 µW eight-channel EEG acquisition ASIC for ambulatory EEG systems. IEEE J. Solid-State Circuits 2008, 43, 3025–3038. [Google Scholar] [CrossRef]
- He, S.; Zhou, Y.; Yu, T.; Zhang, R.; Huang, Q.; Chuai, L.; Mustafa, M.-U.; Gu, Z.; Yu, Z.L.; Tan, H.; et al. EEG- and EOG-Based Asynchronous Hybrid BCI: A System Integrating a Speller, a Web Browser, an E-Mail Client, and a File Explorer. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.; Zhang, T.; Zhao, S. Brain–Robot Interface-Based Navigation Control of a Mobile Robot in Corridor Environments. IEEE Trans. Syst. Man Cybern. Syst. 2020, 50, 3047–3058. [Google Scholar] [CrossRef]
- Kaufmann, T.; Herweg, A.; Kübler, A. Toward brain-computer interface based wheelchair control utilizing tactually-evoked event-related potentials. J. Neuroeng. Rehabil. 2014, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Wu, W.C. An FPGA-based BCI system with SSVEP and phased coding techniques. J. Technol. 2018, 33, 53–62. [Google Scholar]
- Arif, R.; Wijaya, S.K.; Gani, H.S. Design of EEG data acquisition system based on Raspberry Pi 3 for acute ischemic stroke identification. In Proceedings of the 2018 International Conference on Signals and Systems (ICSigSys), Bali, Indonesia, 1–3 May 2018; pp. 271–275. [Google Scholar]
- Kwiatkowski, P. Digital-to-time converter for test equipment implemented using FPGA DSP blocks. Measurement 2021, 177, 109267. [Google Scholar] [CrossRef]
- Kwiatkowski, K.P. Employing FPGA DSP blocks for time-to-digital conversion. Metrol. Meas. Syst. 2023, 26, 631–643. [Google Scholar]
- Moroz, L.; Samotyy, V.; Gepner, P.; Węgrzyn, M.; Nowakowski, G. Power Function Algorithms Implemented in Microcontrollers and FPGAs. Electronics 2023, 12, 3399. [Google Scholar] [CrossRef]
- Song, H.; Luo, G.; Ji, Z.; Bo, R.; Xue, Z.; Yan, D.; Zhang, F.; Bai, K.; Liu, J.; Cheng, X. Highly-integrated, miniaturized, stretchable electronic systems based on stacked multilayer network materials. Sci. Adv. 2022, 8, eabm3785. [Google Scholar] [CrossRef]
- Mannatunga, K.S.; Ali, S.H.M.; Crespo, M.L.; Cicuttin, A.; Samarawikrama, J.G. High Performance 128-Channel Acquisition System for Electrophysiological Signals. IEEE Access 2020, 8, 122366–122383. [Google Scholar] [CrossRef]
- Kuo-Kai, S.; Po-Lei, L.; Ming-Huan, L.; Ming-Hong, L.; Ren-Jie, L.; Yun-Jen, C. Development of a Low-Cost FPGA-Based SSVEP BCI Multimedia Control System. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 125–132. [Google Scholar]
- Prazenica, M.; Resutik, P.; Kascak, S. Practical Implementation of the Indirect Control to the Direct 3 × 5 Matrix Converter Using DSP and Low-Cost FPGA. Sensors 2023, 23, 3581. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, S.; Shao, M.; Liang, C. A Low-Cost Portable Real-Time EEG Signal Acquisition System Based on DSP. Int. J. Signal Process. Image Process. Pattern Recognit. 2016, 9, 239–246. [Google Scholar] [CrossRef]
- Hinss, M.F.; Jahanpour, E.S.; Somon, B.; Pluchon, L.; Dehais, F.; Roy, R.N. Open multi-session and multi-task EEG cognitive Dataset for passive brain-computer Interface Applications. Sci. Data 2023, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Schwilden, H.; Schüttler, J.; Stoeckel, H. Closed-loop feedback control of methohexital anesthesia by quantitative EEG analysis in humans. Anesthesiology 1987, 67, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, M.; Zhu, J.; Zheng, W.; Ma, G.; Deng, X.; Huang, W.; Serdijn, W.A. System Design of a Closed-Loop Vagus Nerve Stimulator Comprising a Wearable EEG Recorder and an Implantable Pulse Generator. IEEE Circuits Syst. Mag. 2022, 22, 22–40. [Google Scholar] [CrossRef]
- Krucoff, M.O.; Rahimpour, S.; Slutzky, M.W.; Edgerton, V.R.; Turner, D.A. Enhancing Nervous System Recovery through Neurobiologics, Neural Interface Training, and Neurorehabilitation. Front. Neurosci. 2016, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Kwak, N.S.; Muller, K.R.; Lee, S.W. A lower limb exoskeleton control system based on steady state visual evoked potentials. J. Neural Eng. 2015, 12, 056009. [Google Scholar] [CrossRef] [PubMed]
- Broniera Junior, P.; Campos, D.P.; Lazzaretti, A.E.; Nohama, P.; Carvalho, A.A.; Krueger, E.; Minhoto Teixeira, M.C. EEG-FES-Force-MMG closed-loop control systems of a volunteer with paraplegia considering motor imagery with fatigue recognition and automatic shut-off. Biomed. Signal Process. Control. 2021, 68, 102662. [Google Scholar] [CrossRef]
- Knierim, M.T.; Bleichner, M.G.; Reali, P. A Systematic Comparison of High-End and Low-Cost EEG Amplifiers for Concealed, Around-the-Ear EEG Recordings. Sensors 2023, 23, 4559. [Google Scholar] [CrossRef]
- Wang, Q.; Mu, Z.; Jin, L. Control method of robot detour obstacle based on EEG. Neural Comput. Appl. 2021, 34, 6745–6752. [Google Scholar] [CrossRef]
- He, W.; Zhao, Y.; Tang, H.; Sun, C.; Fu, W. A Wireless BCI and BMI System for Wearable Robots. IEEE Trans. Syst. Man Cybern. Syst. 2016, 46, 936–946. [Google Scholar] [CrossRef]
- Miah, M.O.; Muhammod, R.; Mamun, K.A.A.; Farid, D.M.; Kumar, S.; Sharma, A.; Dehzangi, A. CluSem: Accurate clustering-based ensemble method to predict motor imagery tasks from multi-channel EEG data. J. Neurosci. Methods 2021, 364, 109373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, H.; Huang, H.; Yi, N.; Shi, X.; Xie, S.; Li, Y.; Ye, Z.; Feng, P.; Lin, T.; et al. Wearable Circuits Sintered at Room Temperature Directly on the Skin Surface for Health Monitoring. ACS Appl. Mater. Interfaces 2020, 12, 45504–45515. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, R.; Dai, Y.; Yuan, D.; Yu, X.; Liu, C.; Feng, L.; Shen, R.; Wang, C.; Dai, S. Infrared Radiation of Graphene Electrothermal Film Triggered Alpha and Theta Brainwaves. Small Sci. 2022, 2, 2200064. [Google Scholar] [CrossRef]
- TajDini, M.; Sokolov, V.; Kuzminykh, I.; Ghita, B. Brainwave-based authentication using features fusion. Comput. Secur. 2023, 129, 103198. [Google Scholar] [CrossRef]
- Swarnalatha, R.; Athiban, S. Analysis of brain wave data to detect epileptic activity using LabVIEW. Soft Comput. 2023, 27, 17231–17241. [Google Scholar] [CrossRef]
- Li, T.-M.; Chao, H.-C.; Zhang, J. Emotion classification based on brain wave: A survey. Hum.-Centric Comput. Inf. Sci. 2019, 9, 345055. [Google Scholar] [CrossRef]
- Akila, N.F.; Nasir, E.M.N.E.M.; Fuad, N.; Helmy Abd Wahab, M.; Zulkarnain Syed Idrus, S. A Review of Human Graphology Analysis and Brainwaves. IOP Conf. Ser. Mater. Sci. Eng. 2020, 917, 012048. [Google Scholar] [CrossRef]
- Yuan, H.; He, B. Brain-computer interfaces using sensorimotor rhythms: Current state and future perspectives. IEEE Trans. Biomed. Eng. 2014, 61, 1425–1435. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kang, H.-B. An analysis of smartphone overuse recognition in terms of emotions using brainwaves and deep learning. Neurocomputing 2018, 275, 1393–1406. [Google Scholar] [CrossRef]
- Willett, F.R.; Kunz, E.M.; Fan, C.; Avansino, D.T.; Wilson, G.H.; Choi, E.Y.; Kamdar, F.; Glasser, M.F.; Hochberg, L.R.; Druckmann, S.; et al. A high-performance speech neuroprosthesis. Nature 2023, 620, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Batzianoulis, I.; Iwane, F.; Wei, S.; Correia, C.G.P.R.; Chavarriaga, R.; Millán, J.d.R.; Billard, A. Customizing skills for assistive robotic manipulators, an inverse reinforcement learning approach with error-related potentials. Commun. Biol. 2021, 4, 1406. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, A.B.; Willett, F.R.; Young, D.R.; Memberg, W.D.; Murphy, B.A.; Miller, J.P.; Walter, B.L.; Sweet, J.A.; Hoyen, H.A.; Keith, M.W.; et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: A proof-of-concept demonstration. Lancet 2017, 389, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Chen, S.; Zhang, X.; Jin, J.; Xu, R.; Daly, I.; Jia, J.; Wang, X.; Cichocki, A.; Jung, T.P. BCI-Based Rehabilitation on the Stroke in Sequela Stage. Neural Plast. 2020, 2020, 8882764. [Google Scholar] [CrossRef] [PubMed]
- Lorach, H.; Galvez, A.; Spagnolo, V.; Martel, F.; Karakas, S.; Intering, N.; Vat, M.; Faivre, O.; Harte, C.; Komi, S.; et al. Walking naturally after spinal cord injury using a brain-spine interface. Nature 2023, 618, 126–133. [Google Scholar] [CrossRef]
- Wang, X.; Ling, Y.; Ling, X.; Li, X.; Li, Z.; Hu, K.; Dai, M.; Zhu, J.; Du, Y.; Yang, Q. A particle swarm algorithm optimization-based SVM–KNN algorithm for epileptic EEG recognition. Int. J. Intell. Syst. 2022, 37, 11233–11249. [Google Scholar] [CrossRef]
- Zhang, J.; Li, K. A multi-view CNN encoding for motor imagery EEG signals. Biomed. Signal Process. Control 2023, 85, 105063. [Google Scholar] [CrossRef]
- Miao, M.; Wang, A.; Liu, F. Application of artificial bee colony algorithm in feature optimization for motor imagery EEG classification. Neural Comput. Appl. 2018, 30, 3677–3691. [Google Scholar] [CrossRef]
- Ma, W.; Xue, H.; Sun, X.; Mao, S.; Wang, L.; Liu, Y.; Wang, Y.; Lin, X. A novel multi-branch hybrid neural network for motor imagery EEG signal classification. Biomed. Signal Process. Control 2022, 77, 103718. [Google Scholar] [CrossRef]
- Djoufack Nkengfack, L.C.; Tchiotsop, D.; Atangana, R.; Louis-Door, V.; Wolf, D. EEG signals analysis for epileptic seizures detection using polynomial transforms, linear discriminant analysis and support vector machines. Biomed. Signal Process. Control 2020, 62, 102141. [Google Scholar] [CrossRef]
- Wijayanto, I.; Hadiyoso, S.; Aulia, S.; Atmojo, B.S. Detecting Ictal and Interictal Condition of EEG Signal using Higuchi Fractal Dimension and Support Vector Machine. J. Phys. Conf. Ser. 2020, 1577, 012016. [Google Scholar] [CrossRef]
- Roy, A.M. An efficient multi-scale CNN model with intrinsic feature integration for motor imagery EEG subject classification in brain-machine interfaces. Biomed. Signal Process. Control 2022, 74, 103496. [Google Scholar] [CrossRef]
- Li, H.; Ding, M.; Zhang, R.; Xiu, C. Motor imagery EEG classification algorithm based on CNN-LSTM feature fusion network. Biomed. Signal Process. Control 2022, 72, 103342. [Google Scholar] [CrossRef]
- Edla, D.R.; Mangalorekar, K.; Dhavalikar, G.; Dodia, S. Classification of EEG data for human mental state analysis using Random Forest Classifier. Procedia Comput. Sci. 2018, 132, 1523–1532. [Google Scholar] [CrossRef]
- Li, X.; Zeng, W. Athletes’ State Monitoring under Data Mining and Random Forest. J. Sens. 2022, 2022, 1966786. [Google Scholar] [CrossRef]
- Bablani, A.; Edla, D.R.; Dodia, S. Classification of EEG data using k-nearest neighbor approach for concealed information test. Procedia Comput. Sci. 2018, 143, 242–249. [Google Scholar] [CrossRef]
- Vempati, R.; Sharma, L.D. EEG rhythm based emotion recognition using multivariate decomposition and ensemble machine learning classifier. J. Neurosci. Methods 2023, 393, 109879. [Google Scholar] [CrossRef]
- Aldayel, M.; Ykhlef, M.; Al-Nafjan, A. Recognition of Consumer Preference by Analysis and Classification EEG Signals. Front. Hum. Neurosci. 2020, 14, 604639. [Google Scholar] [CrossRef]
- Wang, G.; Deng, Z.; Choi, K.-S. Detection of epilepsy with Electroencephalogram using rule-based classifiers. Neurocomputing 2017, 228, 283–290. [Google Scholar] [CrossRef]
- Ma, P.; Dong, C.; Lin, R.; Ma, S.; Liu, H.; Lei, D.; Chen, X. Effect of Local Network Characteristics on the Performance of the SSVEP Brain-Computer Interface. IRBM 2023, 44, 100781. [Google Scholar] [CrossRef]
- Li, C.T.; Chen, C.S.; Cheng, C.M.; Chen, C.P.; Chen, J.P.; Chen, M.H.; Bai, Y.M.; Tsai, S.J. Prediction of antidepressant responses to non-invasive brain stimulation using frontal electroencephalogram signals: Cross-dataset comparisons and validation. J. Affect. Disord. 2023, 343, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Miras, J.; Ibáñez-Molina, A.J.; Soriano, M.F.; Iglesias-Parro, S. Schizophrenia classification using machine learning on resting state EEG signal. Biomed. Signal Process. Control 2023, 79, 104233. [Google Scholar] [CrossRef]
- Subasi, A.; Tuncer, T.; Dogan, S.; Tanko, D.; Sakoglu, U. EEG-based emotion recognition using tunable Q wavelet transform and rotation forest ensemble classifier. Biomed. Signal Process. Control 2021, 68, 102648. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, L.; Zhang, Z.; Gong, X.; Sun, Y.; Wang, H. Short time Fourier transformation and deep neural networks for motor imagery brain computer interface recognition. Concurr. Comput. Pract. Exp. 2018, 30, e4413. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, S.; Tian, J.; Gao, Y. A 2D CNN-LSTM hybrid algorithm using time series segments of EEG data for motor imagery classification. Biomed. Signal Process. Control 2023, 83, 104627. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Zhang, Y.; Zhang, Y.; Liu, S.; Guo, X. Application and Development of EEG Acquisition and Feedback Technology: A Review. Biosensors 2023, 13, 930. https://doi.org/10.3390/bios13100930
Qin Y, Zhang Y, Zhang Y, Liu S, Guo X. Application and Development of EEG Acquisition and Feedback Technology: A Review. Biosensors. 2023; 13(10):930. https://doi.org/10.3390/bios13100930
Chicago/Turabian StyleQin, Yong, Yanpeng Zhang, Yan Zhang, Sheng Liu, and Xiaogang Guo. 2023. "Application and Development of EEG Acquisition and Feedback Technology: A Review" Biosensors 13, no. 10: 930. https://doi.org/10.3390/bios13100930
APA StyleQin, Y., Zhang, Y., Zhang, Y., Liu, S., & Guo, X. (2023). Application and Development of EEG Acquisition and Feedback Technology: A Review. Biosensors, 13(10), 930. https://doi.org/10.3390/bios13100930




