Functionalized-Graphene Field Effect Transistor-Based Biosensor for Ultrasensitive and Label-Free Detection of β-Galactosidase Produced by Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Graphene Device
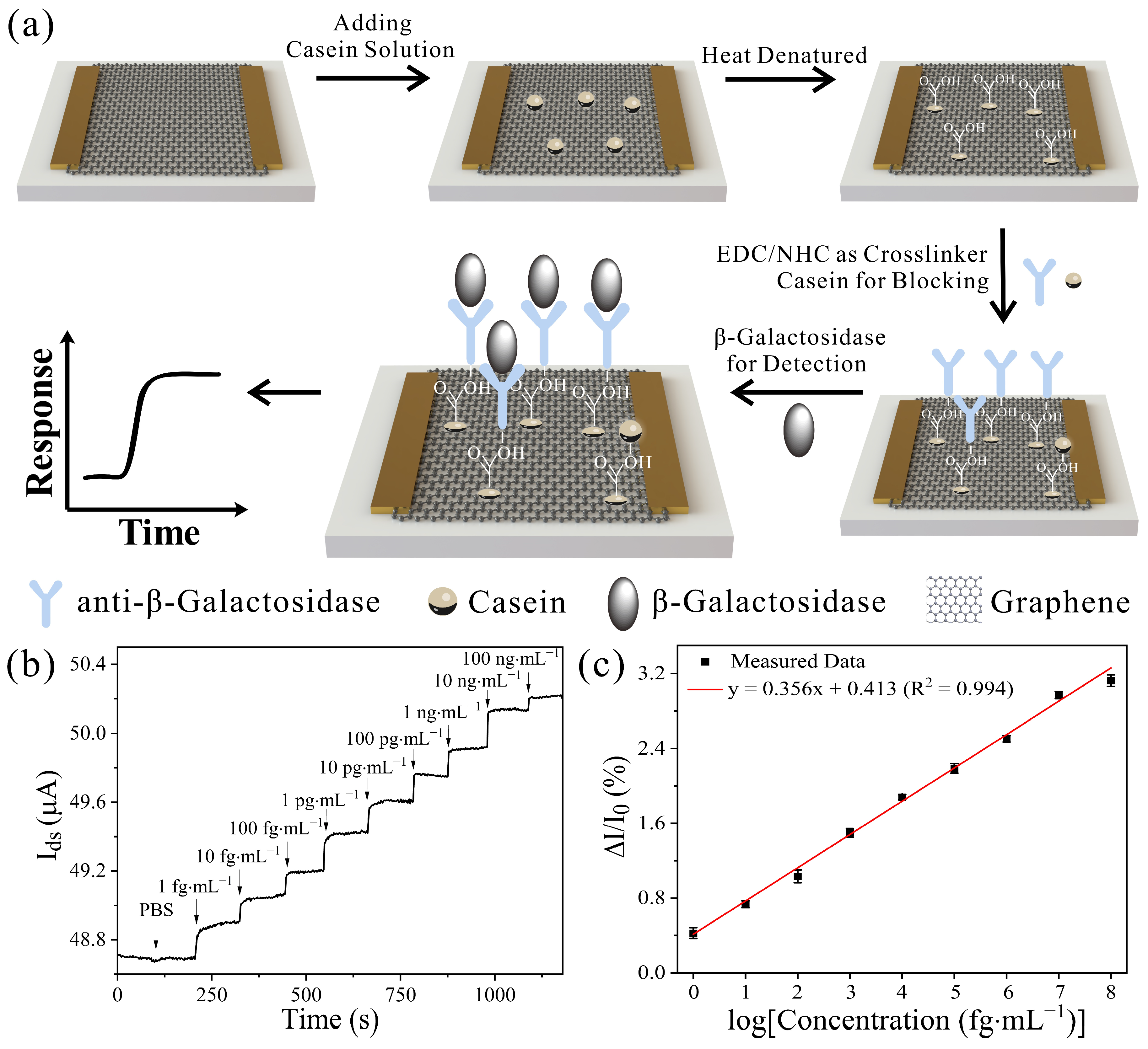
2.3. Functionalization of Graphene Surface
2.4. Characterization and Measurement
3. Results and Discussion
3.1. Verification of GFET
3.2. Characterization of Functionalized GFET
3.3. Sensing Capability of GFET to Detect β-Gal Produced by E. coli
3.4. Specificity of GFET and Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, J.; Gomez, T.; Doyle, M.P.; Wells, J.G.; Zhao, T.; Tauxe, R.V.; Griffin, P.M. Lessons from a large outbreak of Escherichia coli O157:H7 infections: Insights into the infectious dose and method of widespread contamination of hamburger patties. Epidemiol. Infect. 1999, 122, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Paschos, A.; Gupta, R.S.; Schellhorn, H.E. Insertion/Deletion-Based Approach for the Detection of Escherichia coli O157:H7 in Freshwater Environments. Environ. Sci. Technol. 2014, 48, 11462–11470. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Laczka, O.; Garcia-Aljaro, C.; del Campo, F.J.; Pascual, F.X.M.; Mas-Gordi, J.; Baldrich, E. Amperometric detection of Enterobacteriaceae in river water by measuring beta-galactosidase activity at interdigitated microelectrode arrays. Anal. Chim. Acta 2010, 677, 156–161. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, F.Y.; Fu, R.J.; Yang, Y.J.; Jiao, B.N.; He, Y. Enzyme-Nanozyme Cascade Reaction-Mediated Etching of Gold Nanorods for the Detection of Escherichia coli. ACS Appl. Nano Mater. 2020, 3, 9016–9025. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Nugen, S.R. Electrochemical Detection of Escherichia coli from Aqueous Samples Using Engineered Phages. Anal. Chem. 2017, 89, 1650–1657. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Etayash, H.; Jiang, K.; Sohrabi, A.; Hassanpourfard, M.; Naicker, S.; Sadrzadeh, M.; Thundat, T. Portable Nanofiber-Light Addressable Potentiometric Sensor for Rapid Escherichia coli Detection in Orange Juice. ACS Sens. 2018, 3, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.B.; Li, R.B.; Sun, X.X.; Zhang, H.; Yu, H.W.; Dong, S.J. Colorimetric and Electrochemical Dual-Signal Method for Water Toxicity Detection Based on Escherichia coli and p-Benzoquinone. ACS Sens. 2021, 6, 2674–2681. [Google Scholar] [CrossRef]
- Dominguez, R.A.S.; Jimenez, M.A.D.; Diaz, A.O. Antibody Immobilization in Zinc Oxide Thin Films as an Easy-Handle Strategy for Escherichia coli Detection. ACS Omega 2020, 5, 20473–20480. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Harrison, M.; Ng, E.K.; Sauvageau, D.; Elias, A. Immobilized Reporter Phage on Electrospun Polymer Fibers for Improved Capture and Detection of Escherichia coli O157:H7. ACS Food Sci. Technol. 2021, 1, 1085–1094. [Google Scholar] [CrossRef]
- Zhao, L.; Rosati, G.; Piper, A.; Silva, C.D.C.E.; Hu, L.M.; Yang, Q.Y.; Pelle, F.D.; Alvarez-Diduk, R.R.; Merkoci, A. Laser Reduced Graphene Oxide Electrode for Pathogenic Escherichia coli Detection. ACS Appl. Mater. Inter. 2023, 15, 9024–9033. [Google Scholar] [CrossRef]
- Zhan, S.N.; Fang, H.; Fu, J.M.; Lai, W.H.; Leng, Y.K.; Huang, X.L.; Xiong, Y.H. Gold Nanoflower-Enhanced Dynamic Light Scattering Immunosensor for the Ultrasensitive No-Wash Detection of Escherichia coli O157:H7 in Milk. J. Agr. Food Chem. 2019, 67, 9104–9111. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Torralba, A.S.; Alvarez-Diduk, R.; Afkhami, A.; Merkoci, A. Lab in a Tube: Point-of-Care Detection of Escherichia coli. Anal. Chem. 2020, 92, 4209–4216. [Google Scholar] [CrossRef]
- Burnham, S.; Hu, J.; Anany, H.; Brovko, L.; Deiss, F.; Derda, R.; Griffiths, M.W. Towards rapid on-site phage-mediated detection of generic Escherichia coli in water using luminescent and visual readout. Anal. Bioanal. Chem. 2014, 406, 5685–5693. [Google Scholar] [CrossRef] [PubMed]
- Ripp, S.; Jegier, P.; Johnson, C.M.; Brigati, J.R.; Sayler, G.S. Bacteriophage-amplified bioluminescent sensing of Escherichia coli O157:H7. Anal. Bioanal. Chem. 2008, 391, 507–514. [Google Scholar] [CrossRef]
- Taylor, A.D.; Ladd, J.; Yu, Q.M.; Chen, S.F.; Homola, J.; Jiang, S.Y. Quantitative and simultaneous detection of four foodborne bacterial pathogens with a multi-channel SPR sensor. Biosens. Bioelectron. 2006, 22, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, H.H.; Wang, W.W.; Sim, J.S. Detection of Escherichia coli O157:H7 using chicken immunoglobulin Y. Immunol. Lett. 2006, 106, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Gao, F.; Lu, C.; Fauconnier, M.L.; Zheng, J.K. Bio-Specific Au/Fe3+ Porous Spongy Nanoclusters for Sensitive SERS Detection of Escherichia coli O157:H7. Biosensors 2021, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Huang, L.; Zhang, H.; Sun, Z.Y.; Zhang, Z.; Zhang, G.J. Fabrication of Ultrasensitive Field-Effect Transistor DNA Biosensors by a Directional Transfer Technique Based on CVD-Grown Graphene. ACS Appl. Mater. Inter. 2015, 7, 16953–16959. [Google Scholar] [CrossRef]
- Gao, J.W.; Gao, Y.K.; Han, Y.K.; Pang, J.B.; Wang, C.; Wang, Y.H.; Liu, H.; Zhang, Y.; Han, L. Ultrasensitive Label-free MiRNA Sensing Based on a Flexible Graphene Field-Effect Transistor without Functionalization. ACS Appl. Electron. Mater. 2020, 2, 1090–1098. [Google Scholar] [CrossRef]
- Dai, X.C.; Vo, R.; Hsu, H.H.; Deng, P.; Zhang, Y.X.; Jiang, X.C. Modularized Field-Effect Transistor Biosensors. Nano Lett. 2019, 19, 6658–6664. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.Y.; Abbott, J.; Qin, L.; Yeung, K.Y.M.; Song, Y.; Yoon, H.; Kong, J.; Ham, D. Electrophoretic and field-effect graphene for all-electrical DNA array technology. Nat. Commun. 2014, 5, 4866. [Google Scholar] [CrossRef]
- Janissen, R.; Sahoo, P.K.; Santos, C.A.; da Silva, A.M.; von Zuben, A.A.G.; Souto, D.E.P.; Costa, A.D.T.; Celedon, P.; Zanchin, N.I.T.; Almeida, D.B.; et al. InP Nanowire Biosensor with Tailored Biofunctionalization: Ultrasensitive and Highly Selective Disease Biomarker Detection. Nano Lett. 2017, 17, 5938–5949. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Hu, J.W.; Liu, J.L.; Li, X.; Sun, S.; Luan, X.F.; Zhao, Y.; Wei, S.H.; Li, M.X.; Zhang, Q.Z.; et al. Si nanowire Bio-FET for electrical and label-free detection of cancer cell-derived exosomes. Microsyst. Nanoeng. 2022, 8, 57. [Google Scholar] [CrossRef]
- Lu, Z.C.; Liu, T.T.; Zhou, X.J.; Yang, Y.; Liu, Y.X.; Zhou, H.; Wei, S.H.; Zhai, Z.M.; Wu, Y.Q.; Sun, F.; et al. Rapid and quantitative detection of tear MMP-9 for dry eye patients using a novel silicon nanowire-based biosensor. Biosens. Bioelectron. 2022, 214, 114498. [Google Scholar] [CrossRef]
- Kuo, C.J.; Chiang, H.C.; Tseng, C.A.; Chang, C.F.; Ulaganathan, R.K.; Ling, T.T.; Chang, Y.J.; Chen, C.C.; Chen, Y.R.; Chen, Y.T. Lipid-Modified Graphene-Transistor Biosensor for Monitoring Amyloid-beta Aggregation. ACS Appl. Mater. Inter. 2018, 10, 12311–12316. [Google Scholar] [CrossRef]
- Wang, Z.; Yi, K.Y.; Lin, Q.Y.; Yang, L.; Chen, X.S.; Chen, H.; Liu, Y.Q.; Wei, D.C. Free radical sensors based on inner-cutting graphene field-effect transistors. Nat. Commun. 2019, 10, 1544. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.T.; Heiranian, M.; Kim, Y.; You, S.; Leem, J.; Taqieddin, A.; Faramarzi, V.; Jing, Y.H.; Park, I.; van der Zande, A.M.; et al. Ultrasensitive detection of nucleic acids using deformed graphene channel field effect biosensors. Nat. Commun. 2020, 11, 1543. [Google Scholar] [CrossRef]
- Fakih, I.; Durnan, O.; Mahvash, F.; Napal, I.; Centeno, A.; Zurutuza, A.; Yargeau, V.; Szkopek, T. Selective ion sensing with high resolution large area graphene field effect transistor arrays. Nat. Commun. 2020, 11, 3226. [Google Scholar] [CrossRef]
- Wang, L.Q.; Wang, X.J.; Wu, Y.G.; Guo, M.Q.; Gu, C.J.; Dai, C.H.; Kong, D.R.; Wang, Y.; Zhang, C.; Qu, D.; et al. Rapid and ultrasensitive electromechanical detection of ions, biomolecules and SARS-CoV-2 RNA in unamplified samples. Nat. Biomed. Eng. 2022, 6, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, M.Y.; Zhang, Y.H.; Ji, H.; Gao, J.W.; Song, S.; Sun, J.; Liu, H.; Zhang, Y.; Han, L. Ultrasensitive Antibiotic Perceiving Based on Aptamer-Functionalized Ultraclean Graphene Field-Effect Transistor Biosensor. Anal. Chem. 2022, 94, 14785–14793. [Google Scholar] [CrossRef]
- Xu, S.C.; Zhan, J.; Man, B.Y.; Jiang, S.Z.; Yue, W.W.; Gao, S.B.; Guo, C.G.; Liu, H.P.; Li, Z.H.; Wang, J.H.; et al. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor. Nat. Commun. 2017, 8, 14902. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, K.; Sun, H.; Zhao, S.M.; Chen, X.F.; Qian, D.H.; Mao, H.J.; Zhao, J.L. Novel Graphene Biosensor Based on the Functionalization of Multifunctional Nano-bovine Serum Albumin for the Highly Sensitive Detection of Cancer Biomarkers. Nano-Micro Lett. 2019, 11, 20. [Google Scholar] [CrossRef]
- Huang, Y.X.; Dong, X.C.; Liu, Y.X.; Li, L.J.; Chen, P. Graphene-based biosensors for detection of bacteria and their metabolic activities. J. Mater. Chem. 2011, 21, 12358–12362. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.J.; Zhu, Y.B.; Zhou, X.H.; Lin, Q.; He, M. High-kappa Solid-Gate Transistor Configured Graphene Biosensor with Fully Integrated Structure and Enhanced Sensitivity. Adv. Funct. Mater. 2016, 26, 7668–7678. [Google Scholar] [CrossRef]
- Xu, H.L.; Zhang, Z.Y.; Peng, L.M. Measurements and microscopic model of quantum capacitance in graphene. Appl. Phys. Lett. 2011, 98, 133122. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Le, H.D.; Nguyen, V.C.; Ngo, T.T.T.; Le, D.Q.; Nguyen, X.N.; Phan, N.M. Synthesis of multi-layer graphene films on copper tape by atmospheric pressure chemical vapor deposition method. Adv. Nat. Sci. Nanosci. 2013, 4, 035012. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.T.; Ho, J.; Nezich, D.; Son, H.B.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Layer Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 3087. [Google Scholar] [CrossRef][Green Version]
- Das, A.; Pisana, S.; Chakraborty, B.; Piscanec, S.; Saha, S.K.; Waghmare, U.V.; Novoselov, K.S.; Krishnamurthy, H.R.; Geim, A.K.; Ferrari, A.C.; et al. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotechnol. 2008, 3, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Song, S.; Dou, Y.; Jia, X.; Song, S.; Ding, X. Methylation specific enzyme-linked oligonucleotide assays (MS-ELONA) for ultrasensitive DNA methylation analysis. Biosens. Bioelectron. 2023, 238, 115587. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Dou, Y.; Li, T. Ultra-sensitive and label-free detection of Escherichia coli O157:H7 using graphene-based field effect transistor modified with heat-denatured casein. Microchem. J. 2023, 193, 109049. [Google Scholar] [CrossRef]



| Sample | Found | Added (ng·mL−1) | Recovered (ng·mL−1) | Recovery |
|---|---|---|---|---|
| River 1 | Not detected | 1 | 1.072 ± 0.317 | 107.200% |
| River 2 | Not detected | 10 | 9.271 ± 0.281 | 92.710% |
| River 3 | Not detected | 100 | 98.729 ± 0.836 | 98.729% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Dou, Y.; Song, S.; Li, T. Functionalized-Graphene Field Effect Transistor-Based Biosensor for Ultrasensitive and Label-Free Detection of β-Galactosidase Produced by Escherichia coli. Biosensors 2023, 13, 925. https://doi.org/10.3390/bios13100925
Wei S, Dou Y, Song S, Li T. Functionalized-Graphene Field Effect Transistor-Based Biosensor for Ultrasensitive and Label-Free Detection of β-Galactosidase Produced by Escherichia coli. Biosensors. 2023; 13(10):925. https://doi.org/10.3390/bios13100925
Chicago/Turabian StyleWei, Shanhong, Yanzhi Dou, Shiping Song, and Tie Li. 2023. "Functionalized-Graphene Field Effect Transistor-Based Biosensor for Ultrasensitive and Label-Free Detection of β-Galactosidase Produced by Escherichia coli" Biosensors 13, no. 10: 925. https://doi.org/10.3390/bios13100925
APA StyleWei, S., Dou, Y., Song, S., & Li, T. (2023). Functionalized-Graphene Field Effect Transistor-Based Biosensor for Ultrasensitive and Label-Free Detection of β-Galactosidase Produced by Escherichia coli. Biosensors, 13(10), 925. https://doi.org/10.3390/bios13100925






