Abstract
Food and waterborne illnesses are still a major concern in health and food safety areas. Every year, almost 0.42 million and 2.2 million deaths related to food and waterborne illness are reported worldwide, respectively. In foodborne pathogens, bacteria such as Salmonella, Shiga-toxin producer Escherichia coli, Campylobacter, and Listeria monocytogenes are considered to be high-concern pathogens. High-concern waterborne pathogens are Vibrio cholerae, leptospirosis, Schistosoma mansoni, and Schistosima japonicum, among others. Despite the major efforts of food and water quality control to monitor the presence of these pathogens of concern in these kinds of sources, foodborne and waterborne illness occurrence is still high globally. For these reasons, the development of novel and faster pathogen-detection methods applicable to real-time surveillance strategies are required. Methods based on biosensor devices have emerged as novel tools for faster detection of food and water pathogens, in contrast to traditional methods that are usually time-consuming and are unsuitable for large-scale monitoring. Biosensor devices can be summarized as devices that use biochemical reactions with a biorecognition section (isolated enzymes, antibodies, tissues, genetic materials, or aptamers) to detect pathogens. In most cases, biosensors are based on the correlation of electrical, thermal, or optical signals in the presence of pathogen biomarkers. The application of nano and molecular technologies allows the identification of pathogens in a faster and high-sensibility manner, at extremely low-pathogen concentrations. In fact, the integration of gold, silver, iron, and magnetic nanoparticles (NP) in biosensors has demonstrated an improvement in their detection functionality. The present review summarizes the principal application of nanomaterials and biosensor-based devices for the detection of pathogens in food and water samples. Additionally, it highlights the improvement of biosensor devices through nanomaterials. Nanomaterials offer unique advantages for pathogen detection. The nanoscale and high specific surface area allows for more effective interaction with pathogenic agents, enhancing the sensitivity and selectivity of the biosensors. Finally, biosensors’ capability to functionalize with specific molecules such as antibodies or nucleic acids facilitates the specific detection of the target pathogens.
1. Introduction
Every year, contaminated food is responsible for 420,000 deaths and 600 million cases of foodborne illnesses caused by spoiled food [1]. This is not just a problem in low–middle-income countries, high-income countries also have several troubles related to foodborne pathogens. In the U.S. alone, there are more than 9.4 million deaths per year due to the ingestion of pathogenic bacteria in food [2]. During 2010, 420,000 people (one-third of them being children under the age of five) died from illnesses related to salmonellosis and Escherichia coli infections [3]. Foodborne illnesses arise from the presence of pathogens, toxins, or contaminants in food products, and are typically associated with gastrointestinal symptoms (diarrhea, vomiting, abdominal pain, and fever), and other adverse effects on human health such as neurological, hepatic, and renal complications, even becoming a life-threatening issue if not appropriately addressed [4,5]. In recent years, the majority of reported foodborne illness outbreaks were caused by pathogens such as Norovirus [5], Campylobacter [6], Salmonella [5,6], Listeria monocytogenes [7], and Shiga toxin-producing E. coli [8]. Less frequently reported but still of concern are the pathogens Staphylococcus aureus [9], Clostridium species [10], Bacillus cereus [11], and Yersinia enterocolitica [12].
Similarly to food safety, the presence of pathogens in water is a major issue for public health [13]. It is estimated that 663 million people consume unsafe water from surface or groundwater sources [14]. More than 2.2 million deaths per year and more cases of illness (diarrhea, gastrointestinal, and systematic diseases) are linked to contaminated water ingestion [15]; the pathogens of greatest concern are Salmonella, Shigella, Campylobacter, S. aureus, and E. coli [16,17]. However, viruses and parasites are becoming a problem for water security [18]. Parasites and viruses linked to waterborne outbreaks include Vibrio cholerae, Leptospira, Schistosoma mansoni, and Schistosoma japonicum [16,19,20].
Monitoring the presence of pathogens in water is particularly important as a disease-preventive measure from waterborne illnesses and to monitor water quality. This can be achieved through applying wastewater-based surveillance protocols, which allow the detection of pathogens using molecular biology tools [21,22], which can be applied to verify the discharged water quality and indicate the treatment required to prevent adverse effects on the environment; ensuring water sustainability for future generations.
Pathogen-detection methods play a crucial role in ensuring food and water safety; however, actual monitoring methods are time-consuming processes that usually take days to obtain a precise result [23], making them ineffective for real-time monitoring [24]. In fact, the identification of pathogens such as bacteria and viruses is carried out by gold-standard methodologies, which are traditional techniques such as viable plate counts, flow cytometry, and staining methods, among others [25,26,27]. Nevertheless, the detection time is one of the major limitations of this technique because these techniques require the growth of the microorganism in laboratory conditions (this has not been a limitation per se), which can take several days to produce a result, hindering the response time for the control of pathogens [26]. Techniques based on molecular biology that are used for pathogen detection involve [28] polymerase chain reaction techniques (PCR) [21,29,30,31], multiplex polymerase chain reaction (mPCR) [32], quantitative polymerase chain reaction (qPCR) [33], digital droplet PCR (ddPCR) [34], fluorescence in situ hybridization (FISH) [31], enzyme-linked immunosorbent assay (ELISA) [35], surface-enhanced Raman spectroscopy (SERS) [36], immunological methods [37], next-generation sequencing [38], whole-genome sequencing [39], flow cytometry [40], and surface plasmon resonance imaging (SPR) [41]; these techniques have already been applied as detection methodologies of pathogens in food and water matrices [5,26].
Despite the application of molecular-biology techniques in food and water security, if we consider the technological development of the health sector related to pathogen detection, this sector has already developed advanced technologies such as biosensors with nanomaterials and the incorporation of informatic technologies [42]. Efforts are being conducted in the hope of bringing about more specific and faster methodologies to produce a rapid-response diagnosis and prevent outbreaks, focusing on nanomaterials such as glyconanomaterials [43], nanoparticles [44], ZnO nanorods, nanoconjugate (Au–Fe3O4), silicon nanonet FET, nanosphere (RNs@Au) in a biosensor device, combined with molecular detection methods (ELISA, qPCR) and also incorporated with informatic technologies, which are used to create more-sensible and appropriate in situ detection systems for pathogens of major concern. This technology has been applied in order to improve the health care system’s response to pathogen-presence emergencies, (as reviewed by Jian et al., 2021) [45] for HIV and Influenza A virus. These technologies have also been applied to Ebola [46], Malaria [47], Dengue virus [43], and in recent years in SARS-CoV-2 monitoring protocols [42]. Considering the advances made in health security and the demands for improved food and water safety, these existing technologies in the health sector should be transferred to other sectors such as food and water security.
For the above mentioned, and the increase in pathogens related to food and water-borne illnesses, the development of pathogen-detection methods is becoming an urgent step to ensuring health and safety [48]. Unfortunately, and despite recent advances in new pathogen-detection approaches, the application of nanomaterials and biosensors is still limited, this is why technologies capable of obtaining better results, in a fast and affordable way, have been studied, resulting in novel technologies, such as biosensor devices, with “rapid, sensitive and specific” protocol for pathogen detection, resolving the priority assignment of ensuring health security, preventing food- and water-ingestion-related outbreaks [49], with even more affordable technology with the inclusion of the use of biosensors and NPs in recent years [44,50,51].
The previously mentioned methods help to perform faster monitoring (real-time surveillance systems) [52], reducing response times of pathogen detection in water [53]. Additionally, the use of biosensors improved with NPs enhanced the detection performance of the device making it a faster, more specific, and portable device [54]. In fact, due to the diversity of the detection capabilities of nanoparticles, they are the subject of many studies that attempt to understand their role when incorporated into pathogen-detection systems [55].
The basic components of a biosensor device are a biorecognition element, a transducer, an amplifier, and a processor component. The biorecognition element recognizes the analyte of interest, the transducer generates a signal from the recognition of the biomarker into a measurable signal, then the signal is processed using the processor and amplifier component, to obtain a signal output [56,57]. In summary, it is a bioanalytical device that detects specific biomarkers using biochemical reactions [58], mediated by isolated enzymes, antibodies, tissues, organelles, or whole cells for pathogen detection, using electrical, thermal, or optical signals [59], which are able to correlate the presence of specific pathogen and signal emission measures [50].
As is already mentioned, the biosensor application has garnered attention in the field of pathogen detection due to their attractive characteristics, such as precision, selectivity and fast analysis [27]. Nevertheless, it is necessary to mention that these methodologies have certain disadvantages, such as the use of expensive enzymes and equipment, including the extensive workflow required for the device’s development. However, these technologies have a promising future due to their potential application in pathogen-rapid-detection methods [53]. Currently, biosensor-based technology has proved its worth due to its unique sensitivity, low detection limit, and simple operation [60].
In the last decade, the biosensors’ structure has been focused on the miniaturization of the devices without affecting the detection efficacy. To achieve this, NPs have been included in the biosensor architecture, resulting in the development of a nanoscale platform. Indeed, in the different sections of the biosensor, NPs are used as signal transducers to convert a biomolecular interaction into an electrical, optical, or magnetic signal [61]. This functionality inside the biosensor is because of unique properties at the nanometric scales (surface area, small size, affinity for some biomolecules, catalytic activity, and autofluorescence) [62,63].
Like traditional biosensor devices, the nanobiosensors are composed of three main sections: a biorecognition probe, transducer, and amplifier [64] (Figure 1). The NPs are often in the transducer’s component, helping to enhance the biochemical, electrical, magnetic, or optical signal transduction [61]. Also, these signals can be read simply and effectively as a result of the incorporation of functionalized NPs into the biorecognition component [65].
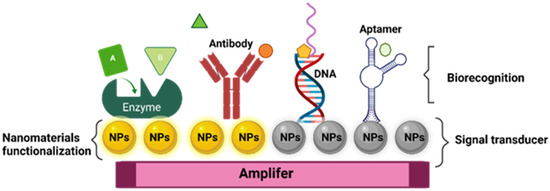
Figure 1.
General structure of nanobiosensor with different agents of biorecognition.
In fact, nanomaterials have been identified as candidates to enhance biosensors’ sensitivity, improving the detection limits and increasing detection specificity [54,55]. The foregoing is based on the fact that the specificity of signal recognition results in the adequate selection of functionalized ligands with NPs, improving the biomarker attraction; also, NPs convert signals from one form to another or act as detectors of the generated signals [66,67]. Biosensors have several methodologies to acquire relevant signals; for example, the electrochemical biosensors work under the method of capitalizing on reactions between immobilized biomolecules and the biomarker, resulting in electron/ion generation/consumption, modifying the electrical properties of the solution, and resulting in a measurable electrical current [68]. On the other hand, optical biosensors work under the method of discerning variations in light properties (absorption, transmission, and reflection), triggered by physical or chemical interactions with biorecognition elements. These biosensors are categorized into two major groups: label-free, where signals arise directly from analyte interactions, and label-based, employing techniques such as calorimetry, fluorescence, or luminescence to produce detectable optical signals. Both methodologies are available to be applied in diverse areas for pathogen detection [69,70,71].
Other possible classifications of biosensors are based on the type of biorecognition immobilized on the nanomaterial [72], which is divided into the following: enzymes [73], antibodies [74], antigens [75], DNA-RNA [76], organelle [77], cell membrane [78], and phage particle [79]. The conversion of this signal can be achieved using different methods, and this can be classified according to the type of conversion used [80]. Finally, the signal conversion section can include the following optical systems: [69] electrochemical nanobiosensors [81], thermoelectric [82], and piezoelectric [83].
2. Biofunctionalization of Nanostructured Surfaces for Interaction with Biorecognition Agents
In order to allow protein adsorption without altering the natural structure of the bioactive molecule, it is essential for the biomaterial surface to be biocompatible [84]. To ensure this, a bioconjugation protocol is applied in the biosensor. Bioconjugation involves the interaction of chemical or functional groups between NPs and biomolecules [85,86]. Additionally, it is important to mention that the properties can affect the efficiency of the connection with the biomarkers, including the optimal distance between biorecognition molecules and the nanostructure, the pH of the storage buffer used, as well as potential modifications to the biological and antigenic properties of biomolecules after conjugation. Hence, it is relevant to develop different approaches to nanostructure conjugation [87,88], using custom functional groups (such as primary amines, carboxylates, cis-diols, and sulfhydryls) on nanosurfaces [62,89].
Therefore, the adsorption methods of bioactive molecules into biosensor and nanobiosensor devices can be classified into the following categories: (i) Non-covalent immobilization strategies, based on electrostatic interaction, hydrophobic adsorption, and coordination bond formation between biomarker and the surface of nanomaterials (Figure 2) [86,90]. (ii) Covalent immobilization strategies, involving chemical bonds, chemically activate and modify biorecognition molecules, achieving a stable binding. They enhance sensitivity and selectivity in pathogen detection, ensuring robust interaction [91]. Finally, (iii) a combination of the above-mentioned techniques [87,92].
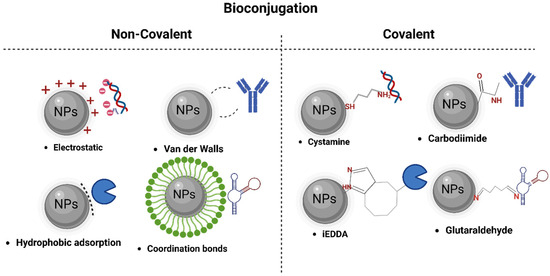
Figure 2.
Different techniques of bioconjugation in nanomaterials.
2.1. Biorecognition Section of Bionsensor Device: Enzymes Applications
Enzymatic reactions in the biodetection processes involve the following steps: Firstly, enzymes recognize and bind to the target molecule in the environment/solution, through specific binding sites or active sites on the enzyme. Enzymes are typically immobilized on the surface, electrode, or substrate of the sensor to provide ideal conditions to react with a target molecule and produce a detectable signal [93]. Subsequently, enzymatic activity can be used as a signal through variations in the concentration of protons, entrance or exit of gases, and heat emission [94]. Finally, the signal generated is detected and quantified using a biosensor-detection component (electrochemical and fluorescence techniques) [93,94]. In fact, a biosensor base in enzymes immobilized on Au-NP was used to detect Campylobacter jejuni in chicken breast samples; in this biosensor, the nuclease, enzymes, and deoxyribozymes were immobilized to detect the pathogen for the reaction of the scission–enzyme, generating a heteroduplex of DNA–RNA, which finally induced a detectable signal based in a fluorescence-detection model with a limit of detection (LOD) of 10 pM. The viability of this method of DNA detection is assessed as an ultra-sensitive analysis; also, the authors remark that the Au-NP-based detection method can reach the lowest LOD (1 pM) of DNA in samples, one fold less than that required by the already mentioned ultra-sensitive fluorescence-detection method [95]. Also, a comparative analysis of the Influenza A virus detection using a biosensor-based technique showed an LOD of 1 pM mL−1 [96], which is extremely low compared to the concentration required in qPCR detection methods [97].
To enhance the biosensor-recognition capacities, the immobilization and stabilization of the enzymes are normal processes. The enzyme immobilization techniques commonly applied to nanostructures are covalent binding, crosslinking, and self-assembled monolayers [98]. However, using enzymes as biorecognition agents has certain disadvantages for in situ applications. Enzymatic activity can be influenced by environmental conditions such as temperature and pH, which affect the stability of the biosensor [99].
2.2. Antibody Applications
Antibodies (immunoglobulins) are proteins produced by cells of the immune system called B lymphocytes [100]. They consist of a basic structure composed of four polypeptide chains: two identical heavy chains and two identical light chains with their typical “Y” shape [100,101]. Antibodies are biological molecules, derived from animals, that have gained importance in pathogen-biomarker detection methods, due to their high specificity and in vivo uniqueness [102], designing monoclonal antibodies to precisely target antigens or receptors [103]. An antibody-based biosensor was presented by Majid et al. (2019) [104]. In this type of biosensor, the immobilization of antibodies in gold-NP or nanomaterial are related to weak electrostatic, hydrophobic, or van de Waals force interactions [105]. On the other hand, the preferred method for immobilizing antibodies on NPs and other surfaces is through covalent bonding, specifically using carbodiimide chemistry and maleimide conjugation. This approach allows for longer-lasting and reusable devices, as well as better control over antibody orientation, resulting in enhanced detection capabilities [105,106].
This type of antibody-functionalized biosensor has been used for different pathogen detection, as reported by Guo et al. (2020) [107], who developed a method using NP etching. These techniques allow for the specific detection of Salmonella Typhimurium, using catalase-modified antibodies that bind to the bacteria and catalyze the conversion of H2O2 to H2O. In the absence of S. Typhimurium, the catalase-modified antibodies do not bind to the bacteria, resulting in a significant accumulation of residual H2O2. Horseradish peroxidase (HRP) triggers the production of •OH, causing a color change in the Au nanowires from dark blue to pink. The linear detection range is between 18 CFU mL−1 and 1.8 × 105 CFU mL−1, with a detection limit of 35 CFU mL−1 [107]. However, antibody-functionalized biosensors have limitations including lack of specificity, long-term stability, high production cost, challenges in antibody immobilization, potential cross-reactivity, limited antibody availability, and batch-to-batch variability [50].
2.3. DNA Applications
Nucleic acid-based biosensors, such as DNA, stand out as biorecognition elements due to their simplicity, speed, and high specificity. For this reason, they are widely used for the detection of pathogens and other substances of interest in various biodetection applications [88]. These characteristics make DNA a powerful and versatile tool in the field of biodetection [108,109]. These molecular probes can be used in different ways in methods such as DNAzyme [110], DNA hairpin [111], DNA hybridization [112], and DNA origami [113]. It is widely recognized that DNA and its assembly structure can be applied to detect specific targets, including nucleic acids, proteins, metal ions, and small biological molecules. Common bioreceptors in this category include deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and peptide nucleic acids (PNA) [114].
These biomarkers have been functionalized with nanomaterials to enhance their selectivity and durability in pathogen biodetection. An application of pathogenic bacteria detection in milk was developed using a combination of photo-induced electron transfer (PET) between a G-quadruplex DNAzyme and silver nanocluster-labeled DNA, along with exponential circular amplification based on the hairpin probe, achieving an ultra-low detection limit of 8 CFU mL−1 for S. Typhimurium. This strategy represents a promising platform for highly sensitive and specific detection of pathogenic bacteria in food analysis [115]. In another study, a fluorescent DNA hairpin template was developed by designing two hairpin probes with Au-NPs for the detection of S. aureus 16S rRNA. HP1 was biofunctionalized with thiol groups and a fluorescent chromophore, and a thiol group was attached to the NP surface. The addition of HP2 causes the target sequence to walk along the surface of the Au-NPs, thus opening the hairpin structure of HP2 and enabling the recycling of the target sequence. They achieved a LOD of 7.73 CFU mL−1 with an FM of 4.36 × 10−5, demonstrating a novel and efficient method for the detection of S. aureus [116].
2.4. Aptamer Applications
Aptamers are short sequences of RNA or DNA (oligonucleotides), capable of folding into unique three-dimensional structures and binding to targets such as proteins, lipids, ions, small-molecular-weight metabolites, even whole cells with high specificity and affinity [117]. To produce aptamers, the SELEX (Systematic Evolution of Ligands by Exponential Enrichment) process is utilized. In this process, aptamers are generated through in vitro synthesis of combinatorial libraries with diverse sequences [53]. Through an iterative selection process, aptamers with higher affinity for the desired target are enriched and amplified, while those with lower affinity are discarded. This enables the generation of highly specific and high-affinity aptamers for various biomolecular targets [118]. The analytes of aptamer-based biosensors can vary in size and complexity as it can detect specific molecules such as proteins or more complex analytes such as whole cells. Aptamers modify their structure once reacting with a specific analyte and the conformational change can be transduced using different types of signals such as optical or electrochemical [49,119].
Some of the applications of aptamer-based sensors were developed for the detection of S. aureus, E. coli and C. jejuni pathogen biomarkers [53]. Another example was the results in S. aureus detection protocols, where they designed an ultra-sensitive magnetic fluorescence aptasensor based on fluorescence-resonance energy transfer, and the aptamers were placed on the surface of Fe3O4 and modified carbon dots (CDs). CDs were used as the fluorescence donor and Fe3O4 as the “off-on” sensor receptor. Due to the strong affinity of the aptamers to bacteria, the presence of target bacteria led to the disassembly of the Fe3O4/CDs aptasensor, resulting in the recovery of CDs fluorescence with a range of detection exhibited between 50 × 107 CFU mL−1 and 8 CFU mL−1 [120].
Another example is the application of E. coli detection using graphene oxide (GO)-modified Au-NPs, enhanced with aptamers; an E8 aptamer was used for E. coli detection. The detection limit was found to be 10 cells/mL in water and coconut water-enriched samples. Furthermore, the aptamer-based nanosensor exhibited selectivity towards its target without any cross-reactivity with other bacteria. The color changes from red to blue, based on aggregation, can be easily seen by the naked eye [121]. Another protocol for E. coli detection in water was the nanobiosensor using QDs functionalized with aptamer II and coated with magnetic NPs. Fluorescence values were recorded for 100, 200, 300, 400, and 500 CFU, each with CdTe-MPA QDs at 100 μg mL−1, resulting in digital signals of 29.3 mV, 34.18 mV, 39.06 mV, 43.94 mV, and 48.82 mV, respectively, demonstrating that CdTe-MPA QDs conjugated with aptamer II were capable of selectively capturing and detecting E. coli [122].
Aptamers exhibit significant advantages to their application in pathogen detection, including lower molecular weight, easier and more cost-effective production methods, and good chemical stability [53]. Moreover, their ability to be generated against a wide range of targets ranging from small molecules to large proteins, and even whole live cells [123], has led to their utilization in various pathogen-detection nanobiosensor-based technologies, combined with different technologies including surface plasmon resonance (SPR), electrochemistry, piezoelectric effect, and chemiluminescence [80].
For example, SPR sensors utilize the reflection of light on a modified metal surface to detect changes using the biomarker binding in the refractive index, resulting in the precise and sensitive detection of the biomarker target [80]. In the case of electrochemical sensors, the analyte interaction is translated into an electrical signal, providing a quantitative means of detection and enabling real-time measurements [124]. Piezoelectric sensors, on the other hand, leverage the piezoelectric effect to convert the mechanical energy generated by analyte interaction into electrical energy, allowing highly sensitive and accurate detection [125]. Furthermore, chemiluminescence is another technology used in biosensors, where the analyte interaction triggers a chemical reaction that generates light. This emitted chemiluminescent light can be measured to detect and quantify the analyte, providing highly sensitive and specific detection [71]. Functionalizing nanomaterials with aptamers has allowed the combination of various signal-transduction strategies for detecting foodborne pathogens.
This detection versality provides the capability to utilize different approaches according to specification of each application, enhancing the sensitivity and selectivity of detection systems. Thus, aptamers are a powerful and promising tool in the fight against food contamination and the protection of public health [126]. These technologies enable the detection and quantification of substances in biological samples, providing versatile and efficient options for applications in fields such as medical diagnosis, food safety, and environmental monitoring (Figure 3).
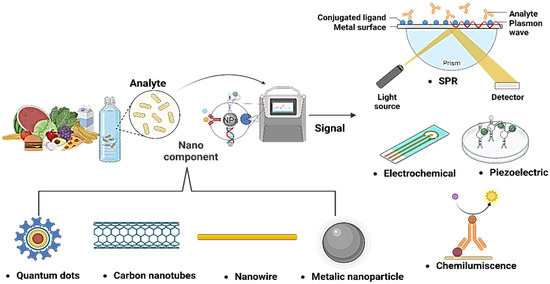
Figure 3.
Combination of different nanomaterials and sensors for the detection of pathogens.
2.5. Molecularly Imprinted Polymers (MIPs)
MIPs are defined as a group of biomimic compounds that replicate the natural interactions between a biorecognition section (antibody, antigen, or enzyme) and a biomarker; these compounds have a “lock and key” bonding mechanism to interact with the molecule of interest [127]. MIP development methods can be divided into the following: (a) covalent, (b) semi-covalent, and (c) non-covalent methods. These are in concordance with the site of action-binding modes; in general, the methods are as follows: bulk, suspension, emulsion, precipitation, multi-step swelling, and surface imprinting electrochemical polymerization [128].
In recent years the application of MIPs-based techniques has been applied to detect pathogens related to foodborne illnesses. In fact, in the case of bacteria detection, MIPs can be divided depending on the detection target (whole cells or cell membrane subunits), and subdivided in to microcontact/stamp imprinting (with a LOD of 70 CFU mL−1 of E. coli) [129], drop coating (with a LOD of 1.6 × 108 cells mL−1 of E. coli strain) [130], Pickering emulsion interfacial imprinting (with a LOD of 1 × 103 CFU mL−1 of L. monocytogenes strain) [131], and electropolymerization (with a LOD of 4 CFU mL−1 of S. aureus strain), among other methods that have been proved, through their LODs, to have a high detection sensitivity for foodborne pathogen [129].
3. Optical and Electrochemical Nanobiosensors
Nanobiosensors play a crucial role in the detection of biomolecules in food and water through two distinct phenomena. Optical nanobiosensors are based on the phenomenon of the interaction of optical nanostructures with light. When specific biomolecules bind to the analyte in the sample, they trigger changes in the optical properties of light, such as absorbance or fluorescence [132]. These changes are detected and quantified to determine the presence and concentration of the analyte. Optical nanobiosensors offer high sensitivity and selectivity by harnessing this phenomenon of light–matter interaction, ensuring precise detection in food and water quality-control applications [69,70]. The Surface Plasmon Resonance (SPR) phenomenon has fundamental applications in the detection of pathogens in food and water. This sensitive and specific optical technique is used to identify the presence of pathogens (bacteria and viruses) in food and water samples. Changes in the SPR resonance angle reveal the interaction between surface biomolecules and pathogens, allowing for rapid and accurate detection of potential microbiological contaminants in these critical products for public health [132,133] (Figure 4A). On the other hand, electrochemical nanobiosensors rely on the phenomenon of electrochemical reactions on nanostructured electrodes. These electrodes provide a large surface area, enabling profoundly sensitive and selective detection. When specific biomolecules in the sample interact with the analyte, changes in electrical current or electrical potential occur on the electrode’s surface, modifying the electrical properties of the solution and generating a detectable signal (Figure 4B) [68].
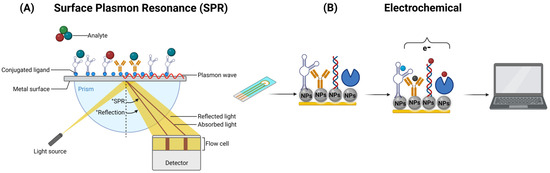
Figure 4.
Operation of an optical SPR (A) and an electrochemical (B) biosensor, respectively.
Both types of nanobiosensors can be miniaturized for portable applications and are essential for ensuring the safety and quality of food and water, with the choice of nanobiosensor type depending on the specific properties of the analyte and the goals of the application at hand. Leticia Tessaro et al. (2022) [133] delve into the utilization of AuNPs in an SPR nanobiosensor designed for SARS-CoV-2 detection. While this method boasts sensitivity and precision on par with traditional RT-qPCR techniques, the cost associated with AuNPs may hinder widespread adoption. Nevertheless, it has achieved a detection time of 100 min and an LOD of 1 ng mL−1 (equivalent to 2.7 × 103 copies per µL), establishing itself as capable of detecting the virus on food surfaces, thus emphasizing its potential in safeguarding food during pandemics.
In a similar applications, Jiayun Hu et al. (2018) [134] demonstrated the exceptional plasmonic properties of AuNPs for LSPR-based detection, offering high sensitivity with an LOD of 10 CFU mL−1 in Pseudomonas aeruginosa detection. The cost aspect remains a concern for large-scale applications. Moreover, the versatile LSPR whole-cell detection scheme demonstrated they can be extended to other microorganisms, including various bacteria and viruses, through the use of different affinity agents. This robust LSPR detection platform holds promise for clinical applications, owing to its rapid detection capability of approximately 3 h, making it suitable for point-of-care and field-based applications. Ajinkya Hariram Dabhade et al. (2023) [135] introduce AgNPs in an electrochemical biosensor for E. coli detection, showcasing cost-effectiveness and simplicity. This sensor demonstrates good selectivity and stability, with an LOD of 150 CFU mL−1. The ease of synthesis and their reproducibility make AgNPs a practical choice for on-site, real-time detection applications. Faezeh Shahdost-Fard et al., 2023 [136], introduce a unique nanocomposite-comprising sponge, copper tungsten oxide hydroxide, and AgNPs. While the synthesis process may be complex, this nanomaterial exhibits impressive performance in S. aureus detection. The nanocomposite-based electrochemical aptasensor offers a low LOD of 1 CFU mL−1 and high specificity. Its applicability in clinical samples underscores its potential for addressing nosocomial infections. The work carried out by Singh et al., 2018 [137], employed gold nanoparticles (GNPs) in a rapid-pathogen-detection assay, capitalizing on colistin’s interaction with lipopolysaccharides and the optical properties of the nanomaterial. This cost-effective approach eliminates tedious sample-preparation steps, offering a rapid, sensitive, visual detection method within 5 min. While GNPs are versatile, their sensitivity for pathogen detection at low concentrations may require further optimization for specific applications, as it exhibited a LOD of 10 cells mL−1 in tap water and 100 cells mL−1 in lake water samples. Zeynep Altintas et al., 2018 [138], present a fully automated microfluidic electrochemical biosensor designed for real-time bacteria detection. It employs immunoassays, including nanomaterial-amplified assays, to quantify E. coli concentrations. The sensor achieved a LOD of 1.99 × 104 CFU mL−1 using nanomaterial amplification.
Srijit Nair et al., 2018 [139], introduce a novel approach for detecting uropathogenic E. coli (UPEC) using crossed surface-relief gratings (CSRGs) as nanometallic sensors. This optical-sensing platform leverages SPR-based light energy exchange for real-time, selective, and label-free UPEC detection. The LOD is reported at 105 CFU mL−1, which is clinically relevant to urinary tract infection (UTI) diagnosis.
Olja Simoska et al., 2019 [140], focus on real-time electrochemical detection of phenazine metabolites produced by P. aeruginosa. Transparent carbon ultramicroelectrode arrays (T-CUAs) are used to monitor the concentrations of pyocyanin (PYO) and other metabolites. Although this work primarily centers on metabolite detection, it offers valuable insights into real-time monitoring. The study provides detailed information about phenazine dynamics over time.
4. Nanomaterials for the Detection of Pathogens in Water and Food
As is mentioned above, one of the major concerns in food and water safety is the precise detection of pathogens, this has led, in combination with novel sensor technologies, to an increasing exploration of nanomaterials in combination with highly efficient aptamers to revolutionize the pathogen detection in water and food. This fusion of nanotechnology and aptamers opens new possibilities for more effective control and quicker responses to potential public health risks. The following Table 1 summarizes the last five years of nanobiosensor production for the detection of viruses, bacteria, and parasites using aptamers in complex matrices.

Table 1.
NPs application for detection of pathogenic bacteria in food and water matrices.
Nanobiosensors, due to their small size and high sensitivity, enable the real-time detection of low concentrations of biomarkers, a crucial characteristic in applications of food and water monitoring. This versatility allows them to adapt to various molecules and technologies, such as artificial intelligence incorporation. Moreover, they are more cost-effective and environmentally friendly than conventional techniques. Their miniaturization capability makes them ideal for portable devices and on-site diagnostic systems, providing quick and efficient access to quality testing and analysis in food and water. This makes them promising tools in various scientific and technological applications (Figure 5).
Figure 5.
Strengths of nanobiosensors.
4.1. Gold Nanopartícles (Au-NPs)
Among the different types of NPs, metallic nanoparticles (MNPs) exhibit many useful characteristics such as high surface-to-volume ratio, conductivity, selectivity, and excellent optical and chemical properties, for their application in the biotechnology field [164,165]. The application can vary depending on the metal used, size, shape, surface properties, and functionalization of the MNPs [166]. On one hand, Au-NPs have been successfully used in pathogen detection because they can easily be conjugated with recognition and biorecognition elements such as aptamers, DNA, antibodies, carbohydrates, and proteins, which can enhance the reactivity and selectivity of the NPs towards specific pathogens [51,167].
In fact, Au-NPs are one of the most stable MNPs, not to mention their unique characteristics such as good chemical reactivity, conductivity, and high resistance, which have attracted attention for their use in biosensor development [168]. The surface of Au-NPs has been functionalized with various biocomponents [169]. These nanobiosensors have a very low LOD for different chemical and biological analytes, not to mention their high stability against oxidation [168]. Also, their characteristics, such as stability, conjugation, amplification properties, and their ability to serve as colorimetric biosensors [170,171,172] are especially relevant in the case of Au-NPs due to their localized surface plasmon resonance, which is a phenomenon that gives unique optical properties to MNPs, particularly Au-NPs. This is due to the interaction of electromagnetic waves with NPs of specific sizes and shapes, resulting in differential absorption of the light spectrum and different colors exhibited by the NPs [50,51]. These properties can be altered in the presence of different analytes, making Au-NPs highly suitable for biosensor development.
4.2. Silver Nanoparticles (Ag-NPs)
Ag-NPs stand out for their wide range of applications. These nanomaterials have been incorporated into textiles, healthcare products, consumer goods, medical devices, and biodetection applications, among others [173]. These materials are highly attractive in diagnostics field due to high conductivity, catalytic activity, and plasmonic properties presented, which may be leveraged to enhance the biosensor’s performance [174]. Sensitivity is a crucial factor for biosensors to detect low concentrations of biomarkers. Ag-NPs have been used to increase the electroactive surface area of electrodes, enhancing the electron-transfer rate and improving biosensor sensitivity [173,174]. In the incorporation of Ag-NPs in biosensor structures, Ag-NPs can amplify signals or improve the detection of nucleic acids. Their plasmonic resonance absorption band, below 500 nm, confers selective absorption in the visible and near-infrared spectrum [168]. In connection with pathogen detection, the phenomenon of surface plasmon resonance (SPR) works using the electrons on the surface of a metal, which are excited by photons of specific wavelengths and incidence angles [175,176] and applied to target detection based on the refractive index [175]. This is achieved when the biomarker is bound to a biorecognition element of the biosensor, the recognition event between the biomarker and the biorecognition element results in a change in the SPR resonance angle [31]. Conjugated polymers, such as those that include silver nanoparticles are promising materials for addressing the current and emerging issues such as pandemic monitoring [177], and pathogen detection both in food [148] and water [146].
4.3. Carbon-Based Nanoparticles
Similar to Au-NPs, carbon-based NPs are useful for the implementation of detection techniques for pathogen monitoring in water [119,178]. Carbon-based NPs such as carbon nanotubes, graphene, and carbon nanodots have great potential in the biosensing of pathogens because of their ability to be coated with different biomolecules for the association of molecular patterns from pathogens and to generate a signal for specific pathogens as functionalized NPs can mimic the specific surface structure of pathogens [179]. Carbon NPs have been used in the fluorescence resonance energy transfer (FRET) technique with quantum dots as donors modified with aptamers for the detection of Vibrio parahaemolyticus and S. Typhimurium in the range of 25 to 35 CFU mL−1 and up to between 50 and 106 CFU mL−1, respectively [180]. Also, these NPs can be used in combination with aptamers to amplify the sensitivity and specificity of the device.
4.4. Magnetic Nanomaterials (MNPs)
Magnetic NPs possess their own versatility when used for biosensing pathogens, because of their specific attributes, particularly fast separation and concentration, that makes them easy tools for pathogen detection [181]. MNPs have been used for detecting pathogens using nucleic acid detection and quantification in devices for point-of-care testing in the detection of the Hepatitis B virus (LOD of 50 IU mL−1) and SARS-CoV-2 (500 copies mL−1) [182]. Magnetic NPs (MNPs) can conform to a section of the transducer part of the biosensor, or be suspended in solution in direct contact with the analyte of interest [183]. When the MNPs are in contact with the sample, they bind to the target molecule through the interaction of the label in the NPs (a functional group) and a protein; once the complex of MNPs and target is formed, an external magnetic field attracts it to the active-detection surface, and after a wash of the unbinding molecules, targets are detected [184].
When talking about magnetic NPs in biosensing, it is important to mention the magnetic relaxation switching mechanism (MRS). This phenomenon describes the incidence when cross-linking occurs between the MNPs in the binding and recognition of targets. When these MNPs clusters are formed, a change in the transverse relaxation of the sample is reflected as motional averaging or static dephasing according to the MNPs cluster size and this change can be monitored using nuclear magnetic resonance [185].
4.5. Silica Nanoparticles (Si-NPs)
Si-NPs have applications in the biomedical field [186], and they present good optical properties and good biocompatibility [187]. NPs are mesoporous, so in combination with other metals, have attractive and profitable characteristics for biosensing purposes [188]. Their uniformity and easily changed pore size among the gating mechanism makes it very useful in biosensing for drug delivery, for example [189]. Another important characteristic of Si-NPs is that they are considered as a GRAS (generally recognized as safe) material by the FDA [190,191]. The mesoporous nature of the Si-NPs is characteristic of a large interest, this feature can be employed to separate bacteria from complex samples even preserving its viability, and colloidal stabilization of magnetic NPs for the same purpose. Also, the silanol functional groups from SiNPs make possible the use and design of various bio-recognition systems that help to increase their sensibility and selectivity while reducing the detection time of different pathogens [190].
4.6. Quantum Dots (QD)
Quantum dots (QD) are colloidal nanocrystalline semiconductors that possess properties such as a quantum confinement effect, allowing them to emit and absorb light at specific wavelengths [191]. Because of this, QDs exhibit excellent optical properties, including a broad absorption spectrum, a narrow emission spectrum, and tunable luminescence, which show great prospects in biodetection [192]. QD-based biosensors include but may not be limited to fluorescence, bioluminescent, chemiluminescent, and photoelectrochemical approaches [193]. Some of the characteristics that make the use of quantum dots attractive for biosensing applications are that they possess high-quantum yield, better photobleaching resistance, wide absorption spectra, a narrow emission spectrum and their specificity with biologic targets in comparison with common fluorophores and dyes [194]. Also, it is very remarkable that its surface is easily functionalized with biologic components in order to integrate QD probes [193]. In the field of nanomaterials, the use of combinations of magnetic compounds displays attractive characteristics for current applications; these nanocomposites, besides maintaining complementary magnetic behavior, add functional proprieties to the final product [159].
As presented above, numerous studies focus their determinations on S. Typhimurium mainly because it is the most common pathogen related to food poisoning in Western countries causing gastroenteritis [195]. If well-used as the model or the target of the experimentations, the modifications in for example primers’ design or binding proteins may allow the replication of studies carried with this strain to any other food pathogens [161,162,196].
5. Prospects and Limitations to Detecting Pathogens with DNA Using Nanobiosensors
The importance of exceptionally responsive devices is essential to advancing biosensors applied in pathogen detection. Insufficient sensitivity and affinity towards biomarkers can significantly impact the performance of the device and prevent the pathogen detection. In fact, some of the biomarkers are at an ultra-low concentration in the samples (pM), and for this reason, it is necessary that the device is available to detect these ultra-low concentrations [197]. These concentrations of pathogen biomarkers in the samples present a limitation to the performance of the detection methods, this is related to the source and nature of the target biomarker itself [198]. However, through using a genetic and whole-cell-based biomarker target, and adequate sensor technologies, some of the biosensors have LOD of three genetic copies per sample [199], 1 CFU mL−1 [200] or have even reached an LOD of 3 × 106 gene copies per sample [9], or 5 × 104 CFU mL−1 biomarker concentration in the sample, this is considered an ultrasensitive detection range [201].
As is mentioned above, the choice of signal recognition technology can determine the sensitivity level required to identify biochemical, genetic, or whole cell biomarker concentration in the sample [132,202] and affect the performance of the device. In fact, the detection’s ultra-low concentration of pathogen-disease biomarkers concentration in samples is a mandatory requirement for early detection in clinical diagnoses [203]. Some of the disadvantages of biosensor-based pathogen detection are as follows: The factor to be determined is the target molecule to be sensed where sensitivity and specificity are compromised by the biomarker choice [109]. The use of genetic markers leads to a more sensitive device. However, it implicates complex systems and laborious sample/re-agent handling procedures [204].
On the other hand, signal emission technology is another important factor in pathogen detection. Colorimetric-based biosensors have several factors that may alter their detection capability, such has colorimetric substrate, incubation time, and even the temperature at which the signal is measured [205]. Particularly in DNA-based biosensors, other factors are lack of ability to form a complex, complication in large-scale patterns, reaction induction by mistake, and high sensitivity to enzymatic degradation and oxidation [109]. However, despite the disadvantages, there are several applied technologies in the biorecognition element of the biosensor device. For example, gene-sequence biomarkers such as CRISPR/Cas9-based technology, where the lowest LOD was three genetic copies per sample, reaching up to 3 × 106 gene copies per sample [9]. In this study, the detection signal was the dose-response intensity. CRISPR/Cas12 based lateral flow, where the lowest LOD was four gene copies in the sample [199]. Other limitations to consider in the application of this device is the ability of the biosensor to discriminate between live/dead cells (LOD 1 CFU mL−1); the use of functionalized NPs with bacteriophages as a biorecognition agent is a solution applied for successful discrimination between live/dead [200].
One of the most astonishing advancements in the field of biosensors is the implementation of artificial intelligence and other informatic technologies in pathogen detection. The combination of artificial intelligence and biosensors has created an interdisciplinary concept of AI biosensors. The basic architecture of AI biosensors consists of three main elements: information gathering, signal conversion, and AI data processing [206]. A nanobiosensor with AI offers advantages in terms of sensitivity, speed, and analytical capability compared to conventional biosensors. This makes it suitable for application where highly precise and rapid detection of biomarkers is required, such as advanced medical diagnostics, environmental monitoring of molecular-level contaminants, and nanoscale quality control in the food industry [206,207]. The study conducted by Taniguchi et al. (2021) [208] revealed that by utilizing nanopores in conjunction with artificial intelligence, the identification of similarly sized coronaviruses is achievable. This capability has the potential to differentiate between various types of coronaviruses, such as HCoV-229E, SARS-CoV, MERS-CoV, and SARS-CoV-2. Furthermore, this technique demonstrated its effectiveness by successfully detecting SARS-CoV-2 in saliva samples. In summary, solid-state virus-immunodetection techniques hold a promising outlook for the development of versatile, adaptable, and cost-effective diagnostic tools in the future [202].
6. Conclusions
Over the years, significant advancements have been made in the field of biosensors, particularly in areas related to food safety and the monitoring of pathogenic microorganisms associated with food and waterborne illnesses. Despite these achievements, progress in technologies for the development of pathogen-detecting biosensors remains a highly promising area of study. This is due to the presence of various nanomaterials (MNPs, QD, carbon nanotubes, among others) with specific properties that enable the identification of specific pathogens and enhance the performance of the devices.
The nanomaterials used in biosensors offer unique advantages for pathogen detection. Thanks to their small size and large specific surface area, they facilitate more effective interaction with pathogen biomarkers, enhancing the sensitivity and selectivity of biosensors. Furthermore, their capacity to be functionalized with specific molecules, such as antibodies, nucleic acids, or aptamers, provides intrinsic advantages in the selectivity and sensitivity of the devices. Particularly with the aptamers, due to their chain-like structure, they offer greater flexibility and ease of design, making them highly selective and sensitive agents for the precise detection of pathogens. In fact, aptamers, functionalized aptamers, and other genetic-based biomarker-detection technologies have promising applications for the enhanced specificity and selectivity that has been proved in pathogen monitoring. Also, this technology compared with antibody techniques has several advantages such as improved specificity and the ability to detect genetic material, rather than proteinic, of structural biomarkers. In addition, pathogen detection through biosensors has a substantial impact on public health. The presented revision shows how nanobiosensors’ technology contributes to the precise and rapid identification of pathogenic agents in food and water, and if applied correctively, can prevent disease outbreaks and prompt appropriate measures to ensure consumer safety.
In summary, the nanomaterials employed in biosensors present diverse advantages for pathogen detection. Their reduced size, high-specific surface area, functionalization capacity, and signal amplification properties contribute to the sensitivity, selectivity, and precision of biosensors. Future possible applications of DNA-based technologies in combination with nanoparticles’ formulation, particularity the application of aptamer technologies and nanoparticles with DNA probes, will have more sensitive and specific detection techniques. In addition, this type of biosensor has the lowest capacity for detection limits by using a genetic fingerprint to discriminate between pathogens, and in the future between nonpathogenic strains, and strains of concern. These qualities, combined with the potential to use detection techniques such as fluorescence, and the application of digital technologies such as IA models, has huge potential to improve the detection capacity of the monitoring methods, creating nanomaterial-based and aptamer-based biosensors, and promising tools for pathogen monitoring and detection, enhancing safety in the food industry and public health overall.
Author Contributions
Conceptualization: H.M.V.-A., A.A.-A., E.R.M.-S., J.E.S.-H. and R.P.-S.; Writing—original draft preparation: H.M.V.-A., A.A.-A., E.R.M.-S., P.G.V.-O., J.E.S.-H. and R.P.-S.; Data curation: H.M.V.-A., A.A.-A., E.R.M.-S., P.G.V.-O., O.d.l.R. and M.A.O.-M.; Visualization: H.M.V.-A., A.A.-A., E.R.M.-S. and P.G.V.-O.; Writing—review and editing: H.M.V.-A., E.R.M.-S.; A.A.-A., O.d.l.R., M.A.O.-M., J.E.S.-H. and R.P.-S.; Administration: A.A.-A., O.d.l.R., M.A.O.-M., J.E.S.-H. and R.P.-S.; Funding acquisition: J.E.S.-H. and R.P.-S.; Supervision. J.E.S.-H. and R.P.-S. All authors have read and agreed to the published version of the manuscript.
Funding
We are thankful for the funding from the Fundación FEMSA with the project entitled “Unidad de respuesta rápida al monitoreo de COVID-19 por agua residual” (grant number NA) and Tecnologico de Monterrey internal funding through the project Challenge-Based Research Funding Program 2022 (Muestreador Pasivo I026-IAMSM005-C4-T1-T).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figures were created with Biorender.com (accessed on 21 August 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Estimating the Burden of Foodborne Diseases. Available online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (accessed on 14 March 2023).
- Riu, J.; Giussani, B. Electrochemical Biosensors for the Detection of Pathogenic Bacteria in Food. TrAC Trends Anal. Chem. 2020, 126, 115863. [Google Scholar] [CrossRef]
- Haughton, P. La OMS Intensifica sus Esfuerzos para Mejorar la Salubridad de los Alimentos y Proteger a la Población de las Enfermedades. Available online: https://www.who.int/es/news/item/07-06-2021-who-steps-up-action-to-improve-food-safety-and-protect-people-from-disease (accessed on 10 May 2023).
- AL-Mamun, M.; Chowdhury, T.; Biswas, B.; Absar, N. Food Poisoning and Intoxication: A Global Leading Concern for Human Health. In Food Safety and Preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 307–352. ISBN 978-0-12-814956-0. [Google Scholar]
- Chin, N.A.; Salihah, N.T.; Shivanand, P.; Ahmed, M.U. Recent Trends and Developments of PCR-Based Methods for the Detection of Food-Borne Salmonella Bacteria and Norovirus. J. Food Sci. Technol. 2022, 59, 4570–4582. [Google Scholar] [CrossRef]
- Thomas, K.M.; De Glanville, W.A.; Barker, G.C.; Benschop, J.; Buza, J.J.; Cleaveland, S.; Davis, M.A.; French, N.P.; Mmbaga, B.T.; Prinsen, G.; et al. Prevalence of Campylobacter and Salmonella in African Food Animals and Meat: A Systematic Review and Meta-Analysis. Int. J. Food Microbiol. 2020, 315, 108382. [Google Scholar] [CrossRef]
- Dos Santos, J.S.; Biduski, B.; Dos Santos, L.R. Listeria Monocytogenes: Health Risk and a Challenge for Food Processing Establishments. Arch. Microbiol. 2021, 203, 5907–5919. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Pathogenicity Assessment of Shiga Toxin-producing Escherichia coli (STEC) and the Public Health Risk Posed by Contamination of Food with STEC. EFS2 2020, 18, e05967. [Google Scholar] [CrossRef]
- Zhou, J.; Yin, L.; Dong, Y.; Peng, L.; Liu, G.; Man, S.; Ma, L. CRISPR-Cas13a Based Bacterial Detection Platform: Sensing Pathogen Staphylococcus Aureus in Food Samples. Anal. Chim. Acta 2020, 1127, 225–233. [Google Scholar] [CrossRef]
- Mora, Z.V.L.; Macías-Rodríguez, M.E.; Arratia-Quijada, J.; Gonzalez-Torres, Y.S.; Nuño, K.; Villarruel-López, A. Clostridium Perfringens as Foodborne Pathogen in Broiler Production: Pathophysiology and Potential Strategies for Controlling Necrotic Enteritis. Animals 2020, 10, 1718. [Google Scholar] [CrossRef]
- Enosi Tuipulotu, D.; Mathur, A.; Ngo, C.; Man, S.M. Bacillus Cereus: Epidemiology, Virulence Factors, and Host–Pathogen Interactions. Trends Microbiol. 2021, 29, 458–471. [Google Scholar] [CrossRef]
- Gupta, V.; Gulati, P.; Bhagat, N.; Dhar, M.S.; Virdi, J.S. Detection of Yersinia Enterocolitica in Food: An Overview. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 641–650. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The Problem of Drinking Water Access: A Review of Disinfection Technologies with an Emphasis on Solar Treatment Methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef]
- Adelodun, B.; Ajibade, F.O.; Ighalo, J.O.; Odey, G.; Ibrahim, R.G.; Kareem, K.Y.; Bakare, H.O.; Tiamiyu, A.O.; Ajibade, T.F.; Abdulkadir, T.S.; et al. Assessment of Socioeconomic Inequality Based on Virus-Contaminated Water Usage in Developing Countries: A Review. Environ. Res. 2021, 192, 110309. [Google Scholar] [CrossRef]
- Ramírez-Castillo, F.; Loera-Muro, A.; Jacques, M.; Garneau, P.; Avelar-González, F.; Harel, J.; Guerrero-Barrera, A. Waterborne Pathogens: Detection Methods and Challenges. Pathogens 2015, 4, 307–334. [Google Scholar] [CrossRef]
- Cissé, G. Food-Borne and Water-Borne Diseases under Climate Change in Low- and Middle-Income Countries: Further Efforts Needed for Reducing Environmental Health Exposure Risks. Acta Trop. 2019, 194, 181–188. [Google Scholar] [CrossRef]
- Mahagamage, M.G.Y.L.; Pathirage, M.V.S.C.; Manage, P.M. Contamination Status of Salmonella Spp., Shigella Spp. and Campylobacter Spp. in Surface and Groundwater of the Kelani River Basin, Sri Lanka. Water 2020, 12, 2187. [Google Scholar] [CrossRef]
- Schoenen, D. Role of Disinfection in Suppressing the Spread of Pathogens with Drinking Water: Possibilities and Limitations. Water Res. 2002, 36, 3874–3888. [Google Scholar] [CrossRef]
- Wen, X.; Chen, F.; Lin, Y.; Zhu, H.; Yuan, F.; Kuang, D.; Jia, Z.; Yuan, Z. Microbial Indicators and Their Use for Monitoring Drinking Water Quality—A Review. Sustainability 2020, 12, 2249. [Google Scholar] [CrossRef]
- Semenza, J.C.; Rocklöv, J.; Ebi, K.L. Climate Change and Cascading Risks from Infectious Disease. Infect. Dis. Ther. 2022, 11, 1371–1390. [Google Scholar] [CrossRef]
- Jahne, M.A.; Schoen, M.E.; Kaufmann, A.; Pecson, B.M.; Olivieri, A.; Sharvelle, S.; Anderson, A.; Ashbolt, N.J.; Garland, J.L. Enteric Pathogen Reduction Targets for Onsite Non-Potable Water Systems: A Critical Evaluation. Water Res. 2023, 233, 119742. [Google Scholar] [CrossRef]
- Parra-Arroyo, L.; Martínez-Ruiz, M.; Lucero, S.; Oyervides-Muñoz, M.A.; Wilkinson, M.; Melchor-Martínez, E.M.; Araújo, R.G.; Coronado-Apodaca, K.G.; Bedran, H.V.; Buitrón, G.; et al. Degradation of Viral RNA in Wastewater Complex Matrix Models and Other Standards for Wastewater-Based Epidemiology: A Review. TrAC Trends Anal. Chem. 2023, 158, 116890. [Google Scholar] [CrossRef]
- Kaya, H.O.; Cetin, A.E.; Azimzadeh, M.; Topkaya, S.N. Pathogen Detection with Electrochemical Biosensors: Advantages, Challenges and Future Perspectives. J. Electroanal. Chem. 2021, 882, 114989. [Google Scholar] [CrossRef]
- Kumar, H.; Kuča, K.; Bhatia, S.K.; Saini, K.; Kaushal, A.; Verma, R.; Bhalla, T.C.; Kumar, D. Applications of Nanotechnology in Sensor-Based Detection of Foodborne Pathogens. Sensors 2020, 20, 1966. [Google Scholar] [CrossRef]
- Kabiraz, M.P.; Majumdar, P.R.; Mahmud, M.M.C.; Bhowmik, S.; Ali, A. Conventional and Advanced Detection Techniques of Foodborne Pathogens: A Comprehensive Review. Heliyon 2023, 9, e15482. [Google Scholar] [CrossRef]
- Clais, S.; Boulet, G.; Van Kerckhoven, M.; Lanckacker, E.; Delputte, P.; Maes, L.; Cos, P. Comparison of Viable Plate Count, Turbidity Measurement and Real-time PCR for Quantification of Porphyromonas Gingivalis. Lett. Appl. Microbiol. 2015, 60, 79–84. [Google Scholar] [CrossRef]
- Rajapaksha, P.; Elbourne, A.; Gangadoo, S.; Brown, R.; Cozzolino, D.; Chapman, J. A Review of Methods for the Detection of Pathogenic Microorganisms. Analyst 2019, 144, 396–411. [Google Scholar] [CrossRef]
- López, M.M.; Ilop, P. Noales Are Molecular Tools Solving the Challenges Posed by Detection of Plant Pathogenic Bacteria and Viruses? Curr. Issues Mol. Biol. 2009, 11, 13–46. [Google Scholar] [CrossRef]
- Aw, T.G.; Rose, J.B. Detection of Pathogens in Water: From Phylochips to qPCR to Pyrosequencing. Curr. Opin. Biotechnol. 2012, 23, 422–430. [Google Scholar] [CrossRef]
- Fu, Y.; Peng, H.; Liu, J.; Nguyen, T.H.; Hashmi, M.Z.; Shen, C. Occurrence and Quantification of Culturable and Viable but Non-Culturable (VBNC) Pathogens in Biofilm on Different Pipes from a Metropolitan Drinking Water Distribution System. Sci. Total Environ. 2021, 764, 142851. [Google Scholar] [CrossRef]
- Srivastava, K.R.; Awasthi, S.; Mishra, P.K.; Srivastava, P.K. Biosensors/Molecular Tools for Detection of Waterborne Pathogens. In Waterborne Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; pp. 237–277. ISBN 978-0-12-818783-8. [Google Scholar]
- Sun, Y.-J.; Chen, G.-F.; Zhang, C.-Y.; Guo, C.-L.; Wang, Y.-Y.; Sun, R. Development of a Multiplex Polymerase Chain Reaction Assay for the Parallel Detection of Harmful Algal Bloom-Forming Species Distributed along the Chinese Coast. Harmful Algae 2019, 84, 36–45. [Google Scholar] [CrossRef]
- Kim, J.-H.; Oh, S.-W. Rapid and Sensitive Detection of E. coli O157:H7 and S. typhimurium in Iceberg Lettuce and Cabbage Using Filtration, DNA Concentration, and qPCR without Enrichment. Food Chem. 2020, 327, 127036. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, Y.; Cao, X.; Liu, C.; Zhang, N.; Shi, D. Droplet Digital PCR as an Emerging Tool in Detecting Pathogens Nucleic Acids in Infectious Diseases. Clin. Chim. Acta 2021, 517, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zeng, D.; Yan, C.; Chen, W.; Ren, J.; Jiang, Y.; Jiang, L.; Xue, F.; Ji, D.; Tang, F.; et al. Rapid and Accurate Detection of Escherichia coli O157:H7 in Beef Using Microfluidic Wax-Printed Paper-Based ELISA. Analyst 2020, 145, 3106–3115. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Q.; Li, C.; Zhang, F.; Gu, H.; Wang, X.; Li, S.; Xue, L.; Madl, T.; Zhang, Y.; et al. Wide-Range, Rapid, and Specific Identification of Pathogenic Bacteria by Surface-Enhanced Raman Spectroscopy. ACS Sens. 2021, 6, 2911–2919. [Google Scholar] [CrossRef]
- Bu, T.; Jia, P.; Liu, J.; Liu, Y.; Sun, X.; Zhang, M.; Tian, Y.; Zhang, D.; Wang, J.; Wang, L. Diversely Positive-Charged Gold Nanoparticles Based Biosensor: A Label-Free and Sensitive Tool for Foodborne Pathogen Detection. Food Chem. X 2019, 3, 100052. [Google Scholar] [CrossRef]
- DeMone, C.; Hwang, M.-H.; Feng, Z.; McClure, J.T.; Greenwood, S.J.; Fung, R.; Kim, M.; Weese, J.S.; Shapiro, K. Application of next Generation Sequencing for Detection of Protozoan Pathogens in Shellfish. Food Waterborne Parasitol. 2020, 21, e00096. [Google Scholar] [CrossRef]
- Eyre, D.W. Infection Prevention and Control Insights from a Decade of Pathogen Whole-Genome Sequencing. J. Hosp. Infect. 2022, 122, 180–186. [Google Scholar] [CrossRef]
- Dąbrowiecki, Z.; Dąbrowiecka, M.; Olszański, R.; Siermontowski, P. Developing a Methodology for Testing and Preliminary Determination of the Presence of and in Environmental Water Samples by Immunomagnetic Separation Combined with Flow Cytometry. Pol. Hyperb. Res. 2019, 68, 71–92. [Google Scholar] [CrossRef]
- Bulard, E.; Bouchet-Spinelli, A.; Chaud, P.; Roget, A.; Calemczuk, R.; Fort, S.; Livache, T. Carbohydrates as New Probes for the Identification of Closely Related Escherichia coli Strains Using Surface Plasmon Resonance Imaging. Available online: https://pubs.acs.org/doi/pdf/10.1021/ac5037704 (accessed on 26 April 2023).
- Ahmed, S.; Ansari, A.; Siddiqui, M.A.; Imran, M.; Kumari, B.; Khan, A.; Ranjan, P. Electrochemical and Optical-Based Systems for SARS-CoV-2 and Various Pathogens Assessment. Adv. Nat. Sci. Nanosci. Nanotechnol. 2023, 14, 033001. [Google Scholar] [CrossRef]
- Losada-Garcia, N.; Garcia-Sanz, C.; Andreu, A.; Velasco-Torrijos, T.; Palomo, J.M. Glyconanomaterials for Human Virus Detection and Inhibition. Nanomaterials 2021, 11, 1684. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Liang, X.; Liu, J.; Zhao, T.-Y.; Li, X.; Zhang, Y.; Guo, G.; Zhang, Z.; Zeng, J. An Achromatic Colorimetric Nanosensor for Sensitive Multiple Pathogen Detection by Coupling Plasmonic Nanoparticles with Magnetic Separation. Talanta 2023, 256, 124271. [Google Scholar] [CrossRef]
- Jain, S.; Nehra, M.; Kumar, R.; Dilbaghi, N.; Hu, T.; Kumar, S.; Kaushik, A.; Li, C. Internet of Medical Things (IoMT)-Integrated Biosensors for Point-of-Care Testing of Infectious Diseases. Biosens. Bioelectron. 2021, 179, 113074. [Google Scholar] [CrossRef]
- Salama, A.M.; Yasin, G.; Zourob, M.; Lu, J. Fluorescent Biosensors for the Detection of Viruses Using Graphene and Two-Dimensional Carbon Nanomaterials. Biosensors 2022, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Nate, Z.; Gill, A.A.S.; Chauhan, R.; Karpoormath, R. Recent Progress in Electrochemical Sensors for Detection and Quantification of Malaria. Anal. Biochem. 2022, 643, 114592. [Google Scholar] [CrossRef] [PubMed]
- Nnachi, R.C.; Sui, N.; Ke, B.; Luo, Z.; Bhalla, N.; He, D.; Yang, Z. Biosensors for Rapid Detection of Bacterial Pathogens in Water, Food and Environment. Environ. Int. 2022, 166, 107357. [Google Scholar] [CrossRef]
- Sharifi, S.; Vahed, S.Z.; Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Khalilov, R.; Ahmadi, M.; Hamidi-Asl, E.; Labib, M. Detection of Pathogenic Bacteria via Nanomaterials-Modified Aptasensors. Biosens. Bioelectron. 2020, 150, 111933. [Google Scholar] [CrossRef]
- Hegde, M.; Pai, P.; Shetty, M.G.; Babitha, K.S. Gold Nanoparticle Based Biosensors for Rapid Pathogen Detection: A Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100756. [Google Scholar] [CrossRef]
- Sadanandan, S.; Meenakshi, V.S.; Ramkumar, K.; Pillai, N.P.; Anuvinda, P.; Sreelekshmi, P.J.; Devika, V.; Ramanunni, K.; Jeevan Sankar, R.; Sreejaya, M.M. Biorecognition Elements Appended Gold Nanoparticle Biosensors for the Detection of Food-Borne Pathogens—A Review. Food Control 2023, 148, 109510. [Google Scholar] [CrossRef]
- Cho, I.-H.; Ku, S. Current Technical Approaches for the Early Detection of Foodborne Pathogens: Challenges and Opportunities. IJMS 2017, 18, 2078. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, L.; Huang, Q.; Tong, T.; Zhou, Y.; Li, Z.; Bai, Q.; Liang, H.; Chen, L. Recent Advances on Aptamer-Based Biosensors for Detection of Pathogenic Bacteria. World J. Microbiol. Biotechnol. 2021, 37, 45. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for Drug Delivery Applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Ghorbani, F.; Abbaszadeh, H.; Mehdizadeh, A.; Ebrahimi-Warkiani, M.; Rashidi, M.-R.; Yousefi, M. Biosensors and Nanobiosensors for Rapid Detection of Autoimmune Diseases: A Review. Microchim. Acta 2019, 186, 838. [Google Scholar] [CrossRef]
- Ali, A.A.; Altemimi, A.B.; Alhelfi, N.; Ibrahim, S.A. Application of Biosensors for Detection of Pathogenic Food Bacteria: A Review. Biosensors 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B. Nanobiosensor Approaches for Pollutant Monitoring. Environ. Chem. Lett. 2019, 17, 975–990. [Google Scholar] [CrossRef]
- Chandra, P.; Prakash, R. (Eds.) Nanobiomaterial Engineering: Concepts and Their Applications in Biomedicine and Diagnostics; Springer: Singapore, 2020; ISBN 978-981-329-839-2. [Google Scholar]
- Fracchiolla, N.; Artuso, S.; Cortelezzi, A. Biosensors in Clinical Practice: Focus on Oncohematology. Sensors 2013, 13, 6423–6447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, J.; Du, X. Electrochemical Biosensors for Detection of Foodborne Pathogens. Micromachines 2019, 10, 222. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Tuteja, S.K.; Mutreja, R.; Neethirajan, S.; Ingebrandt, S. Bioconjugation of Different Nanosurfaces With Biorecognition Molecules for the Development of Selective Nanosensor Platforms. In Advances in Nanosensors for Biological and Environmental Analysis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–94. ISBN 978-0-12-817456-2. [Google Scholar]
- Zhao, X.; Smith, G.; Javed, B.; Dee, G.; Gun’ko, Y.K.; Curtin, J.; Byrne, H.J.; O’Connor, C.; Tian, F. Design and Development of Magnetic Iron Core Gold Nanoparticle-Based Fluorescent Multiplex Assay to Detect Salmonella. Nanomaterials 2022, 12, 3917. [Google Scholar] [CrossRef]
- Verma, M.L.; Rani, V. Biosensors for Toxic Metals, Polychlorinated Biphenyls, Biological Oxygen Demand, Endocrine Disruptors, Hormones, Dioxin, Phenolic and Organophosphorus Compounds: A Review. Environ. Chem. Lett. 2021, 19, 1657–1666. [Google Scholar] [CrossRef]
- de Morais Mirres, A.C.; da Silva, B.E.P.d.M.; Tessaro, L.; Galvan, D.; de Andrade, J.C.; Aquino, A.; Joshi, N.; Conte-Junior, C.A. Recent Advances in Nanomaterial-Based Biosensors for Pesticide Detection in Foods. Biosensors 2022, 12, 572. [Google Scholar] [CrossRef]
- Munawar, A.; Ong, Y.; Schirhagl, R.; Tahir, M.A.; Khan, W.S.; Bajwa, S.Z. Nanosensors for Diagnosis with Optical, Electric and Mechanical Transducers. RSC Adv. 2019, 9, 6793–6803. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Rab, S.; Suman, R. Exploring the Potential of Nanosensors: A Brief Overview. Sens. Int. 2021, 2, 100130. [Google Scholar] [CrossRef]
- Irkham, I.; Ibrahim, A.U.; Pwavodi, P.C.; Al-Turjman, F.; Hartati, Y.W. Smart Graphene-Based Electrochemical Nanobiosensor for Clinical Diagnosis: Review. Sensors 2023, 23, 2240. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Yang, M.; Hao, J. Pathogenic Virus Detection by Optical Nanobiosensors. Cell Rep. Phys. Sci. 2021, 2, 100288. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Saleh Ibrahim, Y.; Alexis Ramírez-Coronel, A.; Kumar Sain, D.; Haleem Al-qaim, Z.; Hassan Jawhar, Z.; Yaseen Mahmood Alabdali, A.; Hayif Jasim Ali, S.; Althomali, R.H.; Fakri Mustafa, Y.; Mireya Romero-Parra, R. Advances in Nanomaterials-Based Chemiluminescence (Bio)Sensor for Specific and Sensitive Determination of Pathogenic Bacteria. Microchem. J. 2023, 191, 108860. [Google Scholar] [CrossRef]
- Selvolini, G.; Marrazza, G. MIP-Based Sensors: Promising New Tools for Cancer Biomarker Determination. Sensors 2017, 17, 718. [Google Scholar] [CrossRef]
- Hroncekova, S.; Lorencova, L.; Bertok, T.; Hires, M.; Jane, E.; Bučko, M.; Kasak, P.; Tkac, J. Amperometric Miniaturised Portable Enzymatic Nanobiosensor for the Ultrasensitive Analysis of a Prostate Cancer Biomarker. JFB 2023, 14, 161. [Google Scholar] [CrossRef]
- Farrokhnia, M.; Amoabediny, G.; Ebrahimi, M.; Ganjali, M.; Arjmand, M. Ultrasensitive Early Detection of Insulin Antibody Employing Novel Electrochemical Nano-Biosensor Based on Controllable Electro-Fabrication Process. Talanta 2022, 238, 122947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mazouzi, Y.; Salmain, M.; Liedberg, B.; Boujday, S. Antibody-Gold Nanoparticle Bioconjugates for Biosensors: Synthesis, Characterization and Selected Applications. Biosens. Bioelectron. 2020, 165, 112370. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Choi, M.Y.; Choi, J.-H. Recent Development in Plasmonic Nanobiosensors for Viral DNA/RNA Biomarkers. Biosensors 2022, 12, 1121. [Google Scholar] [CrossRef]
- Rawat, N.K.; Ghosh, R. Chapter 8—Conducting Polymer–Based Nanobiosensors. In Nanosensors for Smart Cities; Han, B., Tomer, V.K., Nguyen, T.A., Farmani, A., Kumar Singh, P., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 129–142. ISBN 978-0-12-819870-4. [Google Scholar]
- Fang, R.H.; Jiang, Y.; Fang, J.C.; Zhang, L. Cell Membrane-Derived Nanomaterials for Biomedical Applications. Biomaterials 2017, 128, 69–83. [Google Scholar] [CrossRef]
- Aliakbar Ahovan, Z.; Hashemi, A.; De Plano, L.M.; Gholipourmalekabadi, M.; Seifalian, A. Bacteriophage Based Biosensors: Trends, Outcomes and Challenges. Nanomaterials 2020, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Solaimuthu, A.; Vijayan, A.N.; Murali, P.; Korrapati, P.S. Nano-Biosensors and Their Relevance in Tissue Engineering. Curr. Opin. Biomed. Eng. 2020, 13, 84–93. [Google Scholar] [CrossRef]
- Negahdary, M.; Angnes, L. Electrochemical Nanobiosensors Equipped with Peptides: A Review. Microchim. Acta 2022, 189, 94. [Google Scholar] [CrossRef]
- Tsao, Y.-H.; Husain, R.A.; Lin, Y.-J.; Khan, I.; Chen, S.-W.; Lin, Z.-H. A Self-Powered Mercury Ion Nanosensor Based on the Thermoelectric Effect and Chemical Transformation Mechanism. Nano Energy 2019, 62, 268–274. [Google Scholar] [CrossRef]
- Yu, R.; Niu, S.; Pan, C.; Wang, Z.L. Piezotronic Effect Enhanced Performance of Schottky-Contacted Optical, Gas, Chemical and Biological Nanosensors. Nano Energy 2015, 14, 312–339. [Google Scholar] [CrossRef]
- Tavakolian, M.; Jafari, S.M.; Van De Ven, T.G.M. A Review on Surface-Functionalized Cellulosic Nanostructures as Biocompatible Antibacterial Materials. Nano-Micro Lett. 2020, 12, 73. [Google Scholar] [CrossRef]
- Borse, V.B.; Konwar, A.N.; Jayant, R.D.; Patil, P.O. Perspectives of Characterization and Bioconjugation of Gold Nanoparticles and Their Application in Lateral Flow Immunosensing. Drug Deliv. Transl. Res. 2020, 10, 878–902. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Fratila, R.M.; Mitchell, S.G.; Del Pino, P.; Grazu, V.; De La Fuente, J.M. Strategies for the Biofunctionalization of Gold and Iron Oxide Nanoparticles. Langmuir 2014, 30, 15057–15071. [Google Scholar] [CrossRef]
- Wang, D.-X.; Wang, J.; Wang, Y.-X.; Du, Y.-C.; Huang, Y.; Tang, A.-N.; Cui, Y.-X.; Kong, D.-M. DNA Nanostructure-Based Nucleic Acid Probes: Construction and Biological Applications. Chem. Sci. 2021, 12, 7602–7622. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Tan, Y.N. Bioconjugation of Different Nanosurfaces With Biorecognition Molecules for the Development of Selective Nanosensor Platforms. Chem. Asian J. 2020, 15, 3180–3208. [Google Scholar] [CrossRef]
- Valenzuela-Amaro, H.M.; Vázquez Ortega, P.G.; Zazueta-Alvarez, D.E.; López-Miranda, J.; Rojas-Contreras, J.A. Síntesis verde de nanopartículas de magnetita (NPs-Fe3O4): Factores y limitaciones. Mundo Nano Rev. Interdiscip. Nanociencias Nanotechnol. 2022, 16, 1e–18e. [Google Scholar] [CrossRef]
- Carnerero, J.M.; Jimenez-Ruiz, A.; Castillo, P.M.; Prado-Gotor, R. Covalent and Non-Covalent DNA–Gold-Nanoparticle Interactions: New Avenues of Research. ChemPhysChem 2017, 18, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Greca, L.G.; Lehtonen, J.; Tardy, B.L.; Guo, J.; Rojas, O.J. Biofabrication of Multifunctional Nanocellulosic 3D Structures: A Facile and Customizable Route. Mater. Horiz. 2018, 5, 408–415. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Rahmawati, I.; Einaga, Y.; Ivandini, T.A.; Fiorani, A. Enzymatic Biosensors with Electrochemiluminescence Transduction. ChemElectroChem 2022, 9, e202200175. [Google Scholar] [CrossRef]
- McVey, C.; Huang, F.; Elliott, C.; Cao, C. Endonuclease Controlled Aggregation of Gold Nanoparticles for the Ultrasensitive Detection of Pathogenic Bacterial DNA. Biosens. Bioelectron. 2017, 92, 502–508. [Google Scholar] [CrossRef]
- Zamora-Gálvez, A.; Morales-Narváez, E.; Mayorga-Martinez, C.C.; Merkoçi, A. Nanomaterials Connected to Antibodies and Molecularly Imprinted Polymers as Bio/Receptors for Bio/Sensor Applications. Appl. Mater. Today 2017, 9, 387–401. [Google Scholar] [CrossRef]
- Jannetto, P.J.; Buchan, B.W.; Vaughan, K.A.; Ledford, J.S.; Anderson, D.K.; Henley, D.C.; Quigley, N.B.; Ledeboer, N.A. Real-Time Detection of Influenza A, Influenza B, and Respiratory Syncytial Virus A and B in Respiratory Specimens by Use of Nanoparticle Probes. J. Clin. Microbiol. 2010, 48, 3997–4002. [Google Scholar] [CrossRef]
- Karthik, V.; Senthil Kumar, P.; Vo, D.-V.N.; Selvakumar, P.; Gokulakrishnan, M.; Keerthana, P.; Audilakshmi, V.; Jeyanthi, J. Enzyme-Loaded Nanoparticles for the Degradation of Wastewater Contaminants: A Review. Environ. Chem. Lett. 2021, 19, 2331–2350. [Google Scholar] [CrossRef]
- Banakar, M.; Hamidi, M.; Khurshid, Z.; Zafar, M.S.; Sapkota, J.; Azizian, R.; Rokaya, D. Electrochemical Biosensors for Pathogen Detection: An Updated Review. Biosensors 2022, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- McKeague, M.; DeRosa, M.C. Challenges and Opportunities for Small Molecule Aptamer Development. J. Nucleic Acids 2012, 2012, 1–20. [Google Scholar] [CrossRef]
- Chavda, V.P.; Balar, P.C.; Teli, D.; Davidson, M.; Bojarska, J.; Apostolopoulos, V. Antibody–Biopolymer Conjugates in Oncology: A Review. Molecules 2023, 28, 2605. [Google Scholar] [CrossRef]
- Majdi, H.; Salehi, R.; Pourhassan-Moghaddam, M.; Mahmoodi, S.; Poursalehi, Z.; Vasilescu, S. Antibody Conjugated Green Synthesized Chitosan-Gold Nanoparticles for Optical Biosensing. Colloid Interface Sci. Commun. 2019, 33, 100207. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing Nanoparticles with Cancer-Targeting Antibodies: A Comparison of Strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Lara, S.; Perez-Potti, A. Applications of Nanomaterials for Immunosensing. Biosensors 2018, 8, 104. [Google Scholar] [CrossRef]
- Guo, R.; Huang, F.; Cai, G.; Zheng, L.; Xue, L.; Li, Y.; Liao, M.; Wang, M.; Lin, J. A Colorimetric Immunosensor for Determination of Foodborne Bacteria Using Rotating Immunomagnetic Separation, Gold Nanorod Indication, and Click Chemistry Amplification. Microchim. Acta 2020, 187, 197. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; Mashinchian, O.; Ayoubi, B.; Jamali, A.A.; Mobed, A.; Losic, D.; Omidi, Y.; De La Guardia, M. Optical and Electrochemical DNA Nanobiosensors. TrAC Trends Anal. Chem. 2011, 30, 459–472. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, J.; Li, D.; Wang, R. DNA-Based Biosensors for the Biochemical Analysis: A Review. Biosensors 2022, 12, 183. [Google Scholar] [CrossRef]
- Ma, X.; Ding, W.; Wang, C.; Wu, H.; Tian, X.; Lyu, M.; Wang, S. DNAzyme Biosensors for the Detection of Pathogenic Bacteria. Sens. Actuators B Chem. 2021, 331, 129422. [Google Scholar] [CrossRef]
- Ahmed, M.; Patel, R. Electrochemical/Voltammetric/Amperometric Nanosensors for the Detection of Pathogenic Bacteria. In Nanosensors for Point-of-Care Diagnostics of Pathogenic Bacteria; Acharya, A., Singhal, N.K., Eds.; Springer Nature: Singapore, 2023; pp. 113–141. ISBN 978-981-9912-18-6. [Google Scholar]
- Wu, Q.; Zhang, Y.; Yang, Q.; Yuan, N.; Zhang, W. Review of Electrochemical DNA Biosensors for Detecting Food Borne Pathogens. Sensors 2019, 19, 4916. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, R.; Bellassai, N.; Jungbluth, V.; Spoto, G. Recent Advances in Antifouling Materials for Surface Plasmon Resonance Biosensing in Clinical Diagnostics and Food Safety. Polymers 2021, 13, 1929. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Bhardwaj, S.K.; Nayak, M.K.; Mehta, J.; Kim, K.-H.; Deep, A. Fluorescent Nanobiosensors for the Targeted Detection of Foodborne Bacteria. TrAC Trends Anal. Chem. 2017, 97, 120–135. [Google Scholar] [CrossRef]
- Leng, X.; Wang, Y.; Li, R.; Liu, S.; Yao, J.; Pei, Q.; Cui, X.; Tu, Y.; Tang, D.; Huang, J. Circular Exponential Amplification of Photoinduced Electron Transfer Using Hairpin Probes, G-Quadruplex DNAzyme and Silver Nanocluster-Labeled DNA for Ultrasensitive Fluorometric Determination of Pathogenic Bacteria. Microchim. Acta 2018, 185, 168. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Zhang, S.; Deng, L. An Ultrasensitive Fluorescence Detection Template of Pathogenic Bacteria Based on Dual Catalytic Hairpin DNA Walker@Gold Nanoparticles Enzyme-Free Amplification. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 277, 121259. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, R.; AlZabn, R.; Abu-Salah, K.M.; Zourob, M. An Aptamer Based Fluorometric Microcystin-LR Assay Using DNA Strand-Based Competitive Displacement. Microchim. Acta 2019, 186, 435. [Google Scholar] [CrossRef]
- Tessaro, L.; Aquino, A.; De Almeida Rodrigues, P.; Joshi, N.; Ferrari, R.G.; Conte-Junior, C.A. Nucleic Acid-Based Nanobiosensor (NAB) Used for Salmonella Detection in Foods: A Systematic Review. Nanomaterials 2022, 12, 821. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Sorboni, S.G.; Haghighi, D.M.; Ahmadi, F.; Dehghani, Z.; Badiei, A. New Analytical Methods Using Carbon-Based Nanomaterials for Detection of Salmonella Species as a Major Food Poisoning Organism in Water and Soil Resources. Chemosphere 2022, 287, 132243. [Google Scholar] [CrossRef]
- Cui, F.; Sun, J.; De Dieu Habimana, J.; Yang, X.; Ji, J.; Zhang, Y.; Lei, H.; Li, Z.; Zheng, J.; Fan, M.; et al. Ultrasensitive Fluorometric Angling Determination of Staphylococcus Aureus in Vitro and Fluorescence Imaging in Vivo Using Carbon Dots with Full-Color Emission. Anal. Chem. 2019, 91, 14681–14690. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kumar, A.; Kumar, S.; Pinnaka, A.K.; Singhal, N.K. Naked Eye Colorimetric Detection of Escherichia coli Using Aptamer Conjugated Graphene Oxide Enclosed Gold Nanoparticles. Sens. Actuators B Chem. 2021, 329, 129100. [Google Scholar] [CrossRef]
- Pandit, C.; Alajangi, H.K.; Singh, J.; Khajuria, A.; Sharma, A.; Hassan, M.S.; Parida, M.; Semwal, A.D.; Gopalan, N.; Sharma, R.K.; et al. Development of Magnetic Nanoparticle Assisted Aptamer-Quantum Dot Based Biosensor for the Detection of Escherichia coli in Water Samples. Sci. Total Environ. 2022, 831, 154857. [Google Scholar] [CrossRef] [PubMed]
- Sande, M.G.; Rodrigues, J.L.; Ferreira, D.; Silva, C.J.; Rodrigues, L.R. Novel Biorecognition Elements against Pathogens in the Design of State-of-the-Art Diagnostics. Biosensors 2021, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef]
- Feyziazar, M.; Amini, M.; Jahanban-Esfahlan, A.; Baradaran, B.; Oroojalian, F.; Kamrani, A.; Mokhtarzadeh, A.; Soleymani, J.; De La Guardia, M. Recent Advances on the Piezoelectric, Electrochemical, and Optical Biosensors for the Detection of Protozoan Pathogens. TrAC Trends Anal. Chem. 2022, 157, 116803. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, J.; Yang, Q.; Wu, W. Quantum Dot Biosensor Combined with Antibody and Aptamer for Tracing Food-Borne Pathogens. Food Qual. Saf. 2021, 5, fyab019. [Google Scholar] [CrossRef]
- Ramajayam, K.; Ganesan, S.; Ramesh, P.; Beena, M.; Kokulnathan, T.; Palaniappan, A. Molecularly Imprinted Polymer-Based Biomimetic Systems for Sensing Environmental Contaminants, Biomarkers, and Bioimaging Applications. Biomimetics 2023, 8, 245. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, X. Preparation and Application of Molecularly Imprinted Polymers for Flavonoids: Review and Perspective. Molecules 2022, 27, 7355. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Lu, X. Molecular Imprinting Technology for Sensing Foodborne Pathogenic Bacteria. Anal. Bioanal. Chem. 2021, 413, 4581–4598. [Google Scholar] [CrossRef]
- Samardzic, R.; Sussitz, H.F.; Jongkon, N.; Lieberzeit, P.A. Quartz Crystal Microbalance In-Line Sensing of Escherichia coli in a Bioreactor Using Molecularly Imprinted Polymers. Available online: https://www.ingentaconnect.com/contentone/asp/senlet/2014/00000012/f0020006/art00040 (accessed on 12 September 2023).
- Zhao, X.; Cui, Y.; Wang, J.; Wang, J. Preparation of Fluorescent Molecularly Imprinted Polymers via Pickering Emulsion Interfaces and the Application for Visual Sensing Analysis of Listeria Monocytogenes. Polymers 2019, 11, 984. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Recent Advances in Airborne Pathogen Detection Using Optical and Electrochemical Biosensors. Anal. Chim. Acta 2022, 1234, 340297. [Google Scholar] [CrossRef]
- Tessaro, L.; Aquino, A.; Panzenhagen, P.; Ochioni, A.C.; Mutz, Y.S.; Raymundo-Pereira, P.A.; Vieira, I.R.S.; Belem, N.K.R.; Conte-Junior, C.A. Development and Application of an SPR Nanobiosensor Based on AuNPs for the Detection of SARS-CoV-2 on Food Surfaces. Biosensors 2022, 12, 1101. [Google Scholar] [CrossRef]
- Hu, J.; Fu, K.; Bohn, P.W. Whole-Cell Pseudomonas Aeruginosa Localized Surface Plasmon Resonance Aptasensor. Anal. Chem. 2018, 90, 2326–2332. [Google Scholar] [CrossRef]
- Dabhade, A.H.; Verma, R.P.; Paramasivan, B.; Kumawat, A.; Saha, B. Development of Silver Nanoparticles and Aptamer Conjugated Biosensor for Rapid Detection of E. coli in a Water Sample. 3 Biotech 2023, 13, 244. [Google Scholar] [CrossRef]
- Shahdost-Fard, F.; Faridfar, S.; Keihan, A.H.; Aghaei, M.; Petrenko, I.; Ahmadi, F.; Ehrlich, H.; Rahimi-Nasrabadi, M. Applicability of a Green Nanocomposite Consisted of Spongin Decorated Cu2WO4(OH)2 and AgNPs as a High-Performance Aptasensing Platform in Staphylococcus Aureus Detection. Biosensors 2023, 13, 271. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, R.; Choudhary, M.; Pinnaka, A.K.; Kumar, R.; Bhalla, V. Drug and Nanoparticle Mediated Rapid Naked Eye Water Test for Pathogens Detection. Sens. Actuators B Chem. 2018, 262, 603–610. [Google Scholar] [CrossRef]
- Altintas, Z.; Akgun, M.; Kokturk, G.; Uludag, Y. A Fully Automated Microfluidic-Based Electrochemical Sensor for Real-Time Bacteria Detection. Biosens. Bioelectron. 2018, 100, 541–548. [Google Scholar] [CrossRef]
- Nair, S.; Gomez-Cruz, J.; Manjarrez-Hernandez, Á.; Ascanio, G.; Sabat, R.G.; Escobedo, C. Selective Uropathogenic E. coli Detection Using Crossed Surface-Relief Gratings. Sensors 2018, 18, 3634. [Google Scholar] [CrossRef]
- Simoska, O.; Sans, M.; Fitzpatrick, M.D.; Crittenden, C.M.; Eberlin, L.S.; Shear, J.B.; Stevenson, K.J. Real-Time Electrochemical Detection of Pseudomonas Aeruginosa Phenazine Metabolites Using Transparent Carbon Ultramicroelectrode Arrays. ACS Sens. 2019, 4, 170–179. [Google Scholar] [CrossRef]
- Sun, R.; Zou, H.; Zhang, Y.; Zhang, X.; Chen, L.; Lv, R.; Sheng, R.; Du, T.; Li, Y.; Wang, H.; et al. Vancomycin Recognition and Induced-Aggregation of the Au Nanoparticles through Freeze-Thaw for Foodborne Pathogen Staphylococcus aureus Detection. Anal. Chim. Acta 2022, 1190, 339253. [Google Scholar] [CrossRef]
- Lim, S.H.; Ryu, Y.C.; Hwang, B.H. Aptamer-Immobilized Gold Nanoparticles Enable Facile and On-Site Detection of Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2021, 26, 107–113. [Google Scholar] [CrossRef]
- Yang, X.; Huang, R.; Xiong, L.; Chen, F.; Sun, W.; Yu, L. A Colorimetric Aptasensor for Ochratoxin A Detection Based on Tetramethylrhodamine Charge Effect-Assisted Silver Enhancement. Biosensors 2023, 13, 468. [Google Scholar] [CrossRef]
- Dester, E.; Kao, K.; Alocilja, E.C. Detection of Unamplified E. coli O157 DNA Extracted from Large Food Samples Using a Gold Nanoparticle Colorimetric Biosensor. Biosensors 2022, 12, 274. [Google Scholar] [CrossRef]
- Kaushal, S.; Pinnaka, A.K.; Soni, S.; Singhal, N.K. Antibody Assisted Graphene Oxide Coated Gold Nanoparticles for Rapid Bacterial Detection and near Infrared Light Enhanced Antibacterial Activity. Sens. Actuators B Chem. 2021, 329, 129141. [Google Scholar] [CrossRef]
- Abbaspour, A.; Norouz-Sarvestani, F.; Noori, A.; Soltani, N. Aptamer-Conjugated Silver Nanoparticles for Electrochemical Dual-Aptamer-Based Sandwich Detection of Staphylococcus aureus. Biosens. Bioelectron. 2015, 68, 149–155. [Google Scholar] [CrossRef]
- Imran, M.; Ehrhardt, C.J.; Bertino, M.F.; Shah, M.R.; Yadavalli, V.K. Chitosan Stabilized Silver Nanoparticles for the Electrochemical Detection of Lipopolysaccharide: A Facile Biosensing Approach for Gram-Negative Bacteria. Micromachines 2020, 11, 413. [Google Scholar] [CrossRef]
- Yin, M.; Liu, C.; Ge, R.; Fang, Y.; Wei, J.; Chen, X.; Chen, Q.; Chen, X. Paper-Supported near-Infrared-Light-Triggered Photoelectrochemical Platform for Monitoring Escherichia coli O157:H7 Based on Silver Nanoparticles-Sensitized-Upconversion Nanophosphors. Biosens. Bioelectron. 2022, 203, 114022. [Google Scholar] [CrossRef]
- Qaanei, M.; Taheri, R.A.; Eskandari, K. Electrochemical Aptasensor for Escherichia coli O157:H7 Bacteria Detection Using a Nanocomposite of Reduced Graphene Oxide, Gold Nanoparticles and Polyvinyl Alcohol. Anal. Methods 2021, 13, 3101–3109. [Google Scholar] [CrossRef]
- Ertaş, T.; Dinç, B.; Üstünsoy, R.; Eraslan, H.; Ergenç, A.F.; Bektaş, M. Novel Electrochemical Biosensor for Escherichia coli Using Gold-Coated Tungsten Wires and Antibody Functionalized Short Multiwalled Carbon Nanotubes. Instrum. Sci. Technol. 2023, 2, 1–16. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.; Wang, R.; Ji, Y.; Yue, T.; Sun, J.; Li, T.; Wang, J.; Zhang, D. Label-Free Strip Sensor Based on Surface Positively Charged Nitrogen-Rich Carbon Nanoparticles for Rapid Detection of Salmonella Enteritidis. Biosens. Bioelectron. 2019, 132, 360–367. [Google Scholar] [CrossRef]
- Kurt, H.; Yüce, M.; Hussain, B.; Budak, H. Dual-Excitation Upconverting Nanoparticle and Quantum Dot Aptasensor for Multiplexed Food Pathogen Detection. Biosens. Bioelectron. 2016, 81, 280–286. [Google Scholar] [CrossRef]
- Mathelié-Guinlet, M.; Cohen-Bouhacina, T.; Gammoudi, I.; Martin, A.; Béven, L.; Delville, M.-H.; Grauby-Heywang, C. Silica Nanoparticles-Assisted Electrochemical Biosensor for the Rapid, Sensitive and Specific Detection of Escherichia coli. Sens. Actuators B Chem. 2019, 292, 314–320. [Google Scholar] [CrossRef]
- Jenie, S.N.A.; Kusumastuti, Y.; Krismastuti, F.S.H.; Untoro, Y.M.; Dewi, R.T.; Udin, L.Z.; Artanti, N. Rapid Fluorescence Quenching Detection of Escherichia coli Using Natural Silica-Based Nanoparticles. Sensors 2021, 21, 881. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, D.-W.; Pu, H.; Wei, Q. A Dual Signal-on Biosensor Based on Dual-Gated Locked Mesoporous Silica Nanoparticles for the Detection of Aflatoxin B1. Talanta 2023, 253, 124027. [Google Scholar] [CrossRef]
- Boodoo, C.; Dester, E.; David, J.; Patel, V.; Kc, R.; Alocilja, E.C. Multi-Probe Nano-Genomic Biosensor to Detect S. aureus from Magnetically-Extracted Food Samples. Biosensors 2023, 13, 608. [Google Scholar] [CrossRef]
- Diouani, M.F.; Sayhi, M.; Djafar, Z.R.; Ben Jomaa, S.; Belgacem, K.; Gharbi, H.; Ghita, M.; Popescu, L.-M.; Piticescu, R.; Laouini, D. Magnetic Separation and Centri-Chronoamperometric Detection of Foodborne Bacteria Using Antibiotic-Coated Metallic Nanoparticles. Biosensors 2021, 11, 205. [Google Scholar] [CrossRef]
- Wen, J.; Ren, L.; He, Q.; Bao, J.; Zhang, X.; Pi, Z.; Chen, Y. Contamination-Free V-Shaped Ultrafast Reaction Cascade Transferase Signal Amplification Driven CRISPR/Cas12a Magnetic Relaxation Switching Biosensor for Bacteria Detection. Biosens. Bioelectron. 2023, 219, 114790. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Chowdhury, A.D.; Khoris, I.M.; Doong, R.; Li, T.-C.; Hara, T.; Abe, F.; Suzuki, T.; Park, E.Y. Hollow Magnetic-Fluorescent Nanoparticles for Dual-Modality Virus Detection. Biosens. Bioelectron. 2020, 170, 112680. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef]
- Liu, L.; Hong, J.; Wang, W.; Xiao, S.; Xie, H.; Wang, Q.; Gan, N. Fluorescent Aptasensor for Detection of Live Foodborne Pathogens Based on Multicolor Perovskite-Quantum-Dot-Encoded DNA Probes and Dual-Stirring-Bar-Assisted Signal Amplification. J. Pharm. Anal. 2022, 12, 913–922. [Google Scholar] [CrossRef]
- Shang, Y.; Cai, S.; Ye, Q.; Wu, Q.; Shao, Y.; Qu, X.; Xiang, X.; Zhou, B.; Ding, Y.; Chen, M.; et al. Quantum Dot Nanobeads-Labelled Lateral Flow Immunoassay Strip for Rapid and Sensitive Detection of Salmonella Typhimurium Based on Strand Displacement Loop-Mediated Isothermal Amplification. Engineering 2022, 19, 62–70. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Liu, T.; Zhang, Z.; Kong, J.; Yu, H.; Yan, J.; Luan, D.; Zhao, Y.; Bian, X. Reusable and Universal Impedimetric Sensing Platform for the Rapid and Sensitive Detection of Pathogenic Bacteria Based on Bacteria-Imprinted Polythiophene Film. Analyst 2022, 147, 4433–4441. [Google Scholar] [CrossRef]
- Alafeef, M.; Moitra, P.; Pan, D. Nano-Enabled Sensing Approaches for Pathogenic Bacterial Detection. Biosens. Bioelectron. 2020, 165, 112276. [Google Scholar] [CrossRef]
- Ayodhya, D. Recent Progress on Detection of Bivalent, Trivalent, and Hexavalent Toxic Heavy Metal Ions in Water Using Metallic Nanoparticles: A Review. Results Chem. 2023, 5, 100874. [Google Scholar] [CrossRef]
- Patel, R.; Mitra, B.; Vinchurkar, M.; Adami, A.; Patkar, R.; Giacomozzi, F.; Lorenzelli, L.; Baghini, M.S. A Review of Recent Advances in Plant-Pathogen Detection Systems. Heliyon 2022, 8, e11855. [Google Scholar] [CrossRef]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef]
- Ibrahim, N.; Jamaluddin, N.D.; Tan, L.L.; Mohd Yusof, N.Y. A Review on the Development of Gold and Silver Nanoparticles-Based Biosensor as a Detection Strategy of Emerging and Pathogenic RNA Virus. Sensors 2021, 21, 5114. [Google Scholar] [CrossRef]
- Yu, X.; Jiao, Y.; Chai, Q. Applications of Gold Nanoparticles in Biosensors. Nano LIFE 2016, 6, 1642001. [Google Scholar] [CrossRef]
- Baetsen-Young, A.M.; Vasher, M.; Matta, L.L.; Colgan, P.; Alocilja, E.C.; Day, B. Direct Colorimetric Detection of Unamplified Pathogen DNA by Dextrin-Capped Gold Nanoparticles. Biosens. Bioelectron. 2018, 101, 29–36. [Google Scholar] [CrossRef]
- Hui, C.; Hu, S.; Yang, X.; Guo, Y. A Panel of Visual Bacterial Biosensors for the Rapid Detection of Genotoxic and Oxidative Damage: A Proof of Concept Study. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2023, 888, 503639. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, J.-Z.; Rao, Z.-M.; Zhang, W.-G. An Enzymatic Colorimetric Whole-Cell Biosensor for High-Throughput Identification of Lysine Overproducers. Biosens. Bioelectron. 2022, 216, 114681. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.R.; Sampaio, I.; Zucolotto, V. Exploring Silver Nanoparticles for Cancer Therapy and Diagnosis. Colloids Surf. B Biointerfaces 2022, 210, 112254. [Google Scholar] [CrossRef] [PubMed]
- Douaki, A.; Demelash Abera, B.; Cantarella, G.; Shkodra, B.; Mushtaq, A.; Ibba, P.; Inam, A.S.; Petti, L.; Lugli, P. Flexible Screen Printed Aptasensor for Rapid Detection of Furaneol: A Comparison of CNTs and AgNPs Effect on Aptasensor Performance. Nanomaterials 2020, 10, 1167. [Google Scholar] [CrossRef]
- Li, G. Nano-Inspired Biosensors for Protein Assay with Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-815054-2. [Google Scholar]
- Varghese Alex, K.; Tamil Pavai, P.; Rugmini, R.; Shiva Prasad, M.; Kamakshi, K.; Sekhar, K.C. Green Synthesized Ag Nanoparticles for Bio-Sensing and Photocatalytic Applications. ACS Omega 2020, 5, 13123–13129. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Phung, V.-D.; Tran, V.V. Recent Advances in Conjugated Polymer-Based Biosensors for Virus Detection. Biosensors 2023, 13, 586. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, N.; Kumari, A.; Lee, H.-J.; Kim, T.; Tripathi, K.M. Nano-Carbon Based Sensors for Bacterial Detection and Discrimination in Clinical Diagnosis: A Junction between Material Science and Biology. Appl. Mater. Today 2020, 18, 100467. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological Interactions of Carbon-Based Nanomaterials: From Coronation to Degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef]
- Duan, N.; Wu, S.; Dai, S.; Miao, T.; Chen, J.; Wang, Z. Simultaneous Detection of Pathogenic Bacteria Using an Aptamer Based Biosensor and Dual Fluorescence Resonance Energy Transfer from Quantum Dots to Carbon Nanoparticles. Microchim. Acta 2015, 182, 917–923. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Gao, Z.F.; Ye, Y.; Wu, Q.; Chen, H.-Y.; Xu, J.-J. Recent Advances in Nanotechnology for Simultaneous Detection of Multiple Pathogenic Bacteria. Nano Today 2021, 38, 101121. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Y.; Zhu, L.; Liu, H.; Su, X.; Liu, Y.; Chen, Z.; Chen, H.; He, N. A Novel Cartridge for Nucleic Acid Extraction, Amplification and Detection of Infectious Disease Pathogens with the Help of Magnetic Nanoparticles. Chin. Chem. Lett. 2023, 34, 108092. [Google Scholar] [CrossRef]
- Hussain, C.M. Analytical Applications of Functionalized Magnetic Nanoparticles; Royal Society of Chemistry: London, UK, 2021; ISBN 978-1-83916-276-3. [Google Scholar]
- Huang, H.T.; Garu, P.; Li, C.H.; Chang, W.C.; Chen, B.W.; Sung, S.Y.; Lee, C.M.; Chen, J.Y.; Hsieh, T.F.; Sheu, W.J.; et al. Magnetoresistive Biosensors for Direct Detection of Magnetic Nanoparticle Conjugated Biomarkers on a Chip. SPIN 2019, 9, 1940002. [Google Scholar] [CrossRef]
- Min, C.; Shao, H.; Liong, M.; Yoon, T.-J.; Weissleder, R.; Lee, H. Mechanism of Magnetic Relaxation Switching Sensing. ACS Nano 2012, 6, 6821–6828. [Google Scholar] [CrossRef]
- Arya, S.; Singh, A.; Sharma, A.; Gupta, V. 11—Silicon-Based Biosensor. In Silicon-Based Hybrid Nanoparticles; Thomas, S., Nguyen, T.A., Ahmadi, M., Yasin, G., Joshi, N., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 247–267. ISBN 978-0-12-824007-6. [Google Scholar]
- Bagheri, E.; Ansari, L.; Sameiyan, E.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Sensors Design Based on Hybrid Gold-Silica Nanostructures. Biosens. Bioelectron. 2020, 153, 112054. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, H.; Gooch, J.; Frascione, N. Nanomaterials for Optical Biosensors in Forensic Analysis. Talanta 2023, 253, 123945. [Google Scholar] [CrossRef] [PubMed]
- Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of Silicon Dioxide (E 551) as a Food Additive. EFS2 2018, 16, e05088. [Google Scholar] [CrossRef]
- Şen Karaman, D.; Pamukçu, A.; Karakaplan, M.B.; Kocaoglu, O.; Rosenholm, J.M. Recent Advances in the Use of Mesoporous Silica Nanoparticles for the Diagnosis of Bacterial Infections. IJN 2021, 16, 6575–6591. [Google Scholar] [CrossRef]
- Wen, L.; Qiu, L.; Wu, Y.; Hu, X.; Zhang, X. Aptamer-Modified Semiconductor Quantum Dots for Biosensing Applications. Sensors 2017, 17, 1736. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, P.; Su, B. Electrochemiluminescence of Semiconductor Quantum Dots and Its Biosensing Applications: A Comprehensive Review. Biosensors 2023, 13, 708. [Google Scholar] [CrossRef]
- Ma, F.; Li, C.; Zhang, C. Development of Quantum Dot-Based Biosensors: Principles and Applications. J. Mater. Chem. B 2018, 6, 6173–6190. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Rajabzadeh-Khosroshahi, M.; Samadi, A.; Behzadmehr, R.; Rahdar, A.; Ferreira, L.F.R. Properties and Application of Carbon Quantum Dots (CQDs) in Biosensors for Disease Detection: A Comprehensive Review. J. Drug Deliv. Sci. Technol. 2023, 80, 104156. [Google Scholar] [CrossRef]
- Smith, R.P.; Barraza, I.; Quinn, R.J.; Fortoul, M.C. Chapter One—The Mechanisms and Cell Signaling Pathways of Programmed Cell Death in the Bacterial World. In Cell Death Regulation in Health and Disease—Part B; Spetz, J.K.E., Galluzzi, L., Eds.; International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2020; Volume 352, pp. 1–53. [Google Scholar]
- Hao, L.; Xue, L.; Huang, F.; Cai, G.; Qi, W.; Zhang, M.; Han, Q.; Wang, Z.; Lin, J. A Microfluidic Biosensor Based on Magnetic Nanoparticle Separation, Quantum Dots Labeling and MnO2 Nanoflower Amplification for Rapid and Sensitive Detection of Salmonella Typhimurium. Micromachines 2020, 11, 281. [Google Scholar] [CrossRef]
- Schmitz, F.R.W.; Valério, A.; De Oliveira, D.; Hotza, D. An Overview and Future Prospects on Aptamers for Food Safety. Appl. Microbiol. Biotechnol. 2020, 104, 6929–6939. [Google Scholar] [CrossRef] [PubMed]
- Pirzada, M.; Altintas, Z. Nanomaterials for Healthcare Biosensing Applications. Sensors 2019, 19, 5311. [Google Scholar] [CrossRef]
- Mukama, O.; Wu, J.; Li, Z.; Liang, Q.; Yi, Z.; Lu, X.; Liu, Y.; Liu, Y.; Hussain, M.; Makafe, G.G.; et al. An Ultrasensitive and Specific Point-of-Care CRISPR/Cas12 Based Lateral Flow Biosensor for the Rapid Detection of Nucleic Acids. Biosens. Bioelectron. 2020, 159, 112143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, G.; Qiu, J.; Zhou, D.; Gou, D.; Tao, Y.; Li, Y.; Chen, H. A New Biosensor Based on the Recognition of Phages and the Signal Amplification of Organic-Inorganic Hybrid Nanoflowers for Discriminating and Quantitating Live Pathogenic Bacteria in Urine. Sens. Actuators B Chem. 2018, 258, 803–812. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Wu, D.; Li, X.; Hu, N.; Chen, J.; Chen, G.; Wu, Y. Recent Progress in the Design Fabrication of Metal-Organic Frameworks-Based Nanozymes and Their Applications to Sensing and Cancer Therapy. Biosens. Bioelectron. 2019, 137, 178–198. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef]
- Echeverri, D.; Orozco, J. Glycan-Based Electrochemical Biosensors: Promising Tools for the Detection of Infectious Diseases and Cancer Biomarkers. Molecules 2022, 27, 8533. [Google Scholar] [CrossRef]
- Puchkova, A.; Vietz, C.; Pibiri, E.; Wünsch, B.; Sanz Paz, M.; Acuna, G.P.; Tinnefeld, P. DNA Origami Nanoantennas with over 5000-Fold Fluorescence Enhancement and Single-Molecule Detection at 25 μM. Nano Lett. 2015, 15, 8354–8359. [Google Scholar] [CrossRef]
- Chen, J.; Alcaine, S.D.; Jiang, Z.; Rotello, V.M.; Nugen, S.R. Detection of Escherichia coli in Drinking Water Using T7 Bacteriophage-Conjugated Magnetic Probe. Anal. Chem. 2015, 87, 8977–8984. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial Intelligence Biosensors: Challenges and Prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, R.; Dangi, A.K.; Kumar, A.; Chhabra, D.; Shukla, P. Futuristic Biosensors for Cardiac Health Care: An Artificial Intelligence Approach. 3 Biotech 2018, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Minami, S.; Ono, C.; Hamajima, R.; Morimura, A.; Hamaguchi, S.; Akeda, Y.; Kanai, Y.; Kobayashi, T.; Kamitani, W.; et al. Combining Machine Learning and Nanopore Construction Creates an Artificial Intelligence Nanopore for Coronavirus Detection. Nat. Commun. 2021, 12, 3726. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).