Bacteria-Infected Artificial Urine Characterization Based on a Combined Approach Using an Electronic Tongue Complemented with 1H-NMR and Flow Cytometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Bacterial Culture
2.3. Sensor Preparation
2.4. Electrochemical Measurements
2.5. 1H NMR Spectroscopy
2.6. Flow Cytometry
2.7. Data Treatment with Principal Component Analysis in SPSS
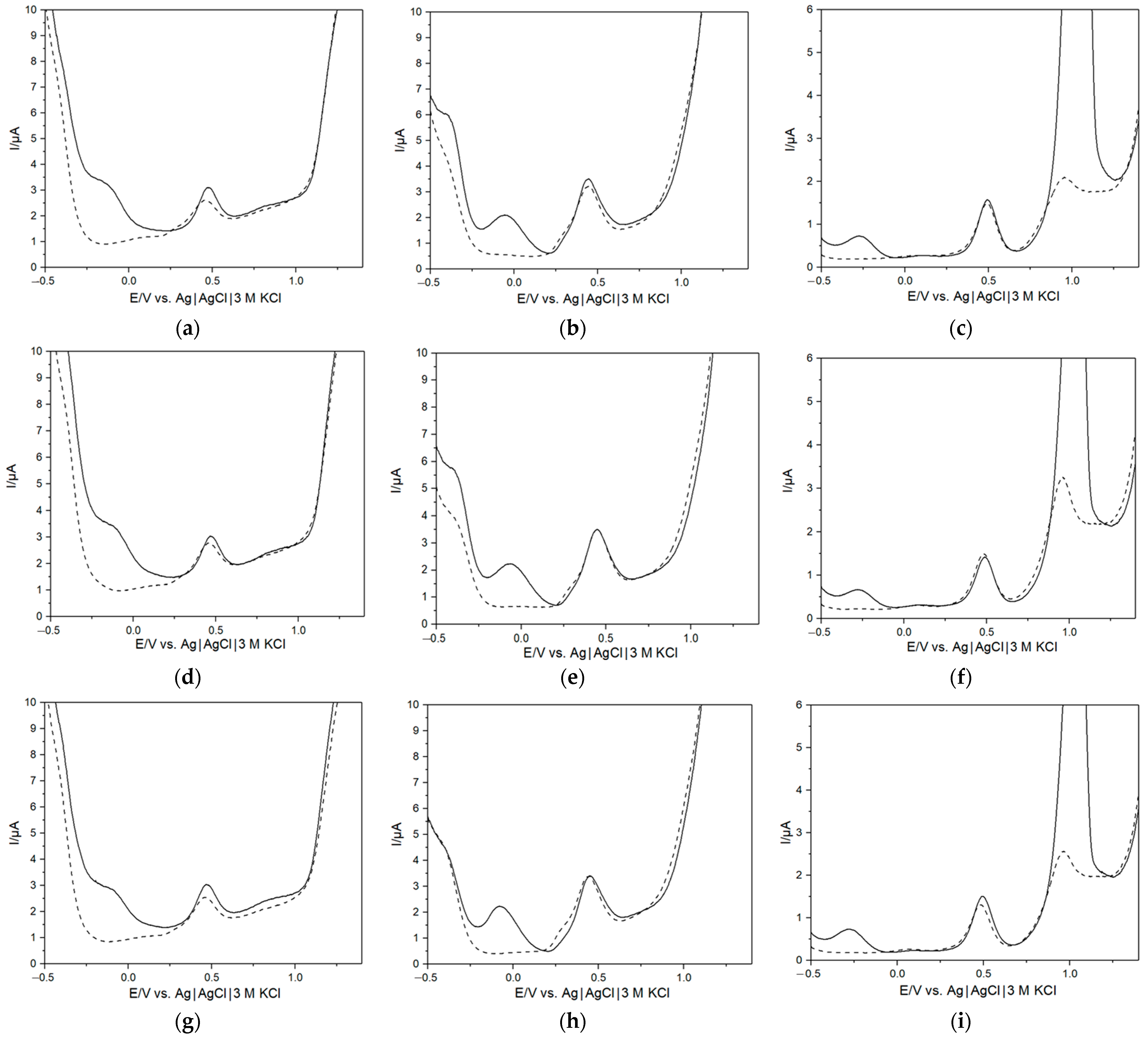
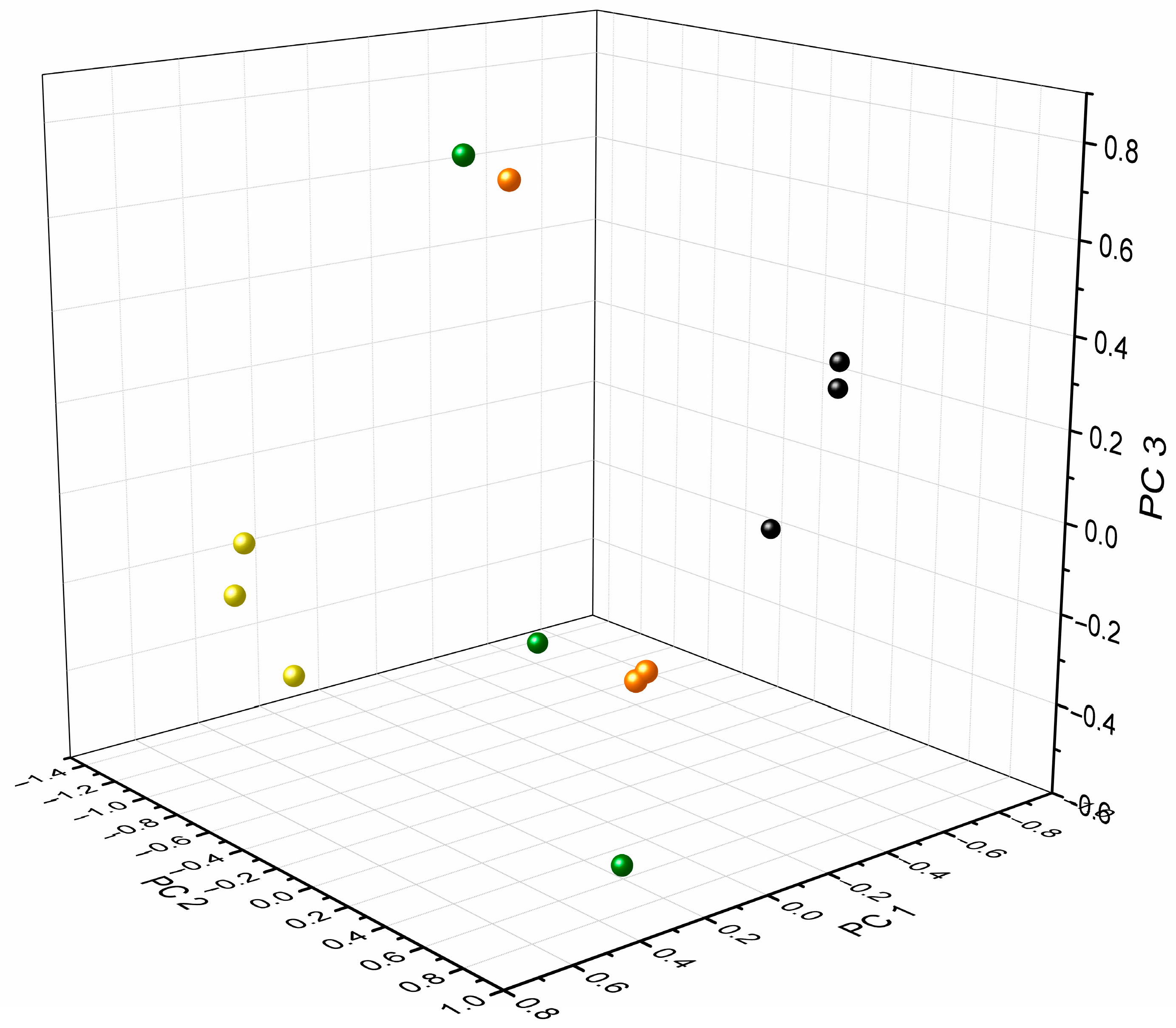
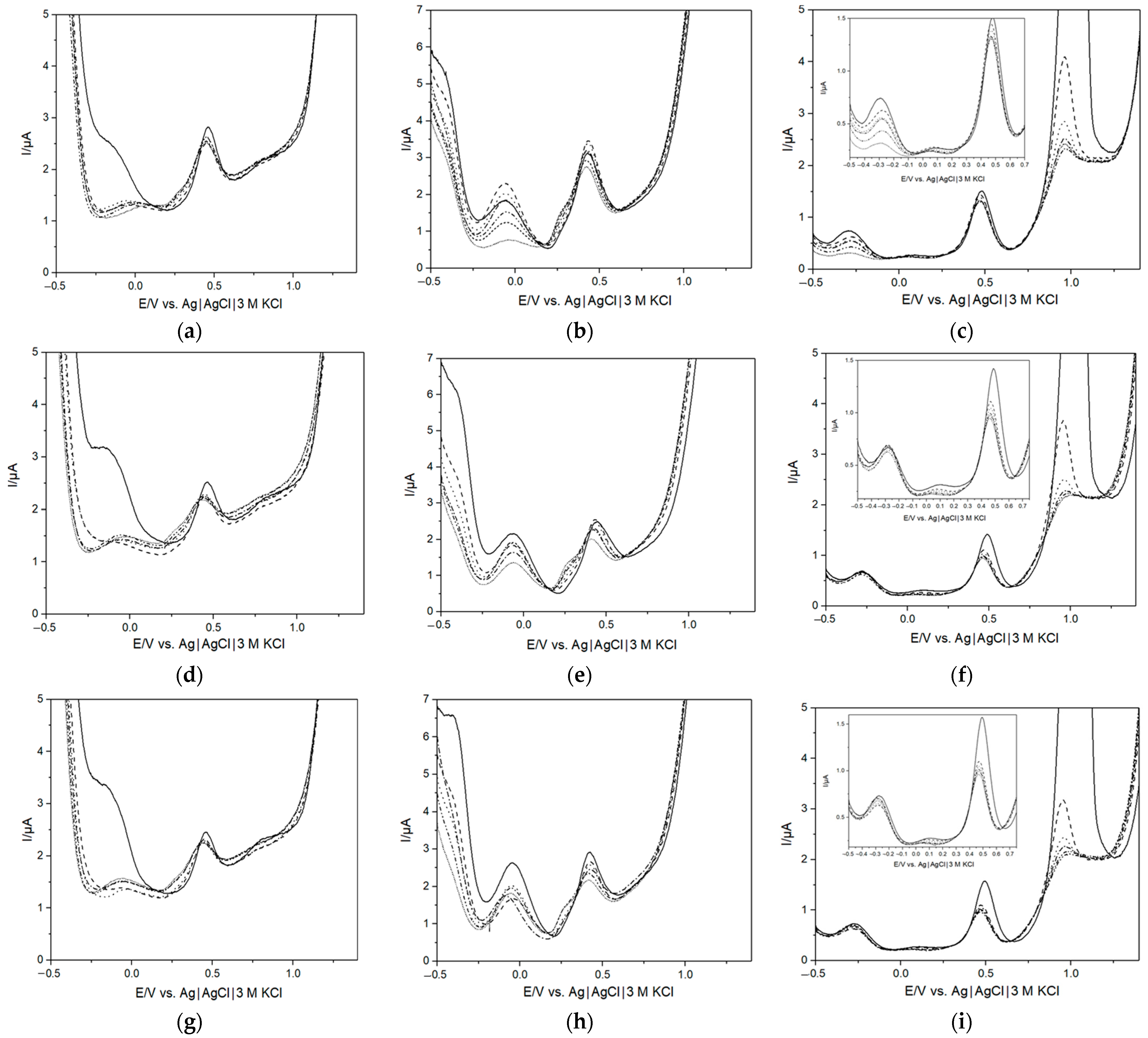
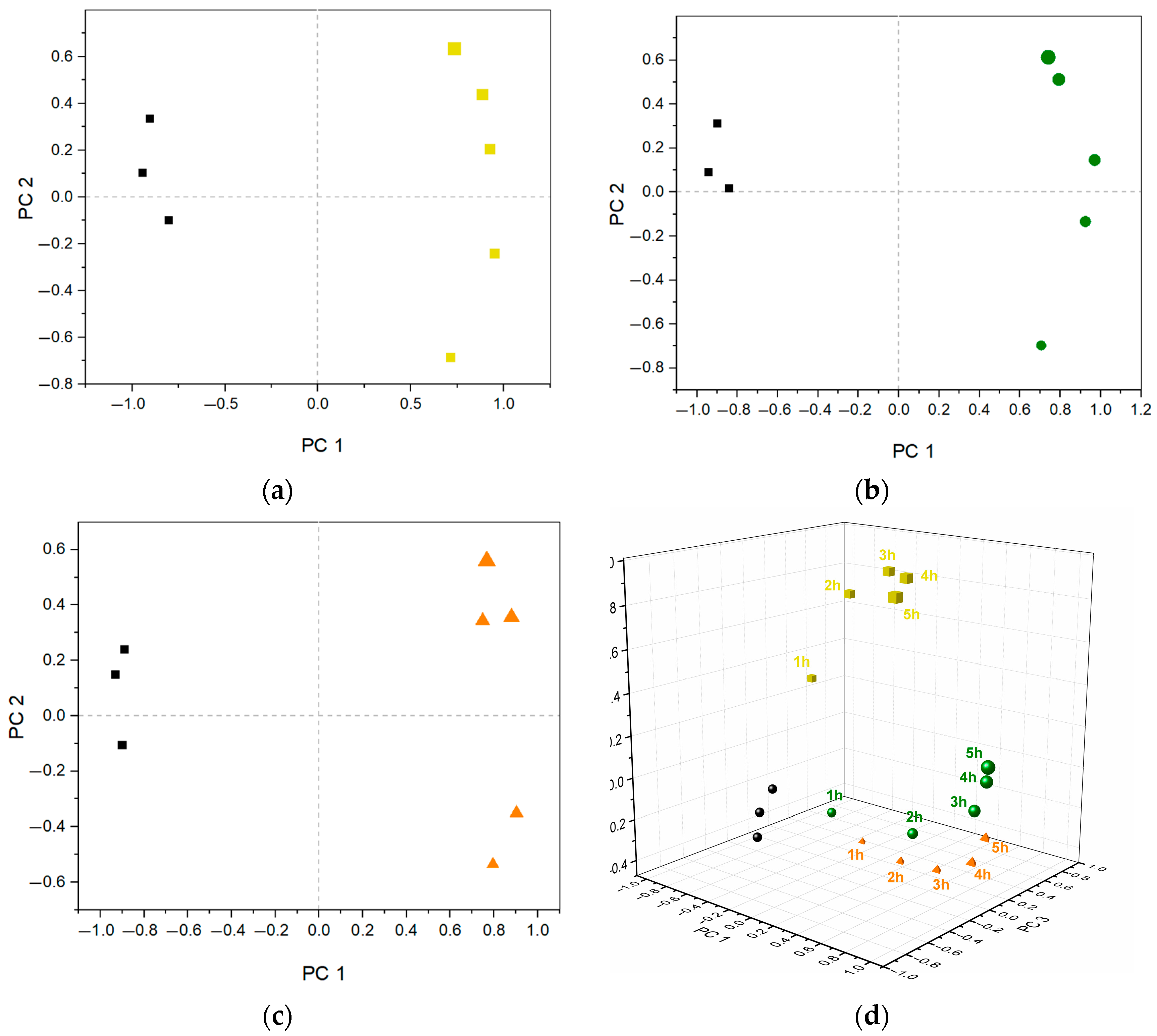
3. Results and Discussion
3.1. Charachterization of Bacterial Growth in AUM
3.2. Quantitative Analysis of Bacterial Growth
3.3. Investigation of Metabolic Changes in AUM Due to Bacterial Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grabe, M.; Bjerklund-Johansen, T.E.; Çek, M.; Naber, K.G.; Pickard, R.S.; Bartoletti, R.; Tenke, P.; Wagenlehner, F.; Wullt, B. Guidelines on Urological Infections; European Association of Urology: Arnhem, The Netherlands, 2015. [Google Scholar]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar]
- Wilson, M.L.; Gaido, L. Laboratory Diagnosis of Urinary Tract Infections in Adult Patients. Med. Microbiol. 2004, 38, 1150–1158. [Google Scholar]
- Alteri, C.J.; Mobley, H.L.T. Metabolism and Fitness of Urinary Tract Pathogens. Microbiol. Spectr. 2015, 3, 215–230. [Google Scholar] [CrossRef]
- Ipe, D.S.; Horton, E.; Ulett, G.C. The Basics of Bacteriuria: Strategies of Microbes for Persistence in Urine. Front. Cell. Infect. Microbiol. 2016, 6, 14. [Google Scholar]
- McLellan, L.K.; Hunstad, D.A. Urinary Tract Infection: Pathogenesis and Outlook. Trends Mol. Med. 2016, 22, 946–957. [Google Scholar]
- Smelov, V.; Naber, K.; Bjerklund Johansen, T.E. Improved Classification of Urinary Tract Infection: Future Considerations. Eur. Urol. Suppl. 2016, 15, 71–80. [Google Scholar]
- Lussu, M.; Camboni, T.; Piras, C.; Serra, C.; Del Carratore, F.; Griffin, J.; Atzori, L.; Manzin, A. (1)H NMR spectroscopy-based metabolomics analysis for the diagnosis of symptomatic E. coli-associated urinary tract infection (UTI). BMC Microbiol. 2017, 17, 201. [Google Scholar]
- Guernion, N.R.; Ratcliffe, N.M.; Spencer-Phillips, P.T.N.; Howe, R.A. Identifying Bacteria in Human Urine: Current Practice and the Potential for Rapid, Near-Patient Diagnosis by Sensing Volatile Organic Compounds. Clin. Chem. Lab. Med. 2001, 39, 893–906. [Google Scholar]
- Simoska, O.; Stevenson, K.J. Electrochemical sensors for rapid diagnosis of pathogens in real time. Analyst 2019, 144, 6461–6478. [Google Scholar]
- Mahoney, E.K.J.; Smieja, M.; Fang, Q. Review—Point-of-Care Urinalysis with Emerging Sensing and Imaging Technologies. J. Electrochem. Soc. 2019, 167, 037518. [Google Scholar]
- Monzo, J.; Insua, I.; Fernandez-Trillo, F.; Rodriguez, P. Fundamentals, achievements and challenges in the electrochemical sensing of pathogens. Analyst 2015, 140, 7116–7128. [Google Scholar]
- Mach, K.E.; Wong, P.K.; Liao, J.C. Biosensor diagnosis of urinary tract infections: A path to better treatment? Trends Pharmacol. Sci. 2011, 32, 330–336. [Google Scholar]
- Ahmed, A.R.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Critical overview on the application of sensors and biosensors for clinical analysis. Trends Anal. Chem. 2016, 85, 36–60. [Google Scholar]
- Cady, P.; Dufour, S.W.; Lawless, P. Impedimetric screening for bacteriuria. J. Clin. Microbiol. 1978, 7, 273–278. [Google Scholar]
- Lam, C.W.; Law, C.Y.; To, K.K.; Cheung, S.K.; Lee, K.; Sze, K.-H.; Leung, K.F.; Yuen, K.-Y. NMR-based metabolomic urinalysis: A rapid screening test for urinary tract infection. Clin. Chim. Acta 2014, 436, 217–223. [Google Scholar]
- Alteri, C.J.; Smith, S.N.; Mobley, H.L. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 2009, 5, e1000448. [Google Scholar]
- Reitzer, L.Z.P. Rapid Growth and Metabolism of Uropathogenic Escherichia coli in Relation to Urine Composition. Clin. Microbiol. Rev. 2019, 33, e00101–e00119. [Google Scholar]
- Mann, R.; Mediati, D.G.; Duggin, I.G.; Harry, E.J.; Bottomley, A.L. Metabolic Adaptations of Uropathogenic E. coli in the Urinary Tract. Front. Cell. Infect. Microbiol. 2017, 7, 241. [Google Scholar]
- Brodehl, J.; Oemar, B.S.; Hoyer, P.F. Renal glucosuria. Pediatr. Nephrol. 1987, 1, 502–508. [Google Scholar]
- Anfora, A.T.; Halladin, D.K.; Haugen, B.J.; Welch, R.A. Uropathogenic Escherichia coli CFT073 Is Adapted to Acetatogenic Growth but Does Not Require Acetate during Murine Urinary Tract Infection. Infect. Immun. 2008, 76, 5760–5767. [Google Scholar]
- Gupta, A.; Dwivedi, M.; Mahdi, A.A.; Khetrapal, C.L.; Bhandari, M. Broad Identification of Bacterial Type in Urinary Tract Infection Using 1H NMR Spectroscopy. J. Proteome Res. 2012, 11, 1844–1854. [Google Scholar] [PubMed]
- Karlsen, H.; Dong, T. Biomarkers of urinary tract infections: State of the art, and promising applications for rapid strip-based chemical sensors. Anal. Methods 2015, 7, 7961–7975. [Google Scholar]
- Goluch, E.D. Microbial Identification Using Electrochemical Detection of Metabolites. Trends Biotechnol. 2017, 35, 1125–1128. [Google Scholar] [PubMed]
- Holmin, S.; Björefors, F.; Eriksson, M.; Krantz-Rülcker, C.; Winquist, F. Investigation of Electrode Materials as Sensors in a Voltammetric Electronic Tongue. Electroanalysis 2002, 14, 839–847. [Google Scholar]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. Bioelectronic tongue: Current status and perspectives. Biosens. Bioelectron. 2020, 150, 111923. [Google Scholar]
- del Valle, M. Electronic Tongues Employing Electrochemical Sensors. Electroanalysis 2010, 22, 1539–1555. [Google Scholar]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Natale, C.D.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar]
- Winquist, F. Voltammetric electronic tongues—Basic principles and applications. Microchim. Acta 2008, 163, 3–10. [Google Scholar]
- Winquist, F.; Krantz-Rülcker, C.; Lundström, I. A Miniaturized Voltammetric Electronic Tongue. Anal. Lett. 2008, 41, 917–924. [Google Scholar]
- del Valle, M. Sensor Arrays and Electronic Tongue Systems. Int. J. Electrochem. 2012, 2012, 986025. [Google Scholar]
- Wold, S. Principal Component Analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar]
- Winquist, F.; Wide, P.; Lundström, I. An electronic tongue based on voltammetry. Anal. Chem. Acta 1997, 357, 21–31. [Google Scholar]
- Falk, M.; Psotta, C.; Cirovic, S.; Ohlsson, L.; Shleev, S. Electronic Tongue for Direct Assessment of SARS-CoV-2-Free and Infected Human Saliva-A Feasibility Study. Biosensors 2023, 13, 717. [Google Scholar]
- Braz, D.C.; Neto, M.P.; Shimizu, F.M.; Sá, A.C.; Lima, R.S.; Gobbi, A.L.; Melendez, M.E.; Arantes, L.M.R.B.; Carvalho, A.L.; Paulovich, F.V.; et al. Using machine learning and an electronic tongue for discriminating saliva samples from oral cavity cancer patients and healthy individuals. Talanta 2022, 243, 123327. [Google Scholar]
- Falk, M.; Nilsson, E.J.; Cirovic, S.; Tudosoiu, B.; Shleev, S. Wearable Electronic Tongue for Non-Invasive Assessment of Human Sweat. Sensors 2021, 21, 7311. [Google Scholar]
- Ciosek, P.; Grabowska, I.; Brzózka, Z.; Wróblewski, W. Analysis of dialysate fluids with the use of a potentiometric electronic tongue. Microchim. Acta 2008, 163, 139–145. [Google Scholar] [CrossRef]
- Gutierrez, M.; Alegret, S.; del Valle, M. Potentiometric bioelectronic tongue for the analysis of urea and alkaline ions in clinical samples. Biosens. Bioelectron. 2007, 22, 2171–2178. [Google Scholar] [PubMed]
- Yaroshenko, I.; Kirsanov, D.; Kartsova, L.; Sidorova, A.; Borisova, I.; Legin, A. Determination of urine ionic composition with potentiometric multisensor system. Talanta 2015, 131, 556–561. [Google Scholar] [PubMed]
- Zniber, M.; Vahdatiyekta, P.; Huynh, T.P. Analysis of urine using electronic tongue towards non-invasive cancer diagnosis. Biosens. Bioelectron. 2022, 219, 114810. [Google Scholar]
- Lvova, L.; Martinelli, E.; Dini, F.; Bergamini, A.; Paolesse, R.; Di Natale, C.; D’Amico, A. Clinical analysis of human urine by means of potentiometric electronic tongue. Talanta 2009, 77, 1097–1104. [Google Scholar] [PubMed]
- Brooks, T.; Keevil, C.W. A simple artificial urine for the growth of urinary pathogens. Lett. Appl. Microbiol. 1997, 24, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, F.M. Short Protocols in Molecular Biology, 5th ed.; Sonns, J.W., Ed.; Wiley: Hoboken, NJ, USA, 2002; Volume 1, pp. 1–6. [Google Scholar]
- Moshaver, B.; de Boer, F.; van Egmond-Kreileman, H.; Kramer, E.; Stegeman, C.; Groeneveld, P. Fast and accurate prediction of positive and negative urine cultures by flow cytometry. BMC Infect. Dis. 2016, 16, 211. [Google Scholar]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar]
- Łukaszewski, M.; Soszko, M.; Czerwiński, A. Electrochemical Methods of Real Surface Area Determination of Noble Metal Electrodes—An Overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar]
- Grdeń, M.; Łukaszewski, M.; Jerkiewicz, G.; Czerwiński, A. Electrochemical behaviour of palladium electrode: Oxidation, electrodissolution and ionic adsorption. Electrochim. Acta 2008, 53, 7583–7598. [Google Scholar]
- Uluc, A.V.; Mol, J.M.C.; Terryn, H.; Böttger, A.J. Hydrogen sorption and desorption related properties of Pd-alloys determined by cyclic voltammetry. J. Electroanal. Chem. 2014, 734, 53–60. [Google Scholar]
- Uosaki, K.; Elumalai, G.; Dinh, H.C.; Lyalin, A.; Taketsugu, T.; Noguchi, H. Highly Efficient Electrochemical Hydrogen Evolution Reaction at Insulating Boron Nitride Nanosheet on Inert Gold Substrate. Sci. Rep. 2016, 6, 32217. [Google Scholar] [CrossRef]
- Kibler, L.A. Hydrogen electrocatalysis. Chemphyschem 2006, 7, 985–991. [Google Scholar] [CrossRef]
- Söderström, C.; Winquist, F.; Krantz-Rülcker, C. Recognition of six microbial species with an electronic tongue. Sens. Actuators B Chem. 2003, 89, 248–255. [Google Scholar] [CrossRef]
- Ernst, H.; Knoll, M. Electrochemical characterisation of uric acid and ascorbic acid at a platinum electrode. Anal. Chem. Acta 2001, 449, 129–134. [Google Scholar] [CrossRef]
- Demirkan, B.; Bozkurt, S.; Cellat, K. Palladium supported on polypyrrole/reduced graphene oxide nanoparticles for simultaneous biosensing application of ascorbic acid, dopamine, and uric acid. Sci. Rep. 2020, 10, 2946. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Behpour, M.; Saeidinejad, F. Electrochemical determination of tryptophan, uric acid and ascorbic acid at a gold nanoparticles modified carbon paste electrode. Anal. Methods 2012, 4, 2447–2453. [Google Scholar] [CrossRef]
- Trettenhahn, G.; Köberl, A. Anodic decomposition of citric acid on gold and stainless steel electrodes: An in situ-FTIR-spectroscopic investigation. Electrochim. Acta 2007, 52, 2716–2722. [Google Scholar] [CrossRef]
- Burke, L.D.; Nugen, P.F. The Electrochemistry of Gold: The Redox Behaviour of the Metal in Aqueous Media. Springer 1997, 30, 43–53. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, C.M.; Bao, S.J.; Lua, Z.; Hong, Y. Direct electrochemistry and electrocatalytic mechanism of evolved Escherichia coli cells in microbial fuel cells. Chem. Commun. 2008, 1290–1292. [Google Scholar] [CrossRef]
- Pankratova, G.; Hederstedt, L.; Gorton, L. Extracellular electron transfer features of Gram-positive bacteria. Anal. Chim. Acta 2019, 1076, 32–47. [Google Scholar] [CrossRef]
- Vebo, H.C.; Solheim, M.; Snipen, L.; Nes, I.F.; Brede, D.A. Comparative genomic analysis of pathogenic and probiotic Enterococcus faecalis isolates, and their transcriptional responses to growth in human urine. PLoS ONE 2010, 5, e12489. [Google Scholar] [CrossRef]
- Robador, A.; LaRowe, D.E.; Finkel, S.E.; Amend, J.P.; Nealson, K.H. Changes in Microbial Energy Metabolism Measured by Nanocalorimetry during Growth Phase Transitions. Front. Microbiol. 2018, 9, 109. [Google Scholar] [CrossRef]
- Cordano, C.; Traverso, E.; Calabrò, V.; Borzone, C.; Stara, S.; Marchese, R.; Marinelli, L. Recurring hypermmonemic encephalopathy induced by bacteria usually not producing urease. BMC Res. Notes 2014, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Withman, B.; Gunasekera, T.S.; Beesetty, P.; Agans, R.; Paliy, O. Transcriptional responses of uropathogenic Escherichia coli to increased environmental osmolality caused by salt or urea. Infect. Immun. 2013, 81, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Juarez, G.E.; Mateyca, C.; Galvan, E.M. Proteus mirabilis outcompetes Klebsiella pneumoniae in artificial urine medium through secretion of ammonia and other volatile compounds. Heliyon 2020, 6, e03361. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D. The selective value of bacterial shape. Microbiol. Mol. Biol. Rev. 2006, 70, 660–703. [Google Scholar] [CrossRef]
- Haslauer, K.E.; Hemmler, D.; Schmitt-Kopplin, P. Heinzmann, S.S. Guidelines for the Use of Deuterium Oxide (D2O) in 1H NMR Metabolomics. Anal. Chem. 2019, 91, 11063–11069. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The Human Urine Metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef]
- Pankratova, G.; Leech, D.; Gorton, L.; Hederstedt, L. Extracellular Electron Transfer by the Gram-Positive Bacterium Enterococcus faecalis. Biochemistry 2018, 57, 4597–4603. [Google Scholar] [CrossRef]
- Ramsey, M.; Hartke, A.; Huycke, M. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. In The Physiology and Metabolism of Enterococci; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; National Library of Medicine, Bookshelf: Boston, MA, USA, 2014. [Google Scholar]
- Sierra-Padilla, A.; García-Guzmán, J.J.; López-Iglesias, D.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. E-Tongues/Noses Based on Conducting Polymers and Composite Materials: Expanding the Possibilities in Complex Analytical Sensing. Sensors 2021, 21, 4976. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Haddi, Z.; Amari, A.; Bouchikhi, B.; Mimendia, A.; Cetó, X.; del Valle, M. Hybrid electronic tongue based on multisensor data fusion for discrimination of beers. Sens. Actuators B Chem. 2013, 177, 989–996. [Google Scholar] [CrossRef]
- Sharma, A.; Agrawal, A.; Awasthi, K.K.; Awasthi, K.; Awasthi, A. Biosensors for diagnosis of urinary tract infections: Advances and future challenges. Mater. Lett. X 2021, 10, 100077. [Google Scholar] [CrossRef]

| Component | Quantity [g] |
|---|---|
| Uric acid | 0.07 |
| Peptone L37 | 1 |
| Citric acid | 0.4 |
| Lactic acid | 0.1 |
| Urea | 10 |
| Creatinine | 0.8 |
| Yeast extract | 0.005 |
| Sodium bicarbonate | 2.1 |
| Calcium chloride | 0.37 |
| Sodium chloride | 5.2 |
| Iron II sulfate | 0.0012 |
| Magnesium sulfate | 0.49 |
| Sodium sulfate | 3.2 |
| Potassium dihydrogen phosphate | 0.95 |
| Di-potassium hydrogen phosphate | 1.2 |
| Ammonium chloride | 1.3 |
| Distilled water | Filled up to 1 L |
| Incubation Time (h) | K. pneumoniae Concentration (Cells/mL) | E. faecalis Concentration (Cells/mL) | E. coli Concentration (Cells/mL) |
|---|---|---|---|
| 0 | 0.8 × 105 | 0.6 × 105 | 2.1 × 105 |
| 1 | 2 × 105 | 1.8 × 105 | 3.8 × 105 |
| 2 | 7.6 × 105 | 4.8 × 105 | 3.9 × 105 |
| 3 | 2 × 106 | 2.4 × 105 | 5.5 × 105 |
| 4 | 5.7 × 106 | 1.4 × 105 | 6 × 105 |
| 5 | 1 × 107 | 1.3 × 105 | 5.3 × 105 |
| Compound (ppm) | AUM (Black) | K. pneumoniae (Yellow) | E. faecalis (Green) | E. coli (Orange) |
|---|---|---|---|---|
| Lactic acid (d; 1.31, 1.32) | 1233 1030 | x | 1629 1474 | x |
| Citric acid (d; 2.50, 2.54, 2.65, 2.69) | 4162 6859 6907 4034 | 1862 3518 3482 2049 | x | 1593 2469 2411 1427 |
| Creatinine (s; 3.03 and 4.05) | 57,212 20,801 | 18,743 17,631 | 34,434 13,077 | 25,369 19,200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psotta, C.; Nilsson, E.J.; Sjöberg, T.; Falk, M. Bacteria-Infected Artificial Urine Characterization Based on a Combined Approach Using an Electronic Tongue Complemented with 1H-NMR and Flow Cytometry. Biosensors 2023, 13, 916. https://doi.org/10.3390/bios13100916
Psotta C, Nilsson EJ, Sjöberg T, Falk M. Bacteria-Infected Artificial Urine Characterization Based on a Combined Approach Using an Electronic Tongue Complemented with 1H-NMR and Flow Cytometry. Biosensors. 2023; 13(10):916. https://doi.org/10.3390/bios13100916
Chicago/Turabian StylePsotta, Carolin, Emelie J. Nilsson, Thomas Sjöberg, and Magnus Falk. 2023. "Bacteria-Infected Artificial Urine Characterization Based on a Combined Approach Using an Electronic Tongue Complemented with 1H-NMR and Flow Cytometry" Biosensors 13, no. 10: 916. https://doi.org/10.3390/bios13100916
APA StylePsotta, C., Nilsson, E. J., Sjöberg, T., & Falk, M. (2023). Bacteria-Infected Artificial Urine Characterization Based on a Combined Approach Using an Electronic Tongue Complemented with 1H-NMR and Flow Cytometry. Biosensors, 13(10), 916. https://doi.org/10.3390/bios13100916






