A Facile Method to Fabricate an Enclosed Paper-Based Analytical Device via Double-Sided Patterning for Ionic Contaminant Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Stamp Molds
2.3. Fabrication of Enclosed 3D-μPAD
2.4. Colorimetric Detection of Heavy Metal Ions and Radioactive Isotope
2.5. Analysis Method
3. Results
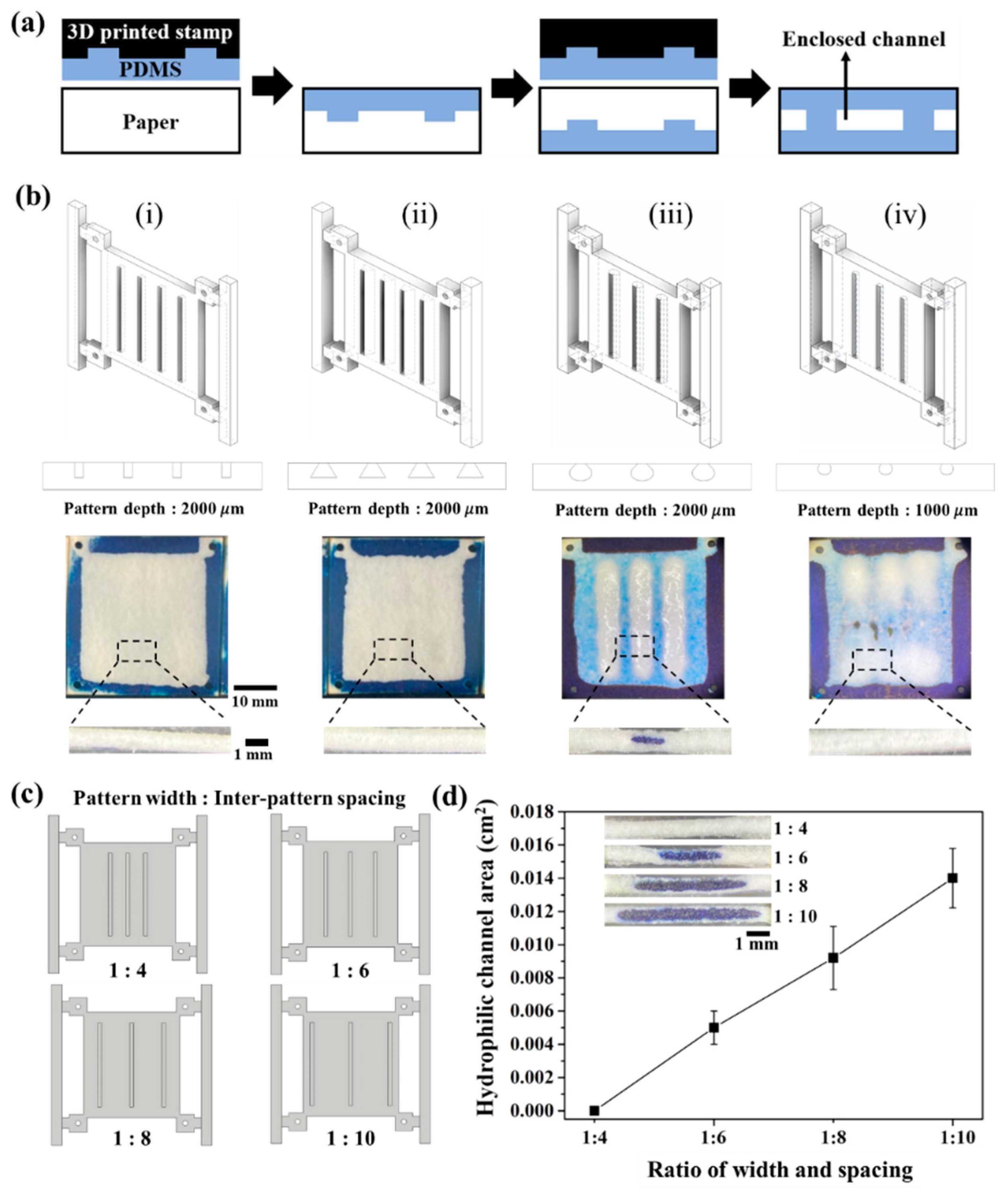
3.1. Fabrication of Enclosed 3D-μPAD
3.2. Effect of Stamp Design on PDMS Penetration of Paper
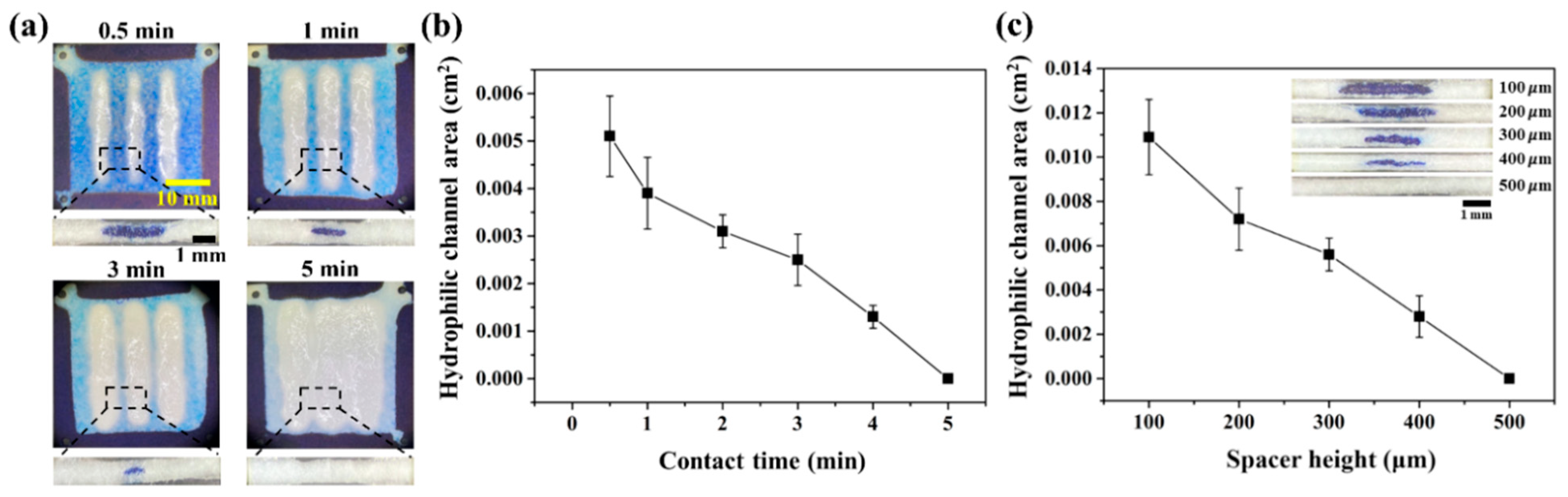
3.3. Controlled Formation of Enclosed Channel Using Double-Sided Patterning
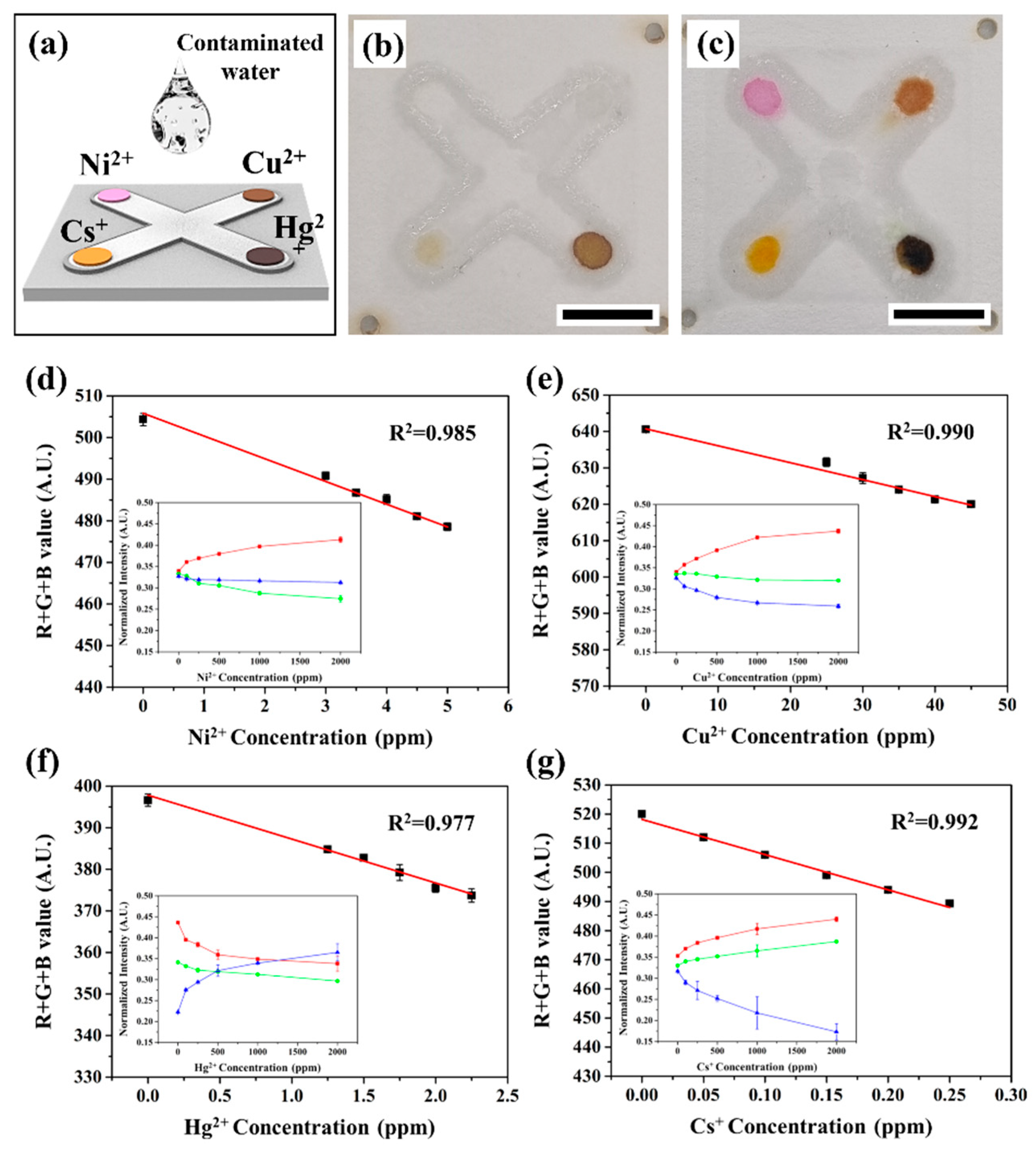
3.4. Application of Colorimetric Sensor for Simultaneous Detection of Heavy Metals and Radioactive Isotope
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinoshita, N.; Sueki, K.; Sasa, K.; Kitagawa, J.I.; Ikarashi, S.; Nishimura, T.; Wong, Y.S.; Satou, Y.; Handa, K.; Takahashi, T.; et al. Assessment of Individual Radionuclide Distributions from the Fukushima Nuclear Accident Covering Central-East Japan. Proc. Natl. Acad. Sci. USA 2011, 108, 19526–19529. [Google Scholar] [CrossRef] [PubMed]
- López, Y.C.; Ortega, G.A.; Reguera, E. Hazardous Ions Decontamination: From the Element to the Material. Chem. Eng. J. Adv. 2022, 11, 100297. [Google Scholar] [CrossRef]
- Iwanade, A.; Kasai, N.; Hoshina, H.; Ueki, Y.; Saiki, S.; Seko, N. Hybrid Grafted Ion Exchanger for Decontamination of Radioactive Cesium in Fukushima Prefecture and Other Contaminated Areas. J. Radioanal. Nucl. Chem. 2012, 293, 703–709. [Google Scholar] [CrossRef]
- Bansod, B.K.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A Review on Various Electrochemical Techniques for Heavy Metal Ions Detection with Different Sensing Platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Voitsekhovitch, O.V.; Håkanson, L.; Hilton, J. A Critical Review of Measures to Reduce Radioactive Doses from Drinking Water and Consumption of Freshwater Foodstuffs. J. Environ. Radioact. 2001, 56, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.M. Heavy Metal Pollution and Human Biotoxic Effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Melnikov, P.; Zanoni, L.Z. Clinical Effects of Cesium Intake. Biol. Trace Elem. Res. 2010, 135, 1–9. [Google Scholar] [CrossRef]
- Centeno, J.A.; Pestaner, J.P.; Omalu, B.I.; Torres, N.L.; Field, F.; Wagner, G.; Mullick, F.G. Blood and Tissue Concentration of Cesium after Exposure to Cesium Chloride A Report of Two Cases. Biol. Trace Elem. Res. 2003, 94, 97–104. [Google Scholar] [CrossRef]
- Daşbaşı, T.; Saçmacı, Ş.; Ülgen, A.; Kartal, Ş. A Solid Phase Extraction Procedure for the Determination of Cd(II) and Pb(II) Ions in Food and Water Samples by Flame Atomic Absorption Spectrometry. Food Chem. 2015, 174, 591–596. [Google Scholar] [CrossRef]
- Mohamed, R.A.; Abdel-Lateef, A.M.; Mahmoud, H.H.; Helal, A.I. Determination of Trace Elements in Water and Sediment Samples from Ismaelia Canal Using Ion Chromatography and Atomic Absorption Spectroscopy. Chem. Speciat. Bioavailab. 2012, 24, 31–38. [Google Scholar] [CrossRef]
- Sharma, H.; Kaur, N.; Singh, A.; Kuwar, A.; Singh, N. Optical Chemosensors for Water Sample Analysis. J. Mater. Chem. C 2016, 4, 5154–5194. [Google Scholar] [CrossRef]
- Kang, S.-M.; Jang, S.C.; Haldorai, Y.; Vilian, A.T.E.; Rethinasabapathy, M.; Roh, C.; Han, Y.K.; Huh, Y.S. Facile Fabrication of Paper-Based Analytical Devices for Rapid and Highly Selective Colorimetric Detection of Cesium in Environmental Samples. RSC Adv. 2017, 7, 48374–48385. [Google Scholar] [CrossRef]
- Ebrahim, A.M.; Eltayeb, M.A.H.; Shaat, M.K.; Mohmed, N.M.A.; Eltayeb, E.A.; Ahmed, A.Y. Study of Selected Trace Elements in Cancerous and Non-Cancerous Human Breast Tissues from Sudanese Subjects Using Instrumental Neutron Activation Analysis. Sci. Total Environ. 2007, 383, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.R.; Gangaiah, T.; Naidu, G.R.K. Determination of Trace Metals in Water by Neutron Activation Analysis after Preconcentration on a Poly( Acrylamidoxime) Resin. Anal. Chim. Acta 1991, 249, 533–537. [Google Scholar] [CrossRef]
- Pujol, L.; Evrard, D.; Groenen-Serrano, K.; Freyssinier, M.; Ruffien-Cizsak, A.; Gros, P. Electrochemical Sensors and Devices for Heavy Metals Assay in Water: The French Groups’ Contribution. Front. Chem. 2014, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Chaiyo, S.; Mehmeti, E.; Žagar, K.; Siangproh, W.; Chailapakul, O.; Kalcher, K. Electrochemical Sensors for the Simultaneous Determination of Zinc, Cadmium and Lead Using a Nafion/Ionic Liquid/Graphene Composite Modified Screen-Printed Carbon Electrode. Anal. Chim. Acta 2016, 918, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; He, M.; Chen, B.; Hu, B. Magnetic Porous Coordination Networks for Preconcentration of Various Metal Ions from Environmental Water Followed by Inductively Coupled Plasma Mass Spectrometry Detection. Talanta 2022, 245, 123470. [Google Scholar] [CrossRef]
- Alizadeh, T.; Amjadi, S. Preparation of Nano-Sized Pb2+ Imprinted Polymer and Its Application as the Chemical Interface of an Electrochemical Sensor for Toxic Lead Determination in Different Real Samples. J. Hazard. Mater. 2011, 190, 451–459. [Google Scholar] [CrossRef]
- Sitko, R.; Janik, P.; Zawisza, B.; Talik, E.; Margui, E.; Queralt, I. Green Approach for Ultratrace Determination of Divalent Metal Ions and Arsenic Species Using Total-Reflection X-ray Fluorescence Spectrometry and Mercapto-Modified Graphene Oxide Nanosheets as a Novel Adsorbent. Anal. Chem. 2015, 87, 3535–3542. [Google Scholar] [CrossRef]
- Marguí, E.; Zawisza, B.; Sitko, R. Trace and Ultratrace Analysis of Liquid Samples by X-ray Fluorescence Spectrometry. TrAC—Trends Anal. Chem. 2014, 53, 73–83. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and Removal of Heavy Metal Ions: A Review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Kumar, N.; Leray, I.; Depauw, A. Chemically Derived Optical Sensors for the Detection of Cesium Ions. Coord. Chem. Rev. 2016, 310, 1–15. [Google Scholar] [CrossRef]
- Choi, J.; Jeong, H.-H. Fabrication of 3D Paper-Based Analytical Device Using Double-Sided Imprinting Method for Metal Ion Detection. Clean Technol. 2022, 28, 323–330. [Google Scholar] [CrossRef]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-H.; Park, C. Fabrication of Paper-based Biosensor Chip Using Polydimethylsiloxane Blade Coating Method. Korean Chem. Eng. Res. 2021, 59, 100–105. [Google Scholar] [CrossRef]
- Costa, M.N.; Veigas, B.; Jacob, J.M.; Santos, D.S.; Gomes, J.; Baptista, P.V.; Inácio, J.; Fortunato, E. A low cost, safe, disposable, rapid and self-sustainable paper-based platform for diagnostic testing: Lab-on-paper. Nanotechnology 2014, 25, 094006. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Wiley, B.J.; Gupta, M.; Whitesides, G.M. FLASH: A Rapid Method for Prototyping Paper-Based Microfluidic Devices. Lab Chip 2008, 8, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.W.; Wang, Z.P.; Huang, G.X.D. Investigation of Wax and Paper Materials for the Fabrication of Paper-Based Microfluidic Devices. Microsyst. Technol. 2012, 18, 649–659. [Google Scholar] [CrossRef]
- He, Q.; Ma, C.; Hu, X.; Chen, H. Method for Fabrication of Paper-Based Microfluidic Devices by Alkylsilane Self-Assembling and UV/O3-Patterning. Anal. Chem. 2013, 85, 1327–1331. [Google Scholar] [CrossRef]
- Asano, H.; Shiraishi, Y. Development of paper-based microfluidic analytical device for iron assay using photomask printed with 3D printer for fabrication of hydrophilic and hydrophobic zones on paper by photolithography. Anal. Chim. Acta. 2015, 883, 55–60. [Google Scholar] [CrossRef]
- Cate, D.M.; Noblitt, S.D.; Volckens, J.; Henry, C.S. Multiplexed Paper Analytical Device for Quantification of Metals Using Distance-Based Detection. Lab Chip 2015, 15, 2808–2818. [Google Scholar] [CrossRef]
- Jayawardane, B.M.; Shen, W.; Mckelvie, I.D.; Kolev, S.D. Microfluidic Paper-Based Analytical Device (µPAD) for the Determination of Nitrite and Nitrate. Anal. Chem. 2014, 86, 7274–7279. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, S.; Cruz-Izquierdo, Á.C.; Scott, J.L.; Di Lorenzo, M. Impedimetric Paper-Based Biosensor for the Detection of Bacterial Contamination in Water. Sens. Actuators B Chem. 2018, 265, 50–58. [Google Scholar] [CrossRef]
- Bedin, F.; Boulet, L.; Voilin, E.; Theillet, G.; Rubens, A.; Rozand, C. Paper-Based Point-of-Care Testing for Cost-Effective Diagnosis of Acute Flavivirus Infections. J. Med. Virol. 2017, 89, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.M.; Chang, C.L.; Hsu, M.Y.; Lin, J.Y.; Kuan, C.M.; Wang, H.K.; Huang, C.T.; Chung, M.C.; Huang, K.C.; Hsu, C.E.; et al. Paper-Based ELISA to Rapidly Detect Escherichia Coli. Talanta 2015, 145, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, F.; Yang, Y.; Zhao, G.; Zhang, C.; Wang, R.; Huang, X. Research Progress and Future Trends of Microfluidic Paper-Based Analytical Devices in In-Vitro Diagnosis. Biosensors 2022, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, T.; Marques, A.C.; Carvalho, P.; Martins, R.; Fortunato, E. Paper Microfluidics and Tailored Gold Nanoparticles for Nonenzymatic, Colorimetric Multiplex Biomarker Detection. ACS Appl. Mater. Interfaces 2021, 13, 3576–3590. [Google Scholar] [CrossRef]
- Zaki, M.F.; Chen, P.C.; Yeh, Y.C.; Lin, P.H.; Xu, M.Y. Engineering a Monolithic 3D Paper-Based Analytical Device (µPAD) by Stereolithography 3D Printing and Sequential Digital Masks for Efficient 3D Mixing and Dopamine Detection. Sens. Actuators A Phys. 2022, 347, 113991. [Google Scholar] [CrossRef]
- Lim, H.; Jafry, A.T.; Lee, J. Fabrication, Flow Control, and Applications of Microfluidic Paper-Based Analytical Devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef]
- Liu, H.; Crooks, R.M. Three-Dimensional Paper Microfluidic Devices Assembled Using the Principles of Origami. J. Am. Chem. Soc. 2011, 133, 17564–17566. [Google Scholar] [CrossRef]
- Park, C.; Han, Y.D.; Kim, H.V.; Lee, J.; Yoon, H.C.; Park, S. Double-sided 3D printing on paper towards mass production of three-dimensional paper-based microfluidic analytical devices (3D-μPADs). Lab Chip 2018, 18, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.G.; Kim, D.H.; Kim, J.; Kim, J.H.; Song, S.; Lee, C.S. Programmable microfluidic flow for automatic multistep digital assay in a single-sheet 3-dimensional paper-based microfluidic device. J. Chem. Eng. 2021, 411, 128429. [Google Scholar] [CrossRef]
- Li, X.; Liu, X. Fabrication of Three-Dimensional Microfluidic Channels in a Single Layer of Cellulose Paper. Microfluid. Nanofluidics 2014, 16, 819–827. [Google Scholar] [CrossRef]
- Wang, C.M.; Chen, C.Y.; Liao, W.S. Enclosed paper-based analytical devices: Concept, variety, and outlook. Anal. Chim. Acta 2021, 1144, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Dornelas, K.L.; Dossi, N.; Piccin, E. A simple method for patterning poly (dimethylsiloxane) barriers in paper using contact-printing with low-cost rubber stamps. Anal. Chim. Acta 2015, 858, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Mohammadi, S.; Maeki, M.; Mohamadi, R.M.; Ishida, A.; Tani, H.; Tokeshi, M. An instrument-free, screen-printed paper microfluidic device that enables bio and chemical sensing. Analyst 2015, 140, 6493–6499. [Google Scholar] [CrossRef]
- Sameenoi, Y.; Nongkai, P.N.; Nouanthavong, S.; Henry, C.S.; Nacapricha, D. One-step polymer screen-printing for microfluidic paper-based analytical device (μPAD) fabrication. Analyst 2014, 139, 6580–6588. [Google Scholar] [CrossRef]
- Xu, M.; Bunes, B.R.; Zang, L. Paper-Based Vapor Detection of Hydrogen Peroxide: Colorimetric Sensing with Tunable Interface. ACS Appl. Mater. Interfaces 2011, 3, 642–647. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Yoon, B.; Park, I.S.; Shin, H.; Park, H.J.; Lee, C.W.; Kim, J.M. A Litmus-Type Colorimetric and Fluorometric Volatile Organic Compound Sensor Based on Inkjet-Printed Polydiacetylenes on Paper Substrates. Macromol. Rapid Commun. 2013, 34, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Jung, J.E.; Lee, H.U.; Bae, J.S.; Rethinasabapathy, M.; Huh, Y.S.; Kang, S.-M. Generation of Controllable Patterned Nanofibrous Networks by Electrospinning Lithography: Simultaneous Detection and Adsorption toward Cesium Ions. ACS Sustain. Chem. Eng. 2023, 11, 3810–3819. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent Developments in Paper-Based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Mendez, S.; Fenton, E.M.; Gallegos, G.R.; Petsev, D.N.; Sibbett, S.S.; Stone, H.A.; Zhang, Y.; López, G.P. Imbibition in Porous Membranes of Complex Shape: Quasi-stationary Flow in Thin Rectangular Segments. Langmuir 2010, 26, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273. [Google Scholar] [CrossRef]
- Sun, X.; Li, B.; Qi, A.; Tian, C.; Han, J.; Shi, Y.; Lin, B.; Chen, L. Improved assessment of accuracy and performance using a rotational paper-based device for multiplexed detection of heavy metals. Talanta 2018, 178, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Amaya, S.; Zhao, M.; Allebach, J.P.; Chiu, G.T.C.; Stanciu, L.A. Ionic strength influences on biofunctional Au-decorated microparticles for enhanced performance in multiplexed colorimetric sensors. ACS Appl. Mater. Interfaces 2020, 12, 32397–32409. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Lee, E.-H.; Kang, S.-M.; Jeong, H.-H. A Facile Method to Fabricate an Enclosed Paper-Based Analytical Device via Double-Sided Patterning for Ionic Contaminant Detection. Biosensors 2023, 13, 915. https://doi.org/10.3390/bios13100915
Choi J, Lee E-H, Kang S-M, Jeong H-H. A Facile Method to Fabricate an Enclosed Paper-Based Analytical Device via Double-Sided Patterning for Ionic Contaminant Detection. Biosensors. 2023; 13(10):915. https://doi.org/10.3390/bios13100915
Chicago/Turabian StyleChoi, Jinsol, Eun-Ho Lee, Sung-Min Kang, and Heon-Ho Jeong. 2023. "A Facile Method to Fabricate an Enclosed Paper-Based Analytical Device via Double-Sided Patterning for Ionic Contaminant Detection" Biosensors 13, no. 10: 915. https://doi.org/10.3390/bios13100915
APA StyleChoi, J., Lee, E.-H., Kang, S.-M., & Jeong, H.-H. (2023). A Facile Method to Fabricate an Enclosed Paper-Based Analytical Device via Double-Sided Patterning for Ionic Contaminant Detection. Biosensors, 13(10), 915. https://doi.org/10.3390/bios13100915





