Nanomaterials and Their Recent Applications in Impedimetric Biosensing
Abstract
:1. Introduction
2. Nanomaterials
2.1. Metal and Metal Oxide Nanoparticles and Two-Dimensional Transition Metal Dichalcogenides
2.2. Graphene and Graphene Oxide (GO)
2.3. Carbon Nanotubes (CNTs)
2.4. Carbon Nanofibers (CNFs)
2.5. Quantum Dots (QDs)
3. Application of Nanomaterials
3.1. Metal and Metal Oxide Nanoparticles and Two-Dimensional Transition Metal Dichalcogenides
| Analyte | Recognition Element | Electrode | Linear Range | LOD | Reference |
|---|---|---|---|---|---|
| CRP | Nitrocellulose membrane | Nano-ZnO/CuO membranes | / | 0.027 ng/mL | [94] |
| Trypsin | Molecularly imprinted polymer and aptamers | GCE/NiO/Apt-ePDA/MIP | 10−3 to 9 × 10−2 ng/mL | 7.5 × 10−4 ng/mL | [95] |
| Leptin | Thiol DNA aptamer | AuNPs/TiO2 NPs/SPE | 10−3 to 10−1 ng/mL, 10−1 to 1 ng/mL | 3.12 × 10−4 ng/mL | [96] |
| Target HBV DNA | Probe HBV DNA | CPE-magnetite-AuNPs | 8.3 (±0.1) × 10−4 to 6.4 (±0.2) × 102 nM | 3.1 (±0.1) × 10−4 nM | [97] |
| AFB M1 | ss-HSDNA | SAM of cysteamine and AuNPs-Au-electrode | 1 to 14 ng/mL | / | [98] |
| D-dimer | DD antibody | AuNPs DHP SPCE | 5 × 102 ng/mL | 8.92 ng/mL | [99] |
| Plasma insulin | ss-DNA aptamer | AuNPs PGE | 10 to 103 nM | 2.7 × 108 nM | [100] |
| NoV-LPs | Anti-NoV antibody | PAni/AuNPs/Au electrode | 10−4 to 103 ng/mL | 1.8 × 10−6 ng/mL | [101] |
| Paraoxon | AChE | MoS2 nanosheets | 106 to 109 ng/mL | 1.3 × 104 ng/mL | [102] |
| Lactate | Lactate oxidase | GC/MoS2 | 5.6 × 104 to 7.7 × 105 nM | 1.7 × 104 nM | [103] |
3.2. Graphene and Graphene Oxide (GO)
3.3. Carbon Nanotubes (CNTs)
3.4. Carbon Nanofibers (CNFs)
3.5. Quantum Dots (QDs)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| Abbreviation | Definition |
| AAB | Anti-apo lipoprotein B |
| AC | Alternating current |
| APTMS | 3-aminopropyltrinemethoxysilane |
| Au-IDE | Gold interdigitated microelectrode |
| AuNPs | Gold nanoparticles |
| Au-SPE | Gold surface-screen printed electrode |
| BN | Boron nitride |
| BPA | Bisphenol A |
| CBZ | Carbendazim |
| Cdl | Double-layer capacitance |
| CE | Counter electrode |
| CNF | Carbon nanofibers |
| CNT | Carbon nanotube |
| ConA | Concanavalin A |
| CPE | Carbon paste electrode |
| CPR | C-reactive protein |
| CQD | Colloidal quantum dots |
| CV | Cyclic voltammetry |
| Cys | Cysteine |
| DD | D-Dimer |
| DHP | Dihexadecyl phosphate |
| EC | Capillary electrophoresis |
| EEC | Equivalent electric circuit |
| EIS | Electrochemical impedance spectroscopy |
| ELISA | Enzyme-linked immunosorbent assay |
| ErGO | Electrochemical-reduced graphene oxide |
| GCE | Glassy carbon electrode |
| GO | Graphene oxide |
| GOx | Glucose oxidase |
| GQD | Graphene quantum dots |
| HBV | Hepatitis B virus |
| HPLC-MS | High-performance liquid chromatography-mass spectroscopy |
| HPR | Horseradish peroxidase |
| ITO | Indium tin oxide |
| LDL | Low-density lipoprotein |
| LOD | Limit of detection |
| MWCNT | Multi-walled carbon nanotube |
| PDDA | Poly diallyl dimethylammonium chloride |
| PGE | Pencil graphite electrode |
| POC | Point-of-care |
| QD | Quantum dots |
| Rct | Charge transfer resistance |
| RE | Reference electrode |
| rGO | Reduced graphene oxide |
| Rs | Ohmic resistance |
| SAM | Self-assembled monolayer |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SPCE | Screen-printed carbon electrode |
| ss-HDNA | Thiol-modified single-stranded DNA |
| SWCNT | Single-walled carbon nanotube |
| SWV | Square wave voltammetry |
| THR | Thrombin |
| TLC | Thin-layer chromatography |
| WE | Working electrode |
| Zw | Warburg impedance |
References
- Štukovnik, Z.; Godec, R.F.; Bren, U. The Use of Yeast Saccharomyces Cerevisiae as a Biorecognition element in the Development of a Model Impedimetric Biosensor for Caffeine Detection. Acta Chim. Slov. 2022, 69, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.-M.; Kim, C.-D.; Ju, F.N.; Kim, H.; Kim, C.-H.; Kim, T.-H. Recent Advances in Electrochemical Biosensors for Monitoring Animal Cell Function and Viability. Biosensors 2022, 12, 1162. [Google Scholar] [CrossRef]
- Kaya, S.I.; Ozcelikay, G.; Mollarasouli, F.; Bakirhan, N.K.; Ozkan, S.A. Recent achievements and challenges on nanomaterial based electrochemical biosensors for the detection of colon and lung cancer biomarkers. Sens. Actuators B Chem. 2022, 351, 130856. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Oh, S.-W. Electrochemical impedimetric biosensors for food safety. Food Sci. Biotechnol. 2020, 29, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Rozman, M.; Štukovnik, Z.; Sušnik, A.; Pakseresht, A.; Hočevar, M.; Drobne, D.; Bren, U. A HepG2 Cell-Based Biosensor That Uses Stainless Steel Electrodes for Hepatotoxin Detection. Biosensors 2022, 12, 160. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Safaei, M.; Zhang, K.; Van Le, Q.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Developments and applications of nanomaterial-based carbon paste electrodes. RSC Adv. 2020, 10, 21561–21581. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; de la Escosura-Muñiz, A. Electrochemical biosensors based on nanomaterials for aflatoxins detection: A review (2015–2021). Anal. Chim. Acta 2022, 1212, 339658. [Google Scholar] [CrossRef]
- Mathew, M.; Radhakrishnan, S.; Vaidyanathan, A.; Chakraborty, B.; Rout, C.S. Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments. Anal. Bioanal. Chem. 2021, 413, 727–762. [Google Scholar] [CrossRef]
- Espinosa-Calderón, A.; Contreras-Medina, L.M.; Muñoz-Huerta, R.F.; Millán-Almaraz, J.R.; González, R.G.G.; Torres-Pacheco, I. Methods for Detection and Quantification of Aflatoxins; InTech: New York, NY, USA, 2011. [Google Scholar]
- Sharma, A.; Goud, K.Y.; Hayat, A.; Bhand, S.; Marty, J.L. Recent Advances in Electrochemical-Based Sensing Platforms for Aflatoxins Detection. Chemosensors 2017, 5, 1. [Google Scholar] [CrossRef]
- Kirchhain, A.; Bonini, A.; Vivaldi, F.; Poma, N.; Di Francesco, F. Latest developments in non-faradic impedimetric biosensors: Towards clinical applications. TrAC Trends Anal. Chem. 2020, 133, 116073. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Suni, I.I.; Bever, C.S.; Hammock, B.D. Impedance biosensors: Applications to sustainability and remaining technical challenges. ACS Sustain. Chem. Eng. 2014, 2, 1649–1655. [Google Scholar] [CrossRef]
- Kabir, S. Characteristics Analysis of Electrochemical Impedance Spectroscopy (Eis) for Different Electrode Patterns. Ph.D. Thesis, The University of Texas Rio Grande Valley, Ann Arbor, MI, USA, 2021. [Google Scholar]
- Hou, Y.; Helali, S.; Zhang, A.; Jaffrezic-Renault, N.; Martelet, C.; Minic, J.; Gorojankina, T.; Persuy, M.-A.; Pajot-Augy, E.; Salesse, R.; et al. Immobilization of rhodopsin on a self-assembled multilayer and its specific detection by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2006, 21, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Probing Biomolecular Interactions at Conductive and Semiconductive Surfaces by Impedance Spectroscopy: Routes to Impedimetric Immunosensors, DNA-Sensors, and Enzyme Biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnková, L.; Skládal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef]
- Furst, A.L.; Francis, M.B. Impedance-Based Detection of Bacteria. Chem. Rev. 2019, 119, 700–726. [Google Scholar] [CrossRef]
- Qi, P.; Wan, Y.; Zhang, D. Impedimetric biosensor based on cell-mediated bioimprinted films for bacterial detection. Biosens. Bioelectron. 2013, 39, 282–288. [Google Scholar] [CrossRef]
- Szekeres, K.J.; Vesztergom, S.; Ujvári, M.; Láng, G.G. Methods for the Determination of Valid Impedance Spectra in Non-stationary Electrochemical Systems: Concepts and Techniques of Practical Importance. ChemElectroChem 2021, 8, 1233–1250. [Google Scholar] [CrossRef]
- El-Azazy, M.; Min, M.; Annus, P. Electrochemical Impedance Spectroscopy; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Congur, G.; Eksin, E.; Erdem, A. Impedimetric Detection of microRNA at Graphene Oxide Modified Sensors. Electrochim. Acta 2015, 172, 20–27. [Google Scholar] [CrossRef]
- Kongsuphol, P.; Ng, H.H.; Pursey, J.P.; Arya, S.K.; Wong, C.C.; Stulz, E.; Park, M.K. EIS-based biosensor for ultra-sensitive detection of TNF-α from non-diluted human serum. Biosens. Bioelectron. 2014, 61, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef] [PubMed]
- Štukovnik, Z.; Bren, U. Recent Developments in Electrochemical-Impedimetric Biosensors for Virus Detection. Int. J. Mol. Sci. 2022, 23, 15922. [Google Scholar] [CrossRef] [PubMed]
- Morali, U.; Erol, S. Analysis of electrochemical impedance spectroscopy response for commercial lithium-ion batteries: Modeling of equivalent circuit elements. Turk. J. Chem. 2020, 44, 602–613. [Google Scholar] [CrossRef]
- Pajkossy, T.; Jurczakowski, R. Electrochemical impedance spectroscopy in interfacial studies. Curr. Opin. Electrochem. 2017, 1, 53–58. [Google Scholar] [CrossRef]
- Fatima, S.; Aarti, T.; Sridhar, S. Chapter 3—Overview of wastewater treatment approaches related to the microbial electrochemical system. In Advanced Nanomaterials and Nanocomposites for Bioelectrochemical Systems; Mubarak, N., Sattar, A., Mazari, S.A., Nizamuddin, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 57–80. [Google Scholar]
- Imanzadeh, H.; Sefid-Sefidehkhan, Y.; Afshary, H.; Afruz, A.; Amiri, M. Nanomaterial-based electrochemical sensors for detection of amino acids. J. Pharm. Biomed. Anal. 2023, 230, 115390. [Google Scholar] [CrossRef]
- de Faria, R.A.D.; Heneine, L.G.D.; Matencio, T.; Messaddeq, Y. Faradaic and non-faradaic electrochemical impedance spectroscopy as transduction techniques for sensing applications. Int. J. Biosens. Bioelectron. 2019, 5, 29–31. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Elancheziyan, M.; Manoj, D.; Saravanakumar, D.; Thenmozhi, K.; Senthilkumar, S. Amperometric sensing of catechol using a glassy carbon electrode modified with ferrocene covalently immobilized on graphene oxide. Microchim. Acta 2017, 184, 2925–2932. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. TrAC Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Goode, J.A.; Rushworth, J.V.; Millner, P.A. Biosensor Regeneration: A Review of Common Techniques and Outcomes. Langmuir 2015, 31, 6267–6276. [Google Scholar] [CrossRef]
- Kim, J.H.; Suh, Y.J.; Park, D.; Yim, H.; Kim, H.; Kim, H.J.; Yoon, D.S.; Hwang, K.S. Technological advances in electrochemical biosensors for the detection of disease biomarkers. Biomed. Eng. Lett. 2021, 11, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.Y.A. Advances in Electrochemical Nano-Biosensors for Biomedical and Environmental Applications: From Current Work to Future Perspectives. Sensors 2022, 22, 7539. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.Y.A.; Mekawy, M.M.; Ramnani, P.; Mulchandani, A. Monitoring of microbial cell viability using nanostructured electrodes modified with Graphene/Alumina nanocomposite. Biosens. Bioelectron. 2017, 91, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.Y.A.; Hassan, H.N.A.; Abdel-Aziz, M.S.; Khaled, E. Nanomaterials-based microbial sensor for direct electrochemical detection of Streptomyces spp. Sens. Actuators B Chem. 2014, 203, 848–853. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Xie, Q.-Z.; Lin, M.-W.; Hsu, W.-E.; Lin, C.-T. Advancements of Nanoscale Structures and Materials in Impedimetric Biosensing Technologies. ECS J. Solid. State Sci. Technol. 2020, 9, 115027. [Google Scholar] [CrossRef]
- Pan, M.; Gu, Y.; Yun, Y.; Li, M.; Jin, X.; Wang, S. Nanomaterials for Electrochemical Immunosensing. Sensors 2017, 17, 1041. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano Biosensors: Properties, applications and electrochemical techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Howes, P.D.; Chandrawati, R.; Stevens, M.M. Colloidal nanoparticles as advanced biological sensors. Science 2014, 346, 1247390. [Google Scholar] [CrossRef] [PubMed]
- Robins, M.M.; Wilde, P.J. COLLOIDS AND EMULSIONS. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 1517–1524. [Google Scholar]
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Ghafori Gorab, M.; Masoud Hashemi, S.; Javanshir, S.; Ahangari Cohan, R.; et al. Applications of carbon-based conductive nanomaterials in biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, B.; Cui, X.; Li, Y.; Tang, J.; Wang, H.; Zhang, D.; Li, Z. Recent advances in aptasensors for mycotoxin detection: On the surface and in the colloid. Talanta 2021, 223, 121729. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.D.; Rahman, A.M.N.A.A. Polymer Colloids for Functional Coating Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Yang, W.; Li, Y.; Bai, Y.; Sun, C. Hydrogen peroxide biosensor based on myoglobin/colloidal gold nanoparticles immobilized on glassy carbon electrode by a Nafion film. Sens. Actuators B Chem. 2006, 115, 42–48. [Google Scholar] [CrossRef]
- Jin, Z.; Hildebrandt, N. Semiconductor quantum dots for in vitro diagnostics and cellular imaging. Trends Biotechnol. 2012, 30, 394–403. [Google Scholar] [CrossRef]
- Kreuzer, M.P.; Quidant, R.; Salvador, J.P.; Marco, M.P.; Badenes, G. Colloidal-based localized surface plasmon resonance (LSPR) biosensor for the quantitative determination of stanozolol. Anal. Bioanal. Chem. 2008, 391, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of Nanoparticles in Electrochemical Sensors and Biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Gupta, S.; Dubey, S.K.; Prakash, R. Genosensor based on a nanostructured, platinum-modified glassy carbon electrode for Listeria detection. Anal. Methods 2015, 7, 2616–2622. [Google Scholar]
- Madianos, L.; Tsekenis, G.; Skotadis, E.; Patsiouras, L.; Tsoukalas, D. A highly sensitive impedimetric aptasensor for the selective detection of acetamiprid and atrazine based on microwires formed by platinum nanoparticles. Biosens. Bioelectron. 2018, 101, 268–274. [Google Scholar] [CrossRef]
- Thouand, G.; Belkin, S.; Daunert, S.; Freemont, P.; Hermans, J.; Karube, I.; Martel, S.; Michelini, E.; Roda, A. Handbook of Cell Biosensors; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Pérez-Fernández, B.; Costa-García, A.; Muñiz, A.D.l.E. Electrochemical (Bio) Sensors for Pesticides Detection Using Screen-Printed Electrodes. Biosensors 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Pourmadadi, M.; Rahmani, E.; Shamsabadipour, A.; Mahtabian, S.; Ahmadi, M.; Rahdar, A.; Díez-Pascual, A.M. Role of Iron Oxide (Fe2O3) Nanocomposites in Advanced Biomedical Applications: A State-of-the-Art Review. Nanomaterials 2022, 12, 3873. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ratinac, K.; Ringer, S.; Thordarson, P.; Gooding, J.; Braet, F. Carbon Nanomaterials in Biosensors: Should You Use Nanotubes or Graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Wu, M.; Zhu, J.; Zhang, X. Multifunctional Carbon-Based Nanomaterials: Applications in Biomolecular Imaging and Therapy. ACS Omega 2018, 3, 9126–9145. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, N.; Spillmann, C.M.; Algar, W.R.; Pons, T.; Stewart, M.H.; Oh, E.; Susumu, K.; Díaz, S.A.; Delehanty, J.B.; Medintz, I.L. Energy Transfer with Semiconductor Quantum Dot Bioconjugates: A Versatile Platform for Biosensing, Energy Harvesting, and Other Developing Applications. Chem. Rev. 2017, 117, 536–711. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Manso, J.; Mena, M.L.; Yáñez-Sedeño, P.; Pingarrón, J. Electrochemical biosensors based on colloidal gold–carbon nanotubes composite electrodes. J. Electroanal. Chem. 2007, 603, 1–7. [Google Scholar] [CrossRef]
- Anik, U. Gold Nanoparticle-Based Electrochemical Biosensors for Medical Applications. In Biosensors Nanotechnology; Wiley: New York, NY, USA, 2014; pp. 263–277. [Google Scholar]
- Belding, S.R.; Campbell, F.W.; Dickinson, E.J.F.; Compton, R.G. Nanoparticle-modified electrodes. Phys. Chem. Chem. Phys. 2010, 12, 11208–11221. [Google Scholar] [CrossRef]
- Tran, T.B.; Son, S.J.; Min, J. Nanomaterials in label-free impedimetric biosensor: Current process and future perspectives. BioChip J. 2016, 10, 318–330. [Google Scholar] [CrossRef]
- Estananto; Septiani, N.L.W.; Iqbal, M.; Suyatman; Nuruddin, A.; Yuliarto, B. Nanocomposite of graphene and WO3 nanowires for carbon monoxide sensors. Nanocomposites 2021, 7, 225–236. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Villalonga, R.; Yáñez-Sedeño, P. Nanoparticle-Modified Electrodes for Sensing; CRC: Boca Raton, FL, USA, 2014. [Google Scholar]
- Patra, D.C.; Deka, N.; Dash, A.; Khan, S.A.; Misra, T.K.; Mondal, S.P. Noninvasive Electrochemical Nitric Oxide Detection in Human Saliva Using Pt and TiO2 Nanoparticle Composite Electrodes. ACS Appl. Electron. Mater. 2023, 5, 832–845. [Google Scholar] [CrossRef]
- Mitrevska, K.; Milosavljevic, V.; Gagic, M.; Richtera, L.; Adam, V. 2D transition metal dichalcogenide nanomaterial-based miRNA biosensors. Appl. Mater. Today 2021, 23, 101043. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Tan, C.; Zhang, X.; Lu, Q.; Sindoro, M.; Huang, X.; Huang, W.; Wang, L.; Zhang, H. Two-dimensional transition metal dichalcogenide nanomaterials for biosensing applications. Mater. Chem. Front. 2017, 1, 24–36. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Hu, H.; Zavabeti, A.; Quan, H.; Zhu, W.; Wei, H.; Chen, D.; Ou, J.Z. Recent advances in two-dimensional transition metal dichalcogenides for biological sensing. Biosens. Bioelectron. 2019, 142, 111573. [Google Scholar] [CrossRef]
- Chang, J.; Zhou, G.; Christensen, E.R.; Heideman, R.; Chen, J. Graphene-based sensors for detection of heavy metals in water: A review. Anal. Bioanal. Chem. 2014, 406, 3957–3975. [Google Scholar] [CrossRef]
- Deng, X.; Tang, H.; Jiang, J. Recent progress in graphene-material-based optical sensors. Anal. Bioanal. Chem. 2014, 406, 6903–6916. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Loh, K.P. Graphene photonics, plasmonics, and broadband optoelectronic devices. ACS Nano 2012, 6, 3677–3694. [Google Scholar] [CrossRef]
- He, Q.; Wu, S.; Yin, Z.; Zhang, H. Graphene-based electronic sensors. Chem. Sci. 2012, 3, 1764–1772. [Google Scholar] [CrossRef]
- Sakellariou, G.; Priftis, D.; Baskaran, D. Surface-initiated polymerization from carbon nanotubes: Strategies and perspectives. Chem. Soc. Rev. 2013, 42, 677–704. [Google Scholar] [CrossRef]
- Giordano, C.; Filatrella, G.; Sarno, M.; Di Bartolomeo, A. Multi-walled carbon nanotube films for the measurement of the alcoholic concentration. Micro Nano Lett. 2019, 14, 304–308. [Google Scholar] [CrossRef]
- Mondal, J.; An, J.M.; Surwase, S.S.; Chakraborty, K.; Sutradhar, S.C.; Hwang, J.; Lee, J.; Lee, Y.-K. Carbon Nanotube and Its Derived Nanomaterials Based High Performance Biosensing Platform. Biosensors 2022, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Zhou, R.; Ping, J.; Ying, Y.; Xie, L. Recent advances in carbon nanotube-based biosensors for biomolecular detection. TrAC Trends Anal. Chem. 2022, 154, 116658. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.; Wei, X.; Sun, J.; Xu, D. Recent advances in morphology, aperture control, functional control and electrochemical sensors applications of carbon nanofibers. Anal. Biochem. 2022, 656, 114882. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Y.; You, T. Carbon nanofiber based electrochemical biosensors: A review. Anal. Methods 2010, 2, 202–211. [Google Scholar] [CrossRef]
- Vamvakaki, V.; Tsagaraki, K.; Chaniotakis, N. Carbon Nanofiber-Based Glucose Biosensor. Anal. Chem. 2006, 78, 5538–5542. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Wang, J.; Yu, A.; Wei, G. Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications. Nanomaterials 2019, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhou, J.; Lu, W.; Liu, Q.; Li, J. Carbon nanofiber-based composites for the construction of mediator-free biosensors. Biosens. Bioelectron. 2008, 23, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, X.; Ju, H. Detection of NADH and Ethanol Based on Catalytic Activity of Soluble Carbon Nanofiber with Low Overpotential. Anal. Chem. 2007, 79, 453–458. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, J.; Huang, Q.; Tao, Y.; Gu, R.; Li, H.-Y.; Liu, H. Electrochemical biosensor employing PbS colloidal quantum dots/Au nanospheres-modified electrode for ultrasensitive glucose detection. Nano Res. 2023, 16, 4085–4092. [Google Scholar] [CrossRef]
- Wu, R.; Feng, Z.; Zhang, J.; Jiang, L.; Zhu, J.-J. Quantum dots for electrochemical cytosensing. TrAC Trends Anal. Chem. 2022, 148, 116531. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Graphene Quantum Dot-Based Electrochemical Immunosensors for Biomedical Applications. Materials 2020, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Alfantazi, A.; Quraishi, M.A. Quantum dots as ecofriendly and aqueous phase substitutes of carbon family for traditional corrosion inhibitors: A perspective. J. Mol. Liq. 2021, 343, 117648. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Rajabzadeh-Khosroshahi, M.; Samadi, A.; Behzadmehr, R.; Rahdar, A.; Ferreira, L.F.R. Properties and application of carbon quantum dots (CQDs) in biosensors for disease detection: A comprehensive review. J. Drug Deliv. Sci. Technol. 2023, 80, 104156. [Google Scholar] [CrossRef]
- Cao, L.; Kiely, J.; Piano, M.; Luxton, R. Nanoparticle-based 3D membrane for impedimetric biosensor applications. Bioelectrochemistry 2020, 136, 107593. [Google Scholar] [CrossRef]
- Roushani, M.; Zalpour, N. Impedimetric ultrasensitive detection of trypsin based on hybrid aptamer-2DMIP using a glassy carbon electrode modified by nickel oxide nanoparticle. Microchem. J. 2022, 172, 106955. [Google Scholar] [CrossRef]
- Erkmen, C.; TiĞ, G.A.; Uslu, B. First label-free impedimetric aptasensor based on Au NPs/TiO2 NPs for the determination of leptin. Sens. Actuators B Chem. 2022, 358, 131420. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Talemi, R.P. Synergistic effect of magnetite and gold nanoparticles onto the response of a label-free impedimetric hepatitis B virus DNA biosensor. Mater. Sci. Eng. C 2016, 59, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Dinçkaya, E.; Kınık, Ö.; Sezgintürk, M.K.; Altuğ, Ç.; Akkoca, A. Development of an impedimetric aflatoxin M1 biosensor based on a DNA probe and gold nanoparticles. Biosens. Bioelectron. 2011, 26, 3806–3811. [Google Scholar] [CrossRef] [PubMed]
- Tasić, N.; Cavalcante, L.; Deffune, E.; Góes, M.S.; Paixão, T.R.L.C.; Gonçalves, L.M. Probeless and label-free impedimetric biosensing of D-dimer using gold nanoparticles conjugated with dihexadecylphosphate on screen-printed carbon electrodes. Electrochim. Acta 2021, 397, 139244. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Khoddami, E.; Rezaei, B. Aptamer@Au-o-phenylenediamine modified pencil graphite electrode: A new selective electrochemical impedance biosensor for the determination of insulin. Colloids Surf. B Biointerfaces 2017, 159, 47–53. [Google Scholar] [CrossRef]
- El Muttaqien, S.; Khoris, I.M.; Widayanti, T.; Pambudi, S.; Park, E.Y. Simple, versatile, and practical impedimetric immunosensor based on gold nanoparticle-polyaniline nanocomposite for clinical dengue virus detection. Biochem. Eng. J. 2023, 198, 109028. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, Y.; Li, X.; Lan, L.; Jiang, C.; Ping, J. Metallic Transition Metal Dichalcogenide Nanosheets as an Effective and Biocompatible Transducer for Electrochemical Detection of Pesticide. Anal. Chem. 2018, 90, 11658–11664. [Google Scholar] [CrossRef]
- Parra-Alfambra, A.M.; Casero, E.; Vázquez, L.; Quintana, C.; del Pozo, M.; Petit-Domínguez, M.D. MoS2 nanosheets for improving analytical performance of lactate biosensors. Sens. Actuators B Chem. 2018, 274, 310–317. [Google Scholar] [CrossRef]
- Yang, H.; Qin, J.; Zhang, M.; Shen, H.; Feng, J.; Hao, H. Label-free Lectin Impedimetric Biosensor Based on a Polyaniline/Graphene Nanocomposite for the Detection of Escherichia coli. Int. J. Electrochem. Sci. 2020, 15, 8913–8927. [Google Scholar] [CrossRef]
- Asadi, H.; Ramasamy, R.P. Graphene-based Electrochemical Biosensor for Impedimetric Detection of miRNAs as Potential Cancer Biomarkers. J. Electrochem. Soc. 2020, 167, 167523. [Google Scholar] [CrossRef]
- Wu, H.; Xi, K.; Xiao, S.; Ngai, S.; Zhou, C.; He, M.; Shi, K.; Yu, Y.; Yang, Y.; Chen, G.; et al. Self-assembled perylenetetracarboxylic acid-reduced graphene oxide film for high-sensitive impedimetric determination of thrombin. Surf. Coat. Technol. 2020, 402, 126491. [Google Scholar] [CrossRef]
- Filip, J.; Zavahir, S.; Klukova, L.; Tkac, J.; Kasak, P. Immobilization of concanavalin A lectin on a reduced graphene oxide-thionine surface by glutaraldehyde crosslinking for the construction of an impedimetric biosensor. J. Electroanal. Chem. 2017, 794, 156–163. [Google Scholar] [CrossRef]
- Bolat, G.; Akbal Vural, O.; Tugce Yaman, Y.; Abaci, S. Label-free impedimetric miRNA-192 genosensor platform using graphene oxide decorated peptide nanotubes composite. Microchem. J. 2021, 166, 106218. [Google Scholar] [CrossRef]
- Prakash, J.; Dey, A.; Uppal, S.; Alexander, R.; Kaushal, A.; Misra, H.S.; Dasgupta, K. Label-free rapid electrochemical detection of DNA hybridization using ultrasensitive standalone CNT aerogel biosensor. Biosens. Bioelectron. 2021, 191, 113480. [Google Scholar] [CrossRef]
- Jiang, H.; Lee, E.-C. Highly selective, reusable electrochemical impedimetric DNA sensors based on carbon nanotube/polymer composite electrode without surface modification. Biosens. Bioelectron. 2018, 118, 16–22. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Amini, M.; Rezaei, B. Impedimetric DNA-biosensor for the study of anti-cancer action of mitomycin C: Comparison between acid and electroreductive activation. Biosens. Bioelectron. 2014, 59, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Jaiswal, N.; Tiwari, I.; Ahmad, M.; Silva, S.R.P. Electrochemical biosensors based on in situ grown carbon nanotubes on gold microelectrode array fabricated on glass substrate for glucose determination. Microchim. Acta 2023, 190, 55. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Congur, G.; Mayer, G. Aptasensor platform based on carbon nanofibers enriched screen printed electrodes for impedimetric detection of thrombin. J. Electroanal. Chem. 2015, 758, 12–19. [Google Scholar] [CrossRef]
- Wang, R.-F.; Wang, R. Modification of polyacrylonitrile-derived carbon nanofibers and bacteriophages on screen-printed electrodes: A portable electrochemical biosensor for rapid detection of Escherichia coli. Bioelectrochemistry 2022, 148, 108229. [Google Scholar] [CrossRef]
- Tsekeli, T.R.; Sebokolodi, T.I.; Sipuka, D.S.; Olorundare, F.O.G.; Akanji, S.P.; Nkosi, D.; Arotiba, O.A. A poly (propylene imine) dendrimer–Carbon nanofiber based aptasensor for bisphenol A in water. J. Electroanal. Chem. 2021, 901, 115783. [Google Scholar] [CrossRef]
- Khandelwal, D.; Jain, D.; Solanki, P.R.; Rakesh Ranjan, K.; Das Mukherjee, M. A novel nanocomposite platform of mercaptopropionic acid stabilized CdSe quantum dots-graphene for impedimetric detection of low density lipoprotein. Mater. Lett. 2022, 308, 131236. [Google Scholar] [CrossRef]
- Moazampour, M.; Zare, H.R.; Shekari, Z. Femtomolar determination of an ovarian cancer biomarker (miR-200a) in blood plasma using a label free electrochemical biosensor based on l-cysteine functionalized ZnS quantum dots. Anal. Methods 2021, 13, 2021–2029. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Hu, Z.; Chen, Y.; Tao, Y.; Wang, L.; Li, L.; Wang, P.; Li, H.-Y.; Zhang, J.; et al. All-solid-state SARS-CoV-2 protein biosensor employing colloidal quantum dots-modified electrode. Biosens. Bioelectron. 2022, 202, 113974. [Google Scholar] [CrossRef]
- Riley, D.J. Electrochemistry in nanoparticle science. Curr. Opin. Colloid. Interface Sci. 2002, 7, 186–192. [Google Scholar] [CrossRef]
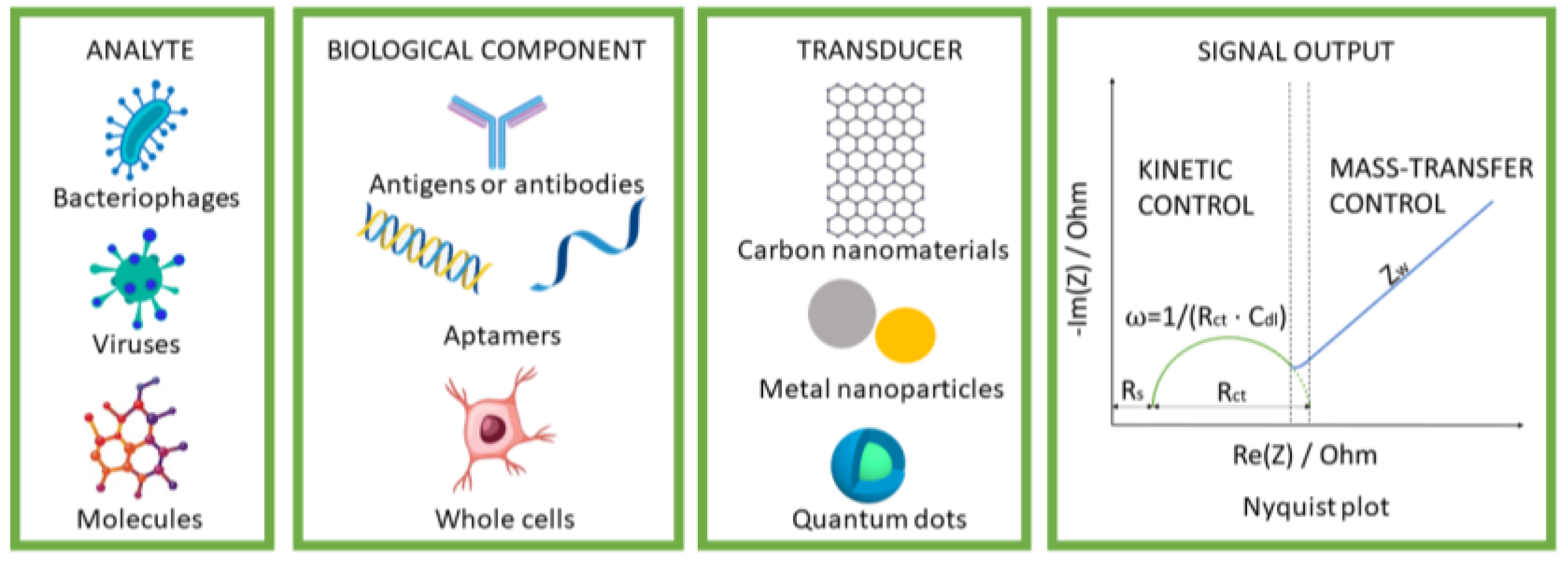

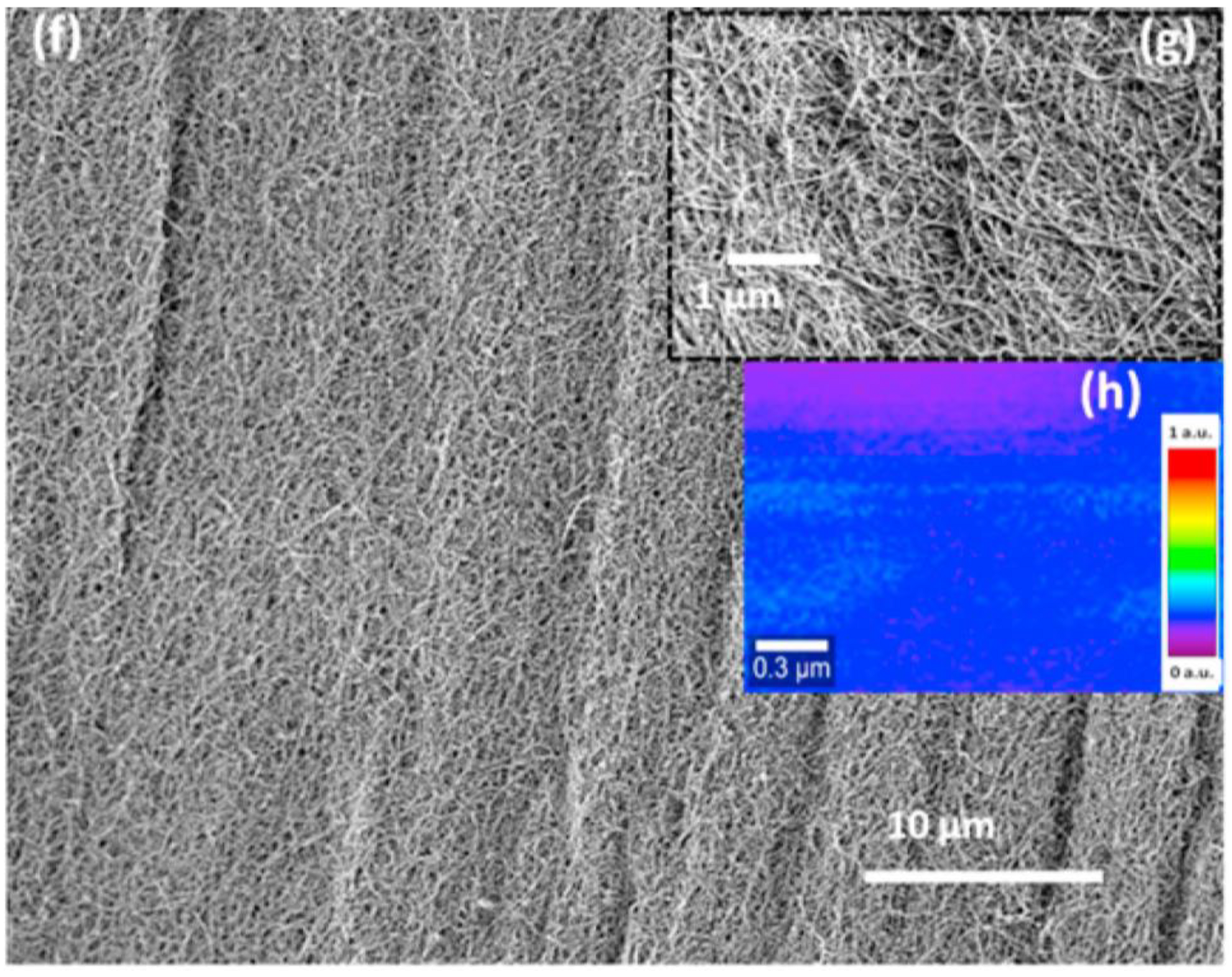
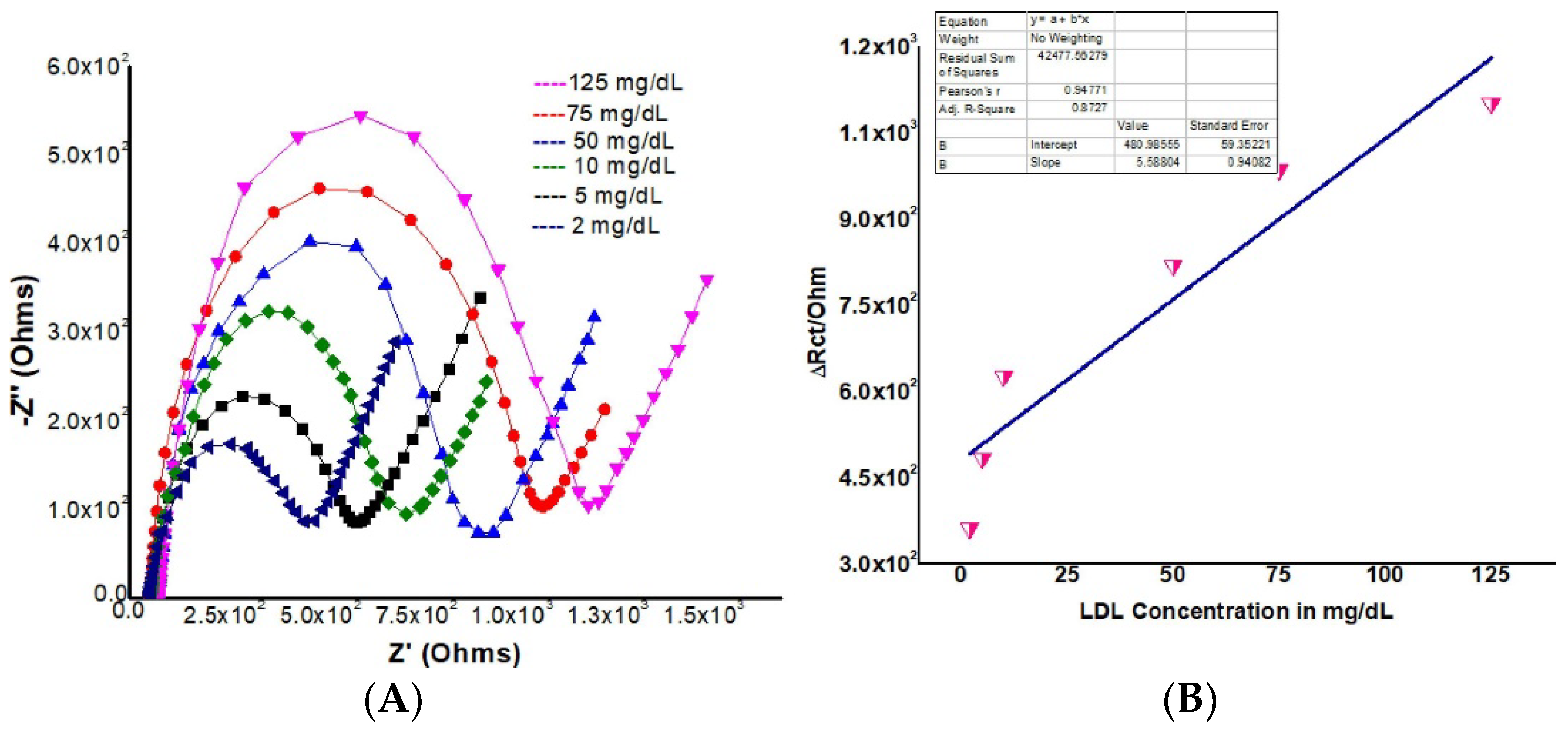
| Analyte | Recognition Element | Electrode | Linear Range | LOD | Reference |
|---|---|---|---|---|---|
| E. coli DH5α | ConA | PANI/G/GCE | 5.0 × 10 to 1.0 × 104 cells/mL | 43 cells/mL | [104] |
| ss-DNA | miRNA-21 | graphene-modified GCE | 10−5 to 10 nM | 3 × 10−6 nM | [105] |
| Thrombin | Aptamer | GCE/rGO/PTCA/TBA | 10−3 nM to 102 nM | 2 × 10−4 nM | [106] |
| INV | ConA | ErGO/Thi | 10−6 to 10 nM | / | [107] |
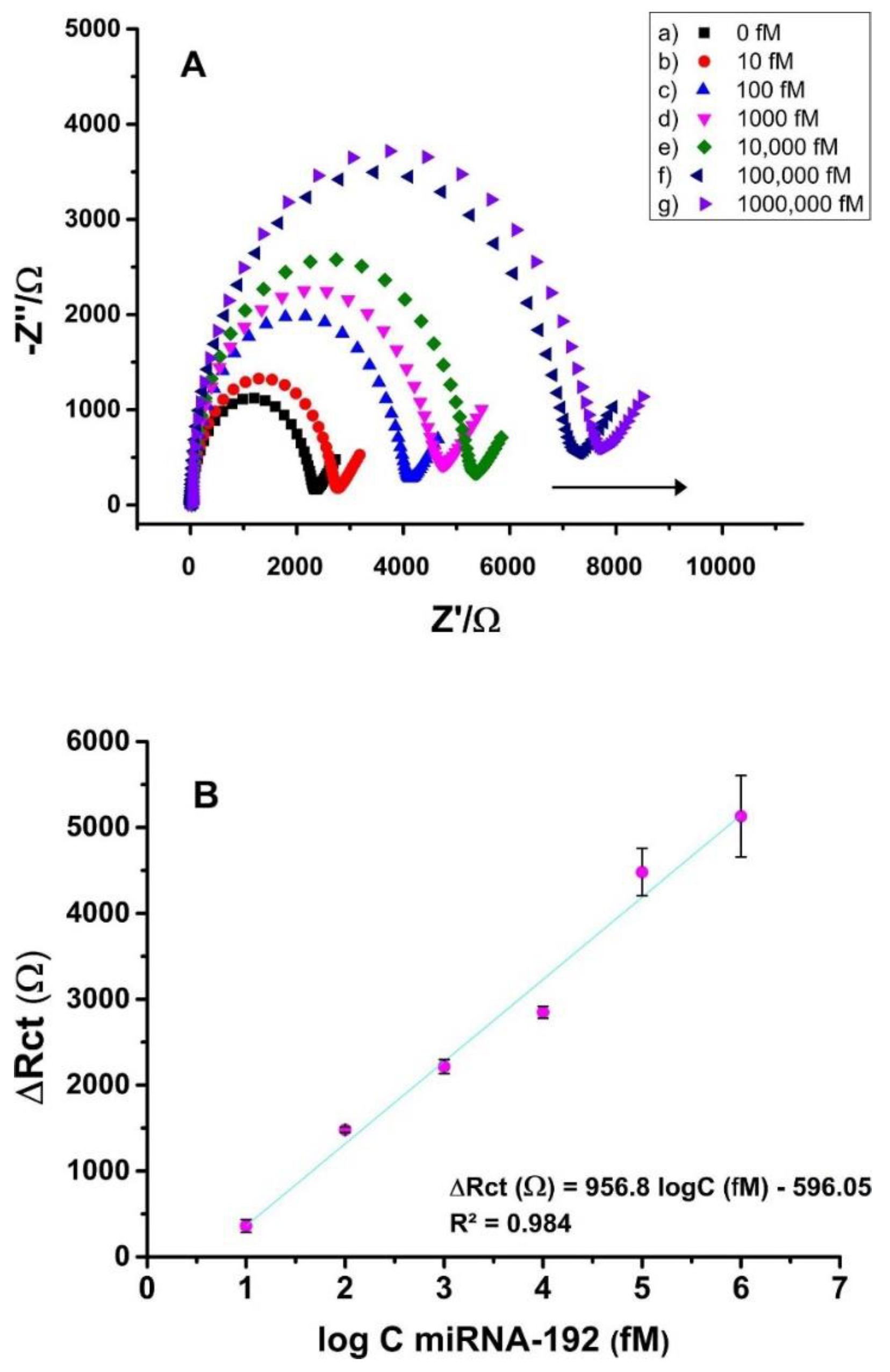
| miRNA-192 | ss-DNA probe | PNT-GO/PGE | 10−5 to 1 nM | 8 × 10−5 nM | [108] |
| Analyte | Recognition Element | Electrode | Linear Range | LOD | Reference |
|---|---|---|---|---|---|
| SARS-CoV-2 ss-DNA | Complimentary ss-DNA | CNT aerogel electrode | 10−1 to 103 nM | 10−3 nM | [109] |
| O-ss-DNA A-ss-DNA | Complimentary ss-DNA | MWCNT-polydimethylsiloxane electrodes | 5 × 10−2 to 10 nM | 2.5 × 10−2 nM | [110] |
| MMC | ds-DNA | MWCNT-PGE | / | / | [111] |
| Glucose | GOx | CNTs/Au MEA | 2 × 102 to 2.75 × 104 nM | 2 × 102 ± 1.4 nM | [112] |
| Analyte | Recognition Element | Electrode | Linear Range | LOD | Reference |
|---|---|---|---|---|---|
| THR | DNA APT | CNF-SPE | 5 × 103 to 2 × 104 ng/mL | 1.8 × 104 ng/mL | [113] |
| E. coli | Bacteriophage | CNF-SPE | 102 to 106 CFU/mL | 36 CFU/mL | [114] |
| BPA | NH-aptamer | GCE/CNFs-PPI | 1 to 10 nM | 6 × 10−2 nM | [115] |
| Analyte | Recognition Element | Electrode | Linear Range | LOD | Reference |
|---|---|---|---|---|---|
| Glucose | Glucose oxidase | PbS CQDs/AuNPs/GOx | 102 to 107 nM | 1.432 nM | [89] |
| LDL | AAB | rGO-CdSe QDs/ITO | 2 × 107 to 1.25 × 105 ng/mL | 3.76 × 107 ng/mL | [116] |
| miR-200a | L-cysteine | Cys-ZnS-QDs | 10−5 to 103 nM | 8.4 × 10−6 nM | [117] |
| SARS-CoV-2 antibodies | SARS-CoV-2 protein | CQDs | 0.969 to 4.99 ng/mL | 7.73 ng/mL | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štukovnik, Z.; Fuchs-Godec, R.; Bren, U. Nanomaterials and Their Recent Applications in Impedimetric Biosensing. Biosensors 2023, 13, 899. https://doi.org/10.3390/bios13100899
Štukovnik Z, Fuchs-Godec R, Bren U. Nanomaterials and Their Recent Applications in Impedimetric Biosensing. Biosensors. 2023; 13(10):899. https://doi.org/10.3390/bios13100899
Chicago/Turabian StyleŠtukovnik, Zala, Regina Fuchs-Godec, and Urban Bren. 2023. "Nanomaterials and Their Recent Applications in Impedimetric Biosensing" Biosensors 13, no. 10: 899. https://doi.org/10.3390/bios13100899
APA StyleŠtukovnik, Z., Fuchs-Godec, R., & Bren, U. (2023). Nanomaterials and Their Recent Applications in Impedimetric Biosensing. Biosensors, 13(10), 899. https://doi.org/10.3390/bios13100899






