Recent Advances in Aptasensing Strategies for Monitoring Phycotoxins: Promising for Food Safety
Abstract
1. Introduction
2. Overview of Developed Aptasensors for Phycotoxins
3. Monitoring of Phycotoxin Pollutants in Environmental and Drinking Water
3.1. Microcystin-Leucine-Arginine (MC-LR)
A Retrospect of Past Studies on Microcystins-LR (MCs) Aptasensor Platforms
3.2. Saxitoxins (STXs)
A Brief Lookat Aptasensing Stages on Saxitoxins (STXs)
| Target | Aptamer Modification | Aptamer Sequences 5′-3′ | Length (nt) | Kd (nM) | Ref |
|---|---|---|---|---|---|
| MC-LR | 5′-SH | TTT TTG GGT CCC GGG GTA GGG ATG GGA GGT ATG GAG GGG TCC TTG TTT CCC TCT TG- | 55 | 50 | [31] |
| 5′-HS-(CH2)6 | GGC GCC AAA CAG GAC CAC CAT GAC AAT TAC CCA TAC CAC CTC ATT ATG CCC CAT CTC CGC | 60 | 28 ± 8 nM | [30] | |
| - | GGC GCC AAA CAG GAC CAC CAT GAC AAT TAC CCA TAC CAC CTC ATT ATG CCC CAT CTC CGC | 60 | 28 ± 8 nM | [16] | |
| 5′-NH2-(CH2) 6 | TTT TTG GGT CCC GGG GTA GGG ATG GGA GGT ATG GAG GGG TCC TTG TTT CCC TCT TG | 56 | 50 | [29] | |
| 5′-NH2-(CH2) 6 | GGC GCC AAA CAG GAC CAC CAT GAC AAT TAC CCA TAC CAC CTC ATT ATG CCC CAT CTC CGC | 60 | 28 ± 8 nM | ||
| 5′-NH2-(CH2) 6 | CAC GCA ACA ACA CAA CAT GCC CAG CGC CTG GAA CAT ATC CTA TGA GTT AGT CCG CCC ACA | 60 | 92 | ||
| 5′-NH2-(CH2) 6 | CAC GCA CAG AAG ACA CCT ACA GGG CCA GAT CAC AAT CGG TTA GTG AAC TCG TAC GGC GCG | 60 | 103 | ||
| 5′-(SH)-(CH2)6- | GGC GCC AAA CAG GAC CAC CAT GAC AAT TAC CCA TAC CAC CTC ATT ATG CCC CAT CTC CGC | 60 | 28 ± 8 nM | [33] | |
| 5′-(SH)-(CH2)6 | GGC GCC AAA CAG GAC CAC CAT GAC AAT TAC CCA TAC CAC CTC ATT ATG CCC CAT CTC CGC | 60 | 28 ± 8 nM | [32] | |
| AAAAAAAAAAAAA | GGC GCC AAA CAG GAC CAC CAT GAC AAT TAC CCA TAC CAC CTC ATT ATG CCC CAT CTC CGC | 60 | 28 ± 8 nM | [20] | |
| FAM | GGC GCC AAA CAG GAC CAC CAT GAC AAT TAC CCA TAC CAC CTC ATT ATG CCC CAT CTC CGC | 60 | 28 ± 8 nM | [35] | |
| QD525 | GGC GCC AAA CAG GAC CAC CAT GAC AAT TAC CCA TAC CAC CTC ATT ATG CCC CAT CTC CGC | 60 | 28 ± 8 nM | [36] | |
| - | GGC GCC AAA CAG GAC CAC CAT GAC AAT TAC CCA TAC CAC CTC ATT ATG CCC CAT CTC CGC | 60 | 28 ± 8 nM | [37] | |
| 5′-SH-TTTTTT | GGC GCC AAA CAG GAC CAC CAT GAC AAT TAC CCA TAC CAC CTC ATT ATG CCC CAT CTC CGC | 60 | 28 ± 8 nM | [38] | |
| STX | 5′-SH-CTTCTTCTTCTT &TTCTTCTTC-3′ | TTG AGG GTC GCA TCC CGT GGA AAC AGG TTC ATT G | 34 | 133 nM | [43] |
| FAM | GGC GGG TTT TGA GGG TCG CAT CCC GTG GAA ACA GGT TCA TTG TTC CCG CC | 50 | 32.8nM | [44] | |
| 5′-Thiol | GGT ATT GAG GGT CGC ATC CCG TGG AAA CAT GTT CAT TGG GCG CAC TCC GCT TTC TGT AGA TGG CTC TAA CTC TCC TCT | 78 | -(long) | [45] | |
| HB-M-30f (HEX 5′-fluorophore (HEX, hexachlorofluorescein) & quencher (BHQ1, black-hole quencher 1)-3′ | TTG AGG GTC GCA TCC CGT GGA AAC AGG TTC ATT G | 34 | 133 nM | [46] |
4. Monitoring of Phycotoxin Pollutants in Fish
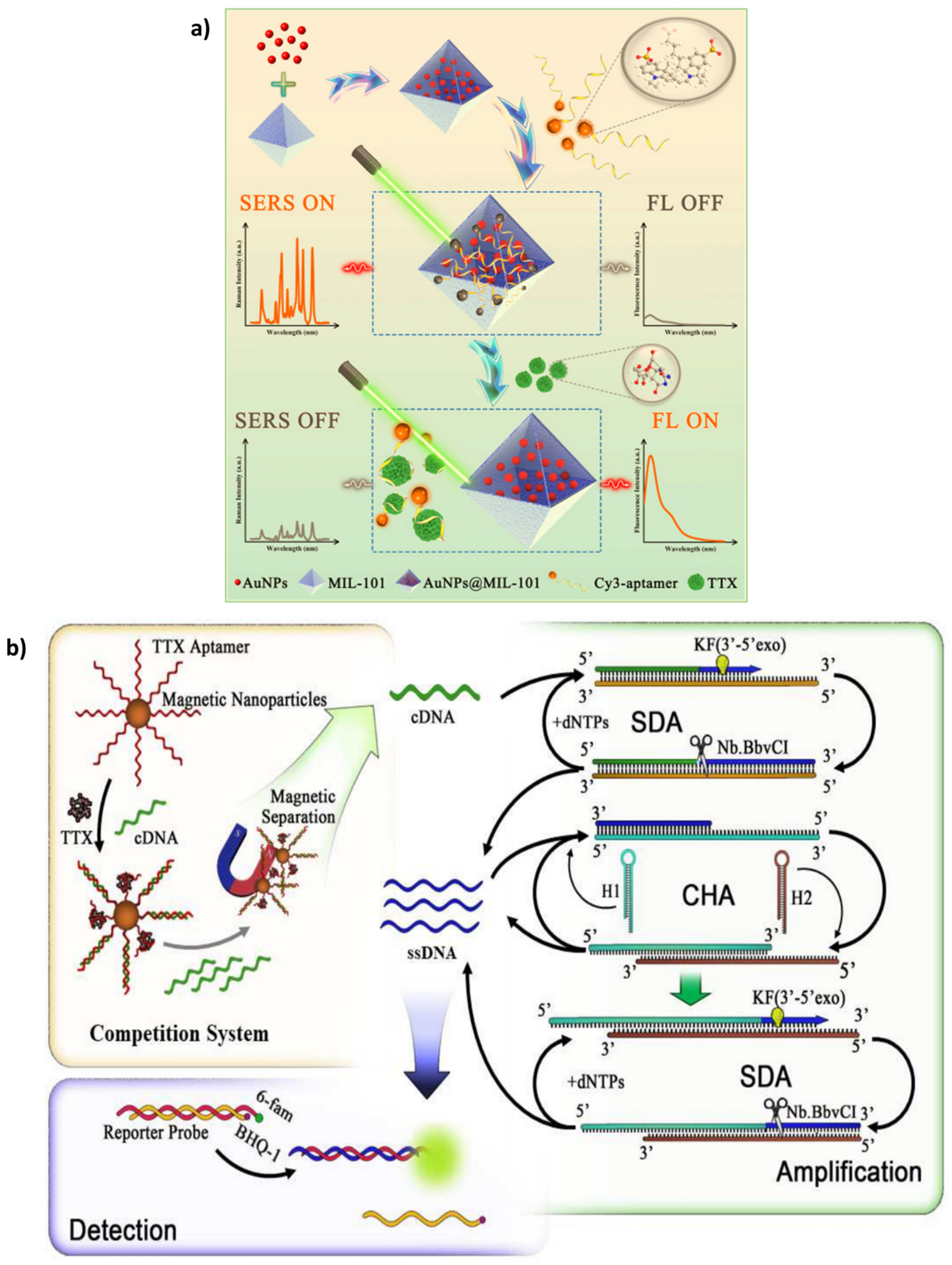
4.1. Tetrodotoxins (TTXs)
Some Aptasensing Platforms on Tetrodotoxins (TTXs)
| Target | Aptamer Modification | Aptamer Sequences 5′-3′ | Length (nt) | Kd (nM) | Ref |
|---|---|---|---|---|---|
| TTX | 5′-cyanine-3 dye | TCA AAT TTT CGT CTA CTC AAT CTT TCT GTC TTA TC | 35 | 1.20 ± 0.25 μM | [39] |
| 5′-HS-(CH2)6 | AAA AAT TTC ACA CGG GTG CCT CGG CTG TCC | 30 | - | [40] | |
| 5′-biotin- | TCA AAT TTT CGT CTA CTC AAT CTT TCT GTC TTA TC | 35 | 1.20 ± 0.25 μM | [42] |
5. Monitoring of Phycotoxin Pollutants in Fish and Shellfishes
5.1. Okadaic Acid (OA)
Recent Development in Aptasensing Platforms on Okadaic Acid (OA)
5.2. Brevetoxins (BTXs)
Aptasensing Platforms on Brevetoxins (BTXs)
5.3. Aptasensing Platforms for the Other Phycotoxins in Fish and Shellfish
5.3.1. Designed Aptasensing Platforms on Gonyautoxin (GTX)
5.3.2. A Developed Aptasensor for the Identity of Palytoxin (PTX)
5.3.3. A platform of Anatoxin-a (ATXs) Aptasensing
| Target | Aptamer Modification | Aptamer Sequences 5′-3′ | Length (nt) | Kd (nM) | Ref |
|---|---|---|---|---|---|
| ATXa | 5′-HO–(CH2)6–S–S–(CH2)6 | TGG CGA CAA GAA GAC GTA CAA ACA CGC ACC AGG CCG GAG TGG AGT ATT CTG AGG TCG G | 58 | 81.3 ± 8 nM | [27] |
| OA | - | GGT CAC CAA CAA CAG GGA GCG CTA CGC GAA GGG TCA ATG TGA CGT CAT GCG GAT GTG TGG | 60 | 23 ± 1.52 nM | [47] |
| 3′-Biotin 3′-FAM | ATT TGA CCA TGT CGA GGG AGA CGC GCA GTC GCT ACC ACC T | 40 | - | [48] | |
| 5′-Thiol- | GGT CAC CAA CAA CAG GGA GCG CTA CGC GAA GGG TCA ATG TGA CGT CAT GCG GAT GTG TGG | 60 | 23 ± 1.52 nM | [70] | |
| - | GGT CAC CAA CAA CAG GGA GCG CTA CGC GAA GGG TCA ATG TGA CGT CAT GCG GAT GTG TGG | 60 | 23 ± 1.52 nM | [49] | |
| 5′-(SH)-(CH2)6- | GGT CAC CAA CAA CAG GGA GCG CTA CGC GAA GGG TCA ATG TGA CGT CAT GCG GAT GTG TGG | 60 | 23 ± 1.52 nM | [7] | |
| 5′-(SH)-(CH2)6 3′-Biotin | GGT CAC CAA CAA CAG GGA GCG CTA CGC GAA GGG TCA ATG TGA CGT CAT GCG GAT GTG TGG | 60 | 23 ± 1.52 nM | ||
| 5′-(SH)-(CH2)6- | GGC CGC GAG AGA GAC AAC AAG GAT ATA TAT TAT ATG TCG GTT GTA GTG TTG GGT TGC G | 58 | 92 nM | ||
| 5′-SH-(CH2)6- | GGT CAC CAA CAA CAG GGA GCG CTA CGC GAA GGG TCA ATG TGA CGT CAT GCG GAT GTG TGG | 60 | 23 ± 1.52 nM | [50] | |
| GTX | - | AAC CTT TGG TCG GGC AAG GTA GGT T | 25 | 17.7nM | [25] |
| BTX | 5′-SH-(CH2)6 | GGC CAC CAA ACC ACA CCG TCG CAA CCG CGA GAA CCG AAG TAG TGA TCA TGT CCC TGC G | 58 | 92 nM | [51] |
| 5′-ThioMC6-D | AT ACC AGC TTA TTC AAT TGG CCA CCA AAC CAC ACC GTC GCA ACC GCG AGA ACC GAA GTA GTG ATC ATG TCC CTG CGT GAG ATA GTA AGT GCA ATC T | 96 | - | [9] | |
| 5′-SH–(CH2)6 | GTG CGT CCC TGT ACT AGT GAT GAA GCC AAG AGC GCC AAC GCT GCC ACA CCA AAC CAC CGG | 60 | 42 nM | [52] | |
| 5′-NH2-(CH2)6 | GTT GCC GTC TCC TTA TCC CAC CAC TGC CGA CAC CAC CCC CCC GCG AGA GCG AGA GAG CAC T | 61 | 96 nM |
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Botana, L.M.; Alfonso, A.; Vale, C.; Vilariño, N.; Rubiolo, J.; Alonso, E.; Cagide, E. Chapter One—The Mechanistic Complexities of Phycotoxins: Toxicology of Azaspiracids and Yessotoxins. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–33. [Google Scholar]
- Butzke, D.; Grune, B.; Kugler, J.; Oelgeschläger, M.; Seiler, A.; Sittner, D.; Luch, A. Chapter 3—The Advent of the Golden Era of Animal Alternatives. In Animal Models for the Study of Human Disease; Conn, P.M., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 49–73. [Google Scholar]
- Zhao, Y.; Li, L.; Yan, X.; Wang, L.; Ma, R.; Qi, X.; Wang, S.; Mao, X. Emerging roles of the aptasensors as superior bioaffinity sensors for monitoring shellfish toxins in marine food chain. J. Hazard. Mater. 2022, 421, 126690. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gautam, S.; Kumar, S. Phycotoxins. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 25–29. [Google Scholar]
- Seymour, B.; Andreosso, A.; Seymour, J. Chapter 7—Cardiovascular Toxicity from Marine Envenomation. In Heart and Toxins; Ramachandran, M., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 203–223. [Google Scholar]
- Gómez-Hens, A. FLUORESCENCE: Food Applications. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 186–194. [Google Scholar]
- Wang, Y.; Rao, D.; Wu, X.; Zhang, Q.; Wu, S. Aptamer-based microcantilever-array biosensor for ultra-sensitive and rapid detection of okadaic acid. Microchem. J. 2021, 160, 105644. [Google Scholar] [CrossRef]
- Kulabhusan, P.K.; Campbell, K. Recent trends in the detection of freshwater cyanotoxins with a critical note on their occurrence in Asia. Trends Environ. Anal. Chem. 2021, 32, e00150. [Google Scholar] [CrossRef]
- Ramalingam, S.; Hayward, G.L.; Singh, A. A reusable QCR aptasensor for the detection of Brevetoxin-2 in shellfish. Talanta 2021, 233, 122503. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, S.M. Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon 2010, 56, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, Y.; Dong, Y.; Han, X.; Wang, S.; Liang, X. Aptamers and Aptasensors for Highly Specific Recognition and Sensitive Detection of Marine Biotoxins: Recent Advances and Perspectives. Toxins 2018, 10, 427. [Google Scholar] [CrossRef]
- Cunha, I.; Biltes, R.; Sales, M.; Vasconcelos, V. Aptamer-Based Biosensors to Detect Aquatic Phycotoxins and Cyanotoxins. Sensors 2018, 18, 2367. [Google Scholar] [CrossRef]
- Ye, W.; Liu, T.; Zhang, W.; Zhu, M.; Liu, Z.; Kong, Y.; Liu, S. Marine Toxins Detection by Biosensors Based on Aptamers. Toxins 2020, 12, 1. [Google Scholar] [CrossRef]
- Eissa, S.; Ng, A.; Siaj, M.; Zourob, M. Label-Free Voltammetric Aptasensor for the Sensitive Detection of Microcystin-LR Using Graphene-Modified Electrodes. Anal. Chem. 2014, 86, 7551–7557. [Google Scholar] [CrossRef]
- Dolati, S.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Recent nucleic acid based biosensors for Pb2+ detection. Sens. Actuators B Chem. 2017, 246, 864–878. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, G.; Zheng, T.; Lin, C.; Lin, X.; Xie, Z. Aptamer-functionalized metal-organic framework-based electrospun nanofibrous composite coating fiber for specific recognition of ultratrace microcystin in water. J. Chromatogr. A 2021, 1656, 462542. [Google Scholar] [CrossRef]
- Kadam, U.S.; Hong, J.C. Advances in aptameric biosensors designed to detect toxic contaminants from food, water, human fluids, and the environment. Trends Environ. Anal. Chem. 2022, 36, e00184. [Google Scholar] [CrossRef]
- Bilibana, M.P.; Citartan, M.; Fuku, X.; Jijana, A.N.; Mathumba, P.; Iwuoha, E. Aptamers functionalized hybrid nanomaterials for algal toxins detection and decontamination in aquatic system: Current progress, opportunities, and challenges. Ecotoxicol. Environ. Saf. 2022, 232, 113249. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, Z.; Housaindokht, M.R.; Verdian, A.; Bozorgmehr, M.R. Simultaneous detection and determination of mercury (II) and lead (II) ions through the achievement of novel functional nucleic acid-based biosensors. Biosens. Bioelectron. 2018, 116, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Abnous, K.; Danesh, N.M.; Nameghi, M.A.; Ramezani, M.; Alibolandi, M.; Lavaee, P.; Taghdisi, S.M. An ultrasensitive electrochemical sensing method for detection of microcystin-LR based on infinity-shaped DNA structure using double aptamer and terminal deoxynucleotidyl transferase. Biosens. Bioelectron. 2019, 144, 111674. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, Z.; Moeenfard, M.; Abnous, K.; Taghdisi, S.M. Nano-gold mediated aptasensor for colorimetric monitoring of acrylamide: Smartphone readout strategy for on-site food control. Food Chem. 2023, 399, 133983. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.; Zaczek-Moczydlowska, M.A.; Edwards, C.; Turner, A.D.; Miller, P.I.; Moore, H.; McKinney, A.; Lawton, L.; Campbell, K. Current Trends and Challenges for Rapid SMART Diagnostics at Point-of-Site Testing for Marine Toxins. Sensors 2021, 21, 2499. [Google Scholar] [CrossRef]
- Majdinasab, M.; Marty, J.L. Recent Advances in Electrochemical Aptasensors for Detection of Biomarkers. Pharmaceuticals 2022, 15, 995. [Google Scholar] [CrossRef]
- Gao, S.; Zheng, X.; Hu, B.; Sun, M.; Wu, J.; Jiao, B.; Wang, L. Enzyme-linked, aptamer-based, competitive biolayer interferometry biosensor for palytoxin. Biosens. Bioelectron. 2017, 89, 952–958. [Google Scholar] [CrossRef]
- Gao, S.; Hu, B.; Zheng, X.; Cao, Y.; Liu, D.; Sun, M.; Binghua, J.; Wang, L. Gonyautoxin 1/4 aptamers with high-affinity and high-specificity: From efficient selection to aptasensor application. Biosens. Bioelectron. 2016, 79, 938–944. [Google Scholar] [CrossRef]
- Handy, S.M.; Yakes, B.J.; DeGrasse, J.A.; Campbell, K.; Elliott, C.T.; Kanyuck, K.M.; DeGrasse, S.L. First report of the use of a saxitoxin–protein conjugate to develop a DNA aptamer to a small molecule toxin. Toxicon 2013, 61, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Elshafey, R.; Siaj, M.; Zourob, M. DNA aptamers selection and characterization for development of label-free impedimetric aptasensor for neurotoxin anatoxin-a. Biosens. Bioelectron. 2015, 68, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Sistla, S.; Veerabhadraiah, S.R.; Bettadaiah, B.K.; Thakur, M.S.; Bhatt, P. DNA aptamer selection and detection of marine biotoxin 20 Methyl Spirolide G. Food Chem. 2021, 363, 130332. [Google Scholar] [CrossRef]
- Xiang, A.; Lei, X.; Ren, F.; Zang, L.; Wang, Q.; Zhang, J.; Wang, Q.; Zhang, J.; Lu, Z.; Guo, Y. An aptamer-based immunoassay in microchannels of a portable analyzer for detection of microcystin-leucine-arginine. Talanta 2014, 130, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, Y.; Liu, P.; Yang, L.; Li, L.; Zhang, Q.; Zayed, Y.; Khan, M.Z.H. A highly sensitive electrochemical aptasensor for detection of microcystin-LR based on a dual signal amplification strategy. Analyst 2019, 144, 1671–1678. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, H.; Xu, Y.; Gao, X.; Qiu, B.; Chen, X.; Chen, G. Determination of microcystin-LR in water by a label-free aptamer based electrochemical impedance biosensor. Talanta 2013, 103, 371–374. [Google Scholar] [CrossRef]
- Xie, W.; He, S.; Fang, S.; Liang, L.; Shi, B.; Wang, D. Visualizing of AuNPs protection aptamer from DNase I enzyme digestion based on Nanopipette and its use for Microcystin-LR detection. Anal. Chim. Acta 2021, 1173, 338698. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Wu, S.; Zhang, Q. Label-free aptamer-based detection of microcystin-LR using a microcantilever array biosensor. Sens. Actuators B Chem. 2018, 260, 42–47. [Google Scholar] [CrossRef]
- Li, X.; Cheng, R.; Shi, H.; Tang, B.; Xiao, H.; Zhao, G. A simple highly sensitive and selective aptamer-based colorimetric sensor for environmental toxins microcystin-LR in water samples. J. Hazard. Mater. 2016, 304, 474–480. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Ghows, N.; Mousavi Shaegh, S.A.; Abnous, K. A novel fluorescent aptasensor for ultrasensitive detection of microcystin-LR based on single-walled carbon nanotubes and dapoxyl. Talanta 2017, 166, 187–192. [Google Scholar] [CrossRef]
- Lee, E.H.; Son, A. Fluorescence resonance energy transfer based quantum dot-Aptasensor for the selective detection of microcystin-LR in eutrophic water. Chem. Eng. J. 2019, 359, 1493–1501. [Google Scholar] [CrossRef]
- Wang, F.; Liu, S.; Lin, M.; Chen, X.; Lin, S.; Du, X.; Li, H.; Ye, H.; Qiu, B.; Lin, Z. Colorimetric detection of microcystin-LR based on disassembly of orient-aggregated gold nanoparticle dimers. Biosens. Bioelectron. 2015, 68, 475–480. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, L.; Zhou, S.; Xie, W.; He, S.; Wang, Y.; Tlili, C.; Tong, S.; Wang, D. Label-Free Sensitive Detection of Microcystin-LR via Aptamer-Conjugated Gold Nanoparticles Based on Solid-State Nanopores. Langmuir 2018, 34, 14825–14833. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huo, Y.; Deng, S.; Li, G.; Li, S.; Huang, L.; Ren, S.; Gao, Z. A facile dual-mode aptasensor based on AuNPs@MIL-101 nanohybrids for ultrasensitive fluorescence and surface-enhanced Raman spectroscopy detection of tetrodotoxin. Biosens. Bioelectron. 2022, 201, 113891. [Google Scholar] [CrossRef]
- Lan, Y.; Qin, G.; Wei, Y.; Dong, C.; Wang, L. Highly sensitive analysis of tetrodotoxin based on free-label fluorescence aptamer sensing system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, M.; Gao, R.; Lu, F.; Liu, J.; Huang, Q. Repurposing of thermally stable nucleic-acid aptamers for targeting tetrodotoxin (TTX). Comput. Struct. Biotechnol. J. 2022, 20, 2134–2142. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Wu, P.; Wang, W.; Cheng, Y.; Huang, L.; Ning, B.; Gao, Z. Development of a highly sensitive detection method for TTX based on a magnetic bead-aptamer competition system under triple cycle amplification. Anal. Chim. Acta 2020, 1119, 18–24. [Google Scholar] [CrossRef]
- Qi, X.; Yan, X.; Zhao, L.; Huang, Y.; Wang, S.; Liang, X. A facile label-free electrochemical aptasensor constructed with nanotetrahedron and aptamer-triplex for sensitive detection of small molecule: Saxitoxin. J. Electroanal. Chem. 2020, 858, 113805. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; Yan, X.; Qi, X.; Wang, L.; Ma, R.; Wang, S.; Mao, X. Development of a terminal-fixed aptamer and a label-free colorimetric aptasensor for highly sensitive detection of saxitoxin. Sens. Actuators B Chem. 2021, 344, 130320. [Google Scholar] [CrossRef]
- Park, J.A.; Kwon, N.; Park, E.; Kim, Y.; Jang, H.; Min, J.; Lee, T. Electrochemical biosensor with aptamer/porous platinum nanoparticle on round-type micro-gap electrode for saxitoxin detection in fresh water. Biosens. Bioelectron. 2022, 210, 114300. [Google Scholar] [CrossRef]
- Cheng, S.; Tang, F.; Yang, C.T.; Zhao, X.; Wang, J.; Thierry, B.; Bansal, V.; Dai, J.; Zhou, X. Study of the binding way between saxitoxin and its aptamer and a fluorescent aptasensor for detection of saxitoxin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Rouhbakhsh, Z.; Ho, T.Y.; Verdian, A.; Chen, C.H. Detection of okadaic acid using a liquid crystal-based aptasensor by exploiting the signal enhancement effect of gold nanoparticles. Biosens. Bioelectron. X 2022, 11, 100148. [Google Scholar]
- Shan, W.; Sun, J.; Liu, R.; Xu, W.; Shao, B. Duplexed aptamer-isothermal amplification-based nucleic acid-templated copper nanoparticles for fluorescent detection of okadaic acid. Sens. Actuators B Chem. 2022, 352, 131035. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, P.; Chen, Y.; Bai, X.; Du, L.; Chen, W.; Wu, C.; Wang, P. Piezoelectric aptasensor with gold nanoparticle amplification for the label-free detection of okadaic acid. Sens. Actuators B Chem. 2021, 346, 130446. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Zhu, P.; Ge, S.; Zhang, L.; Zhang, Y.; Yu, J. Multiple cooperative amplification paper SERS aptasensor based on AuNPs/3D succulent-like silver for okadaic acid quantization. Sens. Actuators B Chem. 2021, 344, 130174. [Google Scholar] [CrossRef]
- Eissa, S.; Siaj, M.; Zourob, M. Aptamer-based competitive electrochemical biosensor for brevetoxin-2. Biosens. Bioelectron. 2015, 69, 148–154. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Üstündağ, Z.; Şahin, S. Spectroscopic ellipsometry methods for brevetoxin detection. Talanta 2022, 237, 122897. [Google Scholar] [CrossRef] [PubMed]
- Akyol, Ç.; Ozbayram, E.G.; Accoroni, S.; Radini, S.; Eusebi, A.L.; Gorbi, S.; Vignaroli, C.; Bacchiocchi, S.; Campacci, D.; Gigli, F.; et al. Monitoring of cyanobacterial blooms and assessing polymer-enhanced microfiltration and ultrafiltration for microcystin removal in an Italian drinking water treatment plant. Environ. Pollut. 2021, 286, 117535. [Google Scholar] [CrossRef]
- Luckas, B.; Krüger, T.; Röder, K. 14—Phycotoxins and food safety. In Chemical Contaminants and Residues in Food; Schrenk, D., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 342–393. [Google Scholar]
- He, X.; Wang, H.; Zhuang, W.; Liang, D.; Ao, Y. Risk prediction of microcystins based on water quality surrogates: A case study in a eutrophicated urban river network. Environ. Pollut. 2021, 275, 116651. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, X.; Liu, Q.; Pang, Y.; Cao, Y.; Zhang, T. Safety assessment of graphene oxide and microcystin-LR complex: A toxicological scenario beyond physical mixture. Part. Fibre Toxicol. 2022, 19, 26. [Google Scholar] [CrossRef]
- Van der Merwe, D. Chapter 31—Freshwater cyanotoxins. In Biomarkers in Toxicology; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 539–548. [Google Scholar]
- Van der Merwe, D. Chapter 31—Cyanobacterial (Blue-Green Algae) Toxins. In Handbook of Toxicology of Chemical Warfare Agents, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 421–429. [Google Scholar]
- Wang, Q.; Cheng, X.; Li, H.; Yu, F.; Wang, Q.; Yu, M.; Lu, D.; Xia, J. A novel DNA quantum dots/aptamer-modified gold nanoparticles probe for detection of Salmonella typhimurium by fluorescent immunoassay. Mater. Today Commun. 2020, 25, 101428. [Google Scholar] [CrossRef]
- Wu, J.; Yu, C.; Yu, Y.; Chen, J.; Zhang, C.; Gao, R.; Mu, X.; Geng, Y.; He, J. Ultra-sensitive detection of microcystin-LR with a new dual-mode aptasensor based on MoS2-PtPd and ZIF-8-Thi-Au. Sens. Actuators B Chem. 2020, 305, 127280. [Google Scholar] [CrossRef]
- Han, S.; Choi, J.S.; Kwon, J. A dual-response ratiometric fluorescent sensor by europium-doped CdTe quantum dots for visual and colorimetric detection of tetracycline. J. Hazard. Mater. 2020, 398, 122894. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Sun, Z.; Sun, Y.; Gui, R. Dual-signal ratiometric platforms: Construction principles and electrochemical biosensing applications at the live cell and small animal levels. TrAC Trends Anal. Chem. 2021, 134, 116124. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, B.; Gao, S.X.; Liu, D.J.; Sun, M.J.; Jiao, B.H.; Wang, L.H. A saxitoxin-binding aptamer with higher affinity and inhibitory activity optimized by rational site-directed mutagenesis and truncation. Toxicon 2015, 101, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, Z.; Abnous, K.; Taghdisi, S.M.; Verdian, A. Liquid crystal-based biosensors as lab-on-chip tools: Promising for future on-site detection test kits. TrAC Trends Anal. Chem. 2021, 142, 116325. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Abnous, K.; Taghdisi, S.M.; Verdian, A. A novel liquid crystal-based aptasensor for ultra-low detection of ochratoxin a using a π-shaped DNA structure: Promising for future on-site detection test strips. Biosens. Bioelectron. 2021, 191, 113457. [Google Scholar] [CrossRef]
- Ng, A.; Chinnappan, R.; Eissa, S.; Liu, H.; Tlili, C.; Zourob, M. Selection, Characterization, and Biosensing Application of High Affinity Congener-Specific Microcystin-Targeting Aptamers. Environ. Sci. Technol. 2012, 46, 10697–10703. [Google Scholar] [CrossRef]
- Cembella, A.D.; Durán-Riveroll, L.M. Chapter One—Marine guanidinium neurotoxins: Biogenic origins and interactions, biosynthesis and pharmacology. In Advances in Neurotoxicology; Novelli, A., Fernández-Sánchez, M.T., Aschner, M., Costa, L.G., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–47. [Google Scholar]
- Novelli, A.; Hernandez-Daranas, A.; Cabrera-García, D.; Ascencio Salazar, F.; Fernández-Sánchez, M.T. Chapter Five—Potential neurotoxins: Okadaic acid and analogs. In Advances in Neurotoxicology; Novelli, A., Fernández-Sánchez, M.T., Aschner, M., Costa, L.G., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 193–221. [Google Scholar]
- Beasley, V.R. Harmful Algal Blooms (Phycotoxins). In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Ramalingam, S.; Chand, R.; Singh, C.B.; Singh, A. Phosphorene-gold nanocomposite based microfluidic aptasensor for the detection of okadaic acid. Biosens. Bioelectron. 2019, 135, 14–21. [Google Scholar] [CrossRef]
- Rhouati, A.; Marty, J.L.; Vasilescu, A. Electrochemical biosensors combining aptamers and enzymatic activity: Challenges and analytical opportunities. Electrochim. Acta 2021, 390, 138863. [Google Scholar] [CrossRef]
- Kuswandi, B.; Futra, D.; Heng, L.Y. Chapter 15—Nanosensors for the Detection of Food Contaminants. In Nanotechnology Applications in Food; Oprea, A.E., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 307–333. [Google Scholar]
- Murray, T.F. Chapter Three—Neurotoxic: Ciguatoxin and brevetoxin—From excitotoxicity to neurotherapeutics. In Advances in Neurotoxicology; Novelli, A., Fernández-Sánchez, T.M., Aschner, M., Costa, L.G., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 89–104. [Google Scholar]
- Haschek, W.M.; Rousseaux, C.G.; Wallig, M.A. Toxicologic Pathology: An Introduction. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 1–9. [Google Scholar]
- Lawrence, D.; McLinskey, N.; Huff, J.S.; Holstege, C.P. CHAPTER 4—Toxin-Induced Neurologic Emergencies. In Clinical Neurotoxicology; Dobbs, M.R., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 30–46. [Google Scholar]
- Ming, T.; Luo, J.; Liu, J.; Sun, S.; Xing, Y.; Wang, H.; Xiao, G.; Deng, Y.; Cheng, Y.; Yang, Z.; et al. Paper-based microfluidic aptasensors. Biosens. Bioelectron. 2020, 170, 112649. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Sekhon, S.S.; Shin, W.R.; Kim, H.C.; Min, J.; Ahn, J.Y.; Kim, Y.H. De novo post-SELEX optimization of a G-quadruplex DNA aptamer binding to marine toxin gonyautoxin 1/4. Comput. Struct. Biotechnol. J. 2020, 18, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.G.; Stahl, F. Aptazymes: Expanding the Specificity of Natural Catalytic Nucleic Acids by Application of In Vitro Selected Oligonucleotides. Adv. Biochem. Eng. Biotechnol. 2020, 170, 107–119. [Google Scholar] [PubMed]
- Hinojosa, M.G.; Prieto, A.I.; Muñoz-Castro, C.; Sánchez-Mico, M.V.; Vitorica, J.; Cameán, A.M.; Jos, Á. Cytotoxicity and Effects on the Synapsis Induced by Pure Cylindrospermopsin in an E17 Embryonic Murine Primary Neuronal Culture in a Concentration- and Time-Dependent Manner. Toxins 2022, 14, 175. [Google Scholar] [CrossRef]
- Otero, P.; Silva, M. Chapter 7—The role of toxins: Impact on human health and aquatic environments. In The Pharmacological Potential of Cyanobacteria; Lopes, G., Silva, M., Vasconcelos, V., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 173–199. [Google Scholar]
- Lin, Y.; Bariya, M.; Javey, A. Wearable Biosensors for Body Computing. Adv. Funct. Mater. 2020, 31, 2008087. [Google Scholar] [CrossRef]



| Target | Method | LOD | Linear Range | Analytical Signal vs. Concentration Slope | Real Sample | Characteristics | Ref |
|---|---|---|---|---|---|---|---|
| MC-LR | AAIA | 0.3 μg/L | 0.5–4.0 μg/L | Y = 9.2288x + 8.1072 R2 = 0.968 | Drinking water | A portable analyzer, assay time: 35 min | [29] |
| Infinity-shaped DNA structure and TdT enzyme | 20 pM in tap water, 35 pM in serum | 70 pM–900 nM for tap water and 100 pM–750 nM in serum | Y = 1.181x − 2.0437 R2 = 0.99924 | Tap water & serum | Assay time: 90 min plus 12 h preparation phase, using DPV analysis | [20] | |
| Electrospinning & seeded growth on MOF of the solid-phase microextraction (SPME) fiber | 0.003 ng/mL | 0.008–1.000 ng/mL | Y = 12.135665x − 33.418 R2 = 0.9996 | Drinking water | Assay time of 20 min plus 72 h preparation phase | [16] | |
| Dual signal amplification system: HRP enzyme & electroactive nanomaterials | 0.002 nM | 0.005–30 nM | Y = 6.28logx + 16.39 R2 = 0.994 | Water from taps, reservoirs & rivers | About 40 h for the synthesis of an AuNP@ MoS2-TiONB nanocomposite and 2 h for the construction | [30] | |
| Electrochemical impedance spectroscopy (EIS) | 1.8 × 10−11 mol/L | 1.0 × 10−7–5 × 10−11 mol/L | Y = 9.9724logx + 111.24 R2 = 0.997 | Water | About 6 h for assay time and preparation phase | [31] | |
| Signal amplification strategy with DNase I | 0.22 nM | 0.25–20 nM | Y = 0.55x − 3.630 R2 = 0.9996 | Drinking water | Directly analysis | [32] | |
| Cantilever array | 1 μg/L | 1–50 μg/L | Y = 2.11x + 9.01 R2 = 0.97 | Buffer | Label-free analysis, About 85 min for assay time and preparation phase | [33] | |
| Gold nanoparticles (AuNPs) and plasma resonance | 0.37 nM | 0.5 nM–7.5 μM | Y = 0.1662logx + 0.2533 R2 = 0.997 | Water | Label-free analyzer, quick detecting: 30 min | [34] | |
| Graphene-modified screen-printed carbon electrodes (SPEs) | 1.9 pM in buffer 1.67 pM inthe spiked sample | 0.1 pM–1.0 nM | Y = 12.20logx + 28.35 R2 = 0.988 | Buffer | Label-free, a stable assay | [14] | |
| Single-walled carbon nanotubes (SWNTs) & dapoxyl dye | 138 pM (0.137 µg/L) | 0.4–1200 nM. | Y = 0.0089x − 2.76 R2 = 0.9929 | Tap water & serum | Label-free analyzer only 75 min for assay time | [35] | |
| Fluorescence resonance energy transfer (FRET)-based quantum dot (QD) | 10−4 μg/L | 10−4–102 ng/mL | Y = 0.15logx + 0.93 R2 = 0.94 | Eutrophic water | Short lifetime unsuitable for on-site analysis of the target | [36] | |
| Oriented formation of gold nanoparticle (AuNP) dimers | 0.05 nM | 0.1–250 nM | R2 = 0.990119 | Water | Achieving results within 5 min | [37] | |
| Solid-state nanopores | 1 μg/L | 0.1 nM–20 μM | denotes | Water | - | [38] | |
| TTX | SERS | 0.006 ng/mL | 0.01–300 ng/mL | Y = 1470.04x + 3386.77 R2 = 0.9958 | Pufferfish and clam meat | Without cumbersome procedures, exhibited signal responses within 1 month, immobilization-free, dual-mode detection | [39] |
| Fluorescence reporter | 0.074 nM | 0.1–500 nM | Y = 0.002x + 2.4713 R2 = 0.9958 | denotes | Label-free direct analysis, environmental and eco-friendly | [40] | |
| Docking and molecular dynamics (MD) simulations and microscale thermophoresis (MST) | denotes | denotes | denotes | Pufferfish | Effective repurposing approach, susceptible | [41] | |
| Triple cycle amplification-based MNPs-apt | 0.265 pg/mL | 0.05–500 ng/mL | Y = 4936.74 + 1327logx R2 = 0.9932 | Clams and shellfish | Isothermal amplification, reliable sensitivity, and stability, practical for the analysis of food | [42] | |
| STX | Electrochemical aptasensor | 0.92 nM | 1–400 nM | Y = 26.7x + 5.48 R2 = 0.9932 | Seawater | Label-free direct analysis, empathetic detection, good practical adaptability and robustness, good recovery, assay time within 30 min | [43] |
| Colorimetric aptasensor | 0.1423 nM | 0.1457–37.30 nM | Y = 42.09logx + 38.70 R2 = 0.9863 | Seawater and Scallop | Label-free direct analysis, a terminal-fixed anti-STX aptamer, assay time of 75 min, high selectivity, and good recoveries | [44] | |
| Square Wave Voltammetry (SWV), Electrochemical Impedance Spectroscopy (EIS) | 4.669 pg/mL | 10 pg/mL–1 μg/mL | Y = 0.05282x + 0.03536 R2 = 0.9713 | Freshwater | High sensitivity, wide detection range, reduce the error rate | [45] | |
| Fluorescent aptasensor | 1.8 ng/mL | 0–24 ng/mL | Y = 5.25x + 587.2 R2 = 0.998 | Shellfish | Assay time of 30 min, simple | [46] | |
| ATX | Impedimetric | 0.5 nM | 1–100 nM | denotes | Drinking water | Label-free direct analysis, assay time 60 min | [27] |
| OA | LC-based aptasensor | 0.42 pM | 0.1–100 pM | Y = 22.5x + 68.6 R2 = 0.988 | Clam | Label-free direct analysis, low-cost detection, rapid | [47] |
| Fluorescent aptasensor | 1.1 ng/L | 1.0 ng/L–50.0 μg/L | Y = 438.3logx + 3344 R2 = 0.9909 | Shellfish | Ultrahigh-sensitivity, a duplexed aptamer-isothermal amplification, used for on-site food safety screening | [48] | |
| Aptamer-based microcantilever-array | 1 pg/ml | 1–5000 pg/ml | Y = 29.2496 × 0.4014/(1 + 0.0892 × 0.4014) R2 = 0.9887 | Clam | Label-free direct analysis, excellent dynamic range, economical | [7] | |
| Microfluidic based aptasensor | 8 pM | 10–250 nM | Y = −0.0142x + 6.1139 R2 = 0.9887 | Mussel | Assay time of 30 min, stable, easy to use | [9] | |
| Piezoelectric aptasensor | 0.32 nM | 0.5–200 nM | Y = 139.0x + 489.3 R2= 0.9826 | Mussel | Label-free direct analysis, ultrasensitive, low-cost | [49] | |
| Paper SERS aptasensor | 0.31 ng/mL | 1.0–2500 ng/mL | Y = 475.55logx + 356.20 R2 = 0.9865 | Shellfish | Rapid on-site analysis, low-cost | [50] | |
| GTX | Optical BLI aptasensor | 50 pg/mL | 0.2–90 ng/mL | Y = 0.0049x + 0.0146 R2 = 0.998 | Shellfish | Label-free direct analysis, stable, good reproducibility, high affinity to GTX1/4, simple detection | [25] |
| PTX | Competitive BLI aptasensor | 0.04 pg/mL | 200–700 pg/mL | Y = (25.05427 − 0.10812)/[1 + (x/0.00180)−1.50701] + 0.10812 R2=0.999 | Shellfish and seawater | Assay time of 30 min, ultra-sensitive, real-time | [24] |
| BTX | EIS | 106 pg/mL | 0.01–2000 ng/mL | Y = 12.2 + (102.35 − 12.2)/(1 + (x/6.66)0.59) R2 = 0.997 | Shellfish | Competitive assay | [51] |
| QCR | 220 nM/mL | 1–1000 nM | Y = 0.0752x + 12.34 R2 = 0.997 | Shellfish | Label-free direct analysis, assay time of 60 min plus preparation phase | [9] | |
| SE | 720 pg/mL in buffer, LOQ~900 pg/mL in real seafood samples | 0.5–2000 nM | Y = 3.023logx + 2.059 R2 = 0.95 | Fish and shrimp | Label-free direct analysis, assay time of 90 min plus preparation phase | [52] | |
| TIRE | 1.32 ng/mL in buffer, LOQ~1.8 ng/mL in real seafood samples |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahraee, H.; Mehrzad, A.; Abnous, K.; Chen, C.-H.; Khoshbin, Z.; Verdian, A. Recent Advances in Aptasensing Strategies for Monitoring Phycotoxins: Promising for Food Safety. Biosensors 2023, 13, 56. https://doi.org/10.3390/bios13010056
Zahraee H, Mehrzad A, Abnous K, Chen C-H, Khoshbin Z, Verdian A. Recent Advances in Aptasensing Strategies for Monitoring Phycotoxins: Promising for Food Safety. Biosensors. 2023; 13(1):56. https://doi.org/10.3390/bios13010056
Chicago/Turabian StyleZahraee, Hamed, Atiyeh Mehrzad, Khalil Abnous, Chih-Hsin Chen, Zahra Khoshbin, and Asma Verdian. 2023. "Recent Advances in Aptasensing Strategies for Monitoring Phycotoxins: Promising for Food Safety" Biosensors 13, no. 1: 56. https://doi.org/10.3390/bios13010056
APA StyleZahraee, H., Mehrzad, A., Abnous, K., Chen, C.-H., Khoshbin, Z., & Verdian, A. (2023). Recent Advances in Aptasensing Strategies for Monitoring Phycotoxins: Promising for Food Safety. Biosensors, 13(1), 56. https://doi.org/10.3390/bios13010056





