Analyte Sensing with Catalytic Micromotors
Abstract
1. Introduction
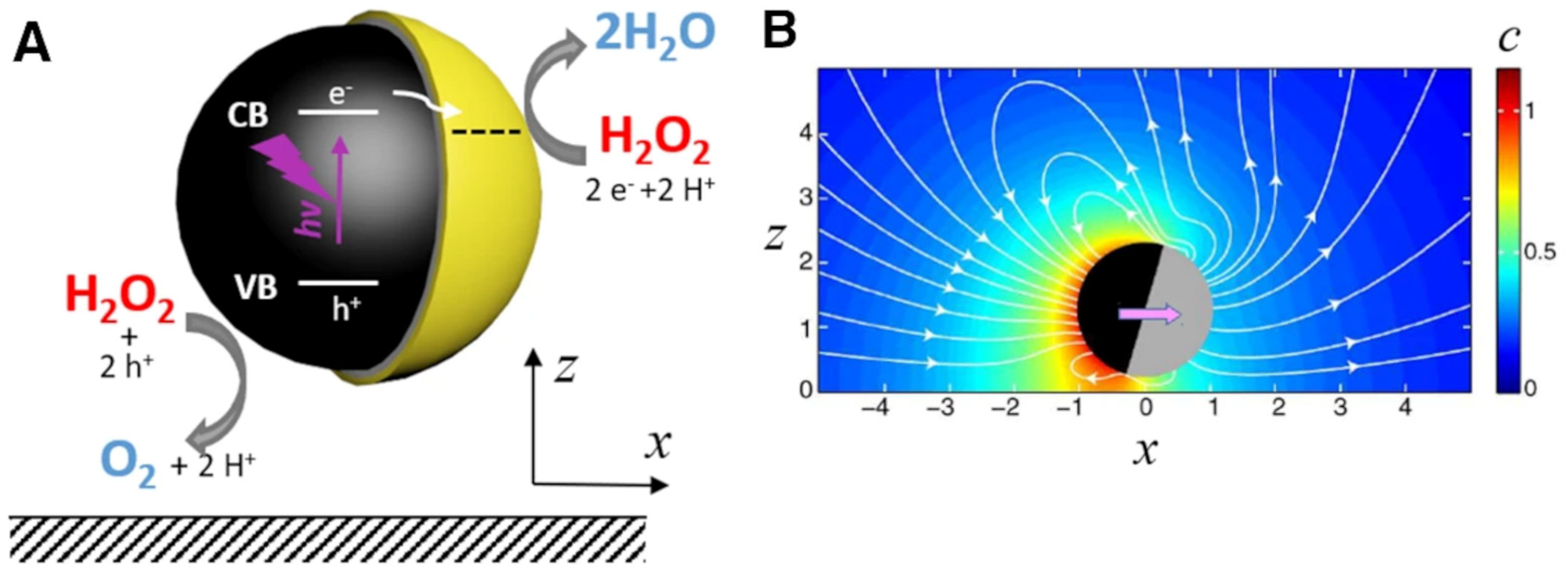
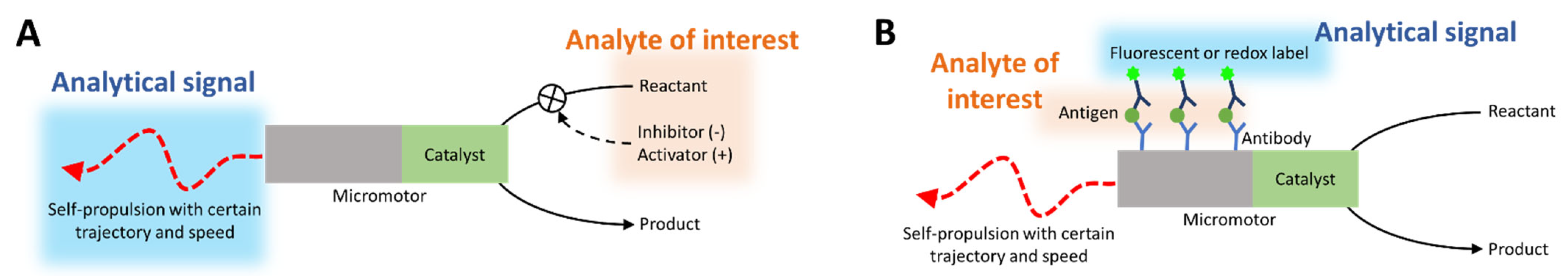
- (i).
- First, catalytic micromotors can analyze tiny samples. They can be suspended and their motion investigated in few microliters of sample. This is useful when large volumes of samples are not available (e.g., blood samples collected from very low birth weight infants [17,18,19]). One should also note that the ability to work with small samples advantageously translates into smaller amounts of chemical/biological waste.
- (ii).
- Second, catalytic micromotors (via their motion) can facilitate enhanced mass transport within the investigated sample without using laboratory equipment (such as stirrers, vortexes, or pumps). This can be important for investigations carried out outside specialized laboratories, in remote areas with limited resources. It can also be important when analyzing a few microliters of sample (e.g., a drop of blood, sweat, tear, or saliva placed on a microscope glass slide). There are very few tools for stirring/mixing within tiny liquid droplets.
- (iii).
- Third, the signal of the catalytic micromotors can be documented using a mobile phone instead of a bulky, expensive laboratory equipment. In turn, this can facilitate sensing outside specialized laboratories and, eventually, by untrained users. While this possibility represents an advantage over classic analytical approaches (which most often require bulky and expensive instrumentation that is used by trained staff in specialized laboratories), only a few times it has been demonstrated. Both the collective behavior of catalytic micromotors [20] and the individual behavior of catalytic micromotors [21,22,23] were already documented using mobile phone cameras and linked to the concentration of the analyte of interest. Reading the fluorescence of catalytic micromotors using a mobile phone was also recently reported [24]. Important to note, some of these mobile phone-based approaches to study micromotors are still relying on image analysis carried out on a computer.
- (iv).
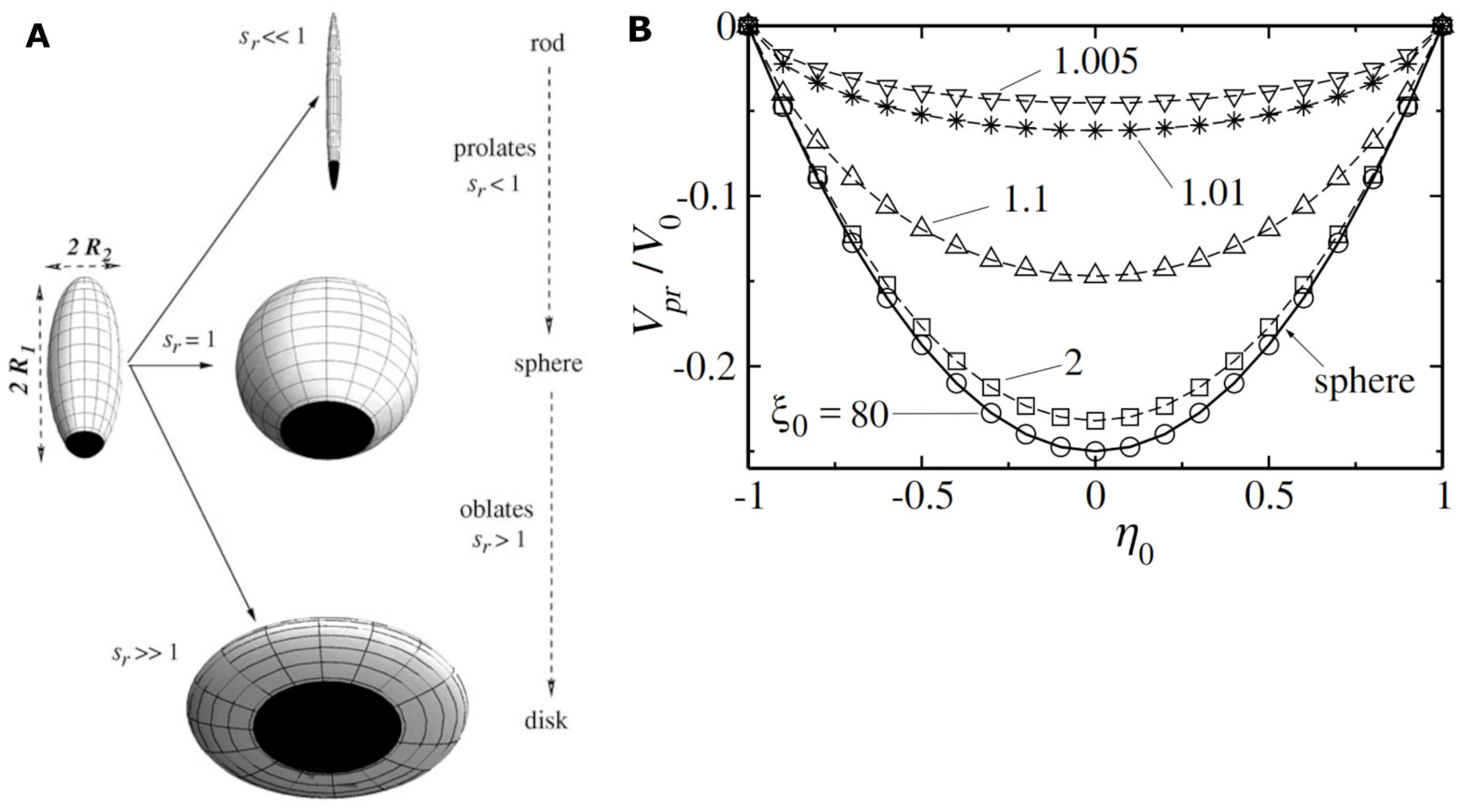
- Forth, sensing with catalytic micromotors is characterized by high spatial resolution because each tiny motor reports on the concentration of the analyte in the solution adjacent to the micromotor. However, achieving sensing with high spatial resolution (i.e., building high resolution chemical 2D/3D maps with catalytic micromotors) is currently still hindered by the heterogeneity of the catalytic micromotors. One cannot be 100% sure that a micromotor self-propels faster/slower than the other because of the local availability/unavailability of the targeted analyte or because of intrinsic, but yet not well understood, heterogeneity from batch to batch, or even within the same batch, in the properties of the individual micromotors (see also Section 2), or for various other reasons related to the experimental setup. For example, it has been observed that hydrazine-fueled micromotors propel faster at the edges of a water droplet than in the middle of it, immediately after the droplet is exposed to hydrazine vapors [25]; this is so because in that setup the hydrazine vapors reach the micromotors faster through the shallow edges of the water droplet [25].
- (v).
2. The Speed (or Diffusion Coefficient) of Catalytic Micromotors as Analytical Signal
3. Sensing Analytes Which Are Also Fuel for the Catalytic Micromotors
4. Sensing Analytes Which Modulate the Speed of Self-Propulsion without Being Fuel for the Catalytic Micromotors
5. Sensing Analytes Which Are Not Involved at All in the Catalytic Process Propelling the Micromotors
- (i).
- By enhancing mass transport, and, thus, enhancing the probability of the biorecognition event to happen. Enhancing mass transport by the self-propulsion of catalytic micromotors comes with some advantages. For example, it does not require laboratory equipment (e.g., magnetic stirrers, shakers, vortex mixers, etc.), and, thus, it is suitable to be used both in specialized laboratories and outside specialized laboratories, in resource poor areas. Enhancing mass transport by the self-propulsion of catalytic micromotors can also be expected to eliminate some previously described inconsistencies [62] which characterize traditional ways of sample agitation. However, no studies have addressed this issue yet. No thorough comparison of mass transport enhancements by catalytic micromotors and by classic approaches was carried out. However, few papers do show that micromotors provide better results than classic stirring/agitation [63,64] (but without providing any explanation for the observed differences). Unlike classic ways to stir and mix samples, catalytic micromotors are also suitable to stir/mix very low volume samples (e.g., 10 µL of serum, saliva, or sweat placed on a glass microscope slide).
- (ii).
- By enhancing the local concentration of optical/electrochemical probes. For example, SiO2-coated Ag nanowires are not only excellent probes for surface-enhanced Raman spectroscopy (SERS) but also show positive phototaxis, that is, they self-propel towards the light source via photocatalytic processes. The latter ability can be used to pre-concentrate the probes and improve the sensitivity and the detection limit of SERS-based detection [65]. Catalytic micromotors were also made using magnetic materials, such as Ni [17,19,46] or Fe3O4 [64]. In turn, these facilitated the magnetic pre-concentration of the catalytic micromotors onto the surface of the electrode for the electrochemical quantification of the analyte (which they have collected during self-propulsion in the investigated sample) [46,64].
- (iii).
- By chemically transforming the targeted analyte. For example, Mg-based catalytic micromotors self-propel in aqueous solution while producing H2 and OH- ions, and the latter species can facilitate the electrochemical detection of diphenyl phthalate by converting this, electrochemically inactive, compound into electrochemically active phenol [49]. A similar concept was also applied for the detection of paraoxon (a cholinesterase inhibitor) [66]. OH- ions produced by catalytic micromotors facilitated also the detection of phenylenediamines by oxidizing these species to colored products [67].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golestanian, R.; Liverpool, T.B.; Ajdari, A. Designing Phoretic Micro- and Nano-Swimmers. New J. Phys. 2007, 9, 126. [Google Scholar] [CrossRef]
- Paxton, W.F.; Kistler, K.C.; Olmeda, C.C.; Sen, A.; Angelo, S.K.S.; Cao, Y.; Mallouk, T.E.; Lammert, P.E.; Crespi, V.H. Catalytic Nanomotors: Autonomous Movement of Striped Nanorods. J. Am. Chem. Soc. 2004, 126, 13424–13431. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.F.; Baker, P.T.; Kline, T.R.; Wang, Y.; Mallouk, T.E.; Sen, A. Catalytically Induced Electrokinetics for Motors and Micropumps. J. Am. Chem. Soc. 2006, 128, 14881–14888. [Google Scholar] [CrossRef]
- Wang, Y.; Hernandez, R.M.; Bartlett, D.J.; Bingham, J.M.; Kline, T.R.; Sen, A.; Mallouk, T.E. Bipolar Electrochemical Mechanism for the Propulsion of Catalytic Nanomotors in Hydrogen Peroxide Solutions. Langmuir 2006, 22, 10451–10456. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Uygun, A.; Wang, J. Hydrogen-Bubble-Propelled Zinc-Based Microrockets in Strongly Acidic Media. J. Am. Chem. Soc. 2012, 134, 897–900. [Google Scholar] [CrossRef]
- Bunea, A.-I.; Pavel, I.-A.; David, S.; Gáspár, S. Sensing Based on the Motion of Enzyme-Modified Nanorods. Biosens. Bioelectron. 2015, 67, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jannasch, A.; Albrecht, U.-R.; Hahn, K.; Miguel-López, A.; Schäffer, E.; Sánchez, S. Enzyme-Powered Hollow Mesoporous Janus Nanomotors. Nano Lett. 2015, 15, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Kline, T.R.; Paxton, W.F.; Mallouk, T.E.; Sen, A. Catalytic Nanomotors: Remote-Controlled Autonomous Movement of Striped Metallic Nanorods. Angew. Chem. Int. Ed. 2005, 44, 744–746. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Hahn, K.; Sánchez, S. Motion Control of Urea-Powered Biocompatible Hollow Microcapsules. ACS Nano 2016, 10, 3597–3605. [Google Scholar] [CrossRef]
- Simmchen, J.; Katuri, J.; Uspal, W.E.; Popescu, M.N.; Tasinkevych, M.; Sánchez, S. Topographical Pathways Guide Chemical Microswimmers. Nat. Commun. 2016, 7, 10598. [Google Scholar] [CrossRef] [PubMed]
- Soler, L.; Sánchez, S. Catalytic Nanomotors for Environmental Monitoring and Water Remediation. Nanoscale 2014, 6, 7175–7182. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, J.; Liu, J.; Dey, K.K.; Li, T.; Chakraborty, R.; Xu, K.; Makarov, D.; Barmin, R.A.; Gorin, D.A.; Tolstoy, V.P.; et al. Micro-Bio-Chemo-Mechanical-Systems: Micromotors, Microfluidics, and Nanozymes for Biomedical Applications. Adv. Mater. 2021, 33, 2007465. [Google Scholar] [CrossRef]
- Wrede, P.; Medina-Sánchez, M.; Fomin, V.M.; Schmidt, O.G. Switching Propulsion Mechanisms of Tubular Catalytic Micromotors. Small 2021, 17, 2006449. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M.; Popescu, M.N.; Domínguez, A.; Simmchen, J. Active Spheres Induce Marangoni Flows That Drive Collective Dynamics. Eur. Phys. J. E 2021, 44, 15. [Google Scholar] [CrossRef] [PubMed]
- Archer, R.J.; Parnell, A.J.; Campbell, A.I.; Howse, J.R.; Ebbens, S.J. A Pickering Emulsion Route to Swimming Active Janus Colloids. Adv. Sci. 2018, 5, 1700528. [Google Scholar] [CrossRef]
- Kaang, B.K.; Mestre, R.; Kang, D.-C.; Sánchez, S.; Kim, D.-P. Scalable and Integrated Flow Synthesis of Triple-Responsive Nano-Motors via Microfluidic Pickering Emulsification. Appl. Mater. Today 2020, 21, 100854. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; Arruza, L.; López, M.Á.; Escarpa, A. On-the-Fly Rapid Immunoassay for Neonatal Sepsis Diagnosis: C-Reactive Protein Accurate Determination Using Magnetic Graphene-Based Micromotors. Biosens. Bioelectron. 2020, 158, 112156. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; López, M.Á.; Escarpa, A. Electrochemical Microfluidic Micromotors-Based Immunoassay for C-Reactive Protein Determination in Preterm Neonatal Samples with Sepsis Suspicion. Anal. Chem. 2020, 92, 5048–5054. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; Moreno-Guzmán, M.; Arruza, L.; López, M.Á.; Escarpa, A. Polymer-Based Micromotor Fluorescence Immunoassay for On-the-Move Sensitive Procalcitonin Determination in Very Low Birth Weight Infants’ Plasma. ACS Sens. 2020, 5, 1336–1344. [Google Scholar] [CrossRef]
- Russell, S.M.; Alba-Patiño, A.; Borges, M.; de la Rica, R. Multifunctional Motion-to-Color Janus Transducers for the Rapid Detection of Sepsis Biomarkers in Whole Blood. Biosens. Bioelectron. 2019, 140, 111346. [Google Scholar] [CrossRef]
- Draz, M.S.; Kochehbyoki, K.M.; Vasan, A.; Battalapalli, D.; Sreeram, A.; Kanakasabapathy, M.K.; Kallakuri, S.; Tsibris, A.; Kuritzkes, D.R.; Shafiee, H. DNA Engineered Micromotors Powered by Metal Nanoparticles for Motion Based Cellphone Diagnostics. Nat. Commun. 2018, 9, 4282. [Google Scholar] [CrossRef]
- Draz, M.S.; Lakshminaraasimulu, N.K.; Krishnakumar, S.; Battalapalli, D.; Vasan, A.; Kanakasabapathy, M.K.; Sreeram, A.; Kallakuri, S.; Thirumalaraju, P.; Li, Y.; et al. Motion-Based Immunological Detection of Zika Virus Using Pt-Nanomotors and a Cellphone. ACS Nano 2018, 12, 5709–5718. [Google Scholar] [CrossRef]
- Yuan, K.; Cuntín-Abal, C.; Jurado-Sánchez, B.; Escarpa, A. Smartphone-Based Janus Micromotors Strategy for Motion-Based Detection of Glutathione. Anal. Chem. 2021, 93, 16385–16392. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; de la Asunción-Nadal, V.; Cuntín-Abal, C.; Jurado-Sánchez, B.; Escarpa, A. On-Board Smartphone Micromotor-Based Fluorescence Assays. Lab Chip 2022, 22, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Li, J.; Rozen, I.; Ezhilan, B.; Xu, T.; Christianson, C.; Gao, W.; Saintillan, D.; Ren, B.; Wang, J. Vapor-Driven Propulsion of Catalytic Micromotors. Sci. Rep. 2015, 5, 13226. [Google Scholar] [CrossRef]
- Villa, K.; Manzanares Palenzuela, C.L.; Sofer, Z.; Matějková, S.; Pumera, M. Metal-Free Visible-Light Photoactivated C3N4 Bubble-Propelled Tubular Micromotors with Inherent Fluorescence and On/Off Capabilities. ACS Nano 2018, 12, 12482–12491. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, J.; Xu, Z.; Yang, J.; Liu, Y.; Liu, L. A Eu-MOF/EDTA-NiAl-CLDH Fluorescent Micromotor for Sensing and Removal of Fe3+ from Water. J. Mater. Chem. C 2019, 7, 10297–10308. [Google Scholar] [CrossRef]
- Chałupniak, A.; Morales-Narváez, E.; Merkoçi, A. Micro and Nanomotors in Diagnostics. Adv. Drug Deliv. Rev. 2015, 95, 104–116. [Google Scholar] [CrossRef]
- Yánez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Janus Particles for (Bio)Sensing. Appl. Mater. Today 2017, 9, 276–288. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B. Nanoscale Biosensors Based on Self-Propelled Objects. Biosensors 2018, 8, 59. [Google Scholar] [CrossRef]
- Kong, L.; Guan, J.; Pumera, M. Micro- and Nanorobots Based Sensing and Biosensing. Curr. Opin. Electrochem. 2018, 10, 174–182. [Google Scholar] [CrossRef]
- Pacheco, M.; López, M.Á.; Jurado-Sánchez, B.; Escarpa, A. Self-Propelled Micromachines for Analytical Sensing: A Critical Review. Anal. Bioanal. Chem. 2019, 411, 6561–6573. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Campuzano, S.; Pingarrón, J.M.; Escarpa, A. Janus Particles and Motors: Unrivaled Devices for Mastering (Bio)Sensing. Microchim. Acta 2021, 188, 416. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, Y.; Xu, D.; Liu, X.; Liu, S.; Ma, X. Design and Fabrication of Micro/Nano-Motors for Environmental and Sensing Applications. Appl. Mater. Today 2021, 23, 101007. [Google Scholar] [CrossRef]
- Duan, W.; Wang, W.; Das, S.; Yadav, V.; Mallouk, T.E.; Sen, A. Synthetic Nano- and Micromachines in Analytical Chemistry: Sensing, Migration, Capture, Delivery, and Separation. Annu. Rev. Anal. Chem. 2015, 8, 311–333. [Google Scholar] [CrossRef]
- Campuzano, S.; Kagan, D.; Orozco, J.; Wang, J. Motion-Driven Sensing and Biosensing Using Electrochemically Propelled Nanomotors. Analyst 2011, 136, 4621–4630. [Google Scholar] [CrossRef]
- Kim, K.; Guo, J.; Liang, Z.; Fan, D. Artificial Micro/Nanomachines for Bioapplications: Biochemical Delivery and Diagnostic Sensing. Adv. Funct. Mater. 2018, 28, 1705867. [Google Scholar] [CrossRef]
- Valles, M.; Pujals, S.; Albertazzi, L.; Sánchez, S. Enzyme Purification Improves the Enzyme Loading, Self-Propulsion, and Endurance Performance of Micromotors. ACS Nano 2022, 16, 5615–5626. [Google Scholar] [CrossRef]
- Kagan, D.; Calvo-Marzal, P.; Balasubramanian, S.; Sattayasamitsathit, S.; Manesh, K.M.; Flechsig, G.-U.; Wang, J. Chemical Sensing Based on Catalytic Nanomotors: Motion-Based Detection of Trace Silver. J. Am. Chem. Soc. 2009, 131, 12082–12083. [Google Scholar] [CrossRef]
- Moran, J.L.; Posner, J.D. Phoretic Self-Propulsion. Annu. Rev. Fluid Mech. 2017, 49, 511–540. [Google Scholar] [CrossRef]
- Popescu, M.N.; Dietrich, S.; Tasinkevych, M.; Ralston, J. Phoretic Motion of Spheroidal Particles Due to Self-Generated Solute Gradients. Eur. Phys. J. E 2010, 31, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.; Kottapalli, S.N.; Fischer, P.; Botin, D.; Palberg, T. Characterization of Active Matter in Dense Suspensions with Heterodyne Laser Doppler Velocimetry. Colloid Polym. Sci. 2021, 299, 269–280. [Google Scholar] [CrossRef]
- Dietrich, K.; Renggli, D.; Zanini, M.; Volpe, G.; Buttinoni, I.; Isa, L. Two-Dimensional Nature of the Active Brownian Motion of Catalytic Microswimmers at Solid and Liquid Interfaces. New J. Phys. 2017, 19, 065008. [Google Scholar] [CrossRef]
- Laocharoensuk, R.; Burdick, J.; Wang, J. Carbon-Nanotube-Induced Acceleration of Catalytic Nanomotors. ACS Nano 2008, 2, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Yadav, A.; Dwivedi, P.; Mangal, R. Interaction of Active Janus Colloids with Tracers. Langmuir 2022, 38, 2686–2698. [Google Scholar] [CrossRef]
- Muñoz, J.; Urso, M.; Pumera, M. Self-Propelled Multifunctional Microrobots Harboring Chiral Supramolecular Selectors for “Enantiorecognition-on-the-Fly”. Angew. Chem. Int. Ed. 2022, 61, e202116090. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, W.; Pan, S.; Fu, Y.; Dong, B.; Wang, H. Fluorescent Self-Propelled Covalent Organic Framework as a Microsensor for Nitro Explosive Detection. Appl. Mater. Today 2020, 19, 100550. [Google Scholar] [CrossRef]
- Arqué, X.; Andrés, X.; Mestre, R.; Ciraulo, B.; Ortega Arroyo, J.; Quidant, R.; Patiño, T.; Sánchez, S. Ionic Species Affect the Self-Propulsion of Urease-Powered Micromotors. Research 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Rojas, D.; Jurado-Sánchez, B.; Escarpa, A. “Shoot and Sense” Janus Micromotors-Based Strategy for the Simultaneous Degradation and Detection of Persistent Organic Pollutants in Food and Biological Samples. Anal. Chem. 2016, 88, 4153–4160. [Google Scholar] [CrossRef]
- Sanchez, S.; Ananth, A.N.; Fomin, V.M.; Viehrig, M.; Schmidt, O.G. Superfast Motion of Catalytic Microjet Engines at Physiological Temperature. J. Am. Chem. Soc. 2011, 133, 14860–14863. [Google Scholar] [CrossRef]
- Van Nguyen, K.; Minteer, S.D. DNA-Functionalized Pt Nanoparticles as Catalysts for Chemically Powered Micromotors: Toward Signal-on Motion-Based DNA Biosensor. Chem. Commun. 2015, 51, 4782–4784. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, X.; Xie, Y.; Wu, J.; Ju, H. An Efficient Enzyme-Powered Micromotor Device Fabricated by Cyclic Alternate Hybridization Assembly for DNA Detection. Nanoscale 2017, 9, 9026–9033. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Wu, J.; Ju, H. Motor-Based Autonomous Microsensor for Motion and Counting Immunoassay of Cancer Biomarker. Anal. Chem. 2014, 86, 4501–4507. [Google Scholar] [CrossRef] [PubMed]
- Maric, T.; Mayorga-Martinez, C.C.; Nasir, M.Z.M.; Pumera, M. Platinum-Halloysite Nanoclay Nanojets as Sensitive and Selective Mobile Nanosensors for Mercury Detection. Adv. Mater. Technol. 2019, 4, 1800502. [Google Scholar] [CrossRef]
- Orozco, J.; García-Gradilla, V.; D’Agostino, M.; Gao, W.; Cortés, A.; Wang, J. Artificial Enzyme-Powered Microfish for Water-Quality Testing. ACS Nano 2013, 7, 818–824. [Google Scholar] [CrossRef]
- Moo, J.G.S.; Wang, H.; Zhao, G.; Pumera, M. Biomimetic Artificial Inorganic Enzyme-Free Self-Propelled Microfish Robot for Selective Detection of Pb2+ in Water. Chem. Eur. J. 2014, 20, 4292–4296. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ge, Y.; Liu, L.; Zhang, L.; Liu, M.; Sun, Y.; Zhang, H.; Dong, B. Motion-Based pH Sensing Based on the Cartridge-Case-like Micromotor. ACS Appl. Mater. Interfaces 2016, 8, 4250–4257. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Wu, J.; Ju, H. Bubble-Propelled Jellyfish-like Micromotors for DNA Sensing. ACS Appl. Mater. Interfaces 2019, 11, 13581–13588. [Google Scholar] [CrossRef]
- Wu, J.; Balasubramanian, S.; Kagan, D.; Manesh, K.M.; Campuzano, S.; Wang, J. Motion-Based DNA Detection Using Catalytic Nanomotors. Nat. Commun. 2010, 1, 36. [Google Scholar] [CrossRef]
- Xie, Y.; Fu, S.; Wu, J.; Lei, J.; Ju, H. Motor-Based Microprobe Powered by Bio-Assembled Catalase for Motion Detection of DNA. Biosens. Bioelectron. 2017, 87, 31–37. [Google Scholar] [CrossRef]
- Singh, V.V.; Kaufmann, K.; de Ávila, B.E.-F.; Uygun, M.; Wang, J. Nanomotors Responsive to Nerve-Agent Vapor Plumes. Chem. Commun. 2016, 52, 3360–3363. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, I.; Fomitcheva-Khartchenko, A.; Kaigala, G.V. Shake It or Shrink It: Mass Transport and Kinetics in Surface Bioassays Using Agitation and Microflidics. Anal. Chem. 2020, 92, 10187–10195. [Google Scholar] [CrossRef] [PubMed]
- Molinero-Fernández, Á.; Jodra, A.; Moreno-Guzmán, M.; López, M.Á.; Escarpa, A. Magnetic Reduced Graphene Oxide/Nickel/Platinum Nanoparticles Micromotors for Mycotoxin Analysis. Chem. Eur. J. 2018, 24, 7172–7176. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Wang, K.; Wang, H. An Immunoassay Based on Nanomotor-Assisted Electrochemical Response for the Detection of Immunoglobulin. Microchim. Acta 2022, 189, 47. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Wang, W.; Xu, D.; Zeng, F.; Zhan, C.; Gu, J.; Li, M.; Zhao, W.; Zhang, J.; et al. Photocatalytically Powered Matchlike Nanomotor for Light-Guided Active SERS Sensing. Angew. Chem. 2018, 130, 13294–13297. [Google Scholar] [CrossRef]
- Cinti, S.; Valdés-Ramírez, G.; Gao, W.; Li, J.; Palleschi, G.; Wang, J. Microengine-Assisted Electrochemical Measurements at Printable Sensor Strips. Chem. Commun. 2015, 51, 8668–8671. [Google Scholar] [CrossRef]
- María-Hormigos, R.; Jurado-Sánchez, B.; Escarpa, A. Self-Propelled Micromotors for Naked-Eye Detection of Phenylenediamines Isomers. Anal. Chem. 2018, 90, 9830–9837. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Pumera, M. Self-Propelled Tags for Protein Detection. Adv. Funct. Mater. 2020, 30, 1906449. [Google Scholar] [CrossRef]
- Pacheco, M.; Jurado-Sánchez, B.; Escarpa, A. Transition Metal Dichalcogenide-Based Janus Micromotors for on-the-Fly Salmonella Detection. Microchim. Acta 2022, 189, 194. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Escarpa, A.; Wang, J. Lighting up Micromotors with Quantum Dots for Smart Chemical Sensing. Chem. Commun. 2015, 51, 14088–14091. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Wang, T.; Ye, Z.; Zhang, H.; Dong, B.; Li, C.Y. A Biodegradable, All-Polymer Micromotor for Gas Sensing Applications. J. Mater. Chem. C 2016, 4, 5945–5952. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Wei, W.; Wang, Y.; Yang, S.; Li, J.; Xing, Y.; Zhou, L.; Dai, W.; Dong, H. Self-Propelled Janus Mesoporous Micromotor for Enhanced MicroRNA Capture and Amplified Detection in Complex Biological Samples. ACS Nano 2022, 16, 5587–5596. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.V.; Kaufmann, K.; de Ávila, B.E.-F.; Karshalev, E.; Wang, J. Molybdenum Disulfide-Based Tubular Microengines: Toward Biomedical Applications. Adv. Funct. Mater. 2016, 26, 6270–6278. [Google Scholar] [CrossRef]
- Cogal, G.C.; Karaca, G.Y.; Uygun, E.; Kuralay, F.; Oksuz, L.; Remskar, M.; Oksuz, A.U. RF Plasma-Enhanced Conducting Polymer/W5O14 Based Self-Propelled Micromotors for MiRNA Detection. Anal. Chim. Acta 2020, 1138, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Báez, D.F.; Ramos, G.; Corvalán, A.; Cordero, M.L.; Bollo, S.; Kogan, M.J. Effects of Preparation on Catalytic, Magnetic and Hybrid Micromotors on Their Functional Features and Application in Gastric Cancer Biomarker Detection. Sens. Actuators B Chem. 2020, 310, 127843. [Google Scholar] [CrossRef]
- Zhang, Z. Magnetic Molecularly Imprinted Microsensor for Selective Recognition and Transport of Fluorescent Phycocyanin in Seawater. J. Mater. Chem. A 2015, 8, 7437–7444. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Lopez-Ramirez, M.A.; Báez, D.F.; Jodra, A.; Singh, V.V.; Kaufmann, K.; Wang, J. Aptamer-Modified Graphene-Based Catalytic Micromotors: Off–On Fluorescent Detection of Ricin. ACS Sens. 2016, 1, 217–221. [Google Scholar] [CrossRef]
- Yuan, K.; López, M.Á.; Jurado-Sánchez, B.; Escarpa, A. Janus Micromotors Coated with 2D Nanomaterials as Dynamic Interfaces for (Bio)-Sensing. ACS Appl. Mater. Interfaces 2020, 12, 46588–46597. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; Moreno-Guzmán, M.; López, M.Á.; Escarpa, A. Biosensing Strategy for Simultaneous and Accurate Quantitative Analysis of Mycotoxins in Food Samples Using Unmodified Graphene Micromotors. Anal. Chem. 2017, 89, 10850–10857. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Pacheco, M.; Rojo, J.; Escarpa, A. Magnetocatalytic Graphene Quantum Dots Janus Micromotors for Bacterial Endotoxin Detection. Angew. Chem. Int. Ed. 2017, 56, 6957–6961. [Google Scholar] [CrossRef]
- Pacheco, M.; de la Asunción-Nadal, V.; Jurado-Sánchez, B.; Escarpa, A. Engineering Janus Micromotors with WS2 and Affinity Peptides for Turn-on Fluorescent Sensing of Bacterial Lipopolysaccharides. Biosens. Bioelectron. 2020, 165, 112286. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Jurado-Sánchez, B.; Escarpa, A. Sensitive Monitoring of Enterobacterial Contamination of Food Using Self-Propelled Janus Microsensors. Anal. Chem. 2018, 90, 2912–2917. [Google Scholar] [CrossRef]
- Singh, V.V.; Kaufmann, K.; Orozco, J.; Li, J.; Galarnyk, M.; Arya, G.; Wang, J. Micromotor-Based on–off Fluorescence Detection of Sarin and Soman Simulants. Chem. Commun. 2015, 51, 11190–11193. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, B.E.-F.; Zhao, M.; Campuzano, S.; Ricci, F.; Pingarrón, J.M.; Mascini, M.; Wang, J. Rapid Micromotor-Based Naked-Eye Immunoassay. Talanta 2017, 167, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Rohaizad, N.; Nasir, M.Z.M.; Guan, J.; Pumera, M. Micromotor-Assisted Human Serum Glucose Biosensing. Anal. Chem. 2019, 91, 5660–5666. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Sanchez, S.; Schmidt, O.G.; Pumera, M. Poisoning of Bubble Propelled Catalytic Micromotors: The Chemical Environment Matters. Nanoscale 2013, 5, 2909–2914. [Google Scholar] [CrossRef]
- Greydanus, B.; Saleheen, M.; Wu, H.; Heyden, A.; Medlin, J.W.; Schwartz, D.K. Probing Surface-Adsorbate Interactions through Active Particle Dynamics. J. Colloid Interface Sci. 2022, 614, 425–435. [Google Scholar] [CrossRef]


| Targeted Analyte | Motor Structure; Self Propulsion Mechanism | Analytical Performances | Ref. |
|---|---|---|---|
| Glucose, glutamate and hypoxanthine | ~2 µm long, half Pt and half poly(pyrrole) nanorods modified with GOx, GluOX or XOD; Self-diffusiophoresis; | DL: 0.05 mM for glucose, 0.03 mM for hypoxanthine, and 0.19 mM for glutamate; LR: up to 1 mM for glucose and glutamate and up to 0.1 mM for hypoxanthine; Selectivity proved in diluted horse serum and cell culture medium; | [6] |
| Hydrazine (vapors) | ~1 µm diameter Au microsphere with one hemisphere covered with a 20 nm thick layer of Ir; Self-electrophoresis; | Vapors from a 2.5 mm diameter droplet containing 5–30% hydrazine induced self-propulsion of motors found in a second 2.5 mm diameter droplet (at 0.5–3 cm from the first); | [25] |
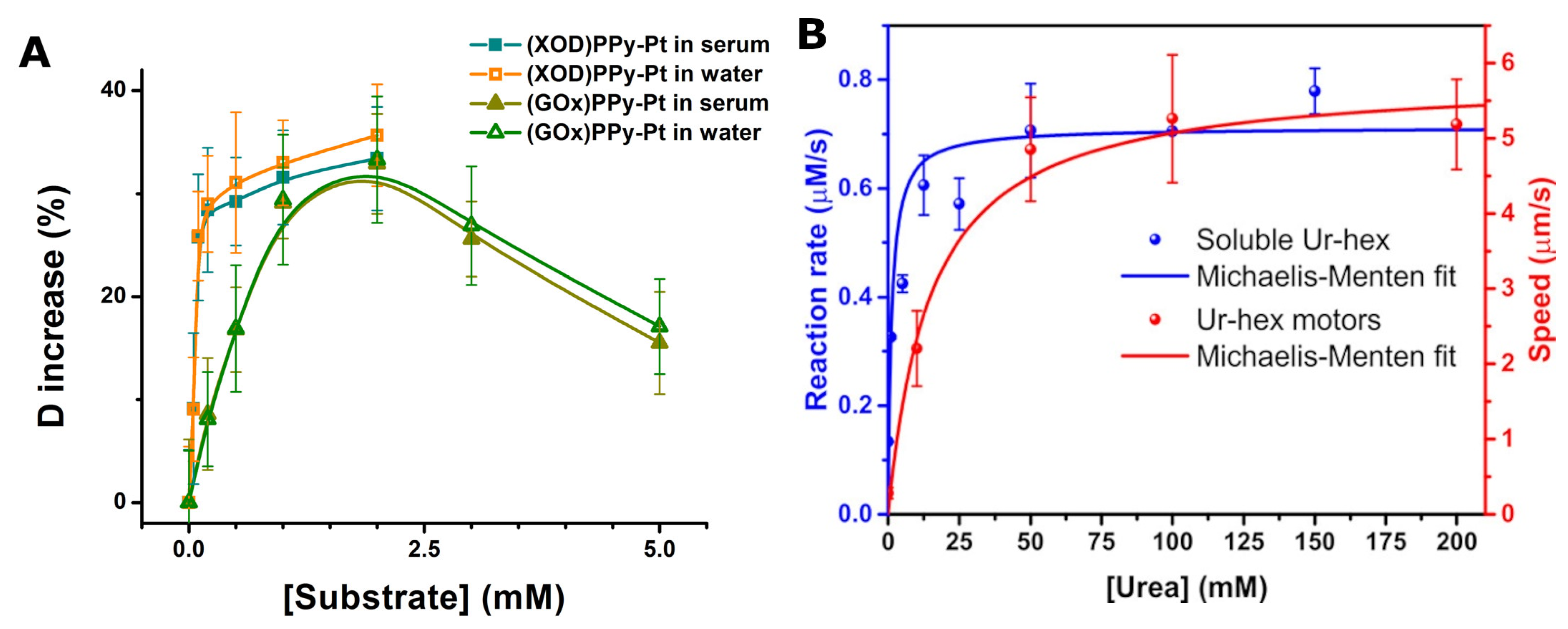
| Urea | ~2 µm diameter hollow silica microcapsules modified with urease; Self-diffusiophoresis; | Self-propulsion was investigated at urea concentrations from 10 mM to 200 mM; Impact of the purity of urease on the speed of self-propulsion was discovered; Some stability problems were also noted; | [38] |
| Targeted Analyte | Micromotor Structure; Self-Propulsion Mechanism | Analytical Performances | Ref. |
|---|---|---|---|
| A. Catalytic micromotors used to detect inorganic compounds: | |||
| Ag+ | Bimetallic nanorod (half Au and half Pt); Self-electrophoresis; | LR: from 0.5 µM to 100 μM; Interference from other ions was observed; | [39] |
| Hg2+ | Halloysite clay nanotube (L ~700 nm, ϕ ~80–100 nm) partially covered with Pt; O2 bubble ejection; | DL: 3.24 ppb; Detected analyte concentrations from 0.25 ppb to 1000 ppb; The speed of the micromotors is also affected by other heavy metals; | [54] |
| Catalase inhibitors (Hg2+, Cu2+, NaN3, and aminotriazole) | PEDOT on Au microtube (L ~8 µm, ϕ ~2µm) with its inner surface modified with catalase; O2 bubble ejection; | Impact on speed observed for 50–200 μM Hg2+, 0.2–1 mM Cu2+, 2.5–25 μM NaN3, and 375–750 mM aminotriazole; The concept does not distinguish in between catalase inhibitors; | [55] |
| Pb2+ | Cu on Pt microtube (ϕ =2 µm); O2 bubble ejection; | Detected concentrations: from 0.48 mM to 1.92 mM; Some selectivity was observed when tested with Cd2+; Interference from compounds able to adsorb onto Pt can be expected; | [56] |
| pH | Cylindrical gelatin cartridge (L ~15 µm, ϕ ~8 µm) with its inner surface decorated with 3 nm Pt nanoparticles; O2 bubble ejection; | Both the speed of self-propulsion and the distance traveled during 5 s were found proportional with pH; Sensitivity to pH from 0 to 14 was observed; | [57] |
| B. Catalytic micromotors used to detect nucleic acids: | |||
| HIV-1 RNA | 6 µm diameter polystyrene microbead modified with a patch of Au nanoparticles (~60 nm in diameter) and with Pt nanoparticles (~3 nm in diameter); O2 bubble ejection; | DL: 1000 virus particles/mL; Selectivity was proved by measurements in human serum; The approach needs RNA amplification; | [21] |
| DNA | PEDOT on Au microtube with its inner surface modified with capture DNA; O2 bubble ejection; | DL: 0.5 μM; The speed of the micromotors increased from 157 μm/s at 0.5 μM target DNA to 222 μm/s at 10 μM target DNA; Good selectivity observed with non-complementary DNA; | [51] |
| DNA | PEDOT on gold microtube (L ~13 µm, ϕ = 5 µm) modified with catalase via cyclic DNA hybridization; O2 bubble ejection; | Detected concentrations: from 10 nM to 1 μM; No selectivity was observed when tested with single-base mismatched DNA; | [52] |
| DNA | Multimetallic (Au/Ag/Ni/Au) shell with an opening of ~20µm and with its concave surface modified with catalase via DNA hybridization; O2 bubble ejection; | LR: from 25 nM to 750 nM and from 0.75 µM to 10 μM; Some interference from single-base and three-base mismatched DNA; 80% of initial speed maintained after 3 weeks; | [58] |
| DNA | Bimetallic nanorod (half Au and half Pt); Self-electrophoresis; | DL: 10 pM; Detected concentrations: from 10 pM to 100 nM; Some interference from two-base mismatched DNA; Relative standard deviation of 5.66%; | [59] |
| DNA | PEDOT on Au microtube (L ~13 µm, ϕ = 5 µm) modified with catalase via DNA conjugation; O2 bubble ejection; | Detected concentrations: from 0.5 µM to 10 μM; No selectivity was observed when tested with single-base mismatched DNA; Good functioning in spiked serum; | [60] |
| C. Catalytic micromotors used to detect proteins: | |||
| Carcinoembryonic antigen | Poly(aniline) on Pt microtube (ϕ = 2 µm) with its outer surface modified first with 13 nm Au nanoparticles and then with antibodies; O2 bubble ejection; | Detected concentrations: from 1 ng/mL to 500 ng/mL; Good selectivity was observed when tested with alfa-fetoprotein and measurements in serum; Analysis time ~5 min; Relative standard deviation 7.8%; | [53] |
| D. Catalytic micromotors used to detect pathogens: | |||
| Zika virus | Antibody-modified, ~5 nm diameter Pt nanoparticle that self-propels with H2O2; This motor attaches and subsequently propels 3 µm diameter, antibody-modified polystyrene microbeads only when the investigated sample contains the virus; O2 bubble ejection; | DL: 10 virus particles/µL; Selectivity was proved by measurements in urine, saliva, and serum samples; | [22] |
| E. Catalytic micromotors used to detect small organic molecules: | |||
| Glutathione | 20 µm diameter polystyrene microbead (covered with a 50 nm thick layer of Au and a layer of graphene oxide) carrying a patch of Pt nanoparticles; O2 bubble ejection; | DL: 0.90 μM; LR: up to 160 µM; Some interference from cysteine and BSA was observed; Good recoveries (>91%) in 100× diluted human serum; | [23] |
| Diethyl chlorophosphate | PEDOT on Au microtube (ϕ = 2 µm) with its inner surface modified with catalase; O2 bubble ejection; | Diethyl chlorophosphate vapors produced by a 0.1 mM solution were detected; Selectivity was observed when tested with non-volatile catalase inhibitors; | [61] |
| Targeted Analyte | Micromotor Structure; Self-Propulsion Mechanism | Analytical Performances | Ref. |
|---|---|---|---|
| A. Catalytic micromotors used to detect inorganic compounds: | |||
| Cu2+ (heavy metals in generally) | Graphitic C3N4 microtube (L = 67 ± 14 µm, ϕ = 9.7 ± 1.5 µm); The micromotor could both sense and remove heavy metals; O2 bubble ejection; | Fluorescence detection; Cu2+ (from 1 ppm to 30 ppm) had the largest impact on the speed and the fluorescence of micromotors; 50% of 15 ppm Cu2+ was removed in 7 min; | [26] |
| Fe3+ | ~15 µm diameter microtube with a metal organic framework- based outer layer (functionalized with EDTA) and a layered double hydroxide- and MnO2-based inner layer (also functionalized with EDTA); O2 bubble ejection; | Fluorescence detection; DL: 0.15 µM; Measured concentrations: from 0.2 µM to 10 mM; LR: up to ~0.2 mM; Interference from other metal ions was observed; Adsorption capacity of 112 mg/g; Self-propulsion was turned on for the removal step while sensing was done in static conditions; | [27] |
| Hg2+ | Layered microtube (L ~18 µm, ϕ ~2 µm) made of an inner layer of Pt and an outer layer of PEDOT modified with CdTe quantum dots; O2 bubble propulsion; | Fluorescence detection; 3 mg/L Hg2+ quenched the fluorescence in 12 s; The approach identifies as positive the samples with Hg2+ content > 0.3 mg/L; No effect of pH, ionic strength, Cu2+, Pb2+, or CH3Pb+; | [70] |
| Gaseous HCl and NH3 | Hexagon-shaped, thiol-terminated polycaprolactone single crystal decorated with catalase; The micromotor also carried pH sensitive fluorescein isothiocyanate; O2 bubble ejection; | Fluorescence detection; DL: 50 ppm; | [71] |
| B. Catalytic micromotors used to detect nucleic acids: | |||
| MicroRNA-155 | ~4.5 µm diameter, mesoporous microsphere partially covered with Pt nanoparticles and also modified with capture DNA probe; Self-diffusiophoresis; | Fluorescence detection; DL: 3.39 fM; LR: from 0.1 fM to 1 nM; Good selectivity observed when tested with single-base mismatched and three-base mismatched miRNA; Sensing functional in cell culture medium and cell lysates; | [72] |
| MicroRNA-21 and thrombin | MoS2 on Pt microtube (ϕ = 5 µm) modified with fluorescently labeled single-strand DNA (for microRNA-21) or aptamer (for thrombin); O2 bubble ejection; | Fluorescence detection; Detection of 0.2 µM miRNA-21 or 0.2 µM thrombin was demonstrated; Some interferences observed from single-base mismatch DNA strands and BSA; | [73] |
| MicroRNA-21 | W5O14 nanowire (L ~10 µm, ϕ = 100 nm) modified with PEDOT, Pt and single stranded DNA; O2 bubble ejection; | Fluorescence detection; DL: 0.028 nM; LR: from 0.1 nM to 100 nM; Tests with single-base mismatch RNA strands highlighted some selectivity problems; | [74] |
| Methylated promoter region of Reprimo (RPRM) gene | Pt microtube (L ~12 µm, ϕ ~4 µm) modified with reduced graphene oxide and complementary single stranded DNA; O2 bubble ejection; | Fluorescence detection; DL: 1.3 µM; LR: from 1 µM to 10 µM; Some interference from non-complementary single stranded DNA was observed; | [75] |
| C. Catalytic micromotors used to detect peptides and proteins: | |||
| C-reactive protein | Ni on Pt microtube (L ~10 µm, ϕ = 5 µm) modified with reduced graphene oxide and antibodies; O2 bubble ejection; | Electrochemical detection; DL: 0.8 µg/mL; LR: from 2 µg/mL to 100 µg/mL; Selectivity was proved in human plasma; Analysis time of 5 min; | [17] |
| C-reactive protein | Ni on Pt microtube (L ~10 µm, ϕ = 5 µm) modified with reduced graphene oxide and antibodies; O2 bubble ejection; | Electrochemical detection; DL: 0.4 µg/mL; LR: from 1 µg/mL to 100 µg/mL; Selectivity was proved in human serum and plasma; Analysis time of 8 min; | [18] |
| Procalcitonin | Ni on Pt microtube (L ~10–20 µm, ϕ = 5 µm) modified with polypyrrole and antibodies; O2 bubble ejection; | Fluorescence detection; DL: 0.07 ng/mL; LR: from 0.5 ng/mL to 150 ng/mL; Selectivity was proved in human serum; Analysis time of 30 min; | [19] |
| Procalcitonin | Dynabeads modified with anti-procalcitonin antibodies (which facilitate a competitive immunoassay with catalase labeled procalcitonin); O2 bubble ejection; | Colorimetric detection; DL: 2 ng/mL; LR: from 1 ng/mL to 20 ng/mL; Selectivity was proved in human whole blood; Analysis time of 13 min; | [20] |
| IgG | ~500 nm Fe3O4 core and SiO2 shell nanoparticle modified with Pt and also with anti-IgG antibodies; Self-diffusiophoresis; | Electrochemical detection; DL: 3.14 pg/mL; LR: from 10 pg/mL to 100 ng/mL; Interference from IgA was observed; ~20% of the signal lost after 15 days of storage at 4 °C; | [64] |
| Rabbit IgG | IrO2 on Pt microtube (L ~10 µm, ϕ ~2.5 µm) modified with anti-rabbit IgG; O2 bubble ejection; | Electrochemical detection; DL: 0.94 pg/mL; LR: from 0.05 ng/mL to 500 ng/mL; Poor selectivity when tested with hemoglobin; Relative standard deviation of ~11%; | [68] |
| Phycocyanin | Microtube (L ~18 µm, ϕ ~2 µm) made of layers of Pt, Ni, and PEDOT imprinted with analyte molecules; O2 bubble ejection; | Fluorescence detection; Measured concentrations: 0.5, 0.75, and 1 mg/mL; BSA and seawater salts did not interfere; | [76] |
| Ricin B | Reduced graphene oxide on Pt microtube (L ~10 µm, ϕ ~5 µm) modified with aptamer; O2 bubble ejection; | Fluorescence detection; LR: from 100 pg/mL to 10 μg/mL; Tests with BSA and saporin indicated good selectivity; | [77] |
| D. Catalytic micromotors used to detect pathogen toxins: | |||
| Cholera toxin B | Graphdiyne on Pt microtube (L = 10–20 µm, ϕ ~5 µm) modified with rhodamine labeled affinity peptide; O2 bubble ejection; | Fluorescence detection; DL: 1.6 ng/mL; LR: from 4.5 ng/mL to 5000 ng/mL; Good selectivity when tested with Escherichia coli toxin and BSA; Good recoveries when tested with serum samples; | [24] |
| Cholera toxin B | 20 µm diameter polystyrene bead covered with graphdiyne oxide and carrying a patch of Pt and Fe2O3 nanoparticles and also recognition peptides; O2 bubble ejection; | Fluorescence detection; DL: 0.002 µg/mL; LR: from 0.008 µg/mL to 10 µg/mL; Selectivity in complex samples (e.g., human serum) was observed; | [78] |
| Fumonosin B1 | Ni on Pt microtube (L ~10 µm, ϕ = 5 µm) modified with reduced graphene oxide; Selectivity assured with labeled aptamer; O2 bubble ejection; | Fluorescence detection; DL: 0.7 ng/mL; LR: from 0.005 µg/mL to 1 µg/mL; Measurements in beer were made; Ochratoxin A was also detected using the same method; | [63] (see also [79]) |
| Salmonella enterica endotoxin | ~10 µm diameter polycaprolactone microspheres loaded with transition metal dichalcogenides carrying fluorescent recognition peptides, 50 nm Pt nanoparticles, and 20 nm Fe2O3 nanoparticles; O2 bubble ejection; | Fluorescence detection; DL: 1.2 μg/mL; LR: from 4 μg/mL to 333 μg/mL; Good selectivity when tested with endotoxins from other bacteria; Recoveries > 93%; Analysis time ~5 min; | [69] |
| Escherichia coli O111:B4 lipopolysaccharide | ~20 µm diameter polycaprolactone microspheres loaded with graphene quantum dots carrying phenylboronic acid, Pt nanoparticles, and Fe2O3 nanoparticles; O2 bubble ejection; | Fluorescence detection; Measured concentrations: from 10 mM to 1 M; No interference from 2 M glucose, fructose, or galactose; Detecting high concentrations of liposaccharide (e.g., 2 M) in urine and serum samples was possible; | [80] |
| Escherichia coli O111:B4 lipopolysaccharide | ~25 µm diameter polycaprolactone microspheres loaded with layered WS2 carrying fluorescent recognition peptides, Pt nanoparticles, and Fe2O3 nanoparticles; O2 bubble ejection; | Fluorescence detection; DL: 120 pM; LR: from 4 ng/mL to 1 mg/mL; Selectivity was proved with non-target lipopolysaccharide endotoxins and measurements in human serum; Analysis time of 5 min; | [81] |
| Salmonella enterica lipopolysaccharide | ~20 µm diameter polycaprolactone microbead containing 100 nm Pt nanoparticles and graphene quantum dots carrying receptor molecules; O2 bubble ejection; | Fluorescence detection; DL: 0.07 ng/mL; LR: up to 1 ng/mL; Analysis time of 15 min; Measurements in milk, mayo, egg yolk, and egg white were made; | [82] |
| E. Catalytic micromotors used to detect small organic molecules: | |||
| L-tryptophan | Ni on Pt microtube (L = 10–20 µm, ϕ = 2–3 µm) modified with CdS quantum dots and with β-cyclodextrin; O2 bubble ejection; | Fluorescence and electrochemical detection; Some preference for L-tryptophan is demonstrated; No other analytical performances were detailed; | [46] |
| 2,4,6-Trinitrophenol | ~30–40 µm diameter polycaprolactone microspheres loaded with fluorescent covalent-organic-frameworks, MnO2 microurchins, and Fe3O4 nanoparticles; O2 bubble ejection; | Fluorescence detection; 2,4,6-trinitrophenol turns off the fluorescence of the micromotors; Detection of 1 ppm of 2,4,6-trinitrophenol was demonstrated; | [47] |
| Diphenyl phthalate | ~20 µm diameter Mg microparticles partially covered with Au; OH- ions, which degrade diphenyl phthalate to phenol, are also produced during self-propulsion; H2 bubble ejection; | Electrochemical detection; DL: 0.039 mM; LR: from 0.12 mM to 1 mM; Recovery > 97 ± 8%; Analysis time ~5 min; Analysis of tap water, whiskey, milk, and human serum samples was successfully carried out; | [49] |
| Crystal violet (as model analyte) | SiO2-coated Ag nanowire (~150 nm in diameter, L ~15 µm) with a spherical AgCl head; Self-electrophoresis; | SERS detection; Measured concentration: 0.1 mM; Positive phototaxis of the micromotors led to an increase in the analytical signal (e.g., 6.2×); | [65] |
| Methyl paraoxon | ~40 µm diameter Mg microbead partially covered with a 80 nm thick layer of Ni and a 10 nm thick layer of Au; H2 bubble ejection; | Electrochemical detection; LR: from ~5 mM to 20 mM; Relative standard deviation < 5%; | [66] |
| Ortho-phenylenediamine | Carbon nanotubes and 50 nm Fe2O3 nanoparticles on MnO2 microtube (L ~12 µm, ϕ ~2 µm); O2 bubble ejection; | Colorimetric detection; DL: 5 µM; LR: from 16.7 µM to 500 μM; Analysis time ~15 min; Interference from 20 µM Cu2+ and 20 µM Fe3+ was observed; Similar results were obtained for para-phenylenediamine; | [67] |
| Diethyl chlorophosphate (nerve agent simulant) | Silica particle covered with fluoresceinamine and then partially covered with Pt; O2 bubble ejection; | Fluorescence detection; 10 mM diethyl chlorophosphate is detected in 1 min while 10 µM diethyl chlorophosphate is detected in about 3 min; Selectivity was proved with ethanol, toluene, acetone, and isopropanol; | [83] |
| Cortisol | Microtube (L ~10 µm, ϕ = 5 µm) made of an outer layer of PEDOT, a middle layer of Ni and an inner layer of Pt; The outer layer was further modified with Au and anti-cortisol antibodies; O2 bubble ejection; | Colorimetric detection; Lowest detected concentration 0.1 μg/mL; Analysis time 2 min; | [84] |
| Glucose | ~30 µm diameter Mg microbead partially covered with Pt; H2 bubble ejection; | Electrochemical detection; DL: 33.2 µM; LR: from 1 mM to 15 mM; Measurements in diluted human serum were made; | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, M.N.; Gáspár, S. Analyte Sensing with Catalytic Micromotors. Biosensors 2023, 13, 45. https://doi.org/10.3390/bios13010045
Popescu MN, Gáspár S. Analyte Sensing with Catalytic Micromotors. Biosensors. 2023; 13(1):45. https://doi.org/10.3390/bios13010045
Chicago/Turabian StylePopescu, Mihail N., and Szilveszter Gáspár. 2023. "Analyte Sensing with Catalytic Micromotors" Biosensors 13, no. 1: 45. https://doi.org/10.3390/bios13010045
APA StylePopescu, M. N., & Gáspár, S. (2023). Analyte Sensing with Catalytic Micromotors. Biosensors, 13(1), 45. https://doi.org/10.3390/bios13010045






