Plier Ligands for Trapping Neurotransmitters into Complexes for Sensitive Analysis by SERS Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Bispidine-Based Bis-Azole Ligands
2.3. SERS Sensor Fabrication
2.4. Immobilization of Cu(II) Ions and Bispidine-Based Bis-Azole Ligands into Chitosan Film
2.5. Material Characterization and Raman Measurements
3. Results and Discussion
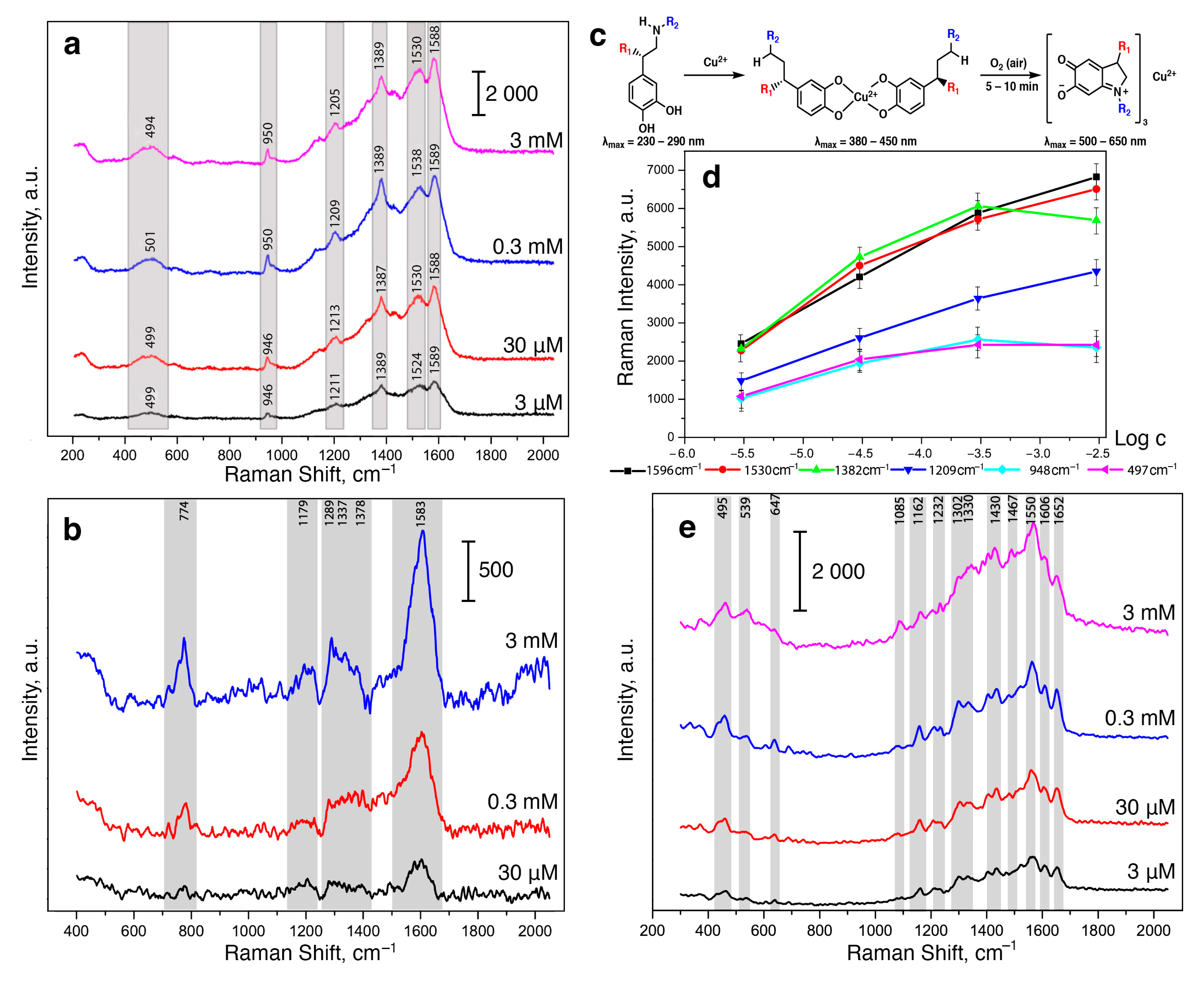
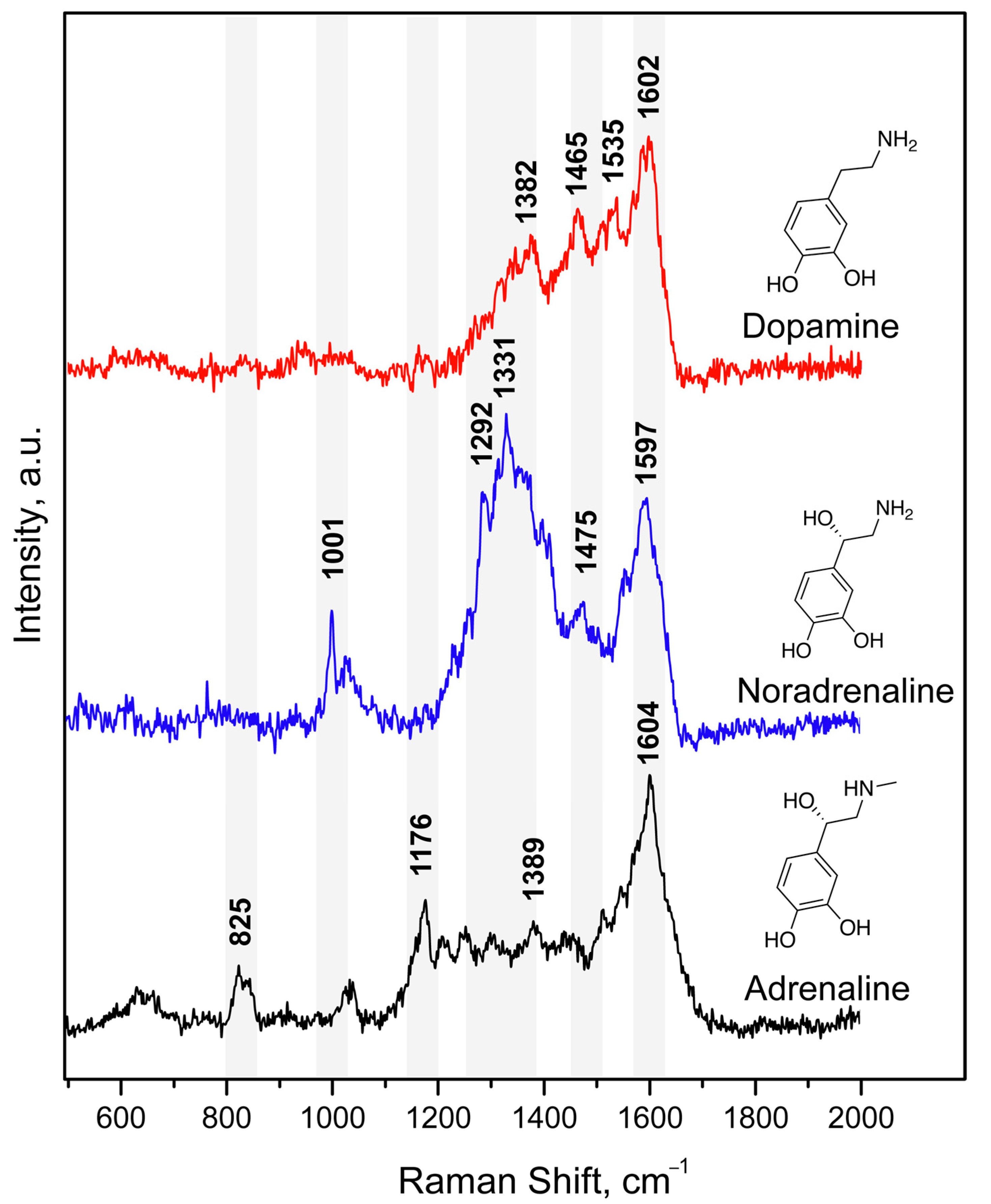
3.1. Complexes of Catecholamines with Cu(II) Ions
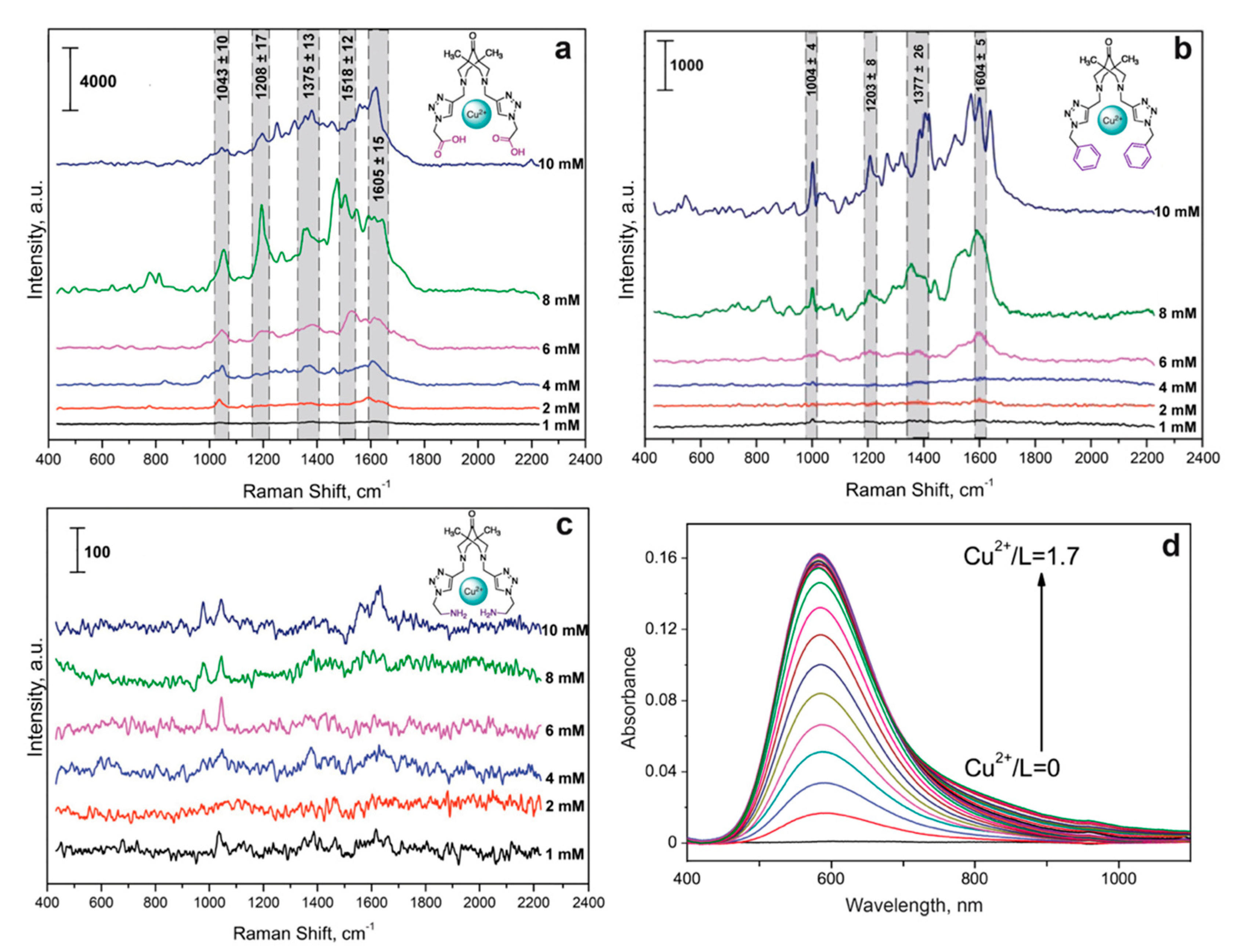
3.2. Complexes of Cu(II) Ions and Bispidine-Based Bis-Azole Ligands
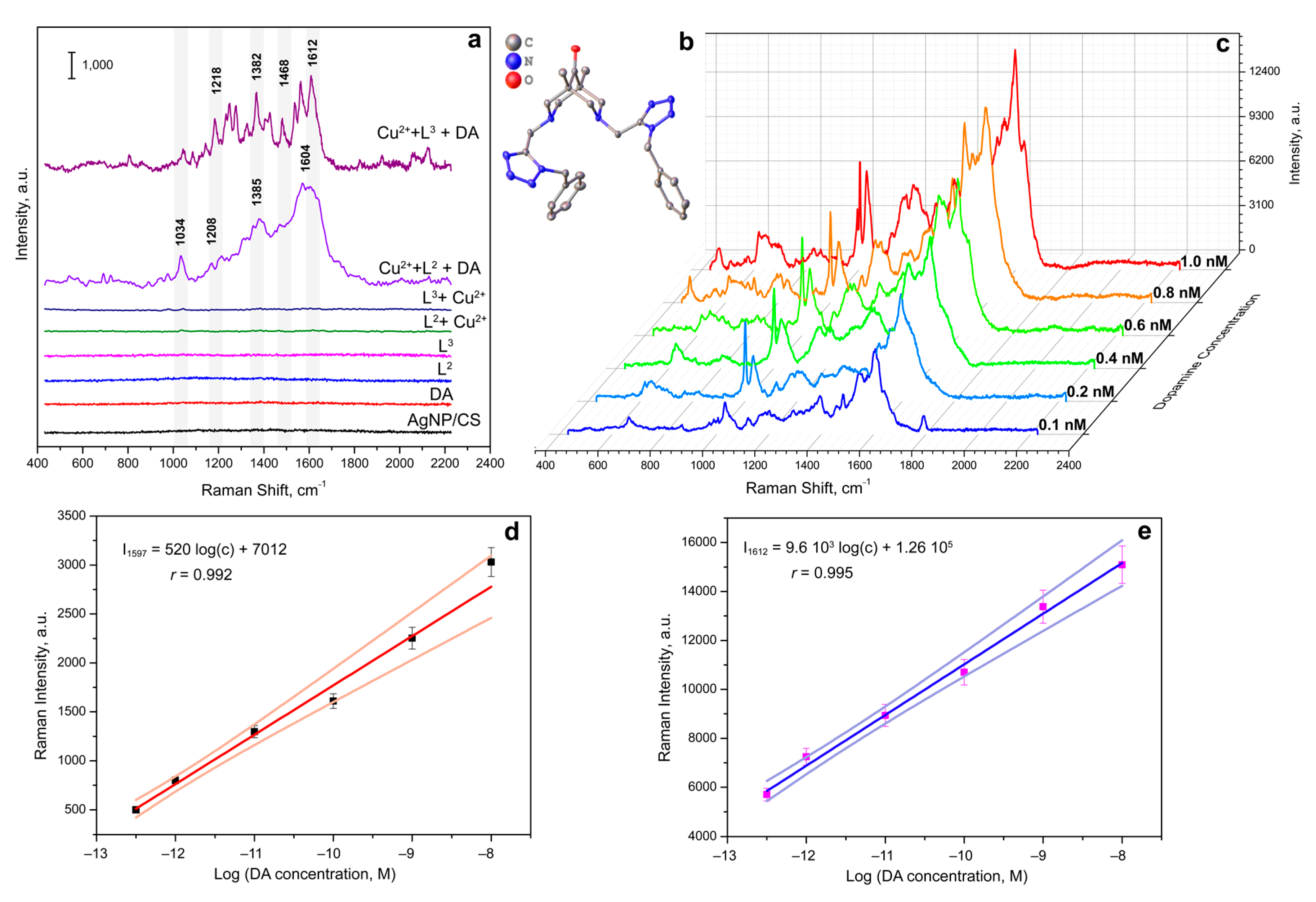
3.3. Complexes of Catecholamines with Cu(II) Ions and Bispidine-Based Bis-Azole Ligands
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veselova, I.A.; Sergeeva, E.A.; Makedonskaya, M.I.; Eremina, O.E.; Kalmykov, S.N.; Shekhovtsova, T.N. Methods for determining neurotransmitter metabolism markers for clinical diagnostics. J. Anal. Chem. 2017, 71, 1155–1168. [Google Scholar] [CrossRef]
- Schroeder, C.; Jordan, J. Norepinephrine transporter function and human cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1273–H1282. [Google Scholar] [CrossRef]
- Miyamoto, S.; Yoshida, Y.; Ozeki, Y.; Okamoto, M.; Gotoh, K.; Masaki, T.; Nishida, H.; Shibuya, T.; Shin, T.; Daa, T.; et al. Dopamine-Secreting Pheochromocytoma and Paraganglioma. J. Endocr. Soc. 2021, 5, bvab163. [Google Scholar] [CrossRef]
- Dwivedi, N.; Shah, J.; Mishra, V.; Tambuwala, M.; Kesharwani, P. Nanoneuromedicine for management of neurodegenerative disorder. J. Drug Deliv. Sci. Technol. 2019, 49, 477–490. [Google Scholar] [CrossRef]
- Liu, S.Q.; Li, B.; Li, J.J.; Sun, S.; Sun, S.R.; Wu, Q. Neuroendocrine regulations in tissue-specific immunity: From mechanism to applications in tumor. Front. Cell Dev. Biol. 2022, 10, 896147. [Google Scholar] [CrossRef] [PubMed]
- Makedonskaya, M.I.; Veselova, I.A.; Kalmykov, S.N.; Shekhovtsova, T.N. Novel biosensing system for the simultaneous multiplex fluorescent determination of catecholamines and their metabolites in biological liquids. J. Pharm. Biomed. Anal. 2018, 156, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chao, M.; Wang, Z. Electrochemical detection of dopamine in the presence of epinephrine, uric acid and ascorbic acid using a graphene-modified electrode. Anal. Methods 2012, 4, 1687–1692. [Google Scholar] [CrossRef]
- Quan, D.; Shin, W. Amperometric detection of catechol and catecholamines by immobilized laccase from DeniLite. Electroanalysis 2004, 16, 1576–1582. [Google Scholar] [CrossRef]
- Yogeswaran, U.; Chen, S.M. Multi-walled carbon nanotubes with poly(methylene blue) composite film for the enhancement and separation of electroanalytical responses of catecholamine and ascorbic acid. Sens. Actuators B Chem. 2008, 130, 739–749. [Google Scholar] [CrossRef]
- Kang, W.J.; Niu, L.M.; Ma, L. 2,3-Dimercaptosuccinic acid self-assembled gold electrode for the simultaneous determination of epinephrine and dopamine. Chin. Chem. Lett. 2009, 20, 221–224. [Google Scholar] [CrossRef]
- Murphy, J.F.; Davies, D.H.; Smith, C.J. The development of enzyme-linked immunosorbent assays (ELISA) for the catecholamines adrenalin and noradrenalin. J. Immunol. Methods 1992, 154, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Da Prada, M.; Zurcher, G. Simultaneous radioenzymatic determination of plasma and tissue adrenaline, noradrenaline and dopamine within the femtomole range. Life Sci. 1976, 19, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, H.; Ryu, J.; Jeon, O.; Paeng, I.R. Competitive enzyme-linked immunosorbent assay for a selective and sensitive determination of dopamine in the presence of ascorbic acid and uric acid. J. Immunoass. Immunochem. 2010, 31, 33–44. [Google Scholar] [CrossRef]
- Ghasemi, F.; Hormozi-Nezhad, M.R.; Mahmoudi, M. Identification of catecholamine neurotransmitters using fluorescence sensor array. Anal. Chim. Acta 2016, 917, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, B.; Xiong, H.; Wen, W.; Cheng, N. A ratiometric fluorometric epinephrine and norepinephrine assay based on carbon dot and CdTe quantum dots nanocomposites. Microchem. J. 2019, 146, 66–72. [Google Scholar] [CrossRef]
- Fotopoulou, M.A.; Ioannou, P.C. Post-column terbium complexation and sensitized fluorescence detection for the determination of norepinephrine, epinephrine and dopamine using high-performance liquid chromatography. Anal. Chim. Acta 2002, 462, 179–185. [Google Scholar] [CrossRef]
- Zheng, J.; Mandal, R.; Wishart, D.S. A sensitive, high-throughput LC-MS/MS method for measuring catecholamines in low volume serum. Anal. Chim. Acta 2018, 1037, 159–167. [Google Scholar] [CrossRef]
- Carrera, V.; Sabater, E.; Vilanova, E.; Sogorb, M.A. A simple and rapid HPLC-MS method for the simultaneous determination of epinephrine, norepinephrine, dopamine and 5-hydroxytryptamine: Application to the secretion of bovine chromaffin cell cultures. J. Chromatogr. B 2007, 847, 88–94. [Google Scholar] [CrossRef]
- Espina-Benitez, M.B.; Marconi, F.; Randon, J.; Demesmay, C.; Dugas, V. Evaluation of boronate affinity solid-phase extraction coupled in-line to capillary isoelectric focusing for the analysis of catecholamines in urine. Anal. Chim. Acta 2018, 1034, 195–203. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Shi, H.; Ma, C.; Cong, B.; Kang, W. Determination of three major catecholamines in human urine by capillary zone electrophoresis with chemiluminescence detection. Anal. Biochem. 2012, 427, 10–17. [Google Scholar] [CrossRef]
- Li, X.; Jin, W.; Weng, Q. Separation and determination of homovanillic acid and vanillylmandelic acid by capillary electrophoresis with electrochemical detection. Anal. Chim. Acta 2002, 461, 123–130. [Google Scholar] [CrossRef]
- Tesoro, C.; Lelario, F.; Ciriello, R.; Bianco, G.; Di Capua, A.; Acquavia, M.A. An Overview of Methods for L-Dopa Extraction and Analytical Determination in Plant Matrices. Separations 2022, 9, 224. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Q.; Wang, L.; Zhang, Y.; Cui, M.; Bi, K.; Li, D.; Li, Q. Nanoconfined liquid phase nanoextraction combined with in-fiber derivatization for simultaneous quantification of seventy amino-containing metabolites in plasma by LC-MS/MS: Exploration of lung cancer screening model. Talanta 2022, 245, 123452. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Satheeshkumar, E.; Karuppaiya, P.; Sivashanmugan, K.; Chao, W.T.; Tsay, H.S.; Yoshimura, M. Biocompatible 3D SERS substrate for trace detection of amino acids and melamine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 181, 91–97. [Google Scholar] [CrossRef]
- Rajapandiyan, P.; Yang, J. Sensitive cylindrical SERS substrate array for rapid microanalysis of nucleobases. Anal. Chem. 2012, 84, 10277–10282. [Google Scholar] [CrossRef]
- Shafer-Peltier, K.E.; Haynes, C.L.; Glucksberg, M.R.; van Duyne, R.P. Toward a glucose biosensor based on surface-enhanced Raman scattering. J. Am. Chem. Soc. 2003, 125, 588–593. [Google Scholar] [CrossRef]
- Raju, N.R.C. Hemoglobin detection on AgO surface enhanced Raman scattering (SERS)-substrates. Mater. Lett. 2014, 130, 274–276. [Google Scholar] [CrossRef]
- Lv, Y.; Qin, Y.; Svec, F.; Tan, T. Molecularly imprinted plasmonic nanosensor for selective SERS detection of protein biomarkers. Biosens. Bioelectron. 2016, 80, 433–441. [Google Scholar] [CrossRef]
- Eremina, O.E.; Semenova, A.A.; Sergeeva, E.A.; Brazhe, N.A.; Maksimov, G.V.; Shekhovtsova, T.N.; Goodilin, E.A.; Veselova, I.A. Surface-enhanced Raman spectroscopy in modern chemical analysis: Advances and prospects. Russ. Chem. Rev. 2018, 87, 741–770. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Dasari, R.R.; Feld, M.S. Near-infrared surface-enhanced Raman scattering (NIR-SERS) of neurotransmitters in colloidal silver solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 481–487. [Google Scholar] [CrossRef]
- Moody, A.S.; Sharma, B. Multi-metal, Multi-wavelength Surface-Enhanced Raman Spectroscopy Detection of Neurotransmitters. ACS Chem. Neurosci. 2018, 9, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, M.; Liang, O.; Sun, K.; Cipriano, A.F.; Schroeder, T.; Liu, H.; Xie, Y.H. Label-Free SERS Selective Detection of Dopamine and Serotonin Using Graphene-Au Nanopyramid Heterostructure. Anal. Chem. 2015, 87, 10255–10261. [Google Scholar] [CrossRef] [PubMed]
- Vatsadze, S.Z.; Medved'ko, A.V.; Bodunov, A.A.; Lyssenko, K.A. Bispidine-based bis-azoles as a new family of supramolecular receptors: The theoretical approach. Mendeleev Commun. 2020, 30, 344–346. [Google Scholar] [CrossRef]
- Semenova, A.A.; Goodilin, E.A.; Brazhe, N.A.; Ivanov, V.K.; Baranchikov, A.E.; Lebedev, V.A.; Goldt, A.E.; Sosnovtseva, O.V.; Savilov, S.V.; Egorov, A.V.; et al. Planar SERS nanostructures with stochastic silver ring morphology for biosensor chips. J. Mater. Chem. 2012, 22, 24530–24544. [Google Scholar] [CrossRef]
- Eremina, O.E.; Sidorov, A.V.; Shekhovtsova, T.N.; Goodilin, E.A.; Veselova, I.A. Novel multilayer nanostructured materials for recognition of polycyclic aromatic sulfur pollutants and express analysis of fuel quality and environmental health by surface enhanced Raman spectroscopy. ACS Appl. Mater. Interfaces 2017, 9, 15058–15067. [Google Scholar] [CrossRef]
- Sidorov, A.V.; Eremina, O.E.; Veselova, I.A.; Goodilin, E.A. Polymer-coated substrates for surface enhanced Raman spectroscopy. Mendeleev Commun. 2015, 25, 460–462. [Google Scholar] [CrossRef]
- Eremina, O.E.; Kapitanova, O.O.; Ferree, M.V.; Lemesh, I.A.; Eremin, D.B.; Goodilin, E.A.; Veselova, I.A. Ultrasensitive and multiplex SERS determination of anthropogenic phenols in oil fuel and environmental samples. Environ. Sci. Nano 2022, 9, 964–974. [Google Scholar] [CrossRef]
- Suzuki, Y. Design and synthesis of fluorescent reagents for selective detection of dopamine. Sens. Actuators B 2017, 239, 383–389. [Google Scholar] [CrossRef]
- Kareem, M.J.; Al-Hamdani, A.A.S.; Jirjees, V.Y.; Khan, M.E.; Allaf, A.W.; Al Zoubi, W. Preparation, spectroscopic study of Schiff base derived from dopamine and metal Ni(II), Pd(II), and Pt(IV) complexes, and activity determination as antioxidants. J. Phys. Org. Chem. 2020, 34, e4156. [Google Scholar] [CrossRef]
- Nagles, E.; Ibarra, L.; Llanos, J.P.; Hurtado, J.; Garcia-Beltrán, O. Development of a novel electrochemical sensor based on cobalt(II) complex useful in the detection of dopamine in presence of ascorbic acid and uric acid. J. Electroanal. Chem. 2017, 788, 38–43. [Google Scholar] [CrossRef]
- Sidorov, A.; Vashkinskaya, O.; Grigorieva, A.; Shekhovtsova, T.; Veselova, I.; Goodilin, E. Entrapment into charge transfer complexes for resonant Raman scattering enhancement. Chem. Commun. 2014, 50, 6468–6470. [Google Scholar] [CrossRef] [PubMed]
- Eremina, O.E.; Yarenkov, N.R.; Kapitanova, O.O.; Zelenetskaya, A.S.; Smirnov, E.A.; Shekhovtsova, T.N.; Goodilin, E.A.; Veselova, I.A. Molecular Immobilization and Resonant Raman Amplification by Complex-Loaded Enhancers (MIRRACLE) on copper (II)-chitosan-modified SERS-active metallic nanostructured substrates for multiplex determination of dopamine, norepinephrine, and epinephrine. Mikrochim. Acta 2022, 189, 211. [Google Scholar] [CrossRef] [PubMed]
- Barreto, W.J.; Barreto, S.R.; Ando, R.A.; Santos, P.S.; DiMauro, E.; Jorge, T. Raman, IR, UV-vis and EPR characterization of two copper dioxolene complexes derived from L-dopa and dopamine. Spectrochim. Acta Part A 2008, 71, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, C.G.; Buchanan, R.M. Transition metal complexes of o-benzoquinone, o-semiquinone, and catecholate ligands. Coord. Chem. Rev. 1981, 38, 45–87. [Google Scholar] [CrossRef]
- Pluto, R.; Burger, P. Normal values of catecholamines in blood plasma determined by high-performance liquid chromatography with amperometric detection. Int. J. Sports Med. 1988, 9, 75–78. [Google Scholar] [CrossRef]
- Peaston, R.T.; Weinkove, C. Measurement of catecholamines and their metabolites. Ann. Clin. Biochem. 2004, 41, 17–38. [Google Scholar] [CrossRef]
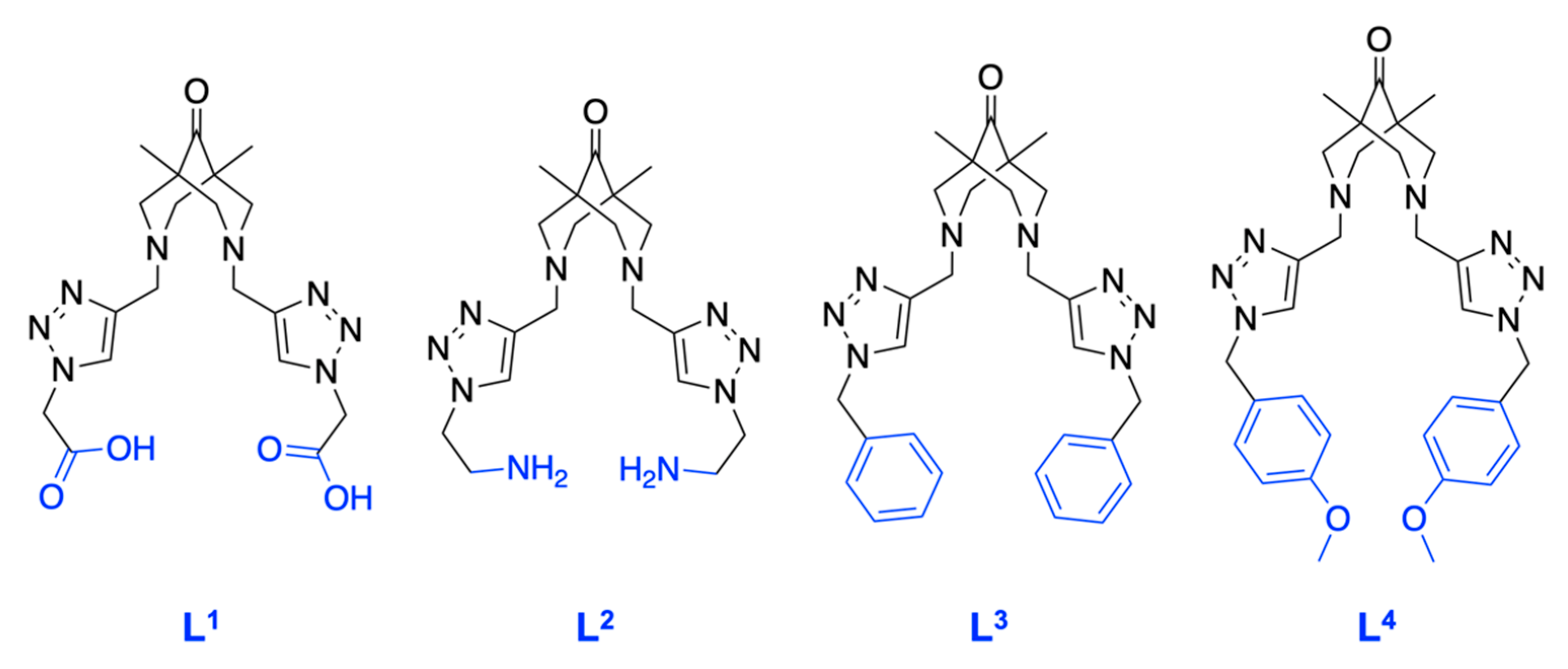

| Ligand, Solvent | L1, H2O | L2, EtOH | L3, MeCN | L4, MeCN |
|---|---|---|---|---|
| lgK(CuL2) | –* | –* | 8.3 ± 0.5 | –* |
| lgK(CuL) | –* | 4.0 ± 0.1 | 6.0 ± 0.5 | 5.2 ± 0.3 |
| lgK(Cu2L3) | 23.0 ± 0.3 (pH 4.0) | –* | –* | –* |
| λmax, nm | 577 | 595 | 583 | 577 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eremina, O.E.; Kapitanova, O.O.; Medved'ko, A.V.; Zelenetskaya, A.S.; Egorova, B.V.; Shekhovtsova, T.N.; Vatsadze, S.Z.; Veselova, I.A. Plier Ligands for Trapping Neurotransmitters into Complexes for Sensitive Analysis by SERS Spectroscopy. Biosensors 2023, 13, 124. https://doi.org/10.3390/bios13010124
Eremina OE, Kapitanova OO, Medved'ko AV, Zelenetskaya AS, Egorova BV, Shekhovtsova TN, Vatsadze SZ, Veselova IA. Plier Ligands for Trapping Neurotransmitters into Complexes for Sensitive Analysis by SERS Spectroscopy. Biosensors. 2023; 13(1):124. https://doi.org/10.3390/bios13010124
Chicago/Turabian StyleEremina, Olga E., Olesya O. Kapitanova, Alexei V. Medved'ko, Alexandra S. Zelenetskaya, Bayirta V. Egorova, Tatyana N. Shekhovtsova, Sergey Z. Vatsadze, and Irina A. Veselova. 2023. "Plier Ligands for Trapping Neurotransmitters into Complexes for Sensitive Analysis by SERS Spectroscopy" Biosensors 13, no. 1: 124. https://doi.org/10.3390/bios13010124
APA StyleEremina, O. E., Kapitanova, O. O., Medved'ko, A. V., Zelenetskaya, A. S., Egorova, B. V., Shekhovtsova, T. N., Vatsadze, S. Z., & Veselova, I. A. (2023). Plier Ligands for Trapping Neurotransmitters into Complexes for Sensitive Analysis by SERS Spectroscopy. Biosensors, 13(1), 124. https://doi.org/10.3390/bios13010124





