Hyaluronic Acid Methacrylate Hydrogel-Modified Electrochemical Device for Adsorptive Removal of Lead(II)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
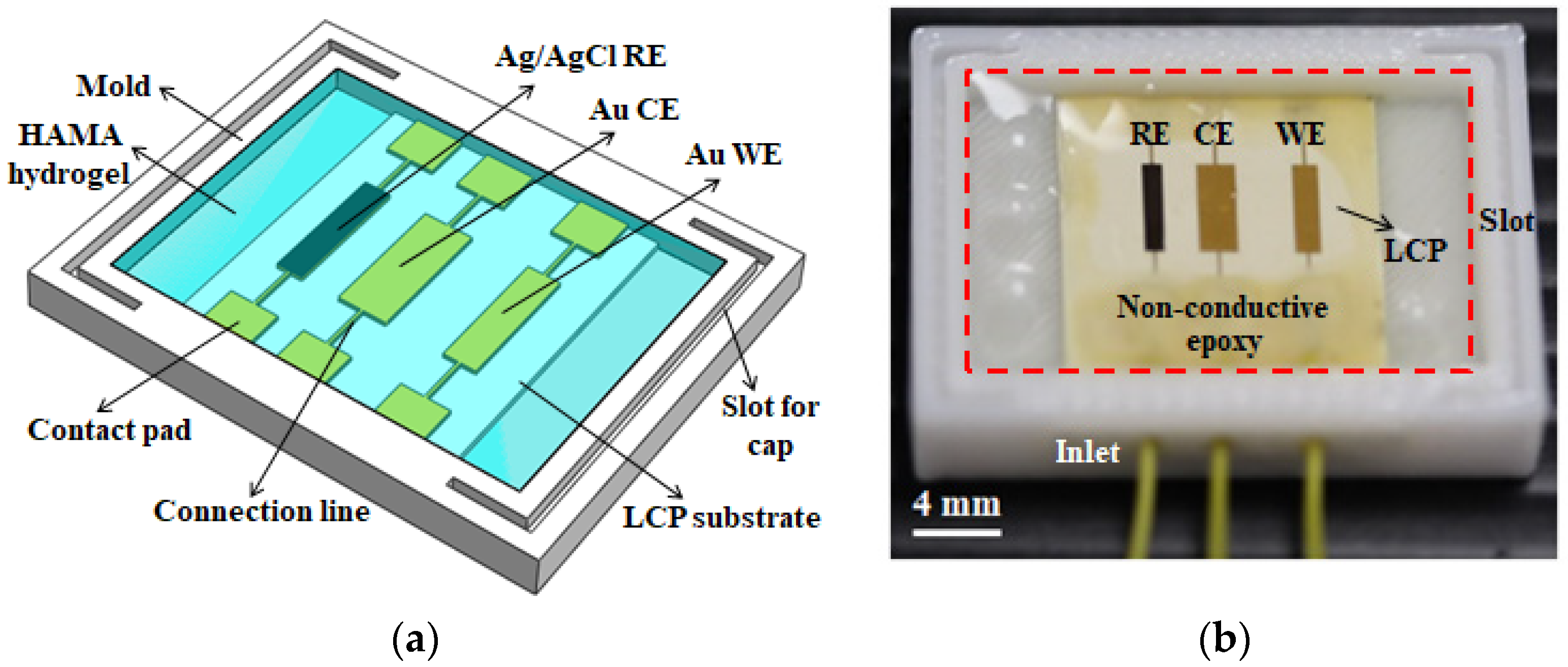
2.2. Device Fabrication
2.3. Device Packaging
2.4. Device Modification
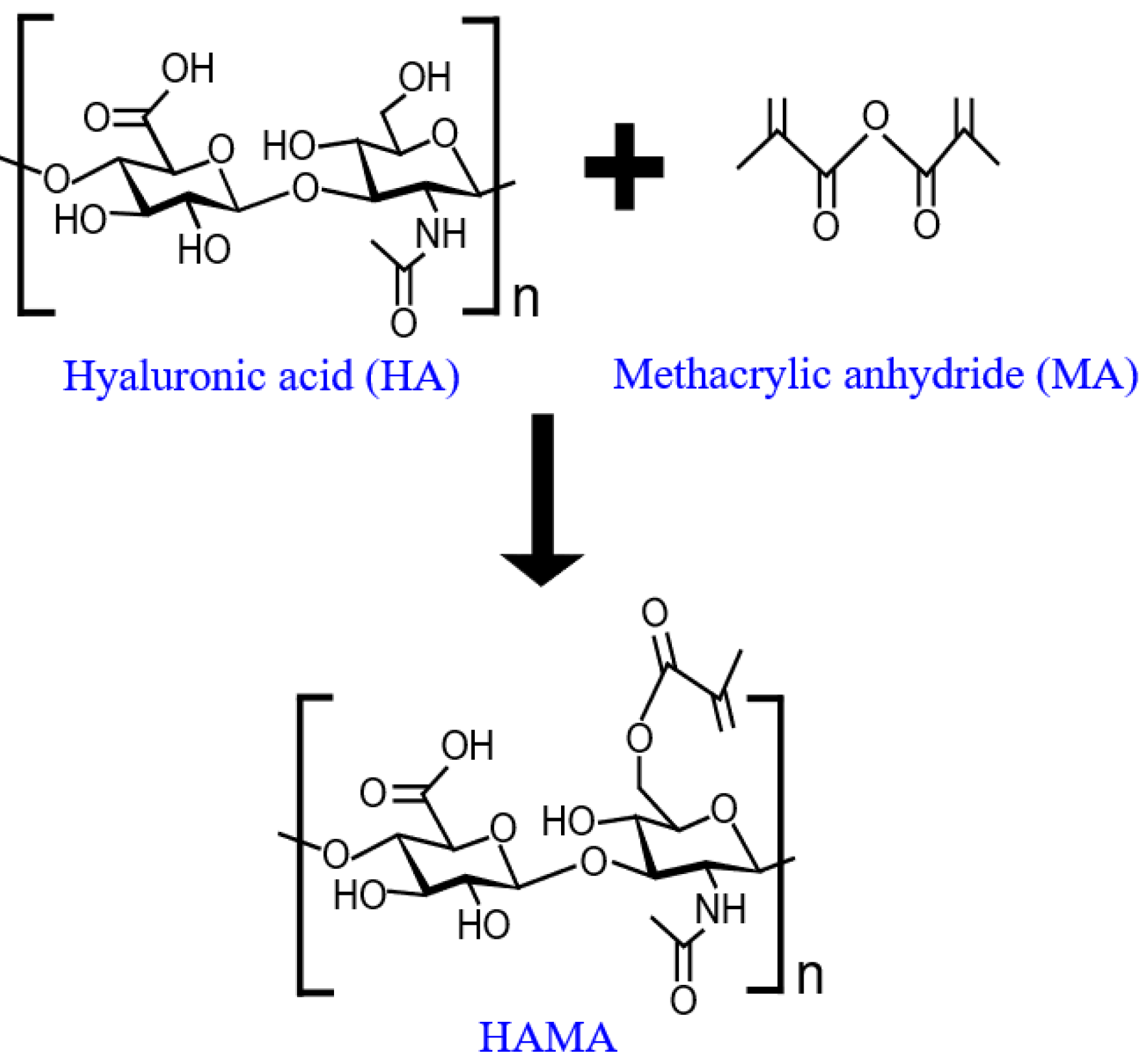
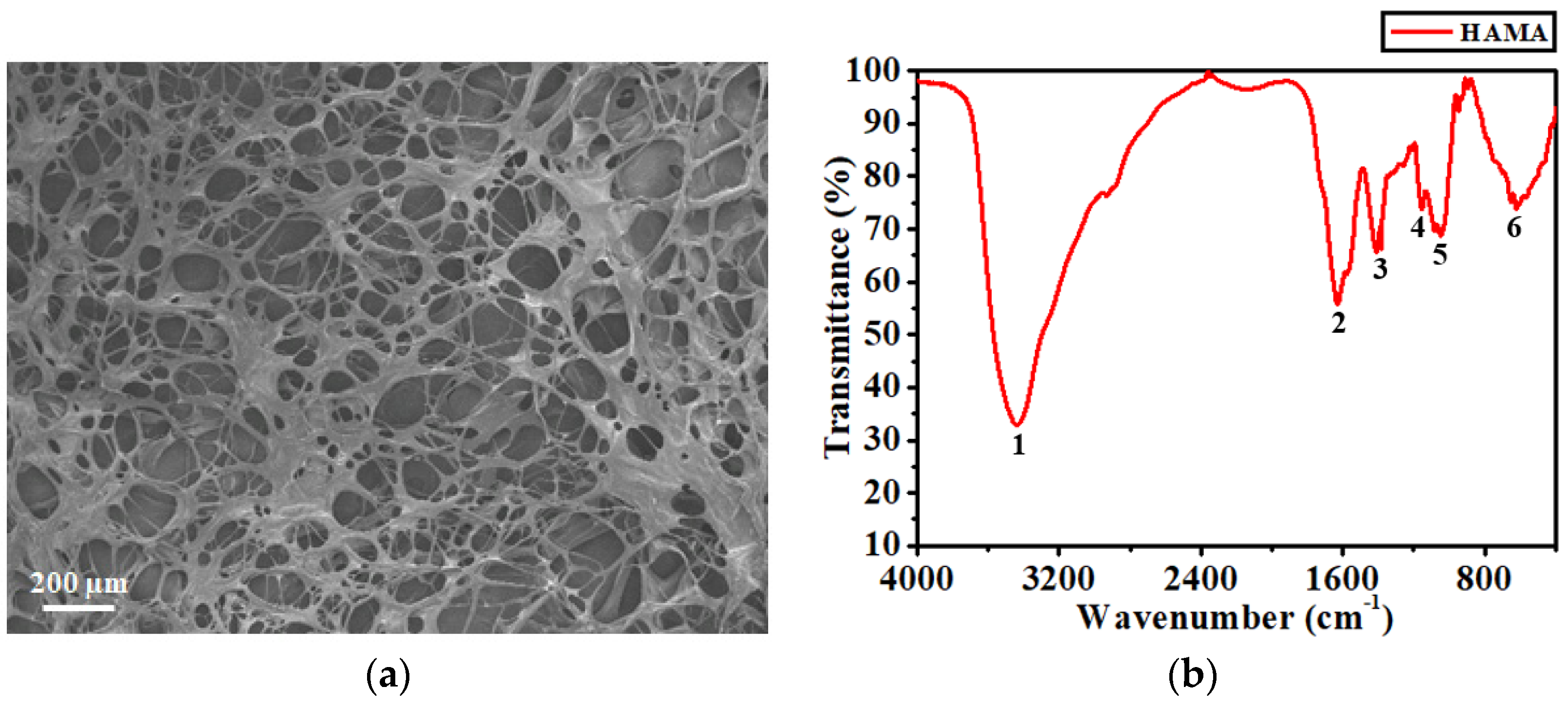
2.5. Investigation of Morphological and Chemical Structures of the Synthesized HAMA Hydrogel
2.6. Investigation of Adsorption Performance of the Modified Device
2.7. Investigation of Adsorption Efficiency of the Modified Device
3. Results and Discussion
3.1. Characterization of the Synthesized HAMA Hydrogel
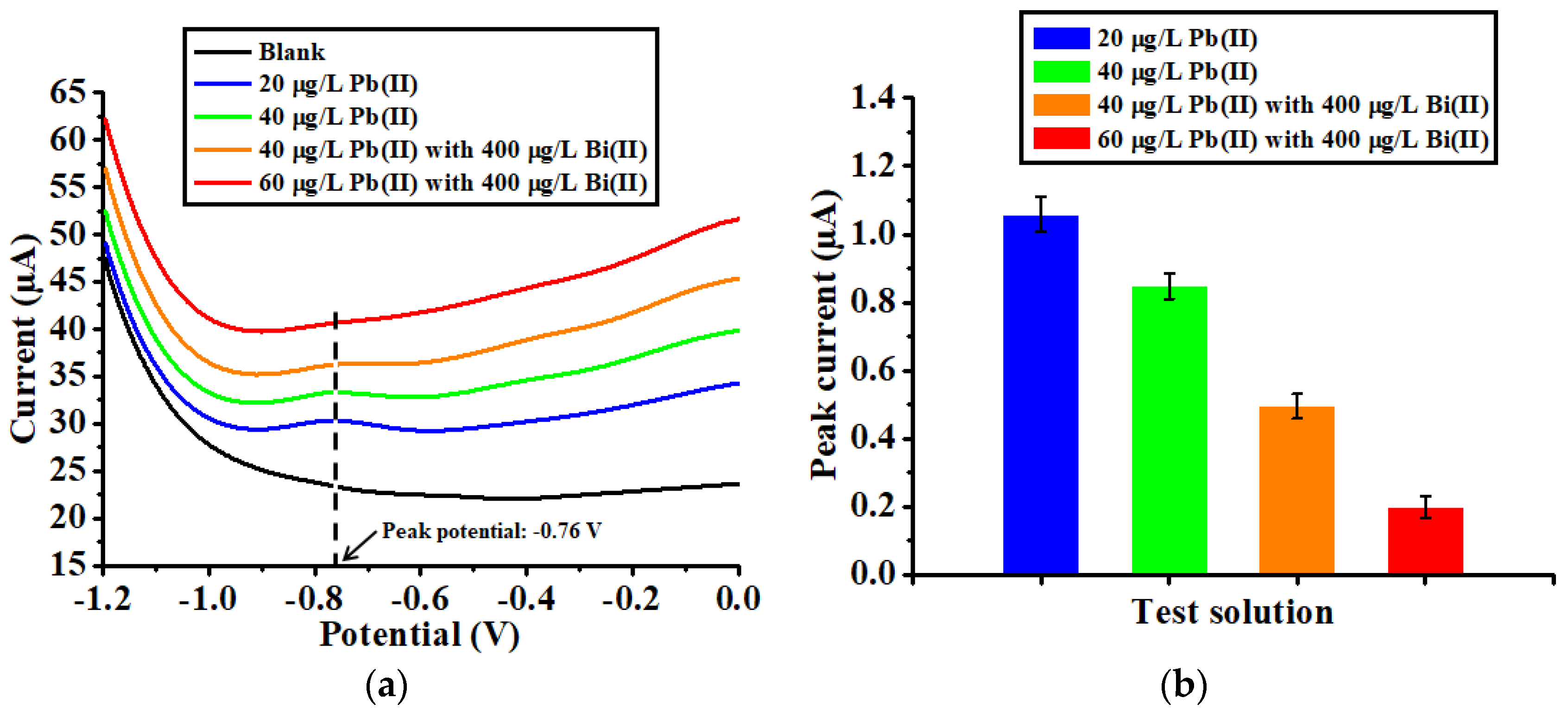
3.2. Electrochemical Investigation of the HAMA Hydrogel-Modified Device
3.3. Adsorption Mechanism of the HAMA Hydrogel-Modified Device
3.4. Validation of the Molecular Interaction
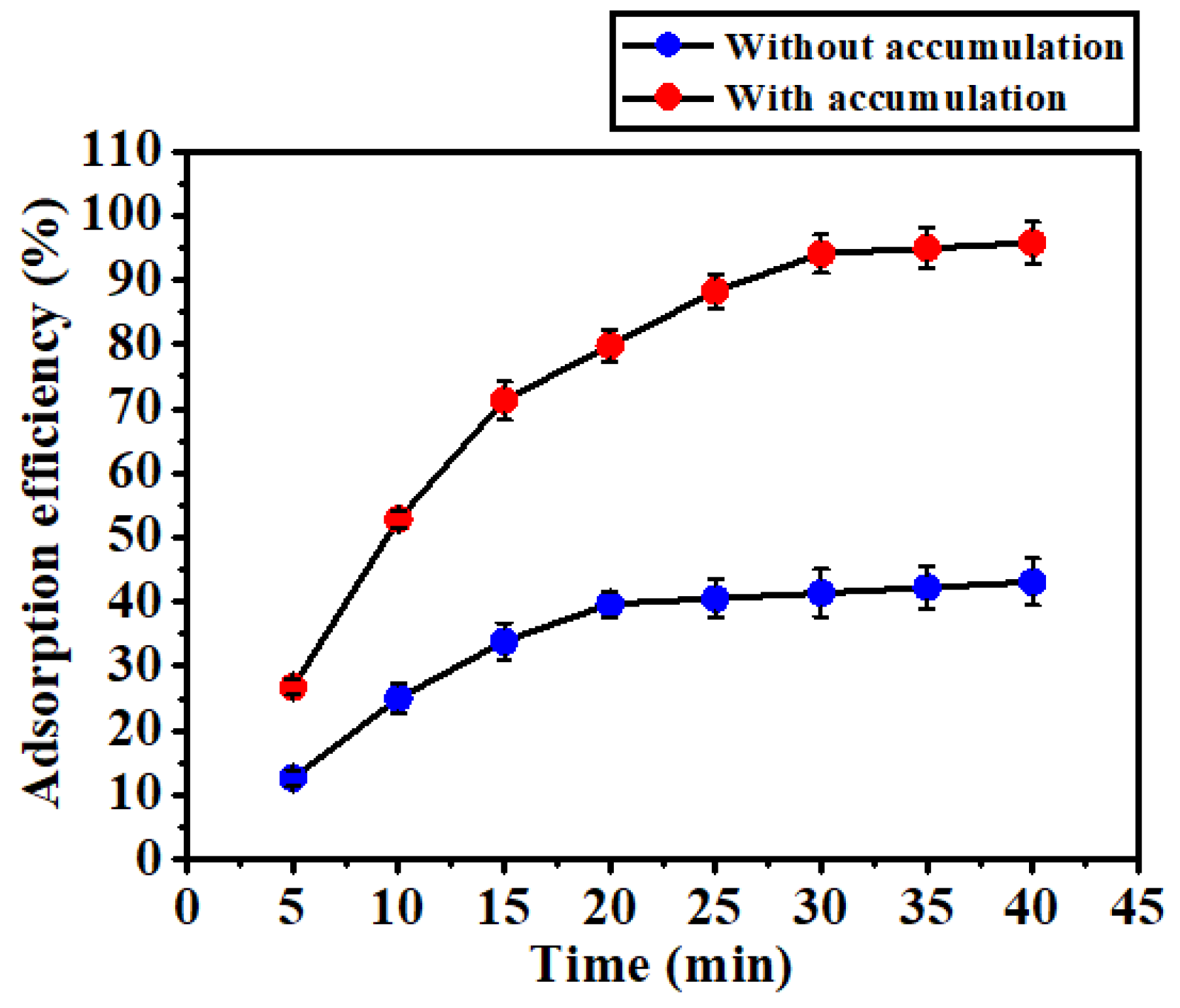
3.5. Validation of the Electrochemical Accumulation
3.6. Applicability of the HAMA Hydrogel-Modified Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Counter, S.A.; Buchanan, L.H.; Ortega, F. Association of hemoglobin levels and brainstem auditory evoked responses in lead-exposed children. Clin. Biochem. 2012, 45, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Hu, H.; Bellinger, D.C.; Mukherjee, B.; Modali, R.; Nasaruddin, K.; Schwartz, J.; Wright, R.O.; Ettinger, A.S.; Palaniapan, K.; et al. Hemoglobin, lead exposure, and intelligence quotient: Effect modification by the DRD2 Taq IA polymorphism. Environ. Health Perspect. 2011, 119, 144–149. [Google Scholar] [CrossRef]
- Philip, A.; Marsden, M.D. Increased body lead burden―cause or consequence of chronic renal insufficiency? N. Engl. J. Med. 2003, 348, 345–347. [Google Scholar]
- Bellinger, D.C. Lead. Pediatrics 2004, 113, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Kordas, K.; Queirolo, E.I.; Ettinger, A.S.; Wright, R.O.; Stoltzfus, R.J. Prevalence and predictors of exposure to multiple metals in preschool children from Montevideo, Uruguay. Sci. Total Environ. 2010, 408, 4488–4494. [Google Scholar] [CrossRef]
- Roy, A.; Ettinger, A.S.; Hu, H.; Bellinger, D.; Schwartz, J.; Modali, R.; Wright, R.O.; Palaniappan, K.; Balakrishnan, K. Effect modification by transferrin C2 polymorphism on lead exposure, hemoglobin levels, and IQ. Neurotoxicology 2013, 38, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Wang, X.; Zhao, D.; Rex, M.M.; Cho, H.J.; Lee, W.H. A novel nanoporous bismuth electrode sensor for in situ heavy metal detection. Electrochim. Acta 2019, 298, 440–448. [Google Scholar] [CrossRef]
- Wang, N.; Kanhere, E.; Miao, J.; Triantafyllou, M.S. Miniaturized chemical sensor with bio-inspired micropillar working electrode array for lead detection. Sens. Actuators B Chem. 2016, 233, 249–256. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Liu, C.; Chang, Y.; Wen, J.; Zhao, H.; Cao, H.; Zhang, Y.; Liu, D. Amino-modification and successive electro-chemical reduction of graphene oxide for highly sensitive electrochemical detection of trace Pb2+. Carbon 2016, 109, 479–486. [Google Scholar] [CrossRef]
- Zhou, G.; Chang, J.; Cui, S.; Pu, H.; Wen, Z.; Chen, J. Real-time, selective detection of Pb2+ in water using a reduced graphene oxide/gold nanoparticle field-effect transistor device. ACS Appl. Mater. Interfaces 2014, 6, 19235–19241. [Google Scholar] [CrossRef]
- Niu, X.; Zhong, Y.; Chen, R.; Wang, F.; Liu, Y.; Luo, D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens. Actuators B Chem. 2018, 255, 1577–1581. [Google Scholar] [CrossRef]
- Saha, S.K.; Ghosh, K.R.; Gao, J.P.; Wang, Z.Y. Highly sensitive dual-mode fluorescence detection of lead ion in water using aggregation-induced emissive polymers. Macromol. Rapid Commun. 2014, 35, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Wu, Y.; Liu, L.; Xing, H.; He, L.; Zhan, X.; Luo, Y.; Zhou, P. A simple fluorescent assay for lead(II) detection based on lead(II)-stabilized G-quadruplex formation. RSC Adv. 2013, 3, 16962–16966. [Google Scholar] [CrossRef]
- Skotadis, E.; Tsekenis, G.; Chatzipetrou, M.; Patsiouras, L.; Madianos, L.; Bousoulas, P.; Zergioti, I.; Tsoukalas, D. Heavy metal ion detection using DNAzyme-modified platinum nanoparticle networks. Sens. Actuators B Chem. 2017, 239, 962–969. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, Y.; Chen, Y.; Wu, X.; Fang, L.; Zhu, Z.; Yang, C.J. Target-responsive DNAzyme cross-linked hydrogel for visual quantitative detection of lead. Anal. Chem. 2014, 86, 11434–11439. [Google Scholar] [CrossRef]
- Wang, Y.; Irudayaraj, J. A SERS DNAzyme biosensor for lead ion detection. Chem. Commun. 2011, 47, 4394–4396. [Google Scholar] [CrossRef]
- Renfrew, D. Lead poisoning and the dangers of pragmatism. Int. J. Environ. Res. Public Health 2018, 15, 1997. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Synthesis and characterization of alumina-coated carbon nanotubes and their ap-plication for lead removal. J. Hazard. Mater. 2011, 185, 17–23. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Zhao, C.; Wang, H.; Chen, J.; Yang, L.; Feng, J.; Yan, W. Study on the synthesis of poly(pyrrole methane)s with the hydroxyl in different substituent position and their selective adsorption for Pb2+. Chem. Eng. J. 2019, 361, 528–537. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, T.G. Removal of Pb(II) ions from aqueous solutions using functionalized cryogels. Chemosphere 2019, 217, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Jacques, A.L.; Lujan-Montelongo, J.A.; Silva-Cuevas, C.; Cortez-Valadez, M.; Estevez, M.; Hernandez-Martinez, A.R. Lead(II) removal by poly(N,N-dimethylacrylamide-co-2-hydroxyethyl methacrylate). Eur. Polym. J. 2018, 101, 262–272. [Google Scholar] [CrossRef]
- Mohammadinezhad, A.; Marandi, G.B.; Farsadrooh, M.; Ja-vadian, H. Synthesis of poly(acrylamide-co-itaconic ac-id)/MWCNTs superabsorbent hydrogel nanocomposite by ultra-sound-assisted technique: Swelling behavior and Pb(II) adsorption capacity. Ultrason. Sonochem. 2018, 49, 1–12. [Google Scholar] [CrossRef]
- Al Hamouz, O.C.S.; Adelabu, I.O.; Saleh, T.A. Novel cross-linked melamine based polyamine/CNT composites for lead ions removal. J. Environ. Manag. 2017, 192, 163–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z. Heavy metals removal using hydrogel-supported nanosized hydrous ferric oxide: Synthesis, characterization, and mechanism. Sci. Total Environ. 2017, 580, 776–786. [Google Scholar] [CrossRef]
- Weidman, J.L.; Mulvenna, R.A.; Boudouris, B.W.; Phillip, W.A. Nanoporous block polymer thin films functionalized with bio-inspired ligands for the efficient capture of heavy metal ions from water. ACS Appl. Mater. Interfaces 2017, 9, 19152–19160. [Google Scholar] [CrossRef]
- Saranya, M.; Latha, S.; Reddi, M.R.G.; Gomathi, T.; Sudha, P.N.; Anil, S. Adsorption studies of Lead(II) from aqueous solution onto Nanochitosan /Polyurethane /Polypropylene glycol ternary blends. Int. J. Biol. Macromol. 2017, 104, 1436–1448. [Google Scholar]
- Chandrasekharan, A.; Seong, K.Y.; Yim, S.G.; Kim, S.; Seo, S.; Yoon, J.; Yang, S.Y. In situ photocrosslinkable hyaluronic ac-id-based surgical glue with tunable mechanical properties and high adhesive strength. J. Polym. Sci. A Polym. Chem. 2019, 57, 522–530. [Google Scholar] [CrossRef]
- Ren, L.; Lim, Y.T. Degradation-regulatable architectured implantable macroporous scaffold for the spatiotemporal modulation of immunosuppressive microenvironment and enhanced combination cancer immunotherapy. Adv. Funct. Mater. 2018, 28, 1804490. [Google Scholar] [CrossRef]
- Yang, G.; Fu, S.; Yao, W.; Wang, X.; Zha, Q.; Tang, R. Hyaluronic acid nanogels prepared via ortho ester linkages show pH-triggered behavior, enhanced penetration and antitumor efficacy in 3-D tumor spheroids. J. Colloid Interface Sci. 2017, 504, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Korogiannaki, M.; Rastegari, B.; Zhang, J.; Chen, M.; Fu, Q.; Sheardown, H.; Filipe, C.D.M.; Hoare, T. “Click” chemistry-tethered hyaluronic acid-based contact lens coatings improve lens wettability and lower protein adsorption. ACS Appl. Mater. Interfaces 2016, 8, 22064–22073. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Yi, H.; Yang, Y.; Chang, H.; Wu, J.; Tang, L.; Yang, Z.; Wang, N.; Hu, L.; Fu, Y.; et al. Origami-inspired electret-based triboelectric generator for biomechanical and ocean wave energy harvesting. Nano Energy 2020, 67, 104197. [Google Scholar] [CrossRef]
- Smeds, K.A.; Pfister-Serres, A.; Miki, D.; Dastgheib, K.; In-oue, M.; Hatchell, D.L.; Grinstaff, M.W. Photocrosslinkable poly-saccharides for in situ hydrogel formation. J. Biomed. Mater. Res. 2001, 54, 115–121. [Google Scholar] [CrossRef]
- Magalhaes, J.; Sousa, R.A.; Mano, J.F.; Reis, R.L.; Blanco, F.J.; Roman, J.S. Synthesis and characterization of sensitive hydrogels based on semi-interpenetrated networks of poly[2-ethyl-(2-pyrrolidone) methacrylate] and hyaluronic acid. J. Biomed. Mater. Res. Part A 2013, 101A, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Nair, S.S.; Nair, A.S. Fabrication of a bioadhesive transdermal device from chitosan and hyaluronic acid for the controlled release of lidocaine. Carbohydr. Polym. 2016, 152, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.J.; Karunakaran, K.T. Purification and characterization of hyaluronic acid produced by Streptococcus zooepidemicus strain 3523-7. J. BioScience Biotechnol. 2013, 2, 173–179. [Google Scholar]
- Wang, N.; Kanhere, E.; Miao, J.; Triantafyllou, M.S. Nanoparticles-modified chemical sensor fabricated on a flexible polymer substrate for cadmium(II) detection. Polymers 2018, 10, 694. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.K.; Choi, E.; Piao, Y. Voltammetric determination of trace heavy metals using an electrochemically deposited graphene/bismuth nanocomposite film-modified glassy carbon electrode. J. Electroanal. Chem. 2016, 766, 120–127. [Google Scholar] [CrossRef]
- Serrano, N.; Alberich, A.; Diaz-Cruz, J.M.; Arino, C.; Esteban, M. Coating methods, modifiers and applications of bismuth screen-printed electrodes. Trends Anal. Chem. 2013, 46, 15–29. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Feng, Y.; Schmidt, A.; Weiss, R.A. Compatibilization of polymer blends by complexation. 1. spectroscopic characterization of ion-amide interactions in ionomer/polyamide blends. Macromolecules 1996, 29, 3909–3917. [Google Scholar] [CrossRef]
- Amiri, S.; Asghari, A.; Vatanpour, V.; Rajabi, M. Fabrication of chitosan-aminopropylsilane graphene oxide nanocomposite hydrogel embedded PES membrane for improved filtration performance and lead separation. J. Environ. Manag. 2021, 294, 112918. [Google Scholar] [CrossRef] [PubMed]
- Aniagor, C.O.; Afifi, M.A.; Hashem, A. Rapid and efficient uptake of aqueous lead pollutant using starch-based superabsorbent hydrogel. Polym. Bull. 2022, 79, 6373–6388. [Google Scholar] [CrossRef]
- Singh, S.; Basu, H.; Bassan, M.K.T.; Singhal, R.K. Thiol functionalised silica microsphere loaded polymeric hydrogel: Development of a novel hybrid sorbent for removal of lead and cadmium. Chemosphere 2022, 286, 131659. [Google Scholar] [CrossRef] [PubMed]

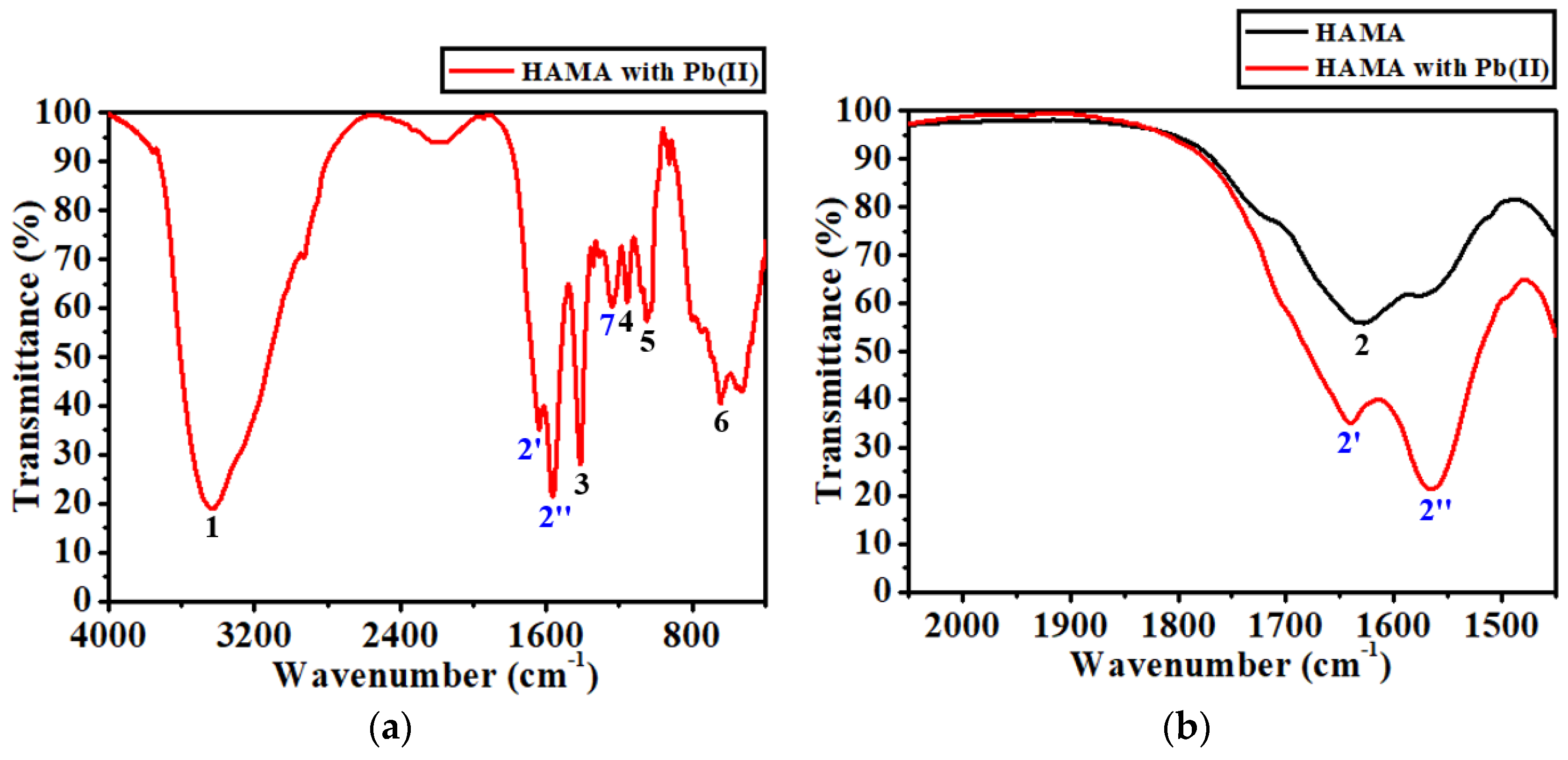


| Peak No. | HAMA (cm−1) | Assignment | Peak No. | HAMA with Pb(II) (cm−1) | Assignment |
|---|---|---|---|---|---|
| 1 | 3436.34 | O-H or N-H | 1 | 3430.24 | O-H or N-H |
| 2 | 1628.07 | C=O | 2′ | 1640.03 | Pb(II)-N |
| 2″ | 1565.72 | Pb(II)-O | |||
| 3 | 1410.64 | O-C=O | 3 | 1413.62 | O-C=O |
| 4 | 1153.41 | C-O | 4 | 1155.69 | C-O |
| 5 | 1046.36 | C-O-C | 5 | 1049.44 | C-O-C |
| 6 | 650.68 | bending | 6 | 644.68 | bending |
| 7 | 1237.61 | C-O or C-N |
| Adsorbent | Removal Efficiency (%) | Adsorption Time (min) | Reference |
|---|---|---|---|
| Poly(allylamine-co-methacrylamide-co-acrylic acid) cryogel | 83.54 | 720 | Kim et al. [22] |
| Poly(N,N-dimethylacrylamide-co-2-hydroxyethyl methacrylate) copolymer | 80.00 | 300 | Ramos-Jacques et al. [23] |
| Melamine-based crosslinked polyamine/CNT composite | 98.63 | 360 | Al Hamouz et al. [25] |
| Polyisoprene-b-polystyrene-b-poly(N,N-dimethyl-acrylamide) polymer | 94.80 | 480 | Weidman et al. [27] |
| Nanochitosan/polyurethane/polypropylene glycol | 95.00 | 60 | Saranya et al. [28] |
| Chitosan-aminopropylsilane graphene oxide nanocomposite hydrogel | 82.30 | 60 | Amiri et al. [43] |
| Copolymerized starch-based hydrogel | 87.00 | 60 | Aniagor et al. [44] |
| Thiol-functionalized silica microsphere-loaded polymeric hydrogel | 97.00 | 1440 | Singh et al. [45] |
| HAMA hydrogel-modified electrochemical device | 94.08 | 30 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Bora, M.; Hao, S.; Tao, K.; Wu, J.; Hu, L.; Liao, J.; Lin, S.; Triantafyllou, M.S.; Li, X. Hyaluronic Acid Methacrylate Hydrogel-Modified Electrochemical Device for Adsorptive Removal of Lead(II). Biosensors 2022, 12, 714. https://doi.org/10.3390/bios12090714
Wang N, Bora M, Hao S, Tao K, Wu J, Hu L, Liao J, Lin S, Triantafyllou MS, Li X. Hyaluronic Acid Methacrylate Hydrogel-Modified Electrochemical Device for Adsorptive Removal of Lead(II). Biosensors. 2022; 12(9):714. https://doi.org/10.3390/bios12090714
Chicago/Turabian StyleWang, Nan, Meghali Bora, Song Hao, Kai Tao, Jin Wu, Liangxing Hu, Jianjun Liao, Shiwei Lin, Michael S. Triantafyllou, and Xiaogan Li. 2022. "Hyaluronic Acid Methacrylate Hydrogel-Modified Electrochemical Device for Adsorptive Removal of Lead(II)" Biosensors 12, no. 9: 714. https://doi.org/10.3390/bios12090714
APA StyleWang, N., Bora, M., Hao, S., Tao, K., Wu, J., Hu, L., Liao, J., Lin, S., Triantafyllou, M. S., & Li, X. (2022). Hyaluronic Acid Methacrylate Hydrogel-Modified Electrochemical Device for Adsorptive Removal of Lead(II). Biosensors, 12(9), 714. https://doi.org/10.3390/bios12090714










