Self-Assembly of Small Organic Molecules into Luminophores for Cancer Theranostic Applications
Abstract
:1. Introduction
2. Self-Assembly of Small Organic Molecules in Theranostics
3. Peptide-Based Assembly for Theranostic Applications
| Name | Target | Theranostic Type | IC50 (Cell Line) | In Vitro or In Vivo | Ref. |
|---|---|---|---|---|---|
| G7CCERGDS | Cancer cell | PDT | - | In vivo | [45] |
| antiCD3-G7-RGD | T cell and Cancer cell | Immunotherapy | - | In vitro | [46] |
| DBT-2FFGYSA | Cancer cell | Imaging and immunotherapy | 38.3 μM (PC-3) | In vivo | [47] |
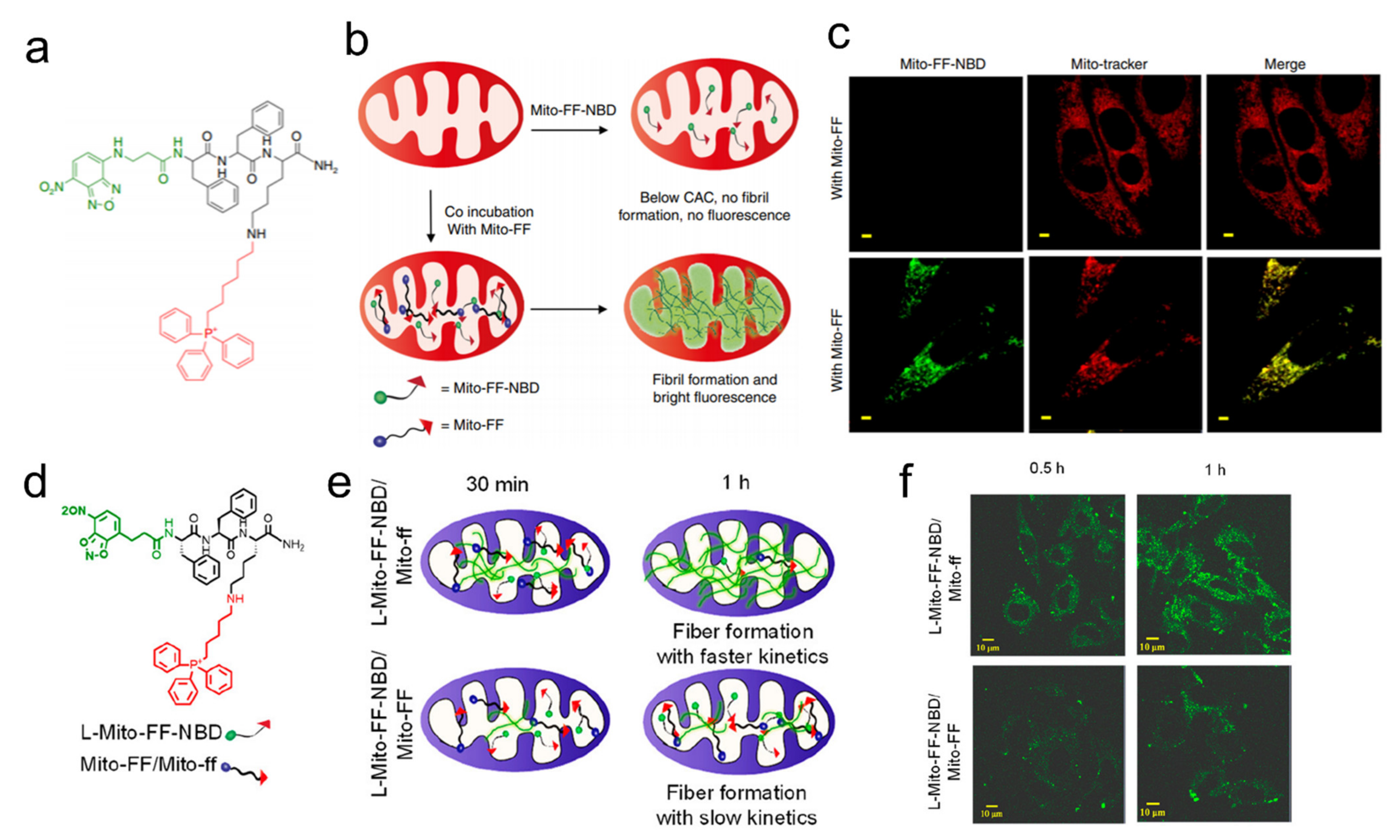
| NBD-FFYK-TPP | Mitochondria | Imaging and mitochondrial dysfunction | 200 μM (HeLa, HepG2, T98G, MCF7) | In vitro | [49] |
| Mito-FF(ff)-pyrene | Mitochondria | Mitochondrial dysfunction | 4–10 μM (HeLa) | In vitro | [50,51] |
| PEAK-DMA | Cancer cell | PDT | - | In vivo | [58] |
| TPP-RRRKLVFFK-Ce6 | Mitochondria | PDT | - | In vivo | [60] |
| LXY30-KLVFFK(Pa) | Cell membrane | PTT and PDT | - | In vivo | [61] |
| Ce6-DEVD-MMAE | Tumor tissues | Light-induced apoptosis | 9–10 nM | In vivo | [63] |
| VPF-FRRG-DOX | Cancer cell | Chemotherapy and PDT | 0.54 μM | In vivo | [64] |
| Sec(Dod)2KGPLGVRGRGD | Tumor | Chemoimmunotherapy | 0.51 μM (MDA-MB-231) | In vivo | [65] |
4. Amphiphile Molecule-Based Assembly for Theranostic Applications
| Name | Target | Theranostic Type | IC50 (Cell Line) | In Vitro or In Vivo | Ref. |
|---|---|---|---|---|---|
| Lips(PTQ/GA/AIPH) | Cancer cell | Imaging and PTT, PTDT | - | In vivo | [73] |
| GNPs@MRM/HAL | Tumor | Imaging and PDT | - | In vivo | [74] |
| IR780@Pt NPs | Mitochondria | Chemophototherapy | 1.2–2.3 μM (143B) | In vivo | [75] |
| Lac-DOX NPs | Tumor | Chemotherapy | - | In vivo | [78] |
| PGCA@PA NPs | Cancer cell | PDT and Immunotherapy | - | In vivo | [79] |
| M(Pt)/V(Pt) | Cancer cell | Imaging and photoactivatable therapy | 20.1 μM (HepG2) 55.9 μM (HeLa) 62.5 μM (A549) | In vivo | [80] |
| Ir-Cb ADDC NPs | Tumor | Chemotherapy | 13–15 μM | In vivo | [81] |
| [Ir(ppy-R)2Cl]2 | Tumor | Imaging and PDT | 1.2–1.3 μM (HepG2 and MCF-7) | In vivo | [82] |
| PTX-DTM-DBCO-chemogene | Tumor | chemo/gene therapy | >10 μM (HeLa) | In vivo | [84] |
| IID-ThTPA NPs | - | PTT and PDT | - | In vivo | [87] |
| TPA-BBT | - | PTT | - | In vivo | [88] |
| FA-PEG-PBLA | Cancer cell | PTT and PDT | - | In vivo | [89] |
| IrDAD-NPs | Tumor | PDT and PTT | 7.1–14.4 μM (A549) | In vivo | [93] |
5. AIEgen-Based Assembly for Theranostic Applications
| Name | Target | Theranostic Type | IC50 (Cell Line) | In Vitro or In Vivo | Ref. |
|---|---|---|---|---|---|
| AIE-mito-TPP | Mitochondria | Imaging and PDT | - | In vitro | [98] |
| DP-PPh3, TPE-PPh3 | Mitochondria | PDT | 1.25 μM 3.60 μM (A549R) | In vivo | [101] |
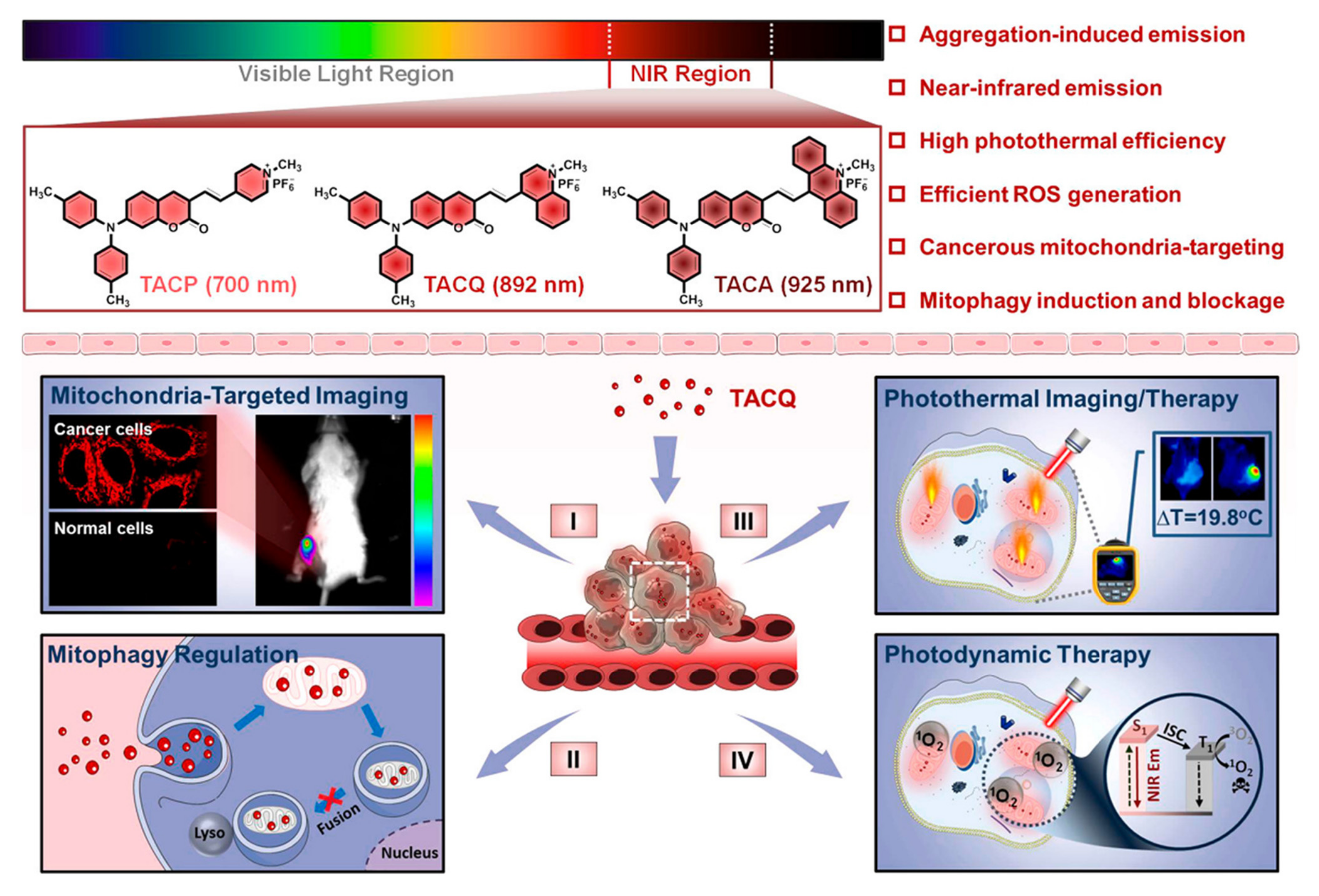
| TACQ | Mitochondria | Imaging and PDT/PTT | - | In vivo | [102] |
| 4TPA-BQ | Bacteria and cancer cells | PDT | - | In vivo | [105] |
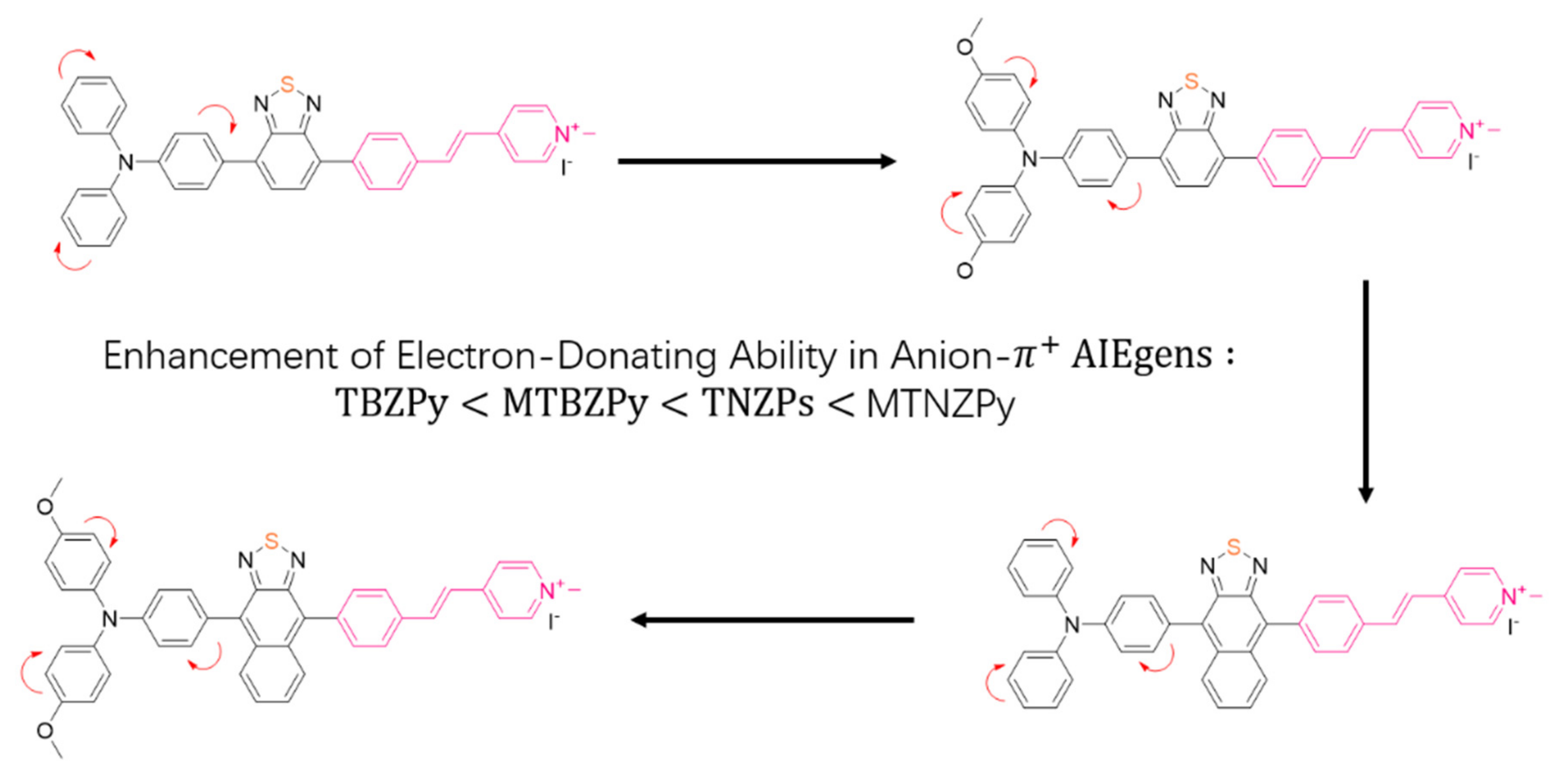
| TNZPy, MTNZPy | Mitochondria and lysosomes | PDT | - | In vivo | [106] |
| TTT-1,2,3,4 | - | Imaging and PDT/PTT | - | In vivo | [108] |
| TFPy, TFVP, TPE-TFPy | Mitochondria, cell membrane, and lysosomes | PDT | 1.40 μM (4T1) 2.72 μM (HeLa) | In vitro | [111] |
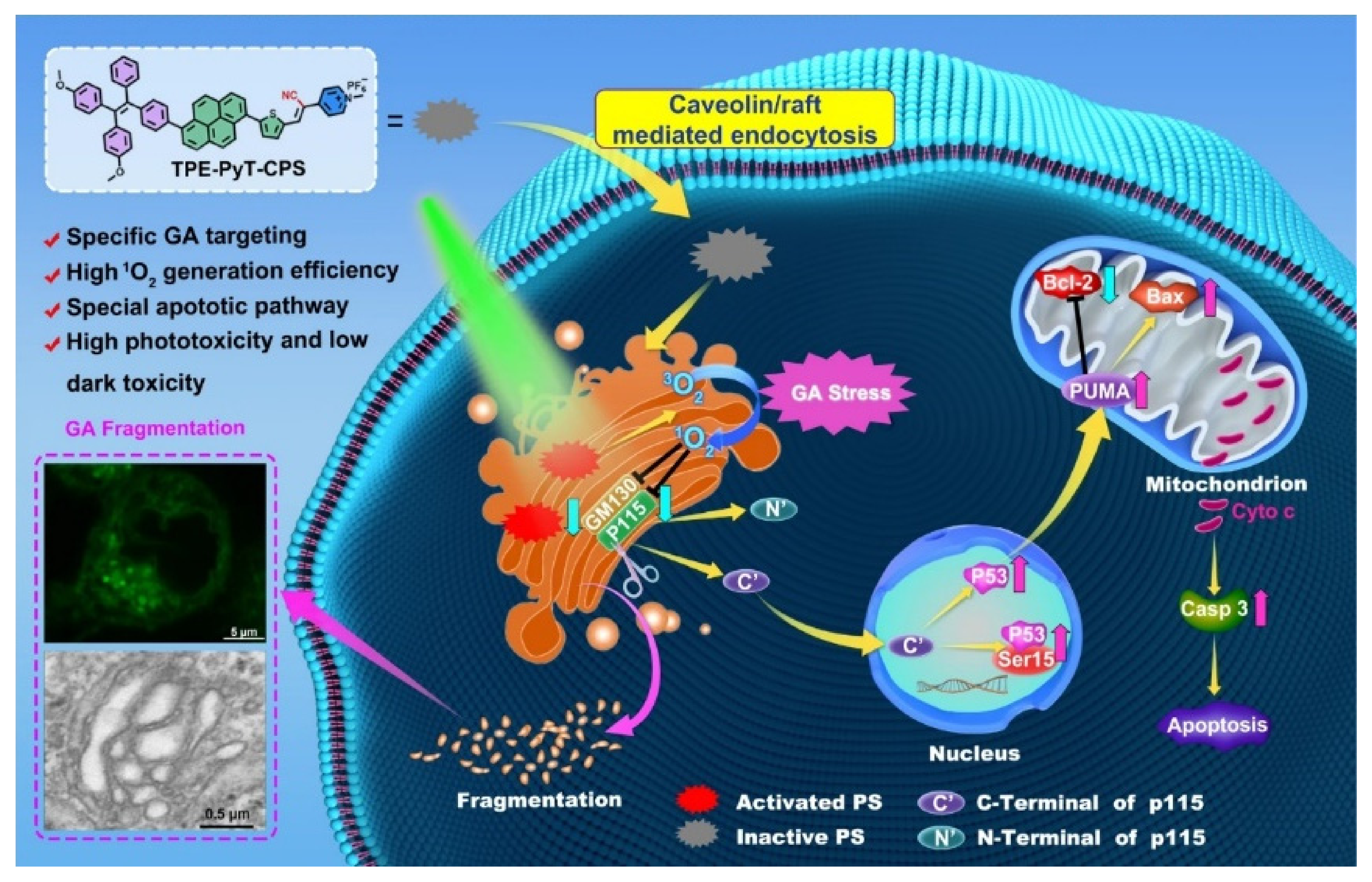
| TPE-PyT-CPS | Golgi apparatus | PDT | 170 nM (HeLa) | In vitro | [112] |
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Delavan, B.; Roberts, R.; Tong, W. Lessons Learned from Two Decades of Anticancer Drugs. Trends Pharmacol. Sci. 2017, 38, 852–872. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-W.; Liu, X.-H.; Fan, J.-X.; Peng, S.-Y.; Wang, J.-W.; Wang, X.-N.; Zhang, C.; Liu, C.-J.; Zhang, X.-Z. Self-mineralized photothermal bacteria hybridizing with mitochondria-targeted metal–organic frameworks for augmenting photothermal tu-mor therapy. Adv. Funct. Mater. 2020, 30, 1909806. [Google Scholar] [CrossRef]
- Singleton, D.C.; Macann, A.; Wilson, W.R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 2021, 18, 751–772. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Choi, S.H. Tumor-associated macrophages in cancer: Recent advancements in cancer nanoimmunotherapies. J. Exp. Clin. Cancer Res. 2022, 41, 68. [Google Scholar] [CrossRef]
- Wang, W.; Yung, T.-L.; Cheng, S.-S.; Chen, F.; Liu, J.-B.; Leung, C.-H.; Ma, D.-L. A long-lived luminogenic iridium(III) complex for acetylacetone detection in environmental samples. Sens. Actuators B Chem. 2020, 321, 128486. [Google Scholar] [CrossRef]
- Li, K.; Xu, S.; Xiong, M.; Huan, S.-Y.; Yuan, L.; Zhang, X.-B. Molecular engineering of organic-based agents for in situ bioim-aging and phototherapeutics. Chem. Soc. Rev. 2021, 50, 11766–11784. [Google Scholar] [CrossRef]
- Bao, G.; Jin, D. Nanoparticles give mice infrared vision. Nat. Photon. 2019, 13, 304–305. [Google Scholar] [CrossRef]
- Bao, G.; Liu, Z.; Luo, Y.; Wong, K.-L.; Tanner, P.A. Effects of europium spectral probe interchange in Ln-dyads with cyclen and phen moieties. Dalton Trans. 2019, 48, 4314–4323. [Google Scholar] [CrossRef]
- Pandith, A.; Siddappa, R.G.; Seo, Y.J. Recent developments in novel blue/green/red/NIR small fluorescent probes for in cellulo tracking of RNA/DNA G-quadruplexes. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 81–116. [Google Scholar] [CrossRef]
- Dasnur Nanjappa, M.; Pandith, A.; Sankaran, S.; Dorairaj, D.P.; Reddy, A.A.; Ramesh, H.P.B. Recent Advancements in De-velopments of Novel Fluorescent Probes: In Cellulo Recognitions of Alkaline Phosphatases. Symmetry 2022, 14, 1634. [Google Scholar] [CrossRef]
- Gao, H.; Duan, X.; Jiao, D.; Zeng, Y.; Zheng, X.; Zhang, J.; Ou, H.; Qi, J.; Ding, D. Boosting photoacoustic effect via intramo-lecular motions amplifying thermal-to-acoustic conversion efficiency for adaptive image-guided cancer surgery. Angew. Chem. Int. Ed. 2021, 60, 21047–21055. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Liu, J.; Lin, C.; Liu, J.; Wang, J. The Integration of Gold Nanoparticles with Polymerase Chain Reaction for Constructing Colorimetric Sensing Platforms for Detection of Health-Related DNA and Proteins. Biosensors 2022, 12, 421. [Google Scholar] [CrossRef]
- Wang, W.; Wu, K.-J.; Vellaisamy, K.; Leung, C.-H.; Ma, D.-L. Peptide-Conjugated Long-Lived Theranostic Imaging for Tar-geting GRPr in Cancer and Immune Cells. Angew. Chem. Int. Ed. 2020, 59, 17897–17902. [Google Scholar] [CrossRef]
- Gao, M.; Yu, F.; Lv, C.; Choo, J.; Chen, L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017, 46, 2237–2271. [Google Scholar] [CrossRef]
- Singh, N.; Son, S.; An, J.; Kim, I.; Choi, M.; Kong, N.; Tao, W.; Kim, J.S. Nanoscale porous organic polymers for drug delivery and advanced cancer theranostics. Chem. Soc. Rev. 2021, 50, 12883–12896. [Google Scholar] [CrossRef]
- Sinha, N.J.; Langenstein, M.G.; Pochan, D.J.; Kloxin, C.J.; Saven, J.G. Peptide Design and Self-assembly into Targeted Nanostructure and Functional Materials. Chem. Rev. 2021, 121, 13915–13935. [Google Scholar] [CrossRef]
- Chen, S.; Costil, R.; Leung, F.K.; Feringa, B.L. Self-Assembly of Photoresponsive Molecular Amphiphiles in Aqueous Media. Angew. Chem. Int. Ed. 2020, 60, 11604–11627. [Google Scholar] [CrossRef]
- Kim, B.J.; Xu, B. Enzyme-Instructed Self-Assembly for Cancer Therapy and Imaging. Bioconjugate Chem. 2020, 31, 492–500. [Google Scholar] [CrossRef]
- Yu, S.-H.; O’Reilly, R.; Jiang, L.; Kotov, N.A. The New Era of Self-Assembled Nanomaterials. Acc. Chem. Res. 2022, 55, 1783–1784. [Google Scholar] [CrossRef]
- Kang, J.; Miyajima, D.; Mori, T.; Inoue, Y.; Itoh, Y.; Aida, T. A rational strategy for the realization of chain-growth supramo-lecular polymerization. Science 2015, 347, 646–651. [Google Scholar] [CrossRef]
- Zhao, P.; Xia, X.; Xu, X.; Leung, K.K.C.; Rai, A.; Deng, Y.; Yang, B.; Lai, H.; Peng, X.; Shi, P.; et al. Nanoparticle-assembled bioadhesive coacervate coating with prolonged gastrointestinal retention for inflammatory bowel disease therapy. Nat. Commun. 2021, 12, 7162. [Google Scholar] [CrossRef]
- Li, G.-L.; Zhuo, Z.; Wang, B.; Cao, X.-L.; Su, H.-F.; Wang, W.; Huang, Y.-G.; Hong, M. Constructing π-Stacked Supramolecular Cage Based Hierarchical Self-Assemblies via π···π Stacking and Hydrogen Bonding. J. Am. Chem. Soc. 2021, 143, 10920–10929. [Google Scholar] [CrossRef]
- Lin, H.; Li, S.; Wang, J.; Chu, C.; Zhang, Y.; Pang, X.; Lv, P.; Wang, X.; Zhao, Q.; Chen, J.; et al. A single-step multi-level supramolecular system for cancer sonotheranostics. Nanoscale Horiz. 2019, 4, 190–195. [Google Scholar] [CrossRef]
- Li, L.-L.; Qiao, Z.-Y.; Wang, L.; Wang, H. Programmable Construction of Peptide-Based Materials in Living Subjects: From Modular Design and Morphological Control to Theranostics. Adv. Mater. 2019, 31, e1804971. [Google Scholar] [CrossRef]
- Mazidi, Z.; Javanmardi, S.; Naghib, S.M.; Mohammadpour, Z. Smart stimuli-responsive implantable drug delivery systems for programmed and on-demand cancer treatment: An overview on the emerging materials. Chem. Eng. J. 2022, 433, 134569. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, J.; Liang, G. Covalently Conjugated Hydrogelators for Imaging and Therapeutic Applications. Bioconjugate Chem. 2020, 31, 448–461. [Google Scholar] [CrossRef]
- Dergham, M.; Lin, S.; Geng, J. Supramolecular Self-Assembly in Living Cells. Angew. Chem. Int. Ed. 2022, 61, e202114267. [Google Scholar] [CrossRef]
- Wang, T.; Ménard-Moyon, C.; Bianco, A. Self-assembly of amphiphilic amino acid derivatives for biomedical applications. Chem. Soc. Rev. 2022, 51, 3535–3560. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, C.; Wang, X.; Yan, M.; Ling, Z.; Chen, Y.; Liu, Z. A Borondifluoride-Complex-Based Photothermal Agent with an 80% Photothermal Conversion Efficiency for Photothermal Therapy in the NIR-II Window. Angew. Chem. Int. Ed. 2021, 60, 22376–22384. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, R. Nanotherapy: Targeting the tumour microenvironment. Nat. Rev. Cancer 2022, 22, 258. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, J.; Pu, K.; James, T.D. Dual-locked spectroscopic probes for sensing and therapy. Nat. Rev. Chem. 2021, 5, 406–421. [Google Scholar] [CrossRef]
- Chen, S.; Chen, M.; Yang, J.; Zeng, X.; Zhou, Y.; Yang, S.; Yang, R.; Yuan, Q.; Zheng, J. Design and Engineering of Hypoxia and Acidic pH Dual-Stimuli-Responsive Intelligent Fluorescent Nanoprobe for Precise Tumor Imaging. Small 2021, 17, 2100243. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Al-Lazikani, B.; Banerji, U.; Workman, P. Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 2012, 30, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Shen, H.; Zhan, J.; Lin, M.; Dai, L.; Ren, C.; Shi, Y.; Liu, J.; Gao, J.; Yang, Z. Supramolecular “Trojan Horse” for Nuclear Delivery of Dual Anticancer Drugs. J. Am. Chem. Soc. 2017, 139, 2876–2879. [Google Scholar] [CrossRef] [PubMed]
- Eccles, S.A.; Welch, D.R. Metastasis: Recent discoveries and novel treatment strategies. Lancet 2007, 369, 1742–1757. [Google Scholar] [CrossRef]
- Mang, D.; Roy, S.R.; Zhang, Q.; Hu, X.; Zhang, Y. Heparan Sulfate-Instructed Self-Assembly Selectively Inhibits Cancer Cell Migration. ACS Appl. Mater. Interfaces 2021, 13, 17236–17242. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Hou, Y.; Li, Y.; Yao, X.; Xue, C.; Fei, Y.; Xiang, Y.; Cai, K.; Zhao, Y.; et al. Tumor-Microenvironment-Activated In Situ Self-Assembly of Sequentially Responsive Biopolymer for Targeted Photodynamic Therapy. Adv. Funct. Mater. 2020, 30, 2000229. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Z.; Xu, B. Assemblies of Peptides in a Complex Environment and their Applications. Angew. Chem. Int. Ed. 2019, 58, 10423–10432. [Google Scholar] [CrossRef]
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A.A. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can. Med. Assoc. J. 1922, 12, 141–146. [Google Scholar]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current ap-plications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Ringler, P.; Schulz, G.E. Self-Assembly of Proteins into Designed Networks. Science 2003, 302, 106–109. [Google Scholar] [CrossRef]
- Paramonov, S.E.; Jun, H.-W.; Hartgerink, J.D. Self-Assembly of Peptide−Amphiphile Nanofibers: The Roles of Hydrogen Bonding and Amphiphilic Packing. J. Am. Chem. Soc. 2006, 128, 7291–7298. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Shi, B.; Shangguan, L.; Tong, W.; Yu, G.; Mao, Z.; Huang, F. Supramolecular peptide constructed by mo-lecular Lego allowing programmable self-assembly for photodynamic therapy. Nat. Commun. 2019, 10, 2412. [Google Scholar] [CrossRef]
- Wang, M.-D.; Lv, G.-T.; An, H.-W.; Zhang, N.-Y.; Wang, H. In Situ Self-Assembly of Bispecific Peptide for Cancer Immuno-therapy. Angew. Chem. Int. Ed. 2022, 61, e202113649. [Google Scholar]
- Li, J.; Fang, Y.; Zhang, Y.; Wang, H.; Yang, Z.; Ding, D. Supramolecular Self-Assembly-Facilitated Aggregation of Tumor-Specific Transmembrane Receptors for Signaling Activation and Converting Immunologically Cold to Hot Tumors. Adv. Mater. 2021, 33, 2008518. [Google Scholar] [CrossRef]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the Autophagy-Inflammation-Cell Death Axis in Organismal Aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Z.; Wang, Y.; Zhou, R.; Yang, Z.; Xu, B. Integrating Enzymatic Self-Assembly and Mitochondria Targeting for Selectively Killing Cancer Cells without Acquired Drug Resistance. J. Am. Chem. Soc. 2016, 138, 16046–16055. [Google Scholar] [CrossRef]
- Jeena, M.T.; Palanikumar, L.; Go, E.M.; Kim, I.; Kang, M.G.; Lee, S.; Park, S.; Choi, H.; Kim, C.; Jin, S.-M.; et al. Mitochondria localization induced self-assembly of peptide amphiphiles for cellular dysfunction. Nat. Commun. 2017, 8, 26. [Google Scholar] [CrossRef]
- Jeena, M.T.; Jeong, K.; Go, E.M.; Cho, Y.; Lee, S.; Jin, S.; Hwang, S.-W.; Jang, J.H.; Kang, C.S.; Bang, W.-Y.; et al. Heterochiral Assembly of Amphiphilic Peptides Inside the Mitochondria for Supramolecular Cancer Therapeutics. ACS Nano 2019, 13, 11022–11033. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, H.; Wang, S.; Zhang, Q.; Zhang, X.; Rodal, A.A.; Xu, B. Enzymatic Assemblies Disrupt the Membrane and Target Endoplasmic Reticulum for Selective Cancer Cell Death. J. Am. Chem. Soc. 2018, 140, 9566–9573. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Banerjee, P.; Shi, L.; Xiao, G.-Y.; Rodriguez, B.L.; Grzeskowiak, C.L.; Liu, X.; Yu, J.; Gibbons, D.L.; Russell, W.K.; et al. p53 loss activates prometastatic secretory vesicle biogenesis in the Golgi. Sci. Adv. 2021, 7, eabf4885. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, Q.; Wang, J.; Yi, M.; He, H.; Xu, B. Enzymatic Assemblies of Thiophosphopeptides Instantly Target Golgi Apparatus and Selectively Kill Cancer Cells. Angew. Chem. Int. Ed. 2021, 60, 12796–12801. [Google Scholar] [CrossRef]
- Hinde, E.; Thammasiraphop, K.; Duong, H.T.T.; Yeow, J.; Karagoz, B.; Boyer, C.; Gooding, J.J.; Gaus, K. Pair correlation mi-croscopy reveals the role of nanoparticle shape in intracellular transport and site of drug release. Nat. Nanotechnol. 2017, 12, 81–89. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Pasqua, L.; Magazù, S. Self-Assembly of Organic Nanomaterials and Biomaterials: The Bottom-Up Approach for Functional Nanostructures Formation and Advanced Applications. Materials 2020, 13, 1048. [Google Scholar] [CrossRef]
- Kolhar, P.; Anselmo, A.C.; Gupta, V.; Pant, K.; Prabhakarpandian, B.; Ruoslahti, E.; Mitragotri, S. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc. Natl. Acad. Sci. USA 2013, 110, 10753–10758. [Google Scholar] [CrossRef]
- Han, K.; Zhang, J.; Zhang, W.; Wang, S.; Xu, L.; Zhang, C.; Zhang, X.; Han, H. Tumor-Triggered Geometrical Shape Switch of Chimeric Peptide for Enhanced In Vivo Tumor Internalization and Photodynamic Therapy. ACS Nano 2017, 11, 3178–3188. [Google Scholar] [CrossRef]
- Cheng, H.; Yuan, P.; Fan, G.; Zhao, L.; Zheng, R.; Yang, B.; Qiu, X.; Yu, X.; Li, S.; Zhang, X. Chimeric peptide nanorods for plasma membrane and nuclear targeted photosensitizer delivery and enhanced photodynamic therapy. Appl. Mater. Today 2019, 16, 120–131. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, D.; Jin, Z.; Xia, C.; Xu, Q.; Fan, M.; Dai, Y.; Liu, J.; Li, Y.; He, Q. Light-triggered nitric oxide release and structure transformation of peptide for enhanced intratumoral retention and sensitized photodynamic therapy. Bioact. Mater. 2022, 12, 303–313. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Yin, X.; Zhu, Z.; Rojalin, T.; Xiao, W.; Zhang, D.; Huang, Y.; Li, L.; Baehr, C.M.; et al. Tumor Receptor-Mediated In Vivo Modulation of the Morphology, Phototherapeutic Properties, and Pharmacokinetics of Smart Nanomaterials. ACS Nano 2020, 15, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Brahma, R.K.; Fang, C.; Yao, S.Q. Stimulus-responsive self-assembled prodrugs in cancer therapy. Chem. Sci. 2022, 13, 4239–4269. [Google Scholar] [CrossRef] [PubMed]
- Um, W.; Park, J.; Ko, H.; Lim, S.; Yoon, H.Y.; Shim, M.K.; Lee, S.; Ko, Y.J.; Kim, M.J.; Park, J.H.; et al. Visible light-induced apoptosis activatable nanoparticles of photosensitizer-DEVD-anticancer drug conjugate for targeted cancer therapy. Biomaterials 2019, 224, 119494. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shim, M.K.; Yang, S.; Hwang, H.S.; Cho, H.; Kim, J.; Yun, W.S.; Moon, Y.; Kim, J.; Yoon, H.Y.; et al. Visible-Light-Triggered Prodrug Nanoparticles Combine Chemotherapy and Photodynamic Therapy to Potentiate Checkpoint Blockade Cancer Immunotherapy. ACS Nano 2021, 15, 12086–12098. [Google Scholar] [CrossRef]
- Wei, Z.; Yi, Y.; Luo, Z.; Gong, X.; Jiang, Y.; Hou, D.; Zhang, L.; Liu, Z.; Wang, M.; Wang, J.; et al. Selenopeptide Nanomedicine Activates Natural Killer Cells for Enhanced Tumor Chemoimmunotherapy. Adv. Mater. 2022, 34, 2108167. [Google Scholar] [CrossRef]
- Edidin, M. Lipids on the frontier: A century of cell-membrane bilayers. Nat. Rev. Mol. Cell Biol. 2003, 4, 414–418. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.-G. Cyclodextrin Polymers: Structure, Synthesis, and Use as Drug Carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, H.; Zhang, R.; Zhang, H.; Huang, W. Nanoparticulation of Prodrug into Medicines for Cancer Therapy. Adv. Sci. 2021, 8, 2101454. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical stimuli-responsive vesicles in drug delivery: Beyond lip-osomes and polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, H.; He, K.; Du, W.; Kong, Y.; Wang, Z.; Li, M.; Shen, Q.; Sun, P.; Fan, Q. NIR-II Excitation Phototheranostic Nanomedicine for Fluorescence/Photoacoustic Tumor Imaging and Targeted Photothermal-Photonic Thermodynamic Therapy. Small 2021, 17, 2102527. [Google Scholar] [CrossRef]
- Lai, Y.; Dang, Y.; Li, F.; Ding, C.; Yu, H.; Zhang, W.; Xu, Z. Reactive Glycolysis Metabolite-Activatable Nanotheranostics for NIR-II Fluorescence Imaging-Guided Phototherapy of Cancer. Adv. Funct. Mater. 2022, 32, 2200016. [Google Scholar] [CrossRef]
- Zhang, W.; Du, X.-F.; Liu, B.; Li, C.; Long, J.; Zhao, M.-X.; Yao, Z.; Liang, X.-J.; Lai, Y. Engineering Supramolecular Nano-medicine for Targeted Near Infrared-triggered Mitochondrial Dysfunction to Potentiate Cisplatin for Efficient Chemophoto-therapy. ACS Nano 2022, 16, 1421–1435. [Google Scholar] [CrossRef]
- Mahato, R.; Tai, W.; Cheng, K. Prodrugs for improving tumor targetability and efficiency. Adv. Drug Deliv. Rev. 2011, 63, 659–670. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Gu, W.; Gao, Y.; Lin, L.; Zhang, Z.; Xi, Y.; Li, Y. The performance of docetaxel-loaded solid lipid nanoparticles targeted to hepatocellular carcinoma. Biomaterials 2009, 30, 226–232. [Google Scholar] [CrossRef]
- Mou, Q.; Ma, Y.; Zhu, X.; Yan, D. A small molecule nanodrug consisting of amphiphilic targeting ligand–chemotherapy drug conjugate for targeted cancer therapy. J. Control. Release 2016, 230, 34–44. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, J.; Liu, X.; Song, H.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. Cascade of reactive oxygen species generation by polyprodrug for combinational photodynamic therapy. Biomaterials 2020, 255, 120210. [Google Scholar] [CrossRef]
- He, S.; Li, C.; Zhang, Q.; Ding, J.; Liang, X.-J.; Chen, X.; Xiao, H.; Chen, X.; Zhou, D.; Huang, Y. Tailoring Platinum(IV) Amphiphiles for Self-Targeting All-in-One Assemblies as Precise Multimodal Theranostic Nanomedicine. ACS Nano 2018, 12, 7272–7281. [Google Scholar] [CrossRef]
- Huang, P.; Wang, D.; Su, Y.; Huang, W.; Zhou, Y.; Cui, D.; Zhu, X.; Yan, D. Combination of Small Molecule Prodrug and Nanodrug Delivery: Amphiphilic Drug–Drug Conjugate for Cancer Therapy. J. Am. Chem. Soc. 2014, 136, 11748–11756. [Google Scholar] [CrossRef]
- Yi, S.; Lu, Z.; Zhang, J.; Wang, J.; Xie, Z.; Hou, L. Amphiphilic Gemini Iridium(III) Complex as a Mitochondria-Targeted Theranostic Agent for Tumor Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 15276–15289. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2021, 55, 2–12. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, Y.; Qian, Q.; Yan, D.; Li, Y.; Zhu, X.; Zhang, C. Carrier-free delivery of precise drug–chemogene conjugates for synergistic treatment of drug-resistant cancer. Angew. Chem. Int. Ed. 2020, 59, 17944–17950. [Google Scholar] [CrossRef]
- Zitzler-Kunkel, A.; Lenze, M.R.; Schnier, T.; Meerholz, K.; Würthner, F. Comparative Studies on Optical, Redox, and Photo-voltaic Properties of a Series of D–A–D and Analogous D–A Chromophores. Adv. Funct. Mater. 2014, 24, 4645–4653. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, M.; Yan, Z.; Zhang, S.; Wang, M.; Xu, H.; Li, H.; Ying, Y.; Qiu, S.; Liu, J.; et al. Ultrastable Near-Infrared Nonlinear Organic Chromophore Nanoparticles with Intramolecular Charge Transfer for Dually Photoinduced Tumor Ablation. Adv. Healthc. Mater. 2020, 9, 2001042. [Google Scholar] [CrossRef]
- Shao, W.; Yang, C.; Li, F.; Wu, J.; Wang, N.; Ding, Q.; Gao, J.; Ling, D. Molecular Design of Conjugated Small Molecule Na-noparticles for Synergistically Enhanced PTT/PDT. Nano Micro Lett. 2020, 12, 147. [Google Scholar] [CrossRef]
- Shao, W.; Wei, Q.; Wang, S.; Li, F.; Wu, J.; Ren, J.; Cao, F.; Liao, H.; Gao, J.; Zhou, M.; et al. Molecular engineering of D–A–D conjugated small molecule nanoparticles for high performance NIR-II photothermal therapy. Mater. Horiz. 2020, 7, 1379–1386. [Google Scholar] [CrossRef]
- He, Z.; Zhao, L.; Zhang, Q.; Chang, M.; Li, C.; Zhang, H.; Lu, Y.; Chen, Y. An Acceptor–Donor–Acceptor Structured Small Molecule for Effective NIR Triggered Dual Phototherapy of Cancer. Adv. Funct. Mater. 2020, 30, 1910301. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, D. Recent advances of transition Ir(III) complexes as photosensitizers for improved photodynamic therapy. View 2021, 2, 20200179. [Google Scholar] [CrossRef]
- Ma, D.-L.; Lin, S.; Wang, W.; Yang, C.; Leung, C.-H. Luminescent chemosensors by using cyclometalated iridium(iii) complexes and their applications. Chem. Sci. 2017, 8, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, C.; Lin, S.; Vellaisamy, K.; Li, G.; Tan, W.; Leung, C.-H.; Ma, D.-L. First Synthesis of an Oridonin-Conjugated Iridium(III) Complex for the Intracellular Tracking of NF-κB in Living Cells. Chem. Eur. J. 2017, 23, 4929–4935. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, K.; Xu, G.; Liu, X.; Zhao, Q.; Xu, C.; Gou, S. An Iridium (III) Complex Bearing a Donor–Acceptor–Donor Type Ligand for NIR-Triggered Dual Phototherapy. Adv. Funct. Mater. 2021, 31, 2008325. [Google Scholar] [CrossRef]
- Reisch, A.; Klymchenko, A.S. Fluorescent Polymer Nanoparticles Based on Dyes: Seeking Brighter Tools for Bioimaging. Small 2016, 12, 1968–1992. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Zhuang, Y.-D.; Chiang, P.-Y.; Wang, C.-W.; Tan, K.-T. Environment-Sensitive Fluorescent Turn-On Probes Targeting Hydrophobic Ligand-Binding Domains for Selective Protein Detection. Angew. Chem. Int. Ed. 2013, 52, 8124–8128. [Google Scholar] [CrossRef]
- Hu, Q.; Gao, M.; Feng, G.; Liu, B. Mitochondria-targeted cancer therapy using a light-up probe with aggregation-induced-emission characteristics. Angew. Chem. Int. Ed. 2014, 53, 14225–14229. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, G.; Hu, F.; Jin, Y.; Zhao, R.; Zhang, D. Emissive nanoparticles from pyridinium-substituted tetra-phenylethylene salts: Imaging and selective cytotoxicity towards cancer cells in vitro and in vivo by varying counter anions. Chem. Sci. 2016, 7, 7013–7019. [Google Scholar] [CrossRef]
- Huang, Y.; You, X.; Wang, L.; Zhang, G.; Gui, S.; Jin, Y.; Zhao, R.; Zhang, D. Pyridinium-Substituted Tetraphenylethylenes Functionalized with Alkyl Chains as Autophagy Modulators for Cancer Therapy. Angew. Chem. Int. Ed. 2020, 59, 10042–10051. [Google Scholar] [CrossRef]
- Su, Y.; Lin, H.; Tu, Y.; Wang, M.-M.; Zhang, G.-D.; Yang, J.; Liu, H.-K.; Su, Z. Fighting metallodrug resistance through alteration of drug metabolism and blockage of autophagic flux by mitochondria-targeting AIEgens. Chem. Sci. 2022, 13, 1428–1439. [Google Scholar] [CrossRef]
- Li, Y.; Zhuang, J.; Lu, Y.; Li, N.; Gu, M.; Xia, J.; Zhao, N.; Tang, B.Z. High-Performance Near-Infrared Aggregation-Induced Emission Luminogen with Mitophagy Regulating Capability for Multimodal Cancer Theranostics. ACS Nano 2021, 15, 20453–20465. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, G.-Q.; Ding, D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. [Google Scholar] [CrossRef]
- Mao, D.; Wu, W.; Ji, S.; Chen, C.; Hu, F.; Kong, D.; Ding, D.; Liu, B. Chemiluminescence-Guided Cancer Therapy Using a Chemiexcited Photosensitizer. Chem 2017, 3, 991–1007. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Min, T.; Gong, J.; Du, L.; Phillips, D.L.; Liu, J.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; et al. Time-Dependent Photodynamic Therapy for Multiple Targets: A Highly Efficient AIE-Active Photosensitizer for Selective Bacterial Elimination and Cancer Cell Ablation. Angew. Chem. Int. Ed. 2019, 59, 9470–9477. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, R.; Zhuang, Z.; Li, Y.; Huang, Y.; Wang, Z.; Zhang, W.; Hou, J.; Tang, B.Z. Molecular Engineering to Boost AIE-Active Free Radical Photogenerators and Enable High-Performance Photodynamic Therapy under Hypoxia. Adv. Funct. Mater. 2020, 30, 2002057. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, X.; Zhang, H.; Ou, H.; Lam, J.W.Y.; Liu, Y.; Shi, L.; Ding, D.; Tang, B.Z. Molecular Motion in Aggregates: Ma-nipulating TICT for Boosting Photothermal Theranostics. J. Am. Chem. Soc. 2019, 141, 5359–5368. [Google Scholar] [CrossRef]
- Wen, H.; Zhang, Z.; Kang, M.; Li, H.; Xu, W.; Guo, H.; Li, Y.; Tan, Y.; Wen, Z.; Wu, Q.; et al. One-for-all phototheranostics: Single component AIE dots as multi-modality theranostic agent for fluores-cence-photoacoustic imaging-guided synergistic cancer therapy. Biomaterials 2021, 274, 120892. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.J.; Chambers, J.E.; Ron, D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 2021, 21, 115–140. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Liu, R.; Chen, C.; Zeng, S.; Liu, Q.; Ding, D. Endoplasmic reticulum targeted AIE bioprobe as a highly efficient inducer of immunogenic cell death. Sci. China Chem. 2020, 63, 1428–1434. [Google Scholar] [CrossRef]
- Xu, W.; Lee, M.M.S.; Nie, J.J.; Zhang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; Xu, F.J.; Wang, D.; Tang, B.Z. Three-Pronged Attack by Homologous Far-red/NIR AIEgens to Achieve 1+1+1>3 Synergistic Enhanced Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 9610–9616. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, Y.; Guo, Y.; Yuan, H.; Cui, T.; Yao, S.; Jin, S.; Fan, H.; Wang, C.; Xie, R.; et al. Golgi apparatus-targeted aggregation-induced emission luminogens for effective cancer photodynamic therapy. Nat. Commun. 2022, 13, 2179. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, X.; Chen, H.; Duan, Y.; Liu, J.; Pan, Y.; Liu, B. Activation of pyroptosis by membrane-anchoring AIE photosen-sitizer design: New prospect for photodynamic cancer cell ablation. Angew. Chem. Int. Ed. 2021, 60, 9093–9098. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, X.; Yang, K.; Hu, S.; Wang, W. Self-Assembly of Small Organic Molecules into Luminophores for Cancer Theranostic Applications. Biosensors 2022, 12, 683. https://doi.org/10.3390/bios12090683
Wang J, Wang X, Yang K, Hu S, Wang W. Self-Assembly of Small Organic Molecules into Luminophores for Cancer Theranostic Applications. Biosensors. 2022; 12(9):683. https://doi.org/10.3390/bios12090683
Chicago/Turabian StyleWang, Jing, Xueliang Wang, Kai Yang, Sijun Hu, and Wanhe Wang. 2022. "Self-Assembly of Small Organic Molecules into Luminophores for Cancer Theranostic Applications" Biosensors 12, no. 9: 683. https://doi.org/10.3390/bios12090683
APA StyleWang, J., Wang, X., Yang, K., Hu, S., & Wang, W. (2022). Self-Assembly of Small Organic Molecules into Luminophores for Cancer Theranostic Applications. Biosensors, 12(9), 683. https://doi.org/10.3390/bios12090683






