Magnetic Bead Handling Using a Paper-Based Device for Quantitative Point-of-Care Testing
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Biocomponents
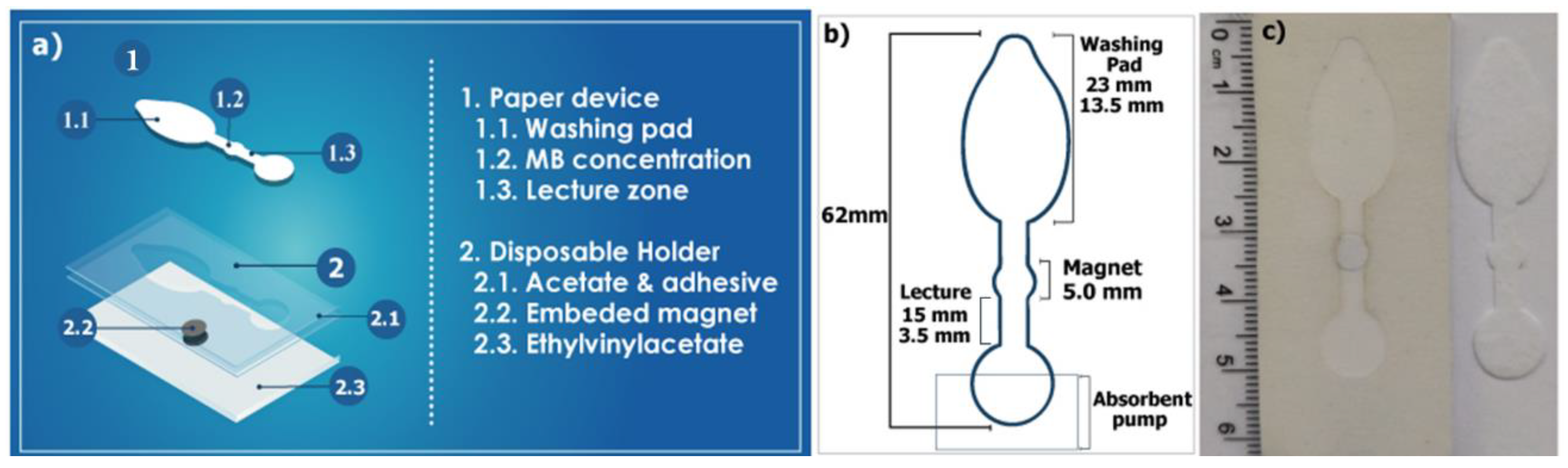
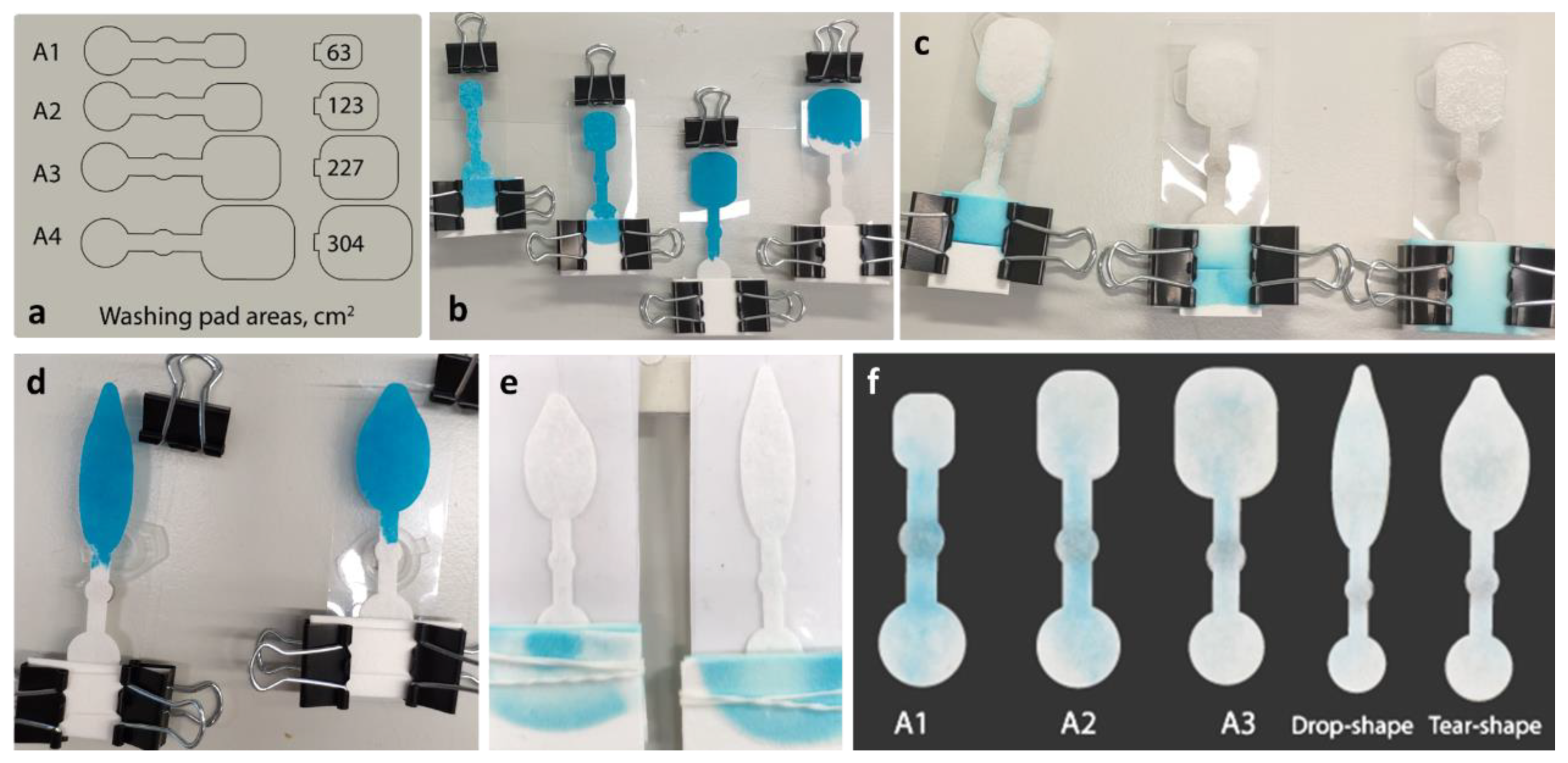
2.2. Device Fabrication
2.3. Smartphone Imaging System
2.4. Pf-LDH Magneto-Immunodetection
2.4.1. Magneto-Immunoassay in Tubes (Classical Approach)
2.4.2. Magneto-Immunoassay Using the μPAD (POC Approach)
2.4.3. Pf-LDH Detection in Whole Blood Clinical Samples
2.5. Data Analysis
3. Results and Discussion
3.1. Production of the µPAD
3.2. Magneto-Immunoassay Handling and Detection Using the µPAD
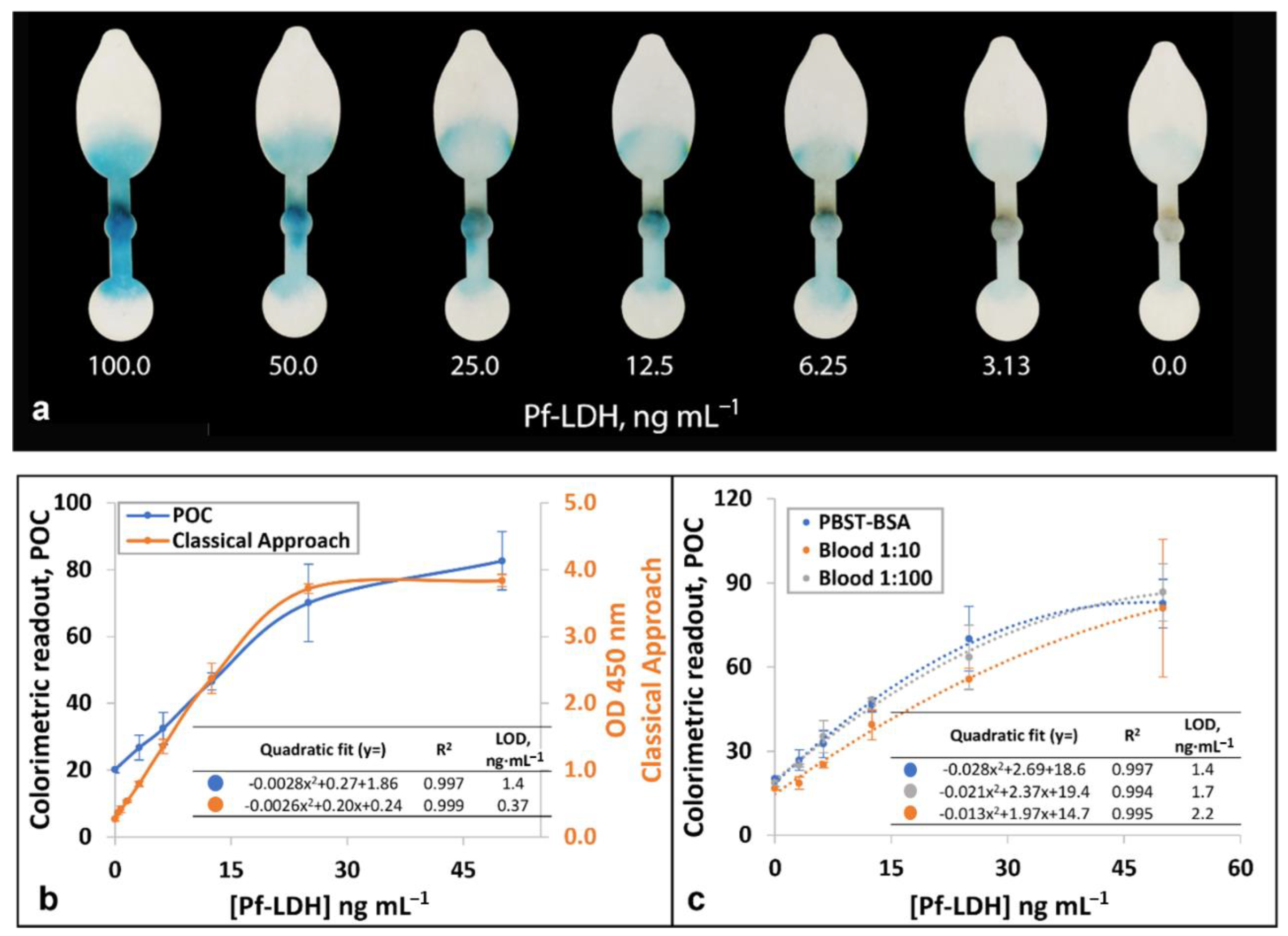
3.3. Quantitative Detection of Pf-LDH Using a Smartphone Detection System
3.4. Analysis of Clinical Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.M.; Chen, C.Y.; Liao, W.S. Enclosed Paper-Based Analytical Devices: Concept, Variety, and Outlook. Anal. Chim. Acta 2021, 1144, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-Based Microfluidics: Simplified Fabrication and Assay Methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Heidt, B.; Siqueira, W.F.; Eersels, K.; Diliën, H.; Van Grinsven, B.; Fujiwara, R.T.; Cleij, T.J. Point of Care Diagnostics in Resource-Limited Settings: A Review of the Present and Future of PoC in Its Most Needed Environment. Biosensors 2020, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Song, S.; Park, S.; Joo, C. Recent Advances in High-Sensitivity Detection Methods for Paper-Based Lateral-Flow Assay. Biosens. Bioelectron. 2020, 152, 112015. [Google Scholar] [CrossRef]
- Shirshahi, V.; Liu, G. Enhancing the Analytical Performance of Paper Lateral Flow Assays: From Chemistry to Engineering. Trends Anal. Chem. 2021, 136, 116200. [Google Scholar] [CrossRef]
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and Disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef]
- WHO. Word Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- WHO. Report on Antimalarial Drug Efficacy, Resistance and Response: 10 Years of Surveillance (2010–2019); World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Monge-Maillo, B.; Jiménez, B.C.; Pérez-Molina, J.A.; Norman, F.; Navarro, M.; Pérez-Ayala, A.; Herrero, J.M.; Zamarrón, P.; López-Vélez, R. Imported Infectious Diseases in Mobile Populations, Spain. Emerg. Infect. Dis. 2009, 15, 1745–1752. [Google Scholar] [CrossRef]
- Caminade, C.; Kovats, S.; Rocklov, J.; Tompkins, A.M.; Morse, A.P.; Colón-González, F.J.; Stenlund, H.; Martens, P.; Lloyd, S.J. Impact of Climate Change on Global Malaria Distribution. Proc. Natl. Acad. Sci. USA 2014, 111, 3286–3291. [Google Scholar] [CrossRef]
- Mouatcho, J.C.; Dean Goldring, J.P. Malaria Rapid Diagnostic Tests: Challenges and Prospects. J. Med. Microbiol. 2013, 62, 1491–1505. [Google Scholar] [CrossRef]
- Wu, L.; Van Den Hoogen, L.L.; Slater, H.; Walker, P.G.T.; Ghani, A.C.; Drakeley, C.J.; Okell, L.C. Comparison of Diagnostics for the Detection of Asymptomatic Plasmodium Falciparum Infections to Inform Control and Elimination Strategies. Nature 2015, 528, S86–S93. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Guidelines for Malaria; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Mukkala, A.N.; Kwan, J.; Lau, R.; Harris, D.; Kain, D.; Boggild, A.K. An Update on Malaria Rapid Diagnostic Tests. Curr. Infect. Dis. Rep. 2018, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Gendrot, M.; Fawaz, R.; Dormoi, J.; Madamet, M.; Pradines, B. Genetic Diversity and Deletion of Plasmodium Falciparum Histidine-Rich Protein 2 and 3: A Threat to Diagnosis of P. Falciparum Malaria. Clin. Microbiol. Infect. 2019, 25, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, S.; Campillo, A.; Gonzalez, I.J. A Systematic Review: Performance of Rapid Diagnostic Tests for the Detection of Plasmodium Knowlesi, Plasmodium Malariae, and Plasmodium Ovale Monoinfections in Human Blood. J. Infect. Dis. 2018, 218, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, S.S. The State of the Malaria RDT Market 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Kavanaugh, M.J.; Azzam, S.E.; Rockabrand, D.M. Malaria Rapid Diagnostic Tests: Literary Review and Recommendation for a Quality Assurance, Quality Control Algorithm. Diagnostics 2021, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Rei Yan, S.L.; Wakasuqui, F.; Wrenger, C. Point-of-Care Tests for Malaria: Speeding up the Diagnostics at the Bedside and Challenges in Malaria Cases Detection. Diagn. Microbiol. Infect. Dis. 2020, 98, 115122. [Google Scholar] [CrossRef]
- Dos Santos, G.P.; Corrêa, C.C.; Kubota, L.T. A Simple, Sensitive and Reduced Cost Paper-Based Device with Low Quantity of Chemicals for the Early Diagnosis of Plasmodium Falciparum Malaria Using an Enzyme-Based Colorimetric Assay. Sens. Actuators B 2018, 255, 2113–2120. [Google Scholar] [CrossRef]
- Nolder, D.; Stewart, L.; Tucker, J.; Ibrahim, A.; Gray, A.; Corrah, T.; Gallagher, C.; John, L.; O’Brien, E.; Aggarwal, D.; et al. Failure of Rapid Diagnostic Tests in Plasmodium Falciparum Malaria Cases in UK Travellers: Identification and Characterisation of the Parasites. Int. J. Infect. Dis. 2021, 108, 137–144. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Atchade, P.S.; Doderer-Lang, C.; Chabi, N.; Perrotey, S.; Abdelrahman, T.; Akpovi, C.D.; Anani, L.; Bigot, A.; Sanni, A.; Candolfi, E. Is a Plasmodium Lactate Dehydrogenase (PLDH) Enzyme-Linked Immunosorbent (ELISA)-Based Assay a Valid Tool for Detecting Risky Malaria Blood Donations in Africa? Malar. J. 2013, 12, 279. [Google Scholar] [CrossRef]
- Nyataya, J.; Waitumbi, J.; Mobegi, V.A.; Noreddin, A.; El Zowalaty, M.E. Plasmodium Falciparum Histidine-Rich Protein 2 and 3 Gene Deletions and Their Implications in Malaria Control. Diseases 2020, 8, 15. [Google Scholar] [CrossRef]
- Deraney, R.N.; Mace, C.R.; Rolland, J.P.; Schonhorn, J.E. Multiplexed, Patterned-Paper Immunoassay for Detection of Malaria and Dengue Fever. Anal. Chem. 2016, 88, 6161–6165. [Google Scholar] [CrossRef]
- Singh, N.K.; Jain, P.; Das, S.; Goswami, P. Dye Coupled Aptamer-Captured Enzyme Catalyzed Reaction for Detection of Pan Malaria and P. falciparum Species in Laboratory Settings and Instrument-Free Paper-Based Platform. Anal. Chem. 2019, 91, 4213–4221. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vega, G.; Arias-Alpízar, K.; de la Serna, E.; Borgheti-Cardoso, L.N.; Sulleiro, E.; Molina, I.; Fernàndez-Busquets, X.; Sánchez-Montalvá, A.; del Campo, F.J.; Baldrich, E. Electrochemical POC Device for Fast Malaria Quantitative Diagnosis in Whole Blood by Using Magnetic Beads, Poly-HRP and Microfluidic Paper Electrodes. Biosens. Bioelectron. 2020, 150, 111925. [Google Scholar] [CrossRef]
- Arias-Alpízar, K.; Sánchez-Cano, A.; Prat-Trunas, J.; de la Serna, E.; Alonso, O.; Sulleiro, E.; Sánchez-Montalvá, A.; Diéguez, A.; Baldrich, E. Malaria quantitative POC testing using magnetic particles, a paper microfluidic device and a hand-held fluorescence reader. Biosens. Bioelectron. 2022, 215, 114513. [Google Scholar] [CrossRef]
- De la Serna, E.; Arias-Alpízar, K.; Borgheti-Cardoso, L.N.; Sanchez-Cano, A.; Sulleiro, E.; Zarzuela, F.; Bosch-Nicolau, P.; Salvador, F.; Molina, I.; Ramírez, M.; et al. Detection of Plasmodium Falciparum Malaria in 1 h Using a Simplified Enzyme-Linked Immunosorbent Assay. Anal. Chim. Acta 2021, 1152, 338254. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.; Rees-Channer, R.R.; Perera, R.; Gamboa, D.; Chiodini, P.L.; González, I.J.; Mayor, A.; Ding, X.C. Analytical Sensitivity of Current Best-in-Class Malaria Rapid Diagnostic Tests. Malar. J. 2017, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.K.; Tyler, A.; Lyman, C.; Kahn, M.; Kalnoky, M.; Rek, J.C.; Arinaitwe, E.; Adrama, H.; Murphy, M.; Imwong, M.; et al. Simultaneous Quantification of Plasmodium Antigens and Host Factor C-Reactive Protein in Asymptomatic Individuals with Confirmed Malaria by Use of a Novel Multiplex Immunoassay. J. Clin. Microbiol. 2019, 57, e00948-18. [Google Scholar] [CrossRef]
- Martiáñez-Vendrell, X.; Jiménez, A.; Vásquez, A.; Campillo, A.; Incardona, S.; González, R.; Gamboa, D.; Torres, K.; Oyibo, W.; Faye, B.; et al. Quantification of Malaria Antigens PfHRP2 and PLDH by Quantitative Suspension Array Technology in Whole Blood, Dried Blood Spot and Plasma. Malar. J. 2020, 19, 12. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Alpízar, K.; Sánchez-Cano, A.; Prat-Trunas, J.; Sulleiro, E.; Bosch-Nicolau, P.; Salvador, F.; Oliveira, I.; Molina, I.; Sánchez-Montalvá, A.; Baldrich, E. Magnetic Bead Handling Using a Paper-Based Device for Quantitative Point-of-Care Testing. Biosensors 2022, 12, 680. https://doi.org/10.3390/bios12090680
Arias-Alpízar K, Sánchez-Cano A, Prat-Trunas J, Sulleiro E, Bosch-Nicolau P, Salvador F, Oliveira I, Molina I, Sánchez-Montalvá A, Baldrich E. Magnetic Bead Handling Using a Paper-Based Device for Quantitative Point-of-Care Testing. Biosensors. 2022; 12(9):680. https://doi.org/10.3390/bios12090680
Chicago/Turabian StyleArias-Alpízar, Kevin, Ana Sánchez-Cano, Judit Prat-Trunas, Elena Sulleiro, Pau Bosch-Nicolau, Fernando Salvador, Inés Oliveira, Israel Molina, Adrián Sánchez-Montalvá, and Eva Baldrich. 2022. "Magnetic Bead Handling Using a Paper-Based Device for Quantitative Point-of-Care Testing" Biosensors 12, no. 9: 680. https://doi.org/10.3390/bios12090680
APA StyleArias-Alpízar, K., Sánchez-Cano, A., Prat-Trunas, J., Sulleiro, E., Bosch-Nicolau, P., Salvador, F., Oliveira, I., Molina, I., Sánchez-Montalvá, A., & Baldrich, E. (2022). Magnetic Bead Handling Using a Paper-Based Device for Quantitative Point-of-Care Testing. Biosensors, 12(9), 680. https://doi.org/10.3390/bios12090680





