Applications of Gelatin in Biosensors: Recent Trends and Progress
Abstract
:1. Introduction
2. Gelatin as a Matrix for Immobilized Biorecognition Materials
2.1. Medical Diagnosis
2.2. Food Testing
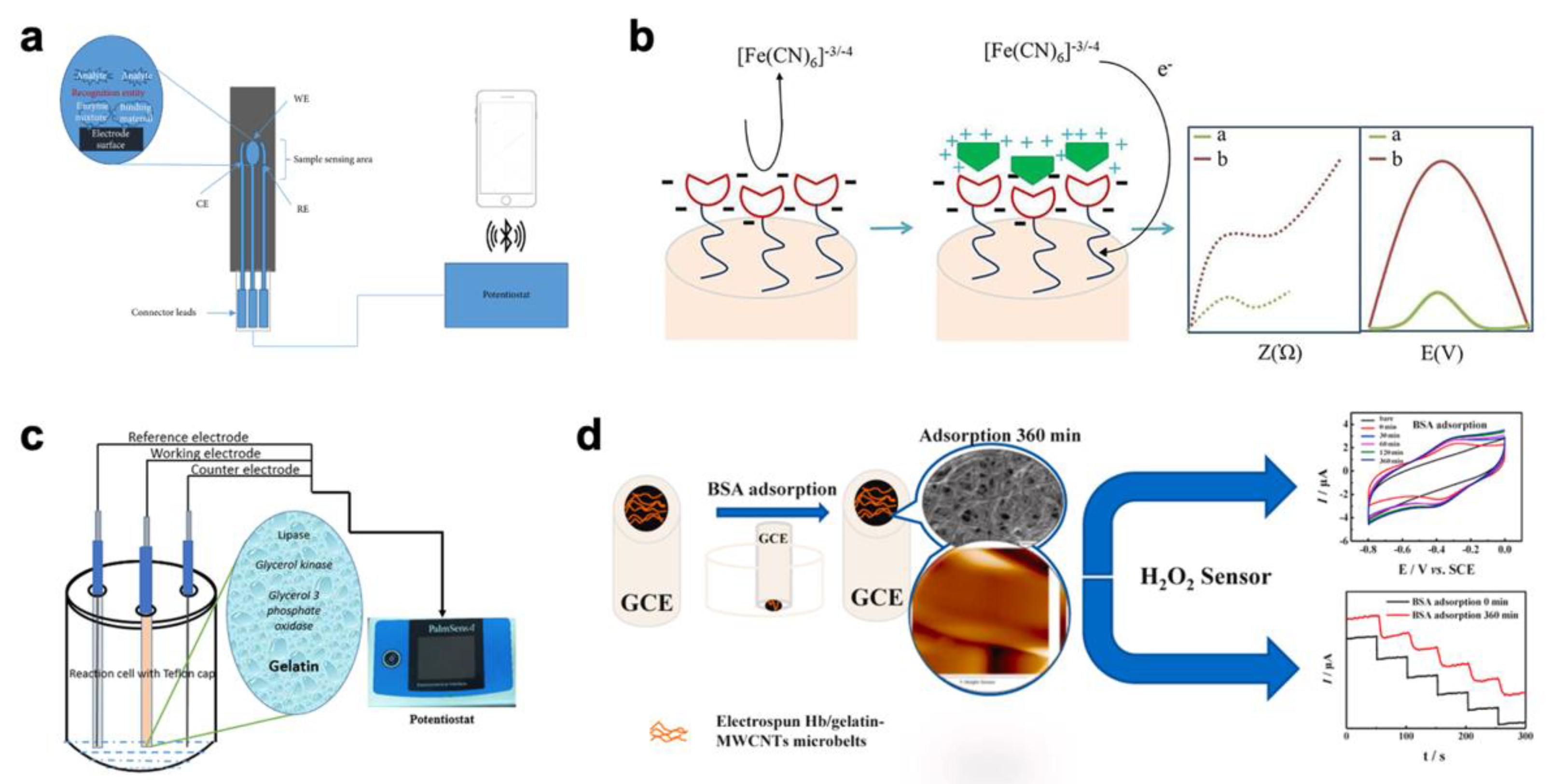
2.3. Environmental Monitoring
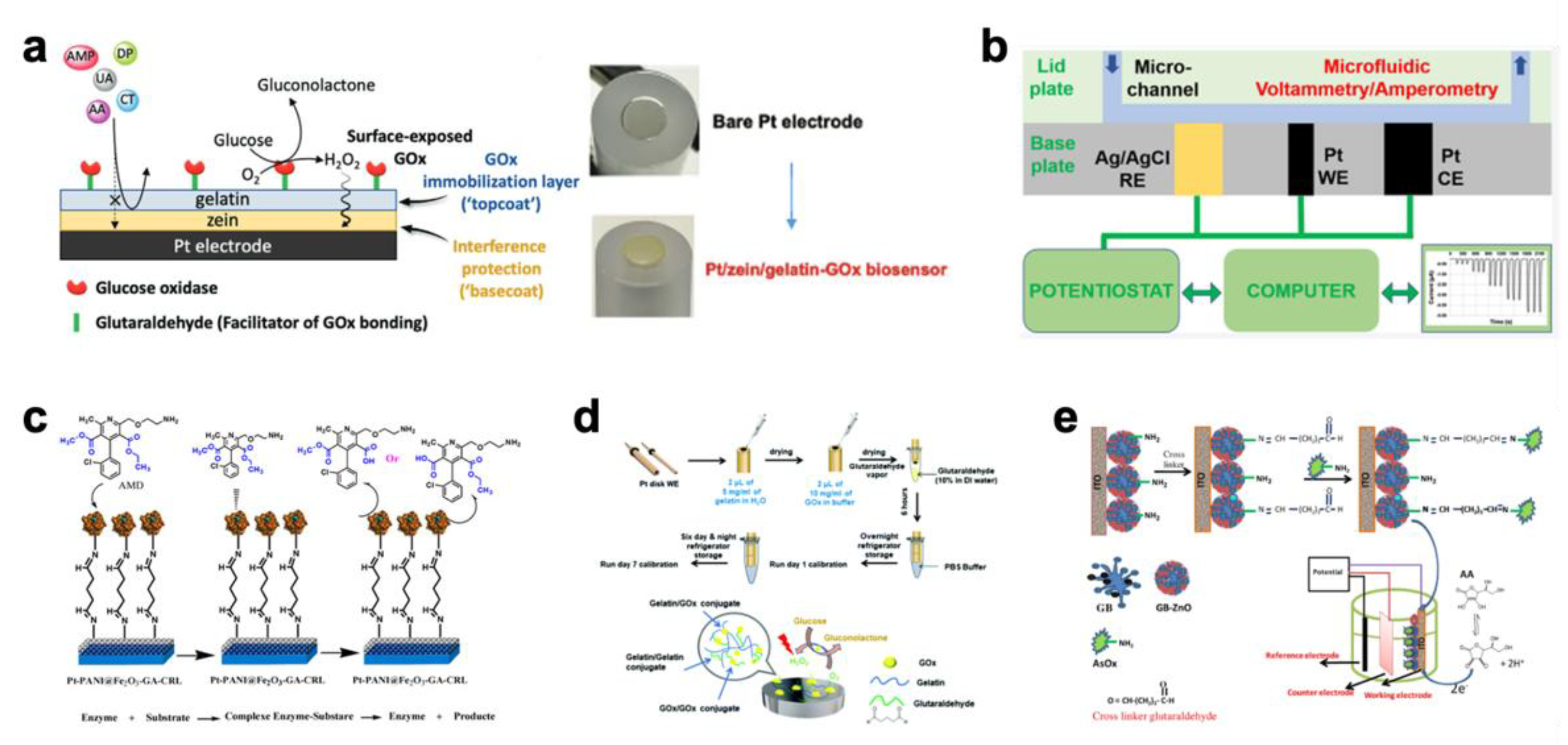
3. Gelatin as a Biorecognition Material for Detecting Target Analytes
3.1. Medical Diagnosis

3.2. Food Testing
3.3. Environmental Monitoring
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Goode, J.A.; Rushworth, J.V.H.; Millner, P.A. Biosensor regeneration: A review of common techniques and outcomes. Langmuir 2015, 31, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biosens. 2016, 60, 1–8. [Google Scholar]
- Demirdogen, B.C. A literature review of biosensors for multiple sclerosis: Towards personalized medicine and point-of-care testing. Mult. Scler. Relat. Disord. 2021, 48, 102675. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Malik, M.; Chaudhary, R. Quantification of pyruvate with special emphasis on biosensors: A review. Microchem. J. 2019, 146, 1102–1112. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. Reagentless Substrate Analysis with Immobilized Enzymes. Science 1962, 158, 270–272. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef]
- Song, S.; Xu, H.; Fan, C. Potential diagnostic applications of biosensors: Current and future directions. Int. J. Nanomed. 2006, 4, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Asal, M.; Ozen, O.; Sahinler, M.; Polatoglu, I. Recent developments in enzyme, DNA and immuno-based biosensors. Sensors 2018, 18, 1924. [Google Scholar] [CrossRef] [Green Version]
- Alipal, J.; Pu’ad, N.A.S.M.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialization. Mater. Today: Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Song, M.; Lin, X.; Peng, Z.; Xu, S.; Jin, L.; Zheng, X.; Luo, H. Materials and Methods of Biosensor Interfaces with Stability. Front. Mater. 2021, 7, 583739. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Chang, Y.; Chen, S. Amperometric glucose sensor based on glucose oxidase immobilized on gelatin-multiwalled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 2011, 80, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, R.E.; Cosnier, S.; Marks, R.S. Protease Amperometric Sensor. Anal. Chem. 2006, 78, 6327–6331. [Google Scholar] [CrossRef]
- Berto, M.; Diacci, C.; Theuer, L.; Lauro, M.D.; Simon, D.T.; Berggren, M.; Biscarini, F.; Beni, V.; Bortolotti, C.A. Label free urea biosensor based on organic electrochemical transistors. Flex. Print. Electron. 2018, 3, 024001. [Google Scholar] [CrossRef]
- Sharma, A.; Rawat, K.; Bohidar, H.B.; Solanki, P.R. Studies on clay-gelatin nanocomposite as urea sensor. Appl. Clay Sci. 2017, 146, 297–305. [Google Scholar] [CrossRef]
- Odaci, D.; Timur, S.; Telefoncu, A. Carboxyl esterase-alcohol oxidase based biosensor for the aspartame determination. Food Chem. 2004, 84, 493–496. [Google Scholar] [CrossRef]
- Zhu, Q.; Yuan, R.; Chai, Y.; Zhuo, Y.; Zhang, Y.; Li, X.; Wang, N. A Novel Amperometric Biosensor for Determination of Hydrogen Peroxide Based on Nafion and Polythionine as Well as Gold Nanoparticles and Gelatin as Matrixes. Anal. Lett. 2006, 39, 483–494. [Google Scholar] [CrossRef]
- Ozdemir, C.; Yeni, F.; Odaci, D.; Timur, S. Electrochemical glucose biosensing by pyranose oxidase immobilized in gold nanoparticle-polyaniline/AgCl/gelatin nanocomposite matrix. Food Chem. 2010, 119, 380–385. [Google Scholar] [CrossRef]
- Dhand, C.; Das, M.; Datta, M.; Malhotra, B.D. Recent advances in polyaniline based biosensors. Biosens. Bioelectron. 2011, 26, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; You, H.; Xu, T.; Bei, H.; Piwko, I.Z.; Kwan, Y.Y.; Zhao, X. Biomedical applications of gelatin methacryloyl hydrogels. Eng. Regen. 2021, 2, 47–56. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Damink, L.H.H.; Dijkstra, P.J.; Luyn, M.J.A.; Wanchem, P.B.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci: Mater. Med. 1995, 6, 460–472. [Google Scholar]
- Sharma, J.; Sharma, S.; Ajay; Sharma, L.K. Role of graphene in biomedical applications. Mater. Today Proc. 2022, 63, 542–546. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Sungur, S.; Emregul, E.; Gunendi, G.; Numanoglu, Y. New glucose biosensor based on glucose oxidase-immobilized gelatin film coated electrodes. J. Biomater. Appl. 2004, 18, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, D.; Zhao, T.; Zeng, B. Development of amperometric glucose biosensor through immobilizing enzyme in a Pt nanoparticles/mesoporous carbon matrix. Talanta 2008, 74, 1586–1591. [Google Scholar] [CrossRef]
- Gouda, M.D.; Kumar, M.A.; Thakur, M.S.; Karanth, N.G. Enhancement of operational stability of an enzyme biosensor for glucose and sucrose using protein based stabilizing agents. Biosens. Bioelectron. 2002, 17, 503–507. [Google Scholar] [CrossRef]
- Karacaoğlu, S.; Timur, S.; Telefoncu, A. Arginine Selective Biosensor Based on Arginase-Urease Immobilized in Gelatin. Artif. Cells Blood Substit. Biotechnol. 2003, 31, 357–363. [Google Scholar] [CrossRef]
- Ozbek, O.; Berkel, C.; Isildak, O.; Isildak, I. Potentiometric urea biosensors. Clin. Chim. Acta 2022, 524, 154–163. [Google Scholar] [CrossRef]
- Pundir, C.S.; Jakhar, S.; Narwal, V. Determination of urea with special emphasis on biosensors: A review. Biosens. Bioelectron. 2019, 123, 36–50. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Kayastha, A.M.; Srinivasan. Characterization of gelatin-immobilized pigeonpea urease and preparation of a new urea biosensor. Biotechnol. Appl. Biochem. 2001, 34, 55–62. [Google Scholar] [CrossRef]
- Panpae, K.; Krintrakul, S.; Chaiyasit, A. Development of a Urea Potentiometric Biosensor Based on Gelatin-Immobilized Urease. Agric. Nat. Resour. 2006, 40, 74–81. [Google Scholar]
- Soysa, H.; Rattanopas, S.; Teanphonkrang, S.; Quek, T.; Phomphrai, K.; Schulte, A. Biopolymer Cooperation for Sustainable High-Performance Oxidase-Based Biosensing with the Simplest Possible Readout of Substrate Conversion. Adv. Mater. Technol. 2021, 6, 2100096. [Google Scholar] [CrossRef]
- Thaweeskulchai, T.; Schulte, A. Sustainable and Efficient: A Reusable DIY Three-Electrode Base Plate for Microfluidic Electroanalysis and Biosensing. Anal. Chem. 2021, 93, 7557–7561. [Google Scholar] [CrossRef]
- Djaalab, E.; Samar, M.; Zougar, S.; Kherrat, R. Electrochemical Biosensor for the Determination of Amlodipine Besylate Based on Gelatin-Polyaniline Iron Oxide Biocomposite Film. Catalysts 2018, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Sakdaphetsiri, K.; Thaweeskulchai, T.; Schulte, A. Rapid sub-micromolar amperometric enzyme biosensing with free substrate access but without nanomaterial signalling support: Oxidase-based glucose detection as a proof-of-principle example. ChemComm Commun. 2020, 56, 7132–7135. [Google Scholar] [CrossRef]
- Rawat, K.; Sharma, A.; Solanki, P.R.; Bohidar, H.B. Potential of Gelatin-Zinc Oxide Nanocomposite as Ascorbic Acid Sensor. Electroanalysis 2015, 27, 2448–2457. [Google Scholar] [CrossRef]
- Yue, K.; Santiago, G.; Alvarez, M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Darvishi, S.; Souissi, M.; Kharaziha, M.; Karimzadeh, F.; Sahara, R.; Ahadian, S. Gelatin methacryloyl hydrogel for glucose biosensing using Ni nanoparticles-reduced graphene oxide: An experimental and modeling study. Electrochim. Acta 2018, 261, 275–283. [Google Scholar] [CrossRef]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant. Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Akyilmaz, E.; Dinckaya, E. Development of a catalase based biosensor for alcohol determination in beer samples. Talanta 2003, 61, 113–118. [Google Scholar] [CrossRef]
- Humphries, P.; Pretorius, E.; Naude, H. Direct and indirect cellular effects of aspartame on the brain. Eur. J. Clin. Nutr. 2008, 62, 451–462. [Google Scholar] [CrossRef] [Green Version]
- Janssen, P.J.C.M.; Heijden, C.A. Aspartame: Review of recent experimental and observational data. Toxiology 1988, 50, 1–26. [Google Scholar] [CrossRef]
- Manoj, D.; Auddy, I.; Nimbkar, S.; Chittibabu, S.; Shanmugasundaram, S. Development of Screen-Printed Electrode Biosensor for Rapid Determination of Triglyceride Content in Coconut Milk. Int. J. Food Sci. 2020, 7, 1696201. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Mehrotra, S.; Sharma, S.K. Challenges in assessing the quality of fruit juices: Intervening role of biosensors. Food Chem. 2022, 386, 132825. [Google Scholar] [CrossRef]
- Dinckaya, E.; Sezginturk, M.K.; Akyılmaz, E.; Ertas, F.N. Sulfite determination using sulfite oxidase biosensor based glassy carbon electrode coated with thin mercury film. Food Chem. 2007, 101, 1540–1544. [Google Scholar] [CrossRef]
- Sezginturk, M.K.; Dinckaya, E. Direct determination of sulfite in food samples by a biosensor based on plant tissue homogenate. Talanta 2005, 65, 998–1002. [Google Scholar] [CrossRef]
- Auddy, I.; Maurikaa, C.S.; Harigaran, N.; Manoj, D.; Nimbkar, S.; Shanmugasundaram, S. Development of amperometric enzyme-based biosensor to evaluate the adulteration in virgin coconut oil. J. Food Process. Preserv. 2022, e16337. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, X.; Yin, L.; Hao, X.; Guo, S.; Huang, Y. AgNPs Modified Glass Carbon Electrode Prepared with Gelatin as an Additive for Hydrogen Peroxide Sensor. Int. J. Electrochem. Sci. 2018, 13, 7696–7705. [Google Scholar] [CrossRef]
- Deng, Z.X.; Tao, J.W.; Zhang, W.; Mu, H.J.; Wu, H.J.; Wang, Y.B.; Xu, X.X.; Zheng, W. Effect of protein adsorption on electrospun hemoglobin/gelatin-MWCNTs microbelts modified electrode: Toward electrochemical measurement of hydrogen peroxide. Mater. Chem. Phys. 2021, 257, 123827. [Google Scholar] [CrossRef]
- Kumaran, A.; Vashishth, R.; Singh, S.; Surendran, U.; James, A.; Chellam, P.V. Biosensors for detection of organophosphate pesticides: Current technologies and future directives. Microchem. J. 2022, 178, 107420. [Google Scholar] [CrossRef]
- Borah, H.; Gogoi, S.; Kalita, S.; Puzari, P. A broad spectrum amperometric pesticide biosensor based on glutathione S-transferase immobilized on graphene oxide-gelatin matrix. J. Electroanal. Chem. 2018, 828, 116–123. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS EST Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Tagad, C.K.; Kulkarni, A.; Aiyer, R.C.; Patil, D.; Sabharwal, S.G. A miniaturized optical biosensor for the detection of Hg2+ based on acid phosphatase inhibition. Optik 2016, 127, 8807–8811. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Qian, J.; Wang, Y.; Zhang, J.; Zhi, J. A Novel Integrated Biosensor Based on Co-Immobilizing the Mediator and Microorganism for Water Biotoxicity Assay. Analyst 2014, 139, 2806–2812. [Google Scholar] [CrossRef]
- Karakaya, U.; Derkus, B.; Emregul, E. Development of Gelatin-Alginate-TiO2-SOD Biosensor for the Detection of Superoxide Radicals. JOTCSA 2020, 7, 571–580. [Google Scholar] [CrossRef]
- Sezginturk, M.K.; Dinckaya, E. A novel amperometric biosensor based on spinach (Spinacia oleracea) tissue homogenate for urinary oxalate determination. Talanta 2003, 59, 545–551. [Google Scholar] [CrossRef]
- Kocabay, O.; Emregul, E.; Aras, S.; Emregul, K.C. Carboxymethylcellulose-gelatin-superoxidase dismutase electrode for amperometric superoxide radical sensing. Bioprocess. Biosyst. Eng. 2012, 35, 923–930. [Google Scholar] [CrossRef]
- Sadak, O. One-pot scalable synthesis of rGO/AuNPs nanocomposite and its application in enzymatic glucose biosensor. Nanocomposites 2021, 7, 44–52. [Google Scholar] [CrossRef]
- Naghib, S.M.; Rahmanian, M.; Majidzadeh-A, K.; Asiaei, S.; Vahidi, O. Novel Magnetic Nanocomposites Comprising Reduced Graphene Oxide/Fe3O4/Gelatin Utilized in Ultrasensitive Non-Enzymatic Biosensing. Int. J. Electrochem. Sci. 2016, 11, 10256–10269. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Yorganci, E.; Asav, E. Do copper ions activate tyrosinase enzyme? A biosensor model for the solution. Bioelectrochemistry 2010, 78, 155–160. [Google Scholar] [CrossRef]
- Erden, P.E.; Kaçar, C.; Öztürk, F.; Kılıç, E. Amperometric uric acid biosensor based on poly(vinylferrocene)-gelatin-carboxylated multiwalled carbon nanotube modified glassy carbon electrode. Talanta 2015, 134, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, D.B.; Kuralay, F. MoS2/Chitosan/GOx-Gelatin modified graphite surface: Preparation, characterization and its use for glucose determination. Mater. Sci. Eng. B 2021, 270, 115215. [Google Scholar] [CrossRef]
- Wael, K.D.; Belder, S.D.; Pilehvar, S.; Steenberge, G.V.; Herrebout, W.; Heering, H.A. Enzyme-Gelatin Electrochemical Biosensors: Scaling Down. Biosensors 2012, 2, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Wang, Y.; Wang, M.; Wang, H.; Huang, Y. Highly Stable Hydrogen Peroxide Biosensor Based on Gelatin-Hierarchical Porous Carbon Obtained from Fish Scales Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2017, 12, 6588–6599. [Google Scholar] [CrossRef]
- Kaçar, C.; Erden, P.E.; Dalkiran, B.; İnal, E.K.; Kiliç, E. Amperometric biogenic amine biosensors based on Prussian blue, indium tin oxide nanoparticles and diamine oxidase-or monoamine oxidase-modified electrodes. Anal. Bioanal. Chem. 2020, 412, 1933–1946. [Google Scholar] [CrossRef]
- Teepoo, S.; Dawan, P.; Barnthip, N. Electrospun Chitosan-Gelatin Biopolymer Composite Nanofibers for Horseradish Peroxidase Immobilization in a Hydrogen Peroxide Biosensor. Biosensors 2017, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.X.; Tao, J.W.; Zhao, L.J.; Zhang, W.; Wang, Y.B.; Mu, H.J.; Wu, H.J.; Xu, X.X.; Zheng, W. Effect of protein adsorption on bioelectrochemistry of electrospun core-shell T MWCNTs/gelatin-Hb nanobelts on electrode surface. Process. Biochem. 2020, 96, 73–79. [Google Scholar] [CrossRef]
- Kacar, C.; Dalkiran, B.; Erden, P.E.; Kilic, E. An amperometric hydrogen peroxide biosensor based on Co3O4 nanoparticles and multiwalled carbon nanotube modified glassy carbon electrode. Appl. Surf. Sci. 2014, 311, 139–146. [Google Scholar] [CrossRef]
- Yashini, M.; Auddy, I.; Shanmugasundaram, S.; Vidyalakshmi, R.; Sunil, C.K. Characterization of Antibody Immobilization on Chitosan/ Gelatin-Modified Electrode and Its Application to Bacillus cereus Detection in Cereal-Based Food. Food Anal. Methods 2022, 15, 2382–2393. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Zhang, W.; Huang, Y. A hydrogen peroxide biosensor with high stability based on gelatin-multiwalled carbon nanotubes modified glassy carbon electrode. J. Solid State Electrochem. 2014, 18, 1981–1987. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Z.; Jing, Y.; Li, J.; Zhan, H. An acetylcholinesterase biosensor based on ionic liquid functionalized graphene-gelatin-modified electrode for sensitive detection of pesticides. Sens. Actuators B 2015, 210, 389–397. [Google Scholar] [CrossRef]
- Pohanka, M.; Jun, D.; Kuca, K. Amperometric Biosensors for Real Time Assays of Organophosphates. Sensors 2008, 8, 5303–5312. [Google Scholar] [CrossRef] [Green Version]
- Tastan, E.; Önder, S.; Kok, F.N. Immobilization of laccase on polymer grafted polytetrafluoroethylene membranes for biosensor construction. Talanta 2011, 84, 524–530. [Google Scholar] [CrossRef]
- Timur, S.; Telefoncu, A. Acetylcholinesterase (AChE) Electrodes Based on Gelatin and Chitosan Matrices for the Pesticide Detection. Artif. Cells Blood Substit. Biotechnol. 2004, 32, 427–442. [Google Scholar] [CrossRef]
- Hutapea, T.P.H.; Rukisah; Mulyadi; Madurani, K.A.; Suprapto; Kurniawan, F. Milkfish (Chanos Chanos) Gelatin as Biosensor Material for Chromium (III) Detection. Int. J. Eng. Technol. 2018, 7, 227–231. [Google Scholar] [CrossRef]
- Wael, K.D.; Bashir, Q.; Vlierberghe, S.V.; Dubruel, P.; Heering, H.A.; Adriaens, A. Electrochemical determination of hydrogen peroxide with cytochrome c peroxidase and horse heart cytochrome c entrapped in a gelatin hydrogel. Bioelectrochemistry 2012, 83, 15–18. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, X.; Gan, T.; He, B.; Wang, X. Preparation of multifunctional biohydrogel sensors with one freeze-thaw process. J. Appl. Polym. Sci. 2022, e52482. [Google Scholar] [CrossRef]
- Won, H.J.; Ryplida, B.; Kim, S.G.; Lee, G.; Ryu, J.H.; Park, S.Y. Diselenide-Bridged Carbon-Dot-Mediated Self-Healing, Conductive, and Adhesive Wireless Hydrogel Sensors for Label-Free Breast Cancer Detection. ACS Nano 2020, 14, 8409–8420. [Google Scholar] [CrossRef]
- Ping, J.; Qi, L.; Wang, Q.; Liu, S.; Jiang, Y.; Yu, L.; Lin, J.; Hu, Q. An integrated liquid crystal sensing device assisted by the surfactant-embedded smart hydrogel. Biosens. Bioelectron. 2021, 187, 113313. [Google Scholar] [CrossRef] [PubMed]
- Candido, S.; Maso, M.D.; Serraino, D.; McCubrey, J.A.; Bortolus, R.; Zanin, M.; Battiston, M.; Salemi, R.; Libra, M.; Polesel, J. Diagnostic value of neutrophil gelatinase-associated lipocalin/matrix metalloproteinase-9 pathway in transitional cell carcinoma of the bladder. Tumor Biol. 2016, 3, 9855–9863. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Roushandeh, A.M.; Ahmadzadeh, E.; Jahanian-Najafabadi, A.; Roudkenar, M.H. Implication and role of neutrophil gelatinase-associated lipocalin in cancer: Lipocalin-2 as a potential novel emerging comprehensive therapeutic target for a variety of cancer types. Mol. Biol. Rep. 2020, 47, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Huang, Y.H.; Au, H.; Wang, L.; Chu, S. The cancer marker neutrophil gelatinase-associated lipocalin is highly expressed in human endometrial hyperplasia. Mol. Biol. Rep. 2012, 39, 1029–1036. [Google Scholar] [CrossRef]
- Duvillard, L.; Ortega-Deballon, P.; Bourredjem, A.; Scherrer, M.; Mantion, G.; Delhorme, J.; Deguelte-Lardière, S.; Petit, J.; Bonithon-Kopp, C.; for the AGARIC study group. A case-control study of pre-operative levels of serum neutrophil gelatinase-associated lipocalin and other potential inflammatory markers in colorectal cancer. BMC cancer 2014, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nossier, A.I.; Mohammed, O.S.; El-deen, R.R.F.; Zaghloul, A.S.; Eissa, S. Gelatin-modified gold nanoparticles for direct detection of urinary total gelatinase activity: Diagnostic value in bladder cancer. Talanta 2016, 161, 511–519. [Google Scholar] [CrossRef]
- Strateva, T.; Yordanov, D. Pseudomonas aeruginosa-a phenomenon of bacterial resistance. J. Med Microbiol. 2009, 58, 1133–1148. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Chen, Y.; Li, M.; Jia, L.; Zhang, L.; Zhu, J. Gelatin-based photonic hydrogels for visual detection of pathogenic Pseudomonas aeruginosa. Sens. Actuators B Chem. 2021, 329, 129137. [Google Scholar] [CrossRef]
- Erturk, G.; Hedstrom, M.; Mattiasson, B. A sensitive and real-time assay of trypsin by using molecular imprinting-based capacitive biosensor. Biosens. Bioelectron. 2016, 86, 557–565. [Google Scholar] [CrossRef]
- Ping, J.; Wu, W.; Qi, L.; Liu, J.; Liu, J.; Zhao, B.; Wang, Q.; Yu, L.; Lin, J.; Hu, Q. Hydrogel-assisted paper-based lateral flow sensor for the detection of trypsin in human serum. Biosens. Bioelectron. 2021, 192, 113548. [Google Scholar] [CrossRef]
- Xia, N.; Sun, Z.; Ding, F.; Wang, Y.; Sun, W.; Liu, L. Protease Biosensor by Conversion of a Homogeneous Assay into a Surface-Tethered Electrochemical Analysis Based on Streptavidin-Biotin Interactions. ACS Sensor 2021, 6, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, P.; Gonzalez-Martinez, J.F.; Ruzgas, T.; Sotres, J. Highly Stable Passive Wireless Sensor for Protease Activity Based on Fatty Acid-Coupled Gelatin Composite Films. Anal. Chem. 2020, 92, 13110–13117. [Google Scholar] [CrossRef]
- Dietrich, R.; Jessberger, N.; Ehling-Schulz, M.; Märtlbauer, E.; Granum, P.E. The Food Poisoning Toxins of Bacillus cereus. Toxins 2021, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Harpaz, D.; Eltzov, E. Development of a portable colorimetric point-of-care device for the detection of Bacillus cereus in food specimens. Sens. Actuators B Chem. 2022, 356, 131354. [Google Scholar] [CrossRef]
- Saum, A.G.E.; Cumming, R.H.; Rowell, F.J. Detection of protease activity in the wetted surface of gelatin-coated electrodes in air by AC impedance spectroscopy. Biosens. Bioelectron. 2000, 15, 305–313. [Google Scholar] [CrossRef]
- Qiao, H.; Soeriyadi, A.H.; Guan, B.; Reece, P.J.; Gooding, J.J. The analytical performance of a porous silicon Bloch surface wave biosensors as protease biosensor. Sens. Actuators B 2015, 211, 469–475. [Google Scholar] [CrossRef]
- Zhang, Y.; Bian, J.; Li, Y.; Lin, T.; Zhang, J.; Huo, K.; Liu, X.; Liu, Y.; Liu, Y. Gel-sol and colorimetric dual-modal sensor for highly selective and sensitive detection of iodide ions based on gelatin fabricated AuNPs. Sens. Actuators B Chem. 2022, 364, 131913. [Google Scholar] [CrossRef]
- Derkus, B.; Emregul, E.; Yucesan, C.; Emregul, K.C. Myelin basic protein immunosensor for multiple sclerosis detection based upon label-free electrochemical impedance spectroscopy. Biosens. Bioelectron. 2013, 46, 53–60. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors-A review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Yang, Y.; Du, W.; Ji, W.; Fang, Z.; Hou, X.; Wu, Q.; Zhang, C.; Li, L. Optical flexible biosensors: From detection principles to biomedical applications. Biosens. Bioelectron. 2022, 210, 114328. [Google Scholar] [CrossRef]
- Dervisevic, M.; Alba, M.B.; Prieto-Simon, N.H. Voelcker, Skin in the diagnostics game: Wearable biosensor nano-and microsystems for medical diagnostics. Nano Today 2020, 30, 100828. [Google Scholar] [CrossRef]
- Kowalczyk, A. Trends and perspectives in DNA biosensors as diagnostic devices. Curr. Opin. Electrochem. 2020, 23, 36–41. [Google Scholar] [CrossRef]
- Ali, M.; Sultana, S.; Hamid, S.; Hossain, M.; Yehya, W.; Kader, M.; Bhargava, S. Gelatin controversies in food, pharmaceuticals, and personal care products: Authentication methods, current status, and future challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 1495–1511. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, B.; Wang, S.; Sangian, D.; Aziz, S.; Gu, Q. Hybrid Gelatin Hydrogels in Nanomedicine Applications. ACS Appl. Bio Mater. 2021, 4, 2886–2906. [Google Scholar] [CrossRef]
- Topkaya, S. Gelatin methacrylate (GelMA) mediated electrochemical DNA biosensor for DNA hybridization. Biosens. Bioelectron. 2015, 64, 456–461. [Google Scholar] [CrossRef]

| Biorecognition Materials | Targeted Analyte | Applications | References |
|---|---|---|---|
| Glucose oxidase | glucose | Medical diagnosis | [12] |
| Urease | urea | Medical diagnosis | [14] |
| ITO | urea | Medical diagnosis | [15] |
| Glucose oxidase | glucose | Medical diagnosis | [25] |
| Glucose oxidase | glucose | Medical diagnosis | [26] |
| Invertase, mutarotase, and Glucose oxidase | glucose and sucrose | Medical diagnosis | [27] |
| Arginase-Urease | arginine | Medical diagnosis | [28] |
| Pigeonpea urease | urea | Medical diagnosis | [31] |
| Urease | urea | Medical diagnosis | [32] |
| Glucose oxidase | glucose | Medical diagnosis | [33] |
| Lipase | amlodipine | Medical diagnosis | [35] |
| Glucose oxidase | glucose | Medical diagnosis | [36] |
| Ascorbate oxidase | ascorbic Acid | Medical diagnosis | [37] |
| Ni-RGO | glucose | Medical diagnosis | [39] |
| SOD enzyme | superoxide radical | Medical diagnosis | [57] |
| Spinach tissue homogenate | oxalate | Medical diagnosis | [58] |
| superoxidase dismutase | superoxide radical | Medical diagnosis | [59] |
| Glucose oxidase | glucose | Medical diagnosis | [60] |
| Fe3O4 | glucose | Medical diagnosis | [61] |
| Tyrosinase | Tyrosine | Medical diagnosis | [62] |
| Uricase enzyme | Uric acid | Medical diagnosis | [63] |
| Glucose oxidase | glucose | Medical diagnosis | [64] |
| Catalase | hydrogen peroxide | Medical diagnosis | [65] |
| Carboxyl esterase-alcohol oxidase | aspartame | Food testing | [16] |
| Catalase | hydrogen peroxide and ethanol | Food testing | [41] |
| lipase, glycerol-3-phosphate, and glycerol kinase | Triglyceride | Food testing | [44] |
| Sulfite oxidase | Sulfite | Food testing | [46] |
| Plant tissue homogenate | sulfites | Food testing | [47] |
| lipase | diglyceride | Food testing | [48] |
| Hemoglobin | hydrogen peroxide | Food testing | [50] |
| Catalase | hydrogen peroxide | Food testing | [66] |
| ITO, diamine oxidase | cadaverine and histamine | Food testing | [67] |
| Horseradish Peroxidase | hydrogen peroxide | Food testing | [68] |
| Hemoglobin | hydrogen peroxide | Food testing | [69] |
| Peroxidase | hydrogen peroxide | Food testing | [70] |
| Anti-Bacillus cereus Polyclonal antibodies | Bacillus cereus | Food testing | [71] |
| Catalase | hydrogen peroxide | Food testing | [72] |
| Glutathione S-transferase | benzamidazole, organochlorine, organothiophosphate, organo-carbamate, polyphenol, and pyrethroid | Environmental monitoring | [52] |
| Acid phosphatase | Hg2+ | Environmental monitoring | [55] |
| E. coli | Hg2+, Cu2+, and Cd2+ | Environmental monitoring | [56] |
| Acetylcholinesterase | carbaryl and monocrotophos | Environmental monitoring | [73] |
| Acetylcholinesterase | organophosphate paraoxon | Environmental monitoring | [74] |
| laccase | phenolic compounds | Environmental monitoring | [75] |
| Acetylcholinesterase | organophosphates | Environmental monitoring | [76] |
| Silver | Chromium (III) | Environmental monitoring | [77] |
| Horseradish peroxidase | hydrogen peroxide | Environmental monitoring | [78] |
| Transglutaminase | - | - | [79] |
| Biorecogination Materials | Targeted Analyte | Applications | References |
|---|---|---|---|
| Glucose oxidase/gelatin | Protease/glucose | Medical diagnosis | [13] |
| pigeonpea urease | urea | Medical diagnosis | [31] |
| Gelatin/CTAB | Trypsin | Medical diagnosis | [81] |
| Gelatin/AuNPs | Gelatinase | Medical diagnosis | [86] |
| Gelatin-based photonic hydrogel | P. aeruginosa | Medical diagnosis | [88] |
| Gelatin | trypsin | Medical diagnosis | [90] |
| Fatty-Acid-Coupled Gelatin Composite Films | Protease | Medical diagnosis | [92] |
| Gelatin | Bacillus cereus/gelatinase | Food testing | [94] |
| Gelatin | protease | Environmental monitoring | [95] |
| Gelatin/porous silicon | protease | Environmental monitoring | [96] |
| Gelatin/AuNPs | iodide ions (I−)/hydrogen peroxide | Medical diagnosis | [97] |
| MBP | Anti-MBP autoantibody | Medical diagnosis | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Huang, Y.; Li, T. Applications of Gelatin in Biosensors: Recent Trends and Progress. Biosensors 2022, 12, 670. https://doi.org/10.3390/bios12090670
Guan Y, Huang Y, Li T. Applications of Gelatin in Biosensors: Recent Trends and Progress. Biosensors. 2022; 12(9):670. https://doi.org/10.3390/bios12090670
Chicago/Turabian StyleGuan, Yuepeng, Yaqin Huang, and Tianyu Li. 2022. "Applications of Gelatin in Biosensors: Recent Trends and Progress" Biosensors 12, no. 9: 670. https://doi.org/10.3390/bios12090670
APA StyleGuan, Y., Huang, Y., & Li, T. (2022). Applications of Gelatin in Biosensors: Recent Trends and Progress. Biosensors, 12(9), 670. https://doi.org/10.3390/bios12090670





