FBG-Based Soft System for Assisted Epidural Anesthesia: Design Optimization and Clinical Assessment
Abstract
:1. Introduction
2. Development of FBG-Based Soft Systems
2.1. FBG Principle of Work
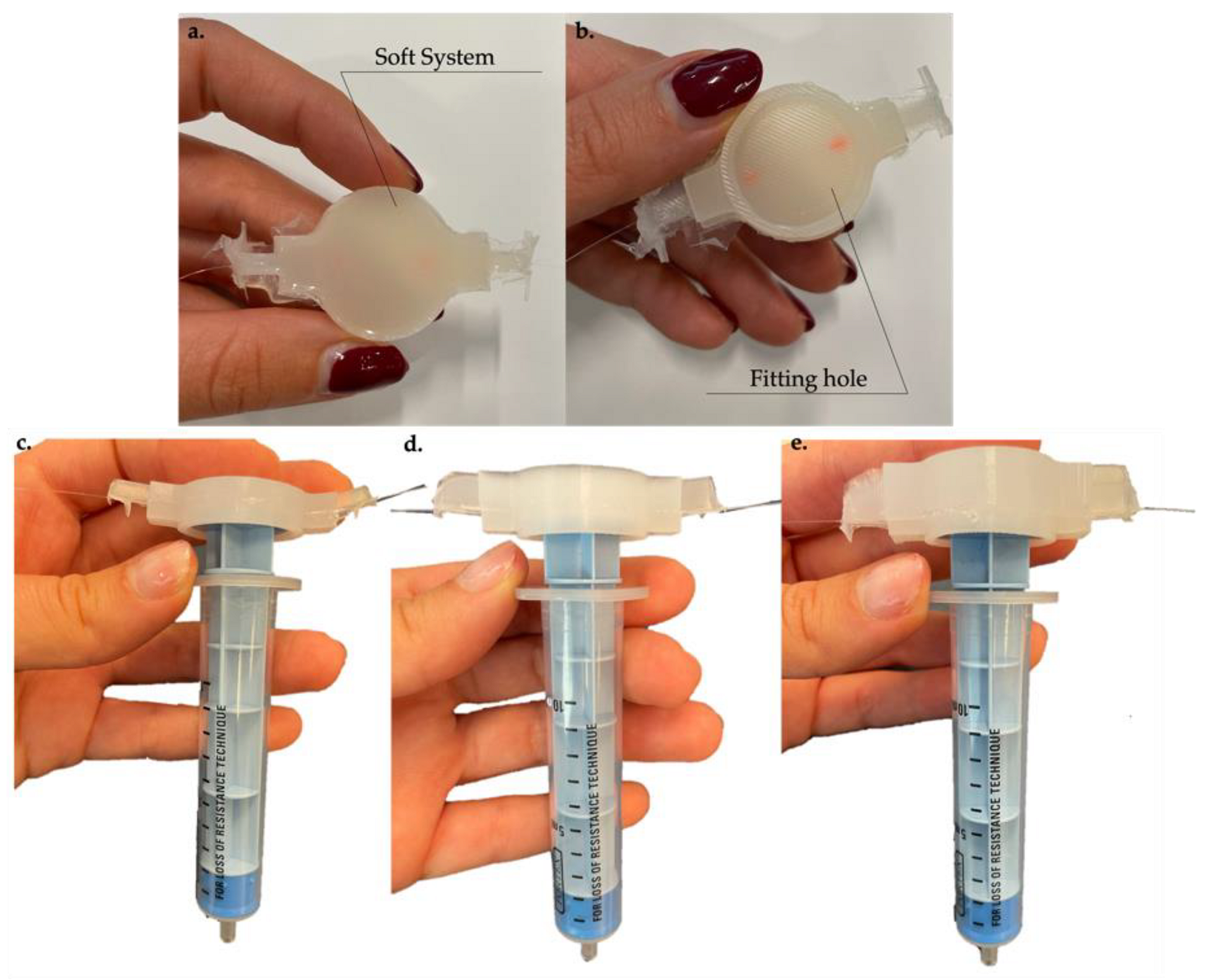
2.2. Materials, Soft Systems Design, and Manufacturing Process
3. Metrological Characterization
3.1. Compression Tests
3.2. Data Analysis
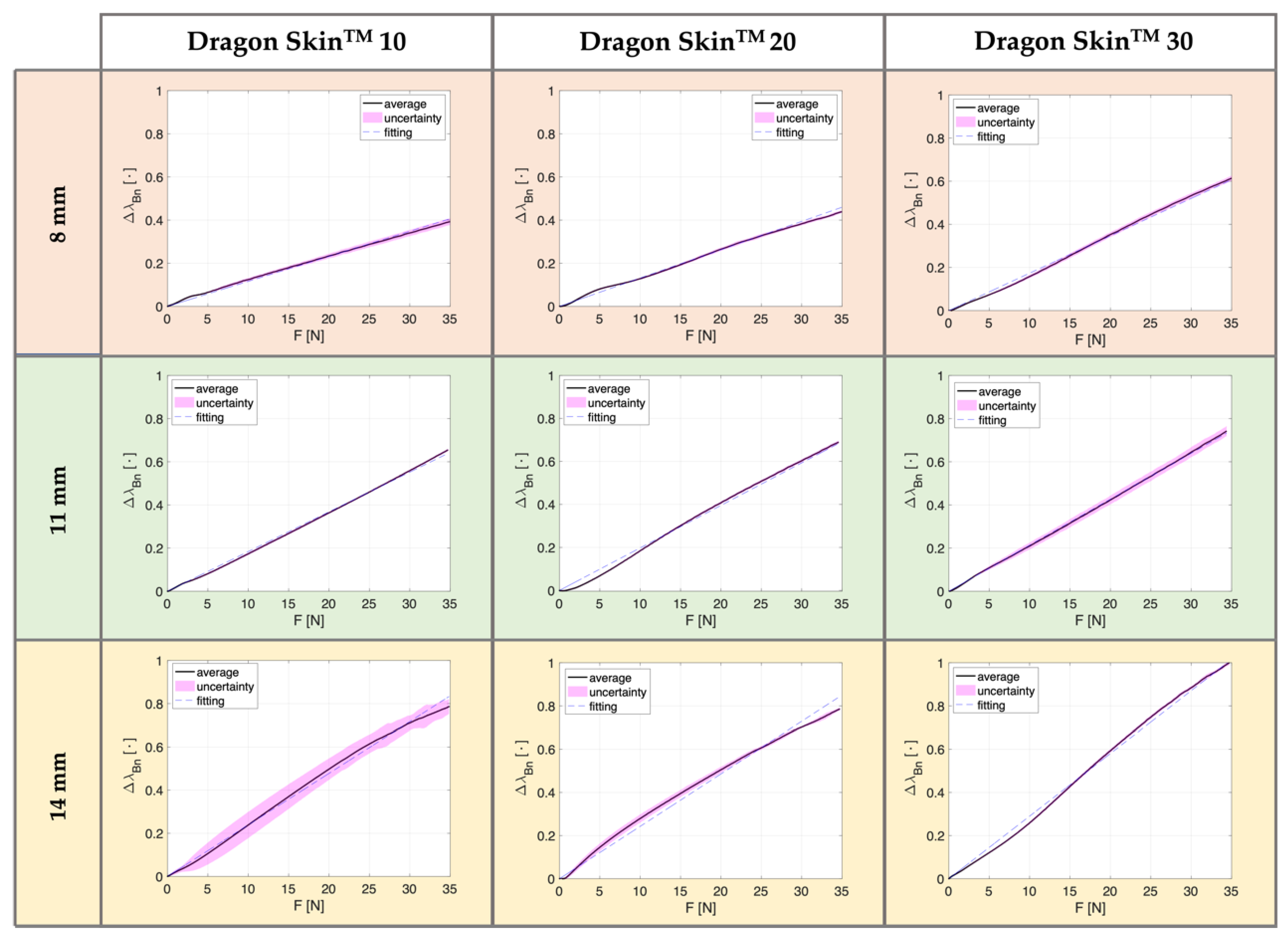
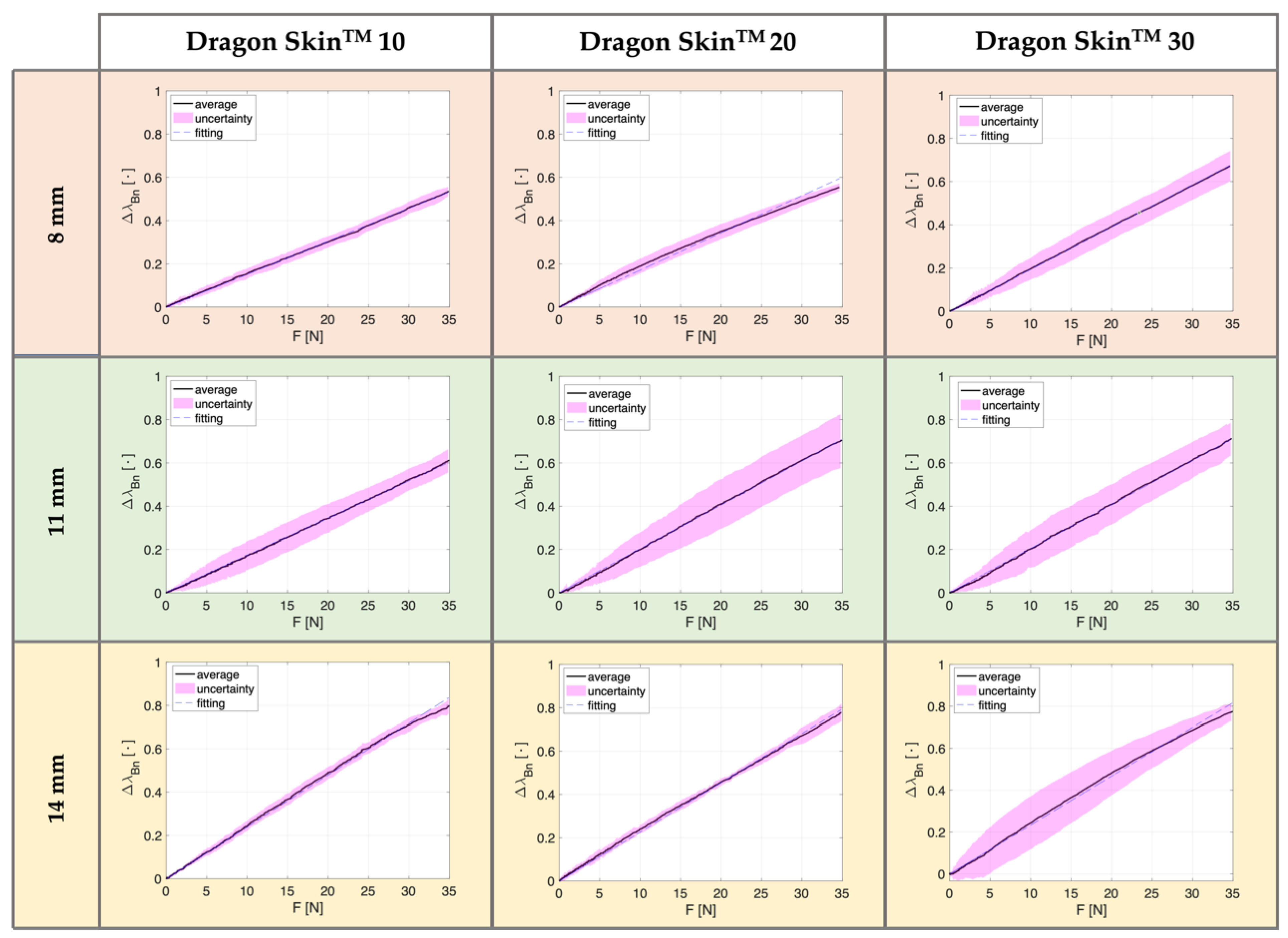
3.3. Results
4. Clinical Assessment of the Optimized System on Patients Affected by Low Back Pain
4.1. Experimental Set-Up
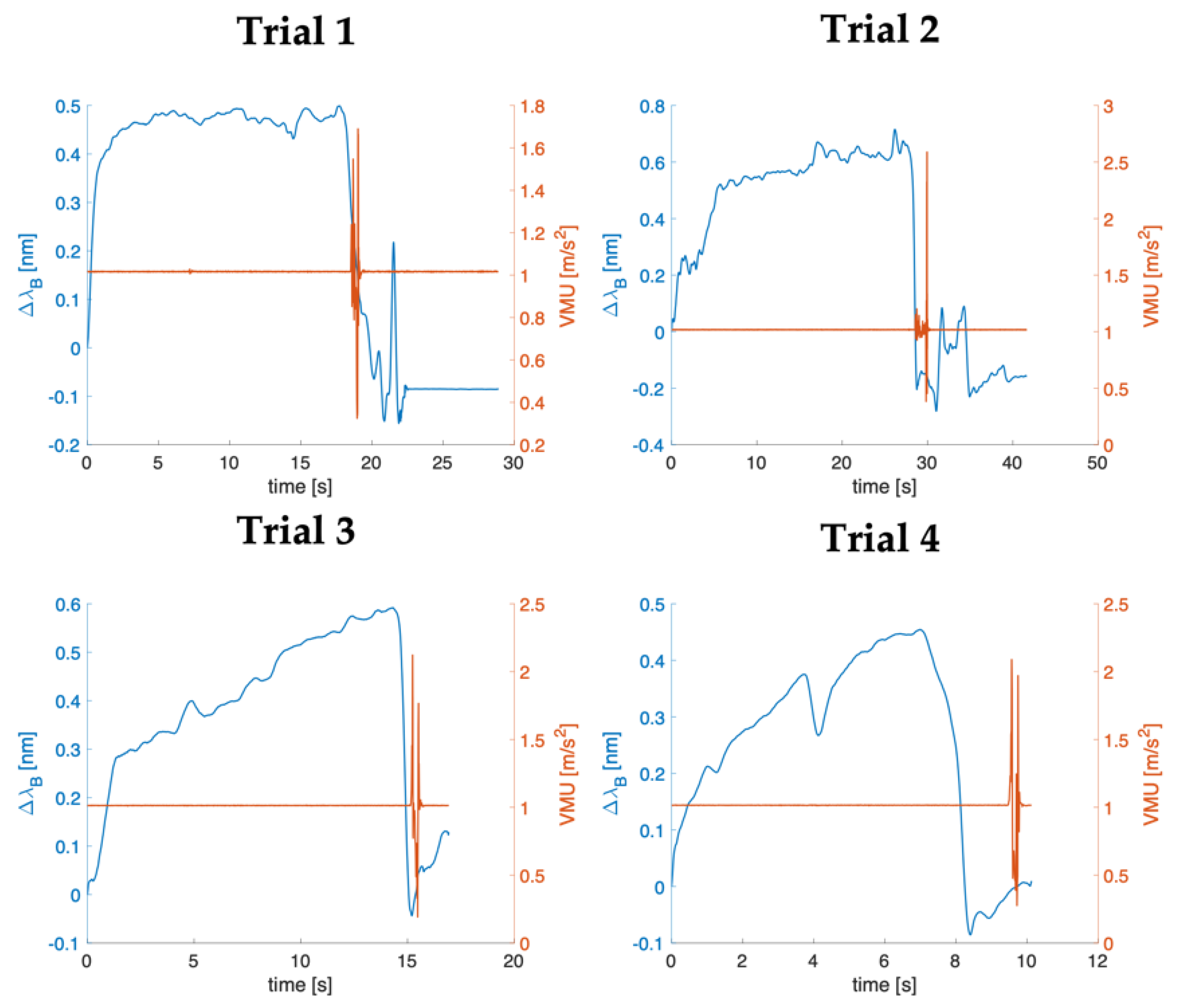
4.2. Data Analysis
4.3. Results
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawkins, J.L. Epidural Analgesia for Labor and Delivery. New Engl. J. Med. 2010, 362, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Anim-Somuah, M.; Smyth, R.; Jones, L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef] [PubMed]
- White, A.H.; Derby, R.; Wynne, G. Epidural injections for the diagnosis and treatment of low-back pain. Spine 1980, 5, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Carassiti, M.; Pascarella, G.; Strumia, A.; Russo, F.; Papalia, G.F.; Cataldo, R.; Gargano, F.; Costa, F.; Pierri, M.; De Tommasi, F.; et al. Epidural Steroid Injections for Low Back Pain: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 231. [Google Scholar] [CrossRef]
- Hoy, D.; Brooks, P.; Blyth, F.; Buchbinder, R. The Epidemiology of low back pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 769–781. [Google Scholar] [CrossRef]
- Latina, R.; De Marinis, M.G.; Giordano, F.; Osborn, J.F.; Giannarelli, D.; Di Biagio, E.; Varrassi, G.; Sansoni, J.; Bertini, L.; Baglio, G.; et al. Epidemiology of Chronic Pain in the Latium Region, Italy: A Cross-Sectional Study on the Clinical Characteristics of Patients Attending Pain Clinics. Pain Manag. Nurs. 2019, 20, 373–381. [Google Scholar] [CrossRef]
- Radiculopathy, L. Epidural steroid injections for low back pain and lumbosacral radiculopathy. J. R. Soc. Med. 1953, 46, 713–716. [Google Scholar] [CrossRef]
- Moraca, R.J.; Sheldon, D.G.; Thirlby, R.C. The role of epidural anesthesia and analgesia in surgical practice. Ann. Surg. 2003, 238, 663. [Google Scholar] [CrossRef]
- Fyneface-Ogan, S. Anatomy and Clinical Importance of the Epidural Space. In Epidural Analgesia—Current Views and Approaches; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Ruppen, W.; Derry, S.; McQuay, H.; Moore, R.A. Incidence of epidural hematoma, infection, and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology 2006, 105, 394–399. [Google Scholar] [CrossRef]
- Segal, S.; Arendt, K.W. A retrospective effectiveness study of loss of resistance to air or saline for identification of the epidural space. Anesth. Analg. 2010, 110, 558–563. [Google Scholar] [CrossRef]
- Rodiera, J.; Calabuig, R.; Aliaga, L.; Espinosa, W.; Hobeich, F.; Oferil, F.; Gual, A. Mathematical analysis of epidural space location. Int. J. Clin. Monit. Comput. 1995, 12, 213–217. [Google Scholar] [CrossRef]
- Sharrock, N.E. Recordings of, and an anatomical explanation for, false positive loss of resistance during lumber extradural analgesia. Br. J. Anaesth. 1979, 51, 253–258. [Google Scholar] [CrossRef]
- Riley, E.T.; Carvalho, B. The EpisureTM syringe: A novel loss of resistance syringe for locating the epidural space. Anesth. Analg. 2007, 105, 1164–1166. [Google Scholar] [CrossRef]
- Lechner, T.J.M.; Van Wijk, M.G.F.; Maas, A.J.J. Clinical results with a new acoustic device to identify the epidural space. Anaesthesia 2002, 57, 768–772. [Google Scholar] [CrossRef]
- Sawada, A.; Kii, N.; Yoshikawa, Y.; Yamakage, M. Epidrum®: A new device to identify the epidural space with an epidural Tuohy needle. J. Anesth. 2012, 26, 292–295. [Google Scholar] [CrossRef]
- Capogna, G.; Camorcia, M.; Coccoluto, A.; Micaglio, M.; Velardo, M. Experimental validation of the CompuFlo® epidural controlled system to identify the epidural space and its clinical use in difficult obstetric cases. Int. J. Obstet. Anesth. 2018, 36, 28–33. [Google Scholar] [CrossRef]
- Tesei, M.; Saccomandi, P.; Massaroni, C.; Quarta, R.; Carassiti, M.; Schena, E.; Setola, R. A cost-effective, non-invasive system for pressure monitoring during epidural needle insertion: Design, development and bench tests. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Orlando, FL, USA, 16–20 August 2016; pp. 194–197. [Google Scholar] [CrossRef]
- Carassiti, M.; Mattei, A.; Quarta, R.; Massaroni, C.; Saccomandi, P.; Tesei, M.; Setola, R.; Schena, E. A New Pressure Guided Management Tool for Epidural Space Detection: Feasibility Assessment on a Simulator. Artif. Organs 2017, 41, E320–E325. [Google Scholar] [CrossRef]
- Carassiti, M.; Quarta, R.; Mattei, A.; Tesei, M.; Saccomandi, P.; Massaroni, C.; Setola, R.; Schena, E. Ex vivo animal-model assessment of a non-invasive system for loss of resistance detection during epidural blockade. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Jeju Island, Korea, 11–15 July 2017; pp. 759–762. [Google Scholar] [CrossRef]
- Carotenuto, B.; Ricciardi, A.; Micco, A.; Amorizzo, E.; Mercieri, M.; Cutolo, A.; Cusano, A. Optical fiber technology enables smart needles for epidurals: An in-vivo swine study. Biomed. Opt. Express 2019, 10, 1351–1364. [Google Scholar] [CrossRef]
- Carotenuto, B.; Micco, A.; Ricciardi, A.; Amorizzo, E.; Mercieri, M.; Cutolo, A.; Cusano, A. Optical guidance systems for epidural space identification. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 371–379. [Google Scholar] [CrossRef]
- Amantayeva, A.; Adilzhanova, N.; Issatayeva, A.; Blanc, W.; Molardi, C.; Tosi, D. Fiber Optic Distributed Sensing Network for Shape Sensing-Assisted Epidural Needle Guidance. Biosensors 2021, 11, 446. [Google Scholar] [CrossRef]
- Ambastha, S.; Umesh, S.; Dabir, S.; Asokan, S. Spinal needle force monitoring during lumbar puncture using fiber Bragg grating force device. J. Biomed. Opt. 2016, 21, 117002. [Google Scholar] [CrossRef]
- De Tommasi, F.; Lo Presti, D.; Virgili, F.; Massaroni, C.; Schena, E.; Carassiti, M. Soft System Based on Fiber Bragg Grating Sensor for Loss of Resistance Detection during Epidural Procedures: In Silico and In Vivo Assessment. Sensors 2021, 21, 5329. [Google Scholar] [CrossRef]
- De Tommasi, F.; Presti, D.L.; Massaroni, C.; Schena, E.; Carassiti, M. FBG-based System for Loss of Resistance Detection During Epidural Injections. In Proceedings of the 2021 IEEE International Workshop on Metrology for Industry 4.0 & IoT (MetroInd4. 0&IoT), Rome, Italy, 7–9 June 2021; pp. 172–176. [Google Scholar]
- Galiano, K.; Obwegeser, A.A.; Walch, C.; Schatzer, R.; Ploner, F.; Gruber, H. Ultrasound-guided versus computed tomography-controlled facet joint injections in the lumbar spine: A prospective randomized clinical trial. Reg. Anesth. Pain Med. 2007, 32, 317–322. [Google Scholar]
- Hogan, Q. Epidural catheter tip position and distribution of injectate evaluated by computed tomography. J. Am. Soc. Anesthesiol. 1999, 90, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Presti, D.L.; Massaroni, C.; Leitao, C.S.J.; Domingues, M.D.F.; Sypabekova, M.; Barrera, D.; Floris, I.; Massari, L.; Oddo, C.M.; Sales, S.; et al. Fiber bragg gratings for medical applications and future challenges: A review. IEEE Access 2020, 8, 156863–156888. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, N.; Tiwari, U.; Kapur, P. Fiber grating sensors in medicine: Current and emerging applications. Sens. Actuators A Phys. 2011, 167, 279–290. [Google Scholar] [CrossRef]
- Mishra, M.; Sahu, P.K. Fiber Bragg Gratings in Healthcare Applications: A Review. IETE Tech. Rev. 2022, 1–18. [Google Scholar] [CrossRef]
- da Silva, A.F.; Goncalves, A.F.; Ferreira, L.A.D.A.; Araujo, F.M.M.; Mendes, P.; Correia, J. PVC smart sensing foil for advanced strain measurements. IEEE Sens. J. 2010, 10, 1149–1155. [Google Scholar] [CrossRef]
- Da Silva, A.F.; Goncalves, A.F.; Ferreira, L.A.D.A.; Araujo, F.M.M.; Mendes, P.M.; Correia, J.H. A smart skin PVC foil based on FBG sensors for monitoring strain and temperature. IEEE Trans. Ind. Electron. 2010, 58, 2728–2735. [Google Scholar] [CrossRef]
- Ge, J.; James, A.; Xu, L.; Chen, Y.; Kwok, K.-W.; Fok, M.P. Bidirectional soft silicone curvature sensor based on off-centered embedded fiber Bragg grating. IEEE Photonics Technol. Lett. 2016, 28, 2237–2240. [Google Scholar] [CrossRef]
- Presti, D.L.; Bianchi, D.; Massaroni, C.; Gizzi, A.; Schena, E. A Soft and Skin-Interfaced Smart Patch Based on Fiber Optics for Cardiorespiratory Monitoring. Biosensors 2022, 12, 363. [Google Scholar] [CrossRef]
- Di Tocco, J.; Presti, D.L.; Zaltieri, M.; D’Alesio, G.; Filosa, M.; Massari, L.; Aliperta, A.; Di Rienzo, M.; Carrozza, M.C.; Ferrarin, M.; et al. A wearable system based on flexible sensors for unobtrusive respiratory monitoring in occupational settings. IEEE Sens. J. 2020, 21, 14369–14378. [Google Scholar] [CrossRef]
- Erdogan, T. Fiber grating spectra. J. Lightwave Technol. 1997, 15, 1277–1294. [Google Scholar] [CrossRef]
- Rao, Y.J. In-fibre Bragg grating sensors. Meas. Sci. Technol. 1997, 8, 355–375. [Google Scholar] [CrossRef]
- Smooth-On. Dragon skinTM Series, High Performance Silicone Rubber. Available online: https://www.smooth-on.com/product-line/dragon-skin/ (accessed on 27 June 2022).
- Presti, D.L.; Dall’Orso, S.; Muceli, S.; Arichi, T.; Neumane, S.; Lukens, A.; Sabbadini, R.; Massaroni, C.; Caponero, M.A.; Formica, D.; et al. An fmri compatible smart device for measuring palmar grasping actions in newborns. Sensors 2020, 20, 6040. [Google Scholar] [CrossRef]
- Figliola, R.S.; Beasley, D.E. Theory and Design for Mechanical Measurements, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1995; Volume 20. [Google Scholar] [CrossRef]
- Vaughan, N.; Dubey, V.N.; Wee, M.Y.K.; Isaacs, R. Towards a realistic in vitro experience of epidural Tuohy needle insertion. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2013, 227, 767–777. [Google Scholar] [CrossRef]



| Silicone Rubber | E [MPa] | Curing Time [h] |
|---|---|---|
| Dragon SkinTM 10 | 0.24 | 5 |
| Dragon SkinTM 20 | 0.47 | 4 |
| Dragon SkinTM 30 | 0.74 | 16 |
| Silicone Rubber | Coating | FBG Length [mm] | λB [nm] | Reflectivity Value [%] |
|---|---|---|---|---|
| Dragon SkinTM 10 | Acrylate | 10 | 1540 | 94 |
| Dragon SkinTM 20 | Acrylate | 10 | 1540 | 94 |
| Dragon SkinTM 30 | Acrylate | 10 | 1536 | 94 |
| Dragon SkinTM 10 | Dragon SkinTM 20 | Dragon SkinTM 30 | ||||
|---|---|---|---|---|---|---|
| AT | PT | AT | PT | AT | PT | |
| 8 mm | 39% | 53% | 44% | 55% | 61% | 67% |
| 11 mm | 66% | 61% | 69% | 70% | 74% | 71% |
| 14 mm | 79% | 80% | 79% | 78% | 100% | 77% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Tommasi, F.; Romano, C.; Lo Presti, D.; Massaroni, C.; Carassiti, M.; Schena, E. FBG-Based Soft System for Assisted Epidural Anesthesia: Design Optimization and Clinical Assessment. Biosensors 2022, 12, 645. https://doi.org/10.3390/bios12080645
De Tommasi F, Romano C, Lo Presti D, Massaroni C, Carassiti M, Schena E. FBG-Based Soft System for Assisted Epidural Anesthesia: Design Optimization and Clinical Assessment. Biosensors. 2022; 12(8):645. https://doi.org/10.3390/bios12080645
Chicago/Turabian StyleDe Tommasi, Francesca, Chiara Romano, Daniela Lo Presti, Carlo Massaroni, Massimiliano Carassiti, and Emiliano Schena. 2022. "FBG-Based Soft System for Assisted Epidural Anesthesia: Design Optimization and Clinical Assessment" Biosensors 12, no. 8: 645. https://doi.org/10.3390/bios12080645
APA StyleDe Tommasi, F., Romano, C., Lo Presti, D., Massaroni, C., Carassiti, M., & Schena, E. (2022). FBG-Based Soft System for Assisted Epidural Anesthesia: Design Optimization and Clinical Assessment. Biosensors, 12(8), 645. https://doi.org/10.3390/bios12080645









