Ultra-Small and Metabolizable Near-Infrared Au/Gd Nanoclusters for Targeted FL/MRI Imaging and Cancer Theranostics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Au/Gd@BSA NCs
2.3. Preparation of Au/Gd@FA NCs
2.4. Characterization of Au/Gd@FA NCs
2.5. MTT Assay
2.6. Laser Confocal Fluorescence Imaging
2.7. Relaxometry and In Vitro MRI
2.8. In Vivo FL Imaging and MRI Analysis
2.8.1. In vivo FL Imaging
2.8.2. In Vivo MRI
2.9. Intracellular ROS Detection
3. Results and Discussion
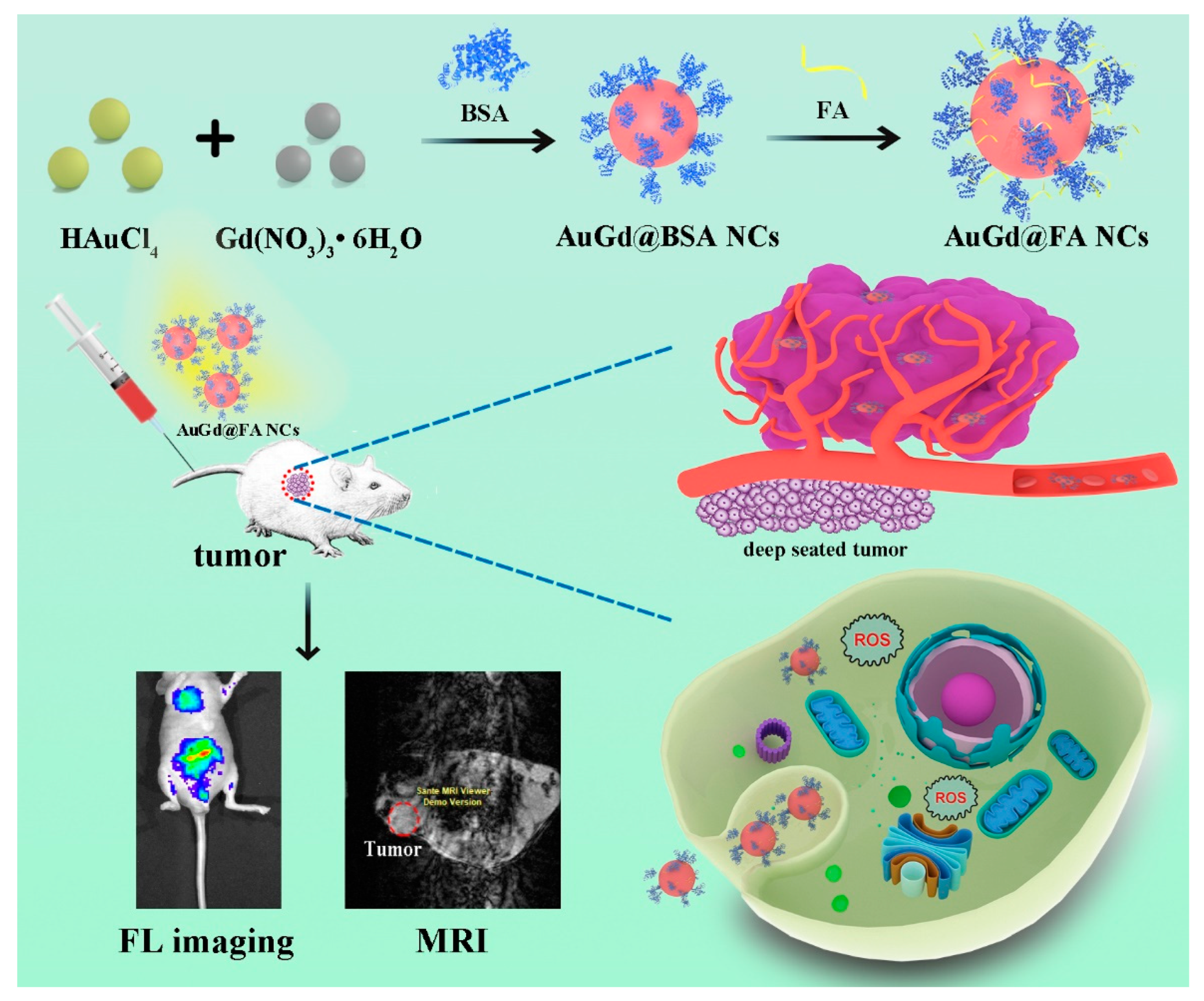
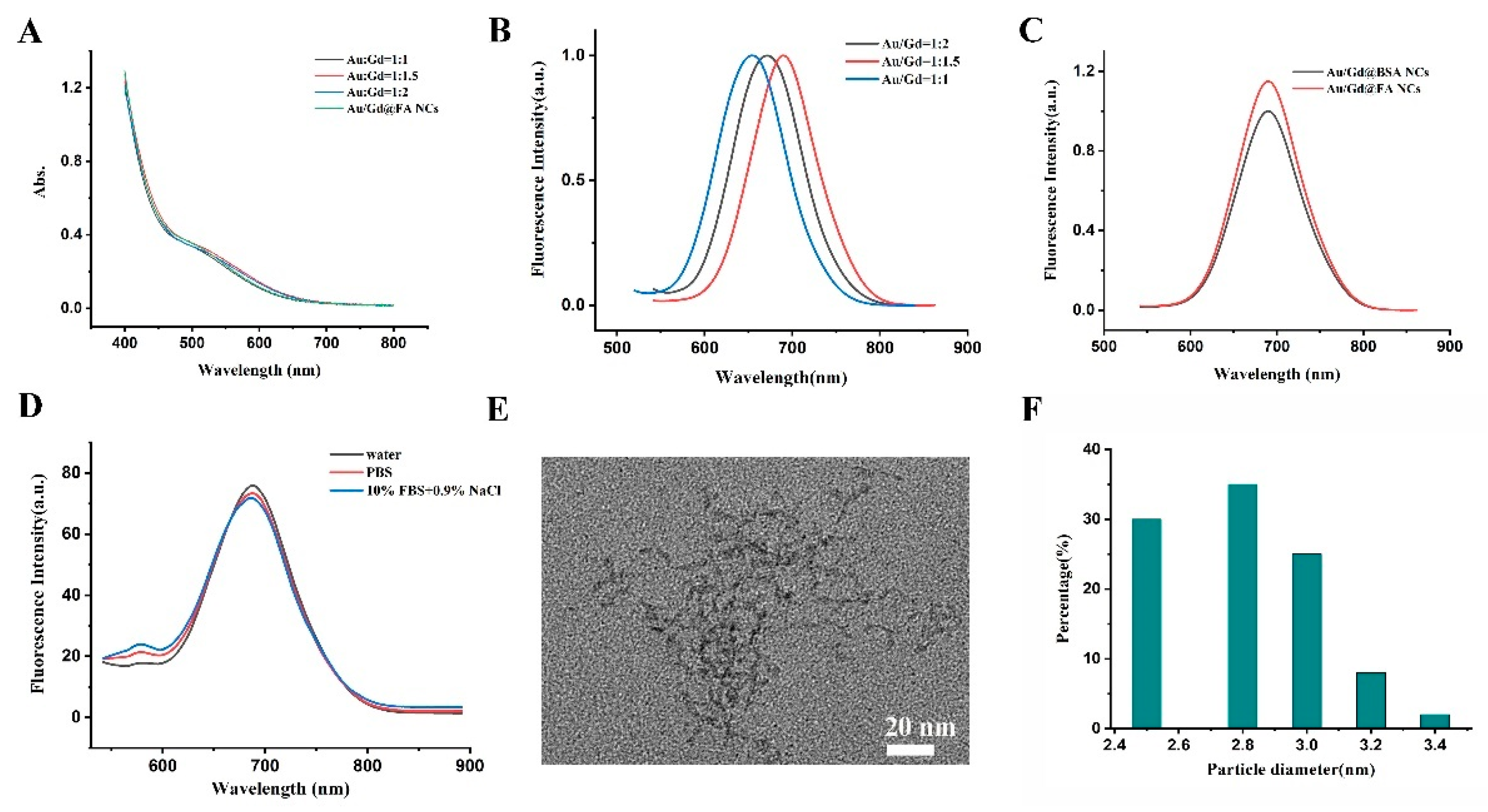
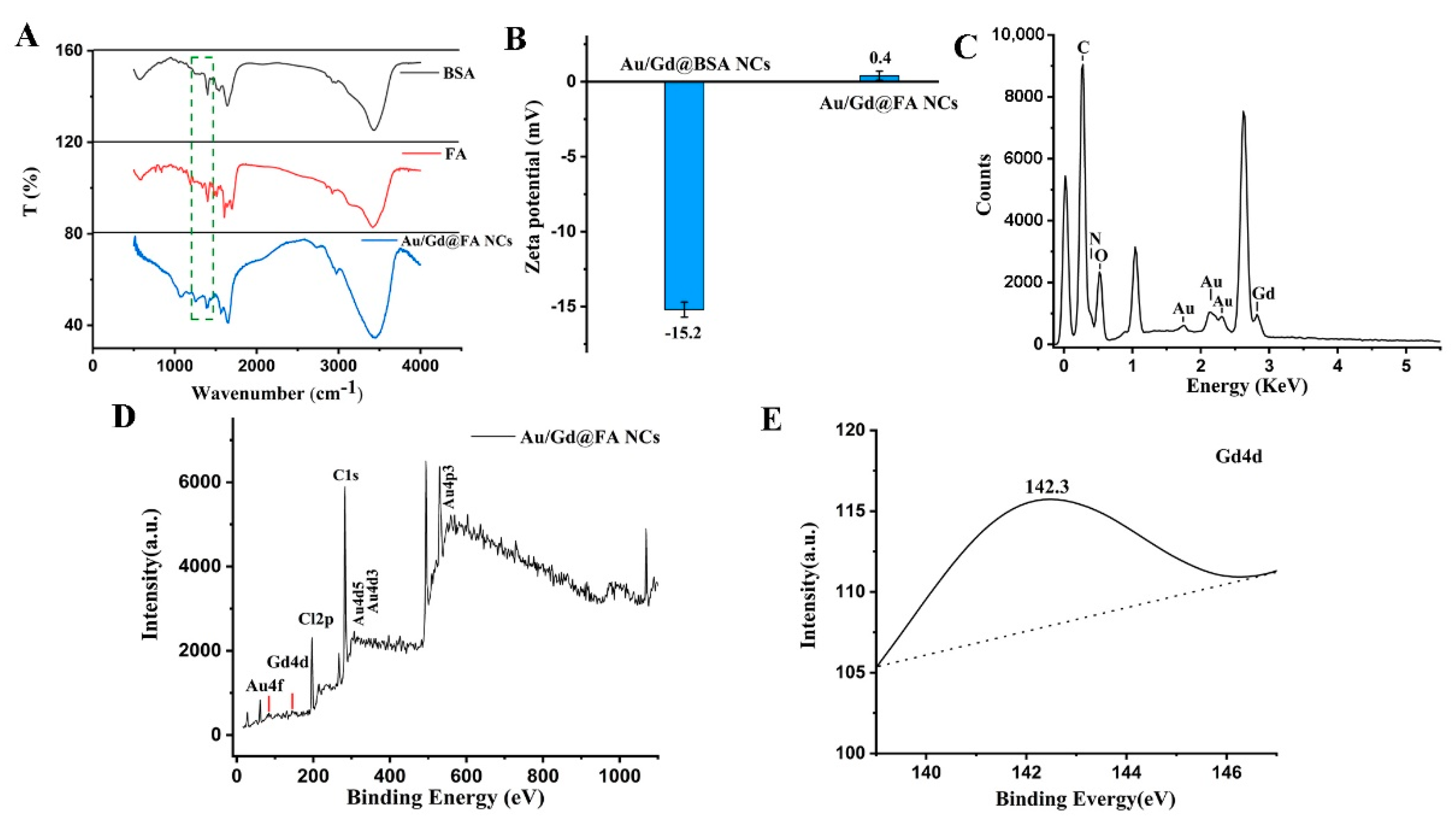
3.1. Synthesis and Characterization of Au/Gd@FA NCs
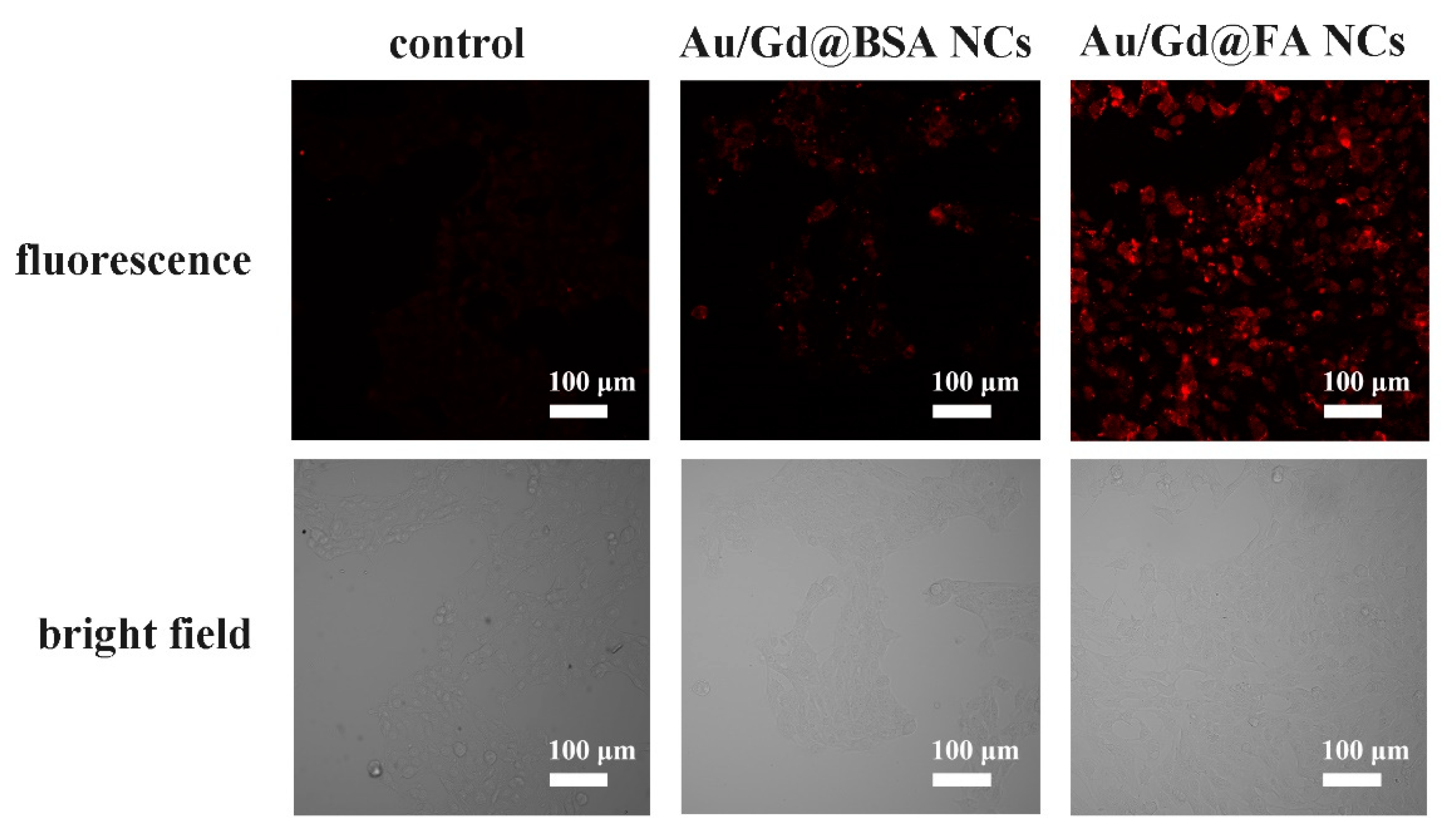
3.2. Cytotoxicity Assay and Cell Uptake
3.3. Intracellular Near-Infrared FL Imaging of Au/Gd@FA NCs
3.4. In Vivo Near-Infrared FL Imaging Metabolism of Au/Gd@FA NCs
3.5. In Vivo T1-Weighted MRI of Au/Gd@FA NCs
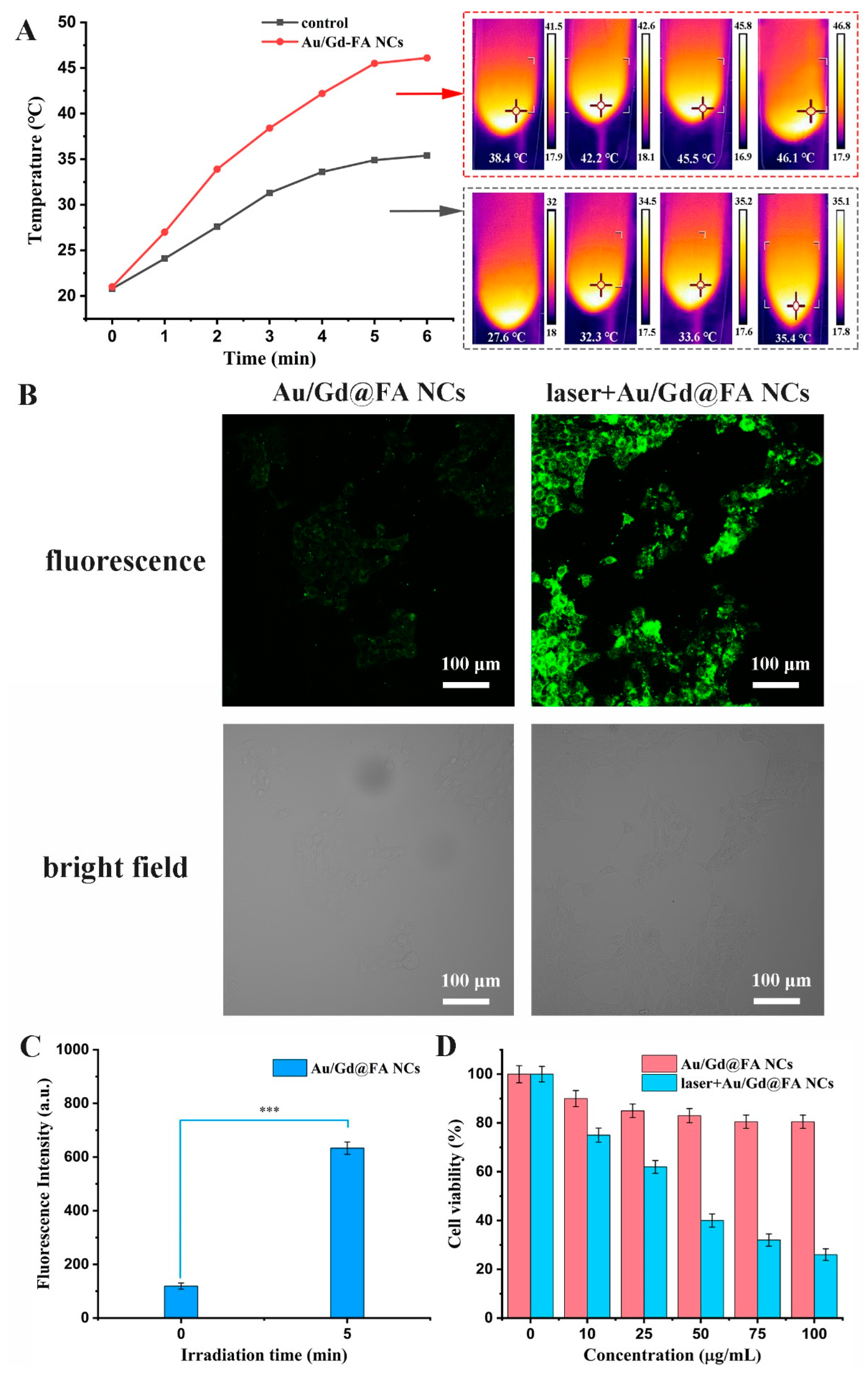
3.6. Intracellular ROS Detection and Analysis by Laser Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, X.D.; Jiang, H.; Wang, X.M. Biodegradable Metal Organic Frameworks for Multimodal Imaging and Targeting Theranostics. Biosensors 2021, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, W.; Ma, Q.; Lu, Y.; Xu, Y.; Bian, K.; Liu, F.; Shi, C.; Wang, H.; Shi, Y.; et al. Hemoglobin-mediated biomimetic synthesis of paramagnetic O2-evolving theranostic nanoprobes for MR imaging-guided enhanced photodynamic therapy of tumor. Theranostics 2020, 10, 11607–11621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, X.; Li, W.; Long, Z.; Ouyang, J.; Na, N. Multi-Dimensionally Extended Functionalization Innovates to an Entropy-Driven Detection of Multi-miRNAs for One-Step Cancer Screening and Diagnosis in Living Cells. Anal. Chem. 2020, 92, 8125–8132. [Google Scholar] [CrossRef]
- Balachandran, Y.L.; Li, X.; Jiang, X. Biodegradable freestanding rare-earth nanosheets promote multimodal imaging and delivers CRISPR-Cas9 plasmid against tumor. Chem. Commun. 2021, 57, 9386–9389. [Google Scholar] [CrossRef]
- Chauhan, V.M.; Elsutohy, M.M.; McClure, C.P.; Irving, W.L.; Roddis, N.; Aylott, J.W. Gold-Oligonucleotide Nanoconstructs Engineered to Detect Conserved Enteroviral Nucleic Acid Sequences. Biosensors 2021, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Omori, N.; Candeo, A.; Mosca, S.; Lezcano-Gonzalez, I.; Robinson, I.K.; Li, L.; Greenaway, A.G.; Collier, P.; Beale, A.M. Multimodal Imaging of Autofluorescent Sites Reveals Varied Chemical Speciation in SSZ-13 Crystals. Angew. Chem. Int. Edit. 2021, 60, 5125–5131. [Google Scholar] [CrossRef]
- Rosenkrans, Z.T.; Ferreira, C.A.; Ni, D.; Cai, W. Internally Responsive Nanomaterials for Activatable Multimodal Imaging of Cancer. Adv. Healthc. Mater. 2021, 10, 2000690. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Guo, C.; Hu, G.; Wang, L. Multiresponsive Nanoprobes for Turn-On Fluorescence/F-19 MRI Dual-Modal Imaging. Anal. Chem. 2020, 92, 11739–11746. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, Z.; Shen, J.; Yan, Z.; Li, W.; Qiu, H. Integrating the second near-infrared fluorescence imaging with clinical techniques for multimodal cancer imaging by neodymiumdoped gadolinium tungstate nanoparticles. Nano Res. 2021, 14, 2160–2170. [Google Scholar] [CrossRef]
- Zhong, H.; Wu, Y.; Yu, S.; Wang, X.; He, K.; Li, D.; Cao, Y.; Gan, N. Two-Photon CQDs-Based Dual-Mode Nanoprobe for Fluorescence Imaging and Magnetic Resonance Imaging of Intracellular Wide pH. Anal. Chem. 2021, 93, 5691–5699. [Google Scholar] [CrossRef]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.K.; Li, K.; Lu, C.Y.; Xie, Y.M.; Liu, Y.H.; Zhou, Q.; Bao, J.K.; Yu, X.Q. Multifunctional gold nanoparticles as smart nanovehicles with enhanced tumour-targeting abilities for intracellular pH mapping and in vivo MR/fluorescence imaging. Nanoscale 2020, 12, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xie, T.; Wang, K.; Jin, S.; Li, K.; Dou, P.; Yu, N.; Xu, K. Development of fluorescence/MR dual-modal manganese-nitrogen-doped carbon nanosheets as an efficient contrast agent for targeted ovarian carcinoma imaging. J. Nanobiotechnol. 2020, 18, 175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, B.; Wang, X.; Meng, Y.; Bai, L.; Zheng, Y. Safe and efficient magnetic resonance imaging of acute myocardial infarction with gadolinium-doped carbon dots. Nanomedicine 2020, 15, 2385–2398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Zhang, Z.; Wang, L.; Wang, D.; Tang, B.Z. Triple-Jump Photodynamic Theranostics: MnO2 Combined Upconversion Nanoplatforms Involving a Type-I Photosensitizer with Aggregation-Induced Emission Characteristics for Potent Cancer Treatment. Adv. Mater. 2021, 33, 2103748. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, B.; Sun, Q.; Feng, L.; He, F.; Yang, P.; Gai, S.; Quan, Z.; Lin, J. Upconverted Metal-Organic Framework Janus Architecture for Near-Infrared and Ultrasound Co-Enhanced High Performance Tumor Therapy. ACS Nano 2021, 15, 12342–12357. [Google Scholar] [CrossRef]
- Zhang, Y.; Garcia-Gabilondo, M.; Rosell, A.; Roig, A. MRI/Photoluminescence Dual-Modal Imaging Magnetic PLGA Nanocapsules for Theranostics. Pharmaceutics 2020, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Guo, M.; Li, J.; Qin, F.; Wang, Y.; Liu, T.; Liu, J.; Sabet, Z.F.; Wang, Y.; Liu, Y.; et al. Hypoxia-Triggered Self-Assembly of Ultrasmall Iron Oxide Nanoparticles to Amplify the Imaging Signal of a Tumor. J. Am. Chem. Soc. 2021, 143, 1846–1853. [Google Scholar] [CrossRef]
- Cai, H.; Dai, X.; Wang, X.; Tan, P.; Gu, L.; Luo, Q.; Zheng, X.; Li, Z.; Zhu, H.; Zhang, H.; et al. A Nanostrategy for Efficient Imaging-Guided Antitumor Therapy through a Stimuli-Responsive Branched Polymeric Prodrug. Adv. Sci. 2020, 7, 1903243. [Google Scholar] [CrossRef]
- Wang, L.; Wan, Q.; Zhang, R.; Situ, B.; Ni, K.; Gao, J.; Feng, X.; Zhang, P.; Wang, Z.; Qin, A.; et al. Synergistic Enhancement of Fluorescence and Magnetic Resonance Signals Assisted by Albumin Aggregate for Dual-Modal Imaging. ACS Nano 2021, 15, 9924–9934. [Google Scholar] [CrossRef]
- Li, D.; Pan, J.; Xu, S.; Fu, S.; Chu, C.; Liu, G. Activatable Second Near-Infrared Fluorescent Probes: A New Accurate Diagnosis Strategy for Diseases. Biosensors 2021, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Ohulchanskyy, T.Y.; Chen, G. Lanthanide-Doped Near-Infrared Nanoparticles for Biophotonics. Adv. Mater. 2021, 33, 2000678. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hattiholi, A.; Selvan, S.T.; Yan, S.X.; Fang, W.; Chandrasekharan, P.; Koteswaraiah, P.; Herold, C.J.; Gulyas, B.; Aw, S.E.; et al. Gadolinium-based bimodal probes to enhance T1-Weighted magnetic resonance/optical imaging. Acta Biomater. 2020, 110, 15–36. [Google Scholar] [CrossRef]
- Wang, X.; Chan, H.N.; Desbois, N.; Gros, C.P.; Bolze, F.; Li, Y.; Li, H.W.; Wong, M.S. Multimodal Theranostic Cyanine-Conjugated Gadolinium (III) Complex for In Vivo Imaging of Amyloid-beta in an Alzheimer’s Disease Mouse Model. ACS Appl. Mater. Interfaces 2021, 13, 18525–18532. [Google Scholar] [CrossRef] [PubMed]
- Koudrina, A.; McConnell, E.M.; Zurakowski, J.A.; Cron, G.O.; Chen, S.Z.; Tsai, E.C.; DeRosa, M.C. Exploring the Unique Contrast Properties of Aptamer-Gadolinium Conjugates in Magnetic Resonance Imaging for Targeted Imaging of Thrombi. ACS Appl. Mater. Interfaces 2021, 13, 9412–9424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, Z.; Di, H.; Zeng, E.; Jiang, Y.; Liu, D. Gadolinium-doped Au@prussian blue nanoparticles as MR/SERS bimodal agents for dendritic cell activating and tracking. Theranostics 2020, 10, 6061–6071. [Google Scholar] [CrossRef]
- Koyama, T.; Shimura, M.; Minemoto, Y.; Nohara, S.; Shibata, S.; Iida, Y.; Iwashita, S.; Hasegawa, M.; Kurabayashi, T.; Hamada, H.; et al. Evaluation of selective tumor detection by clinical magnetic resonance imaging using antibody-conjugated superparamagnetic iron oxide. J. Control. Release 2012, 159, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Richard, S.; Boucher, M.; Herbet, A.; Lalatonne, Y.; Meriaux, S.; Boquet, D.; Motte, L. Endothelin B receptors targeted by iron oxide nanoparticles functionalized with a specific antibody: Toward immunoimaging of brain tumors. J. Mater. Chem. B 2015, 3, 2939–2942. [Google Scholar] [CrossRef]
- Kim, Y.; Yang, J.; Hur, H.; Oh, S.; Lee, H.H. Highly Sensitive Colorimetric Assay of Cortisol Using Cortisol Antibody and Aptamer Sandwich Assay. Biosensors 2021, 11, 163. [Google Scholar] [CrossRef]
- Li, L.; Xing, H.; Zhang, J.; Lu, Y. Functional DNA Molecules Enable Selective and Stimuli-Responsive Nanoparticles for Biomedical Applications. Acc. Chem. Res. 2019, 52, 2415–2426. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Yue, X.; Dai, Z. Cyanine Conjugate-Based Biomedical Imaging Probes. Adv. Healthc. Mater. 2020, 9, 2001327. [Google Scholar] [CrossRef] [PubMed]
- Miron-Merida, V.A.; Gonzalez-Espinosa, Y.; Collado-Gonzalez, M.; Gong, Y.Y.; Guo, Y.; Goycoolea, F.M. Aptamer-Target-Gold Nanoparticle Conjugates for the Quantification of Fumonisin B1. Biosensors 2021, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, Z.; Liu, B.; Jia, T.; Wang, C.; Yang, D.; He, F.; Gai, S.; Yang, P. Self-generation of oxygen and simultaneously enhancing photodynamic therapy and MRI effect: An intelligent nanoplatform to conquer tumor hypoxia for enhanced phototherapy. Chem. Eng. J. 2020, 390, 124624. [Google Scholar] [CrossRef]
- Yang, B.; Dai, Z.; Zhang, G.; Hu, Z.; Yao, X.; Wang, S.; Liu, Q.; Zheng, X. Ultrasmall Ternary FePtMn Nanocrystals with Acidity-Triggered Dual-Ions Release and Hypoxia Relief for Multimodal Synergistic Chemodynamic/Photodynamic/Photothermal Cancer Therapy. Adv. Healthc. Mater. 2020, 9, 1901634. [Google Scholar] [CrossRef]
- Sun, S.; Dong, L.; Cao, Y.; Sun, H.; Yan, X. Fabrication of Multifunctional Gd2O3/Au Hybrid Nanoprobe via a One-Step Approach for Near-Infrared Fluorescence and Magnetic Resonance Multimodal Imaging in Vivo. Anal. Chem. 2013, 85, 8436–8441. [Google Scholar] [CrossRef]
- Hada, A.; Craciun, A.; Focsan, M.; Borlan, R.; Soritau, O.; Todea, M.; Astilean, S. Folic acid functionalized gold nanoclusters for enabling targeted fluorescence imaging of human ovarian cancer cells. Talanta 2021, 225, 121960. [Google Scholar] [CrossRef]
- Huang, X.; Fan, C.; Zhu, H.; Le, W.; Cui, S.; Chen, X.; Li, W.; Zhang, F.; Huang, Y.; Shi, D.; et al. Glypican-I-antibody-conjugated Gd-Au nanoclusters for FI/MRI dual-modal targeted detection of pancreatic cancer. Int. J. Nanomed. 2018, 13, 2585–2599. [Google Scholar] [CrossRef] [Green Version]
- Hoshyar, N.; Gray, S.; Han, H.B.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; He, X.; Zhang, Z.; Zhao, Y.; Feng, W. Metabolism of Nanomaterials in Vivo: Blood Circulation and Organ Clearance. Acc. Chem. Res. 2013, 46, 761–769. [Google Scholar] [CrossRef]
- Hu, H.; Yang, Q.; Baroni, S.; Yang, H.; Aime, S.; Steinmetz, N.F. Polydopamine-decorated tobacco mosaic virus for photoacoustic/magnetic resonance bimodal imaging and photothermal cancer therapy. Nanoscale 2019, 11, 9760–9768. [Google Scholar] [CrossRef]
- Rad, J.K.; Mahdavian, A.R.; Khoei, S.; Shirvalilou, S. Enhanced Photogeneration of Reactive Oxygen Species and Targeted Photothermal Therapy of C6 Glioma Brain Cancer Cells by Folate-Conjugated Gold-Photoactive Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 19483–19493. [Google Scholar]
- Chen, G.; Yang, Y.; Xu, Q.; Ling, M.; Lin, H.; Ma, W.; Sun, R.; Xu, Y.; Liu, X.; Li, N.; et al. Self-Amplification of Tumor Oxidative Stress with Degradable Metallic Complexes for Synergistic Cascade Tumor Therapy. Nano Lett. 2020, 20, 8141–8150. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Cai, Z.; Zhao, X.; Li, L.; Zhao, M. Evaluation of the Biological Activity of Folic Acid-Modified Paclitaxel-Loaded Gold Nanoparticles. Int. J. Nanomed. 2021, 16, 7023–7033. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Ye, J.; Wang, Y.; Xiong, H.; Jiang, H.; Lu, H.; Liu, X.; Wang, X. Ultra-Small and Metabolizable Near-Infrared Au/Gd Nanoclusters for Targeted FL/MRI Imaging and Cancer Theranostics. Biosensors 2022, 12, 558. https://doi.org/10.3390/bios12080558
Dong X, Ye J, Wang Y, Xiong H, Jiang H, Lu H, Liu X, Wang X. Ultra-Small and Metabolizable Near-Infrared Au/Gd Nanoclusters for Targeted FL/MRI Imaging and Cancer Theranostics. Biosensors. 2022; 12(8):558. https://doi.org/10.3390/bios12080558
Chicago/Turabian StyleDong, Xiawei, Jing Ye, Yihan Wang, Hongjie Xiong, Hui Jiang, Hongbing Lu, Xiaohui Liu, and Xuemei Wang. 2022. "Ultra-Small and Metabolizable Near-Infrared Au/Gd Nanoclusters for Targeted FL/MRI Imaging and Cancer Theranostics" Biosensors 12, no. 8: 558. https://doi.org/10.3390/bios12080558
APA StyleDong, X., Ye, J., Wang, Y., Xiong, H., Jiang, H., Lu, H., Liu, X., & Wang, X. (2022). Ultra-Small and Metabolizable Near-Infrared Au/Gd Nanoclusters for Targeted FL/MRI Imaging and Cancer Theranostics. Biosensors, 12(8), 558. https://doi.org/10.3390/bios12080558






