An Immunocolorimetric Sensing System for Highly Sensitive and High-Throughput Detection of BNP with Carbon-Gold Nanocomposites Amplification
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Apparatus
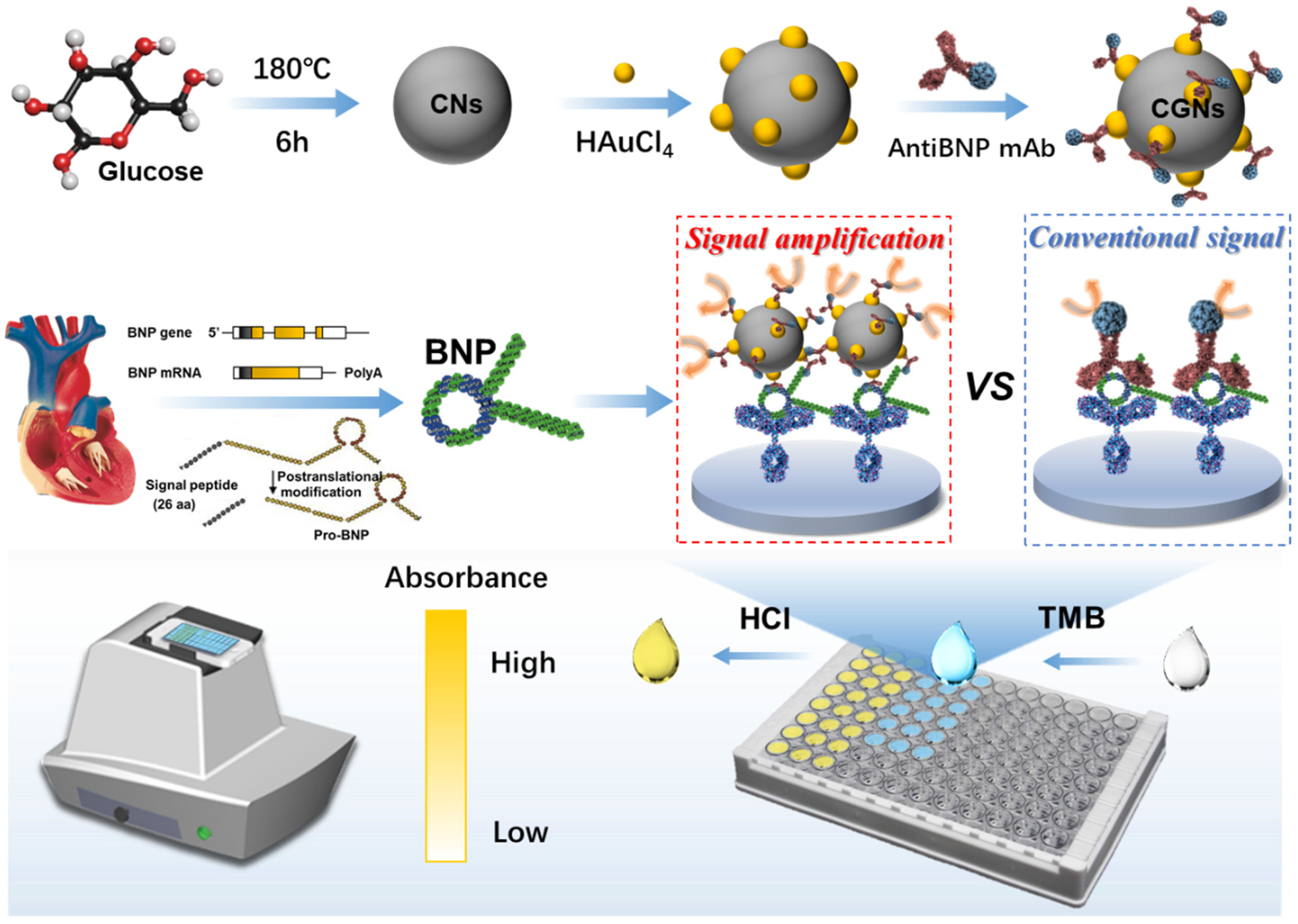
2.3. Synthesis of Carbon Nanospheres
2.4. Synthesis of Carbon-Gold Nanocomposites
2.5. Synthesis of CGNs@AntiBNP-HRP Immunoprobe
2.6. Fabrication of GCN-Based ELISA
2.7. Serum Sample Preparation
3. Results and Discussion
3.1. Optimization of CNs Synthesis
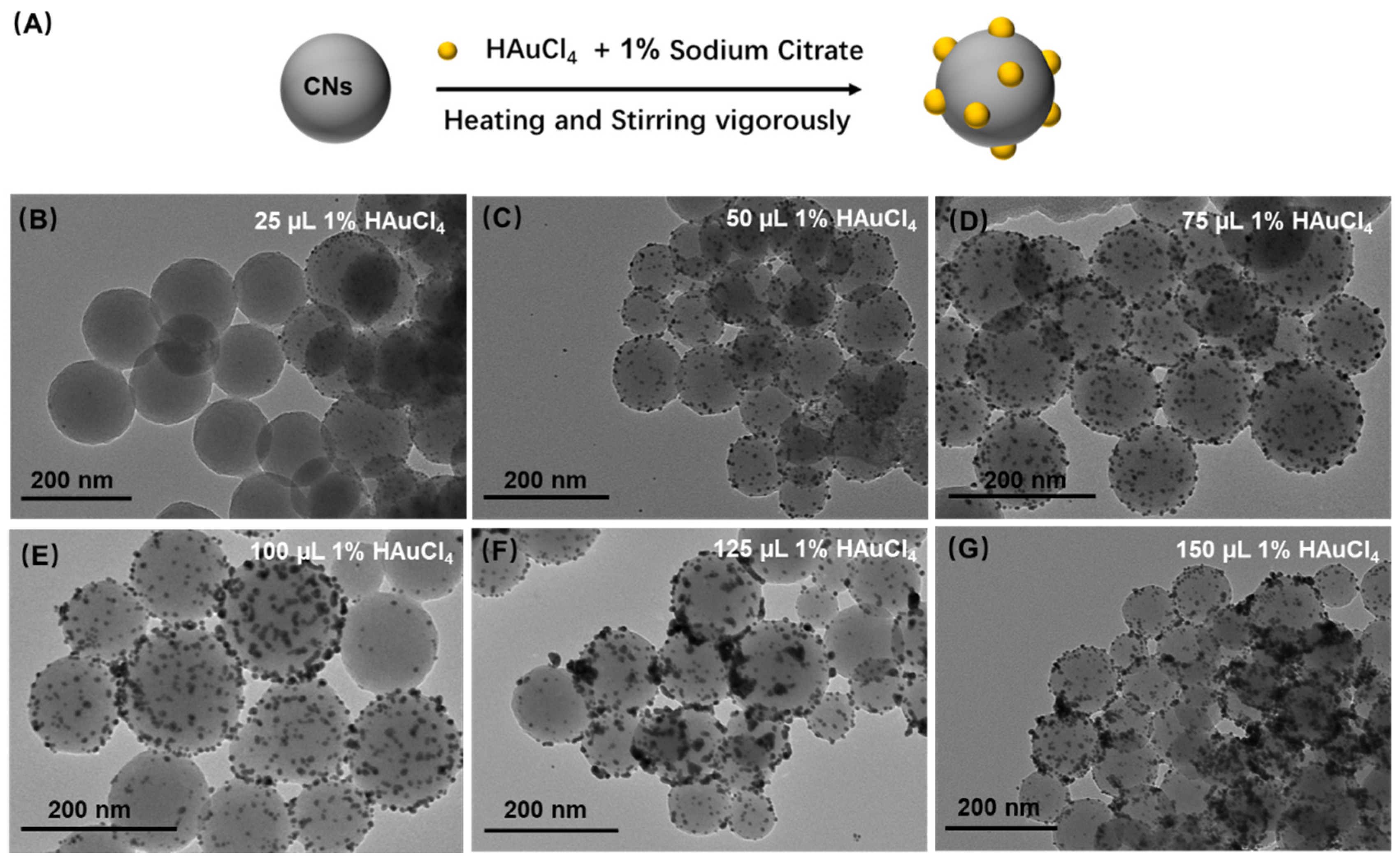
3.2. Growth of AuNPs on CNs (CGNs)
3.3. Characterization of CGNs and CGNs@AntiBNP-HRP Immunoprobe
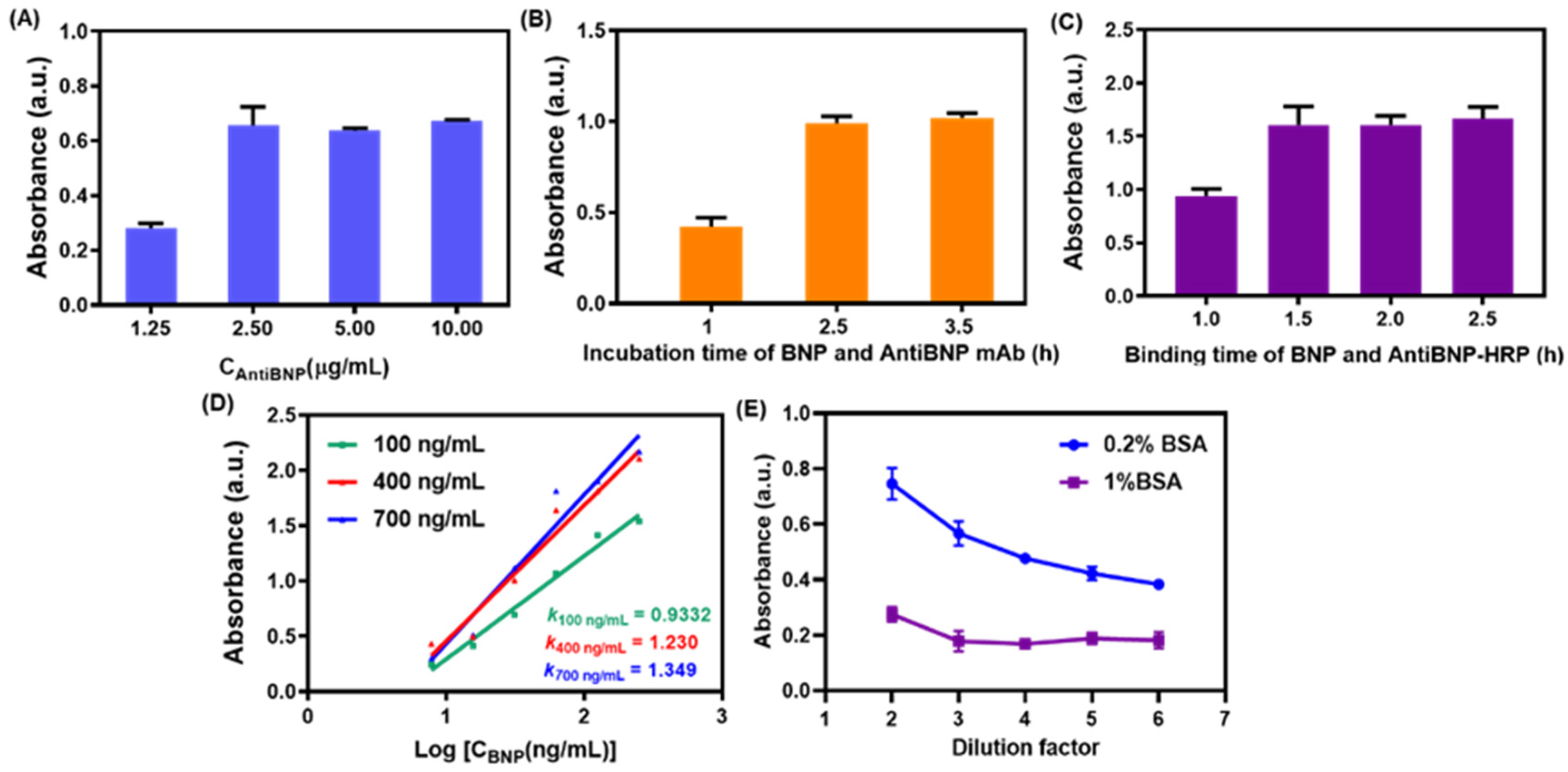
3.4. Optimization of Conditions for the Immunocolorimetric Probe
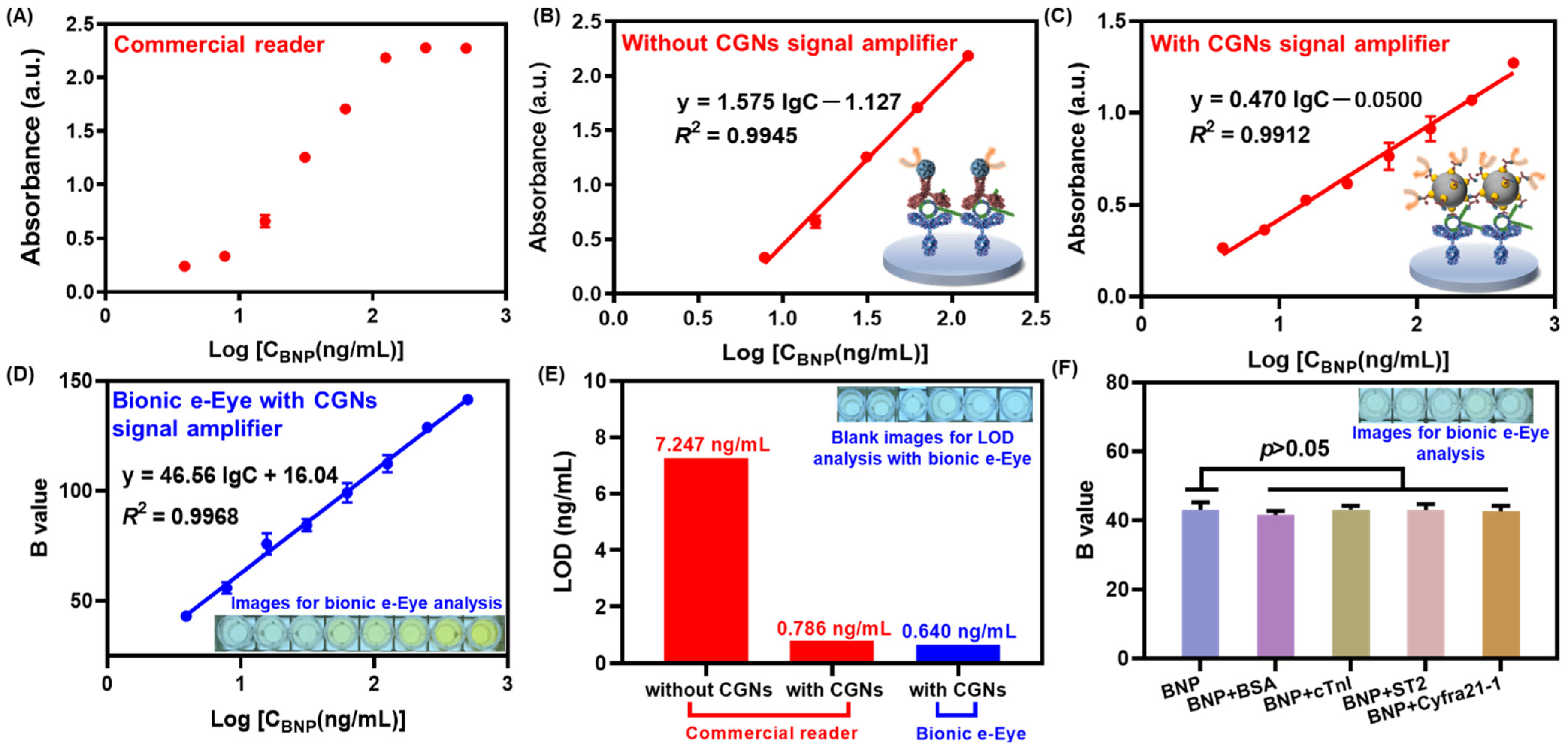
3.5. Validation of the Immunoprobe Signal Amplification
3.6. Analytical Performance of the Immunocolorimetric Sensor Based on Bionic e-Eye
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Early stage screening of breast cancer using electrochemical biomarker detection. TrAC Trends Anal. Chem. 2017, 91, 67–76. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; Leng, Y.; Lai, W.; Huang, X.; Xiong, Y. Emerging design strategies for constructing multiplex lateral flow test strip sensors. Biosens. Bioelectron. 2020, 157, 112168. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Gao, T.; Yang, X.; Dai, X.; Zhou, W.; Zhang, A.; Lieber, C.M. Specific detection of biomolecules in physiological solutions using graphene transistor biosensors. Proc. Natl. Acad. Sci. USA 2016, 113, 14633–14638. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing based on field-effect transistors (FET): Recent progress and challenges. Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef]
- Xue, T.-Y.; Mei, L.-P.; Xu, Y.-T.; Liu, Y.-L.; Fan, G.-C.; Li, H.-Y.; Ye, D.; Zhao, W.-W. Nanoporous semiconductor electrode captures the quantum dots: Toward ultrasensitive signal-on liposomal photoelectrochemical immunoassay. Anal. Chem. 2019, 91, 3795–3799. [Google Scholar] [CrossRef]
- Dong, W.; Mo, X.; Wang, Y.; Lei, Q.; Li, H. Photoelectrochemical immunosensor based on ZnIn2S4/Bi2Se3 nanocomposite for the determination of cardiac troponin I. Anal. Lett. 2020, 53, 1888–1901. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Eom, G.; Hwang, A.; Kim, H.; Moon, J.; Kang, H.; Jung, J.; Lim, E.-K.; Jeong, J.; Park, H.G.; Kang, T. Ultrasensitive Detection of Ovarian Cancer Biomarker Using Au Nanoplate SERS Immunoassay. BioChip J. 2021, 15, 348–355. [Google Scholar] [CrossRef]
- Shi, C.; Xie, H.; Ma, Y.; Yang, Z.; Zhang, J. Nanoscale Technologies in Highly Sensitive Diagnosis of Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2020, 8, 531. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, L.; Du, W.; Li, H.; Yang, D.; Zhu, C. Au@Pt nanodendrites enhanced multimodal enzyme-linked immunosorbent assay. Nanoscale 2019, 11, 8798–8802. [Google Scholar] [CrossRef]
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of nano-ELISA in food analysis: Recent advances and challenges. TrAC Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Montagut, E.J.; Vilaplana, L.; Martin-Gomez, M.T.; Marco, M.P. High-Throughput Immunochemical Method to Assess the 2-Heptyl-4-quinolone Quorum Sensing Molecule as a Potential Biomarker of Pseudomonas aeruginosa Infections. ACS Infect. Dis. 2020, 6, 3237–3246. [Google Scholar] [CrossRef]
- Jaria, G.; Calisto, V.; Otero, M.; Esteves, V.I. Monitoring pharmaceuticals in the aquatic environment using enzyme-linked immunosorbent assay (ELISA)—A practical overview. Anal. Bioanal. Chem. 2020, 412, 3983–4008. [Google Scholar] [CrossRef]
- Kreimer, S.; Gao, Y.; Ray, S.; Jin, M.; Tan, Z.; Mussa, N.A.; Tao, L.; Li, Z.; Ivanov, A.R.; Karger, B.L. Host cell protein profiling by targeted and untargeted analysis of data independent acquisition mass spectrometry data with parallel reaction monitoring verification. Anal. Chem. 2017, 89, 5294–5302. [Google Scholar] [CrossRef]
- Ye, H.; Yang, K.; Tao, J.; Liu, Y.; Zhang, Q.; Habibi, S.; Nie, Z.; Xia, X. An enzyme-free signal amplification technique for ultrasensitive colorimetric assay of disease biomarkers. ACS Nano 2017, 11, 2052–2059. [Google Scholar] [CrossRef]
- De La Rica, R.; Stevens, M.M. Plasmonic ELISA for the detection of analytes at ultralow concentrations with the naked eye. Nat. Protoc. 2013, 8, 1759–1764. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Romero, R.; Romero, A.; Valverde, J.L. Carbon nanospheres: Synthesis, physicochemical properties and applications. J. Mater. Chem. 2011, 21, 1664–1672. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. 2004, 116, 607–611. [Google Scholar] [CrossRef]
- Xu, T.; Liu, N.; Yuan, J.; Ma, Z. Triple tumor markers assay based on carbon–gold nanocomposite. Biosens. Bioelectron. 2015, 70, 161–166. [Google Scholar] [CrossRef]
- Cui, R.; Liu, C.; Shen, J.; Gao, D.; Zhu, J.J.; Chen, H.Y. Gold nanoparticle–colloidal carbon nanosphere hybrid material: Preparation, characterization, and application for an amplified electrochemical immunoassay. Adv. Funct. Mater. 2008, 18, 2197–2204. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Wan, H.; Gan, Y.; Sun, J.; Liang, T.; Zhou, S.; Wang, P. High sensitive reduced graphene oxide-based room temperature ionic liquid electrochemical gas sensor with carbon-gold nanocomposites amplification. Sens. Actuators B Chem. 2019, 299, 126952. [Google Scholar] [CrossRef]
- Shrivastava, A.; Haase, T.; Zeller, T.; Schulte, C. Biomarkers for Heart Failure Prognosis: Proteins, Genetic Scores and Non-coding RNAs. Front. Cardiovasc. Med. 2020, 7, 601364. [Google Scholar] [CrossRef]
- Szunerits, S.; Mishyn, V.; Grabowska, I.; Boukherroub, R. Electrochemical cardiovascular platforms: Current state of the art and beyond. Biosens. Bioelectron. 2019, 131, 287–298. [Google Scholar] [CrossRef]
- Savonnet, M.; Rolland, T.; Cubizolles, M.; Roupioz, Y.; Buhot, A. Recent advances in cardiac biomarkers detection: From commercial devices to emerging technologies. J. Pharm. Biomed. Anal. 2021, 194, 113777. [Google Scholar] [CrossRef]
- Ding, Y.D.; Lei, J.Y.; Chen, Y.; Jin, J. A sandwich ELISA for assessment of pharmacokinetics of HSA-(BNP)2 fusion protein in mouse plasma. J. Pharm. Biomed. Anal. 2010, 51, 658–663. [Google Scholar] [CrossRef]
- Su, K.; Zou, Q.; Zhou, J.; Zou, L.; Li, H.; Wang, T.; Hu, N.; Wang, P. High-sensitive and high-efficient biochemical analysis method using a bionic electronic eye in combination with a smartphone-based colorimetric reader system. Sens. Actuators B Chem. 2015, 216, 134–140. [Google Scholar] [CrossRef]
- Gan, Y.; Hu, N.; He, C.; Zhou, S.; Tu, J.; Liang, T.; Pan, Y.; Kirsanov, D.; Legin, A.; Wan, H.; et al. MnO2 nanosheets as the biomimetic oxidase for rapid and sensitive oxalate detection combining with bionic E-eye. Biosens. Bioelectron. 2019, 130, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, T.; Jia, X.; Meng, L.; Mao, X. Gold nanoparticles decorated carbon nanotube probe based immunochromatographic assay on cotton thread. Sens. Actuators B Chem. 2017, 251, 1112–1118. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Baeck, S.-H.; Cuenya, B.R.; McFarland, E.W. Catalytic activity of supported Au nanoparticles deposited from block copolymer micelles. J. Am. Chem. Soc. 2003, 125, 7148–7149. [Google Scholar] [CrossRef] [PubMed]
- Desimoni, E.; Brunetti, B. About Estimating the Limit of Detection by the Signal to Noise Approach. Pharm. Anal. Acta 2015, 6, 2153–2435. [Google Scholar]
- Long, G.L.; Winefordner, J.D. Limit of detection: A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar]



| Mass | AntiBNP-HRP (µg) | CGNs (mg) |
|---|---|---|
| mInput | 5.6 a | 1.1 |
| mSupernatant | 4.136 b | 0 |
| mOutput | 1.464 | 1.1 |
| mAntiBNP-HRP/mCGNs | 1.331 µg/mg | |
| Sample | Spike Level (ng/mL) | Found b | Recovery (%) b | CV (%) c |
|---|---|---|---|---|
| Healthy human serum a | 200 | 183.91 ± 3.64 | 91.96 ± 1.82 | 1.98 |
| 50 | 49.97 ± 7.29 | 99.94 ± 14.58 | 14.59 | |
| 25 | 24.55 ± 3.15 | 98.21 ± 12.61 | 12.84 | |
| 12.5 | 14.01 ± 1.36 | 112.07 ± 10.87 | 9.70 | |
| 6.25 | 6.86 ± 0.63 | 109.81 ± 10.02 | 9.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Gan, Y.; Li, F.; Qiu, Y.; Pan, Y.; Wan, H.; Wang, P. An Immunocolorimetric Sensing System for Highly Sensitive and High-Throughput Detection of BNP with Carbon-Gold Nanocomposites Amplification. Biosensors 2022, 12, 619. https://doi.org/10.3390/bios12080619
Liu X, Gan Y, Li F, Qiu Y, Pan Y, Wan H, Wang P. An Immunocolorimetric Sensing System for Highly Sensitive and High-Throughput Detection of BNP with Carbon-Gold Nanocomposites Amplification. Biosensors. 2022; 12(8):619. https://doi.org/10.3390/bios12080619
Chicago/Turabian StyleLiu, Xin, Ying Gan, Fengheng Li, Yong Qiu, Yuxiang Pan, Hao Wan, and Ping Wang. 2022. "An Immunocolorimetric Sensing System for Highly Sensitive and High-Throughput Detection of BNP with Carbon-Gold Nanocomposites Amplification" Biosensors 12, no. 8: 619. https://doi.org/10.3390/bios12080619
APA StyleLiu, X., Gan, Y., Li, F., Qiu, Y., Pan, Y., Wan, H., & Wang, P. (2022). An Immunocolorimetric Sensing System for Highly Sensitive and High-Throughput Detection of BNP with Carbon-Gold Nanocomposites Amplification. Biosensors, 12(8), 619. https://doi.org/10.3390/bios12080619






