Selective Amplification of Plasmonic Sensor Signal for Cortisol Detection Using Gold Nanoparticles
Abstract
:1. Introduction
2. Experimental Studies
2.1. Chemical Materials and Optical System
2.2. Synthesis and Characterization of Functional Monomer and Gold Nanoparticles
2.3. Modification and Preparation of Plasmonic Sensors
2.4. Characterization of Plasmonic Sensors
2.5. Kinetic Analyses with Plasmonic Sensors
2.6. Selectivity and Reusability Analyses
2.7. Complex Environment Analyses
2.8. Validation Analyses
3. Results and Discussion
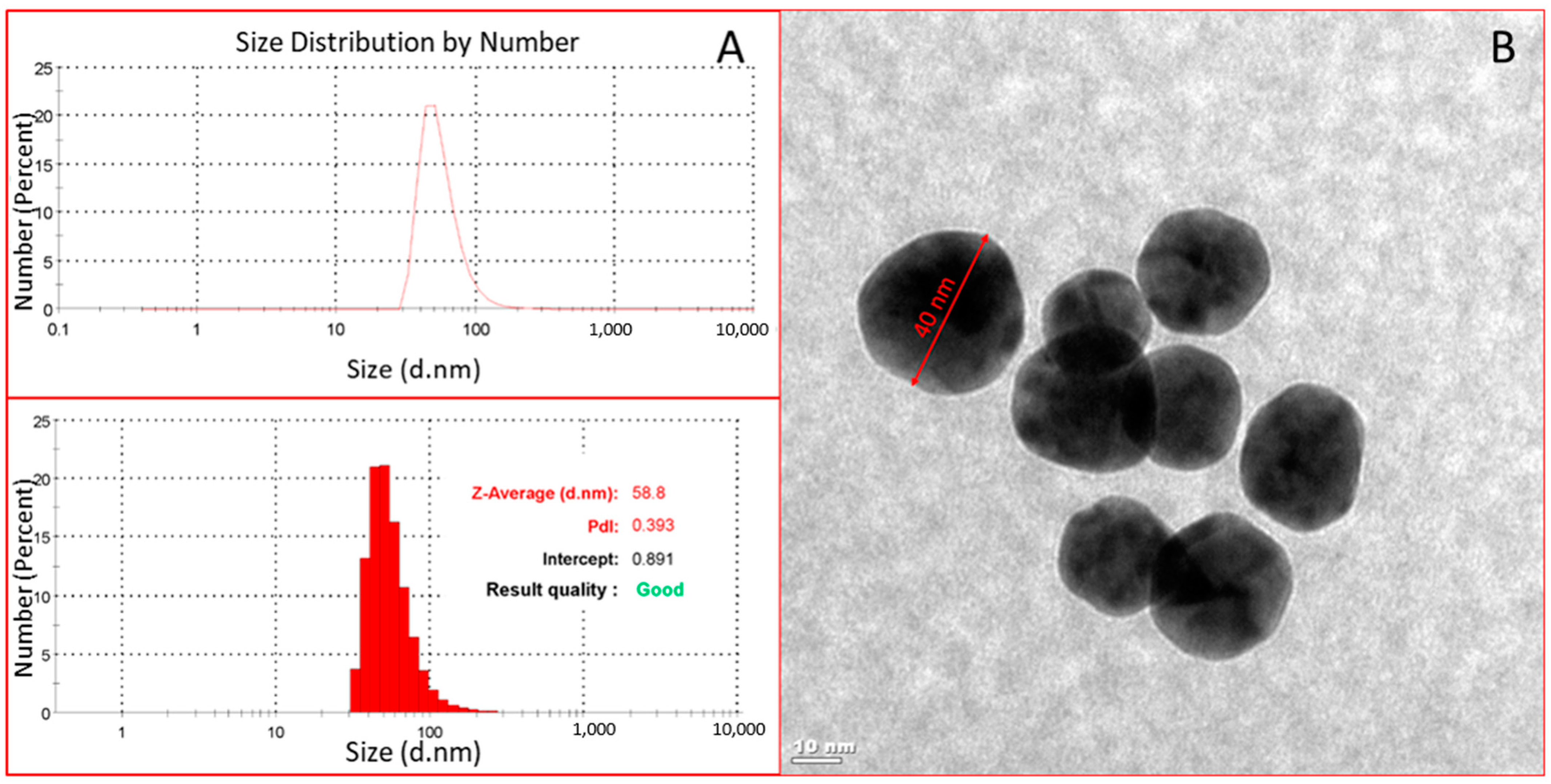
3.1. Characterization of Functional Monomer and Gold Nanoparticles
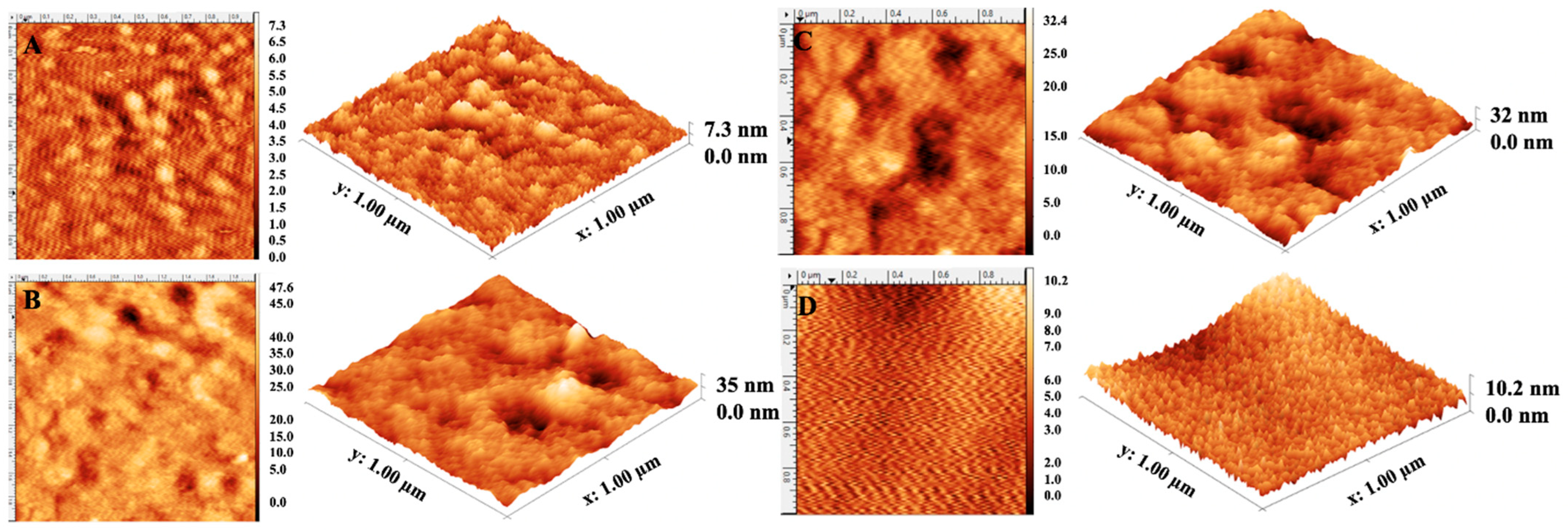
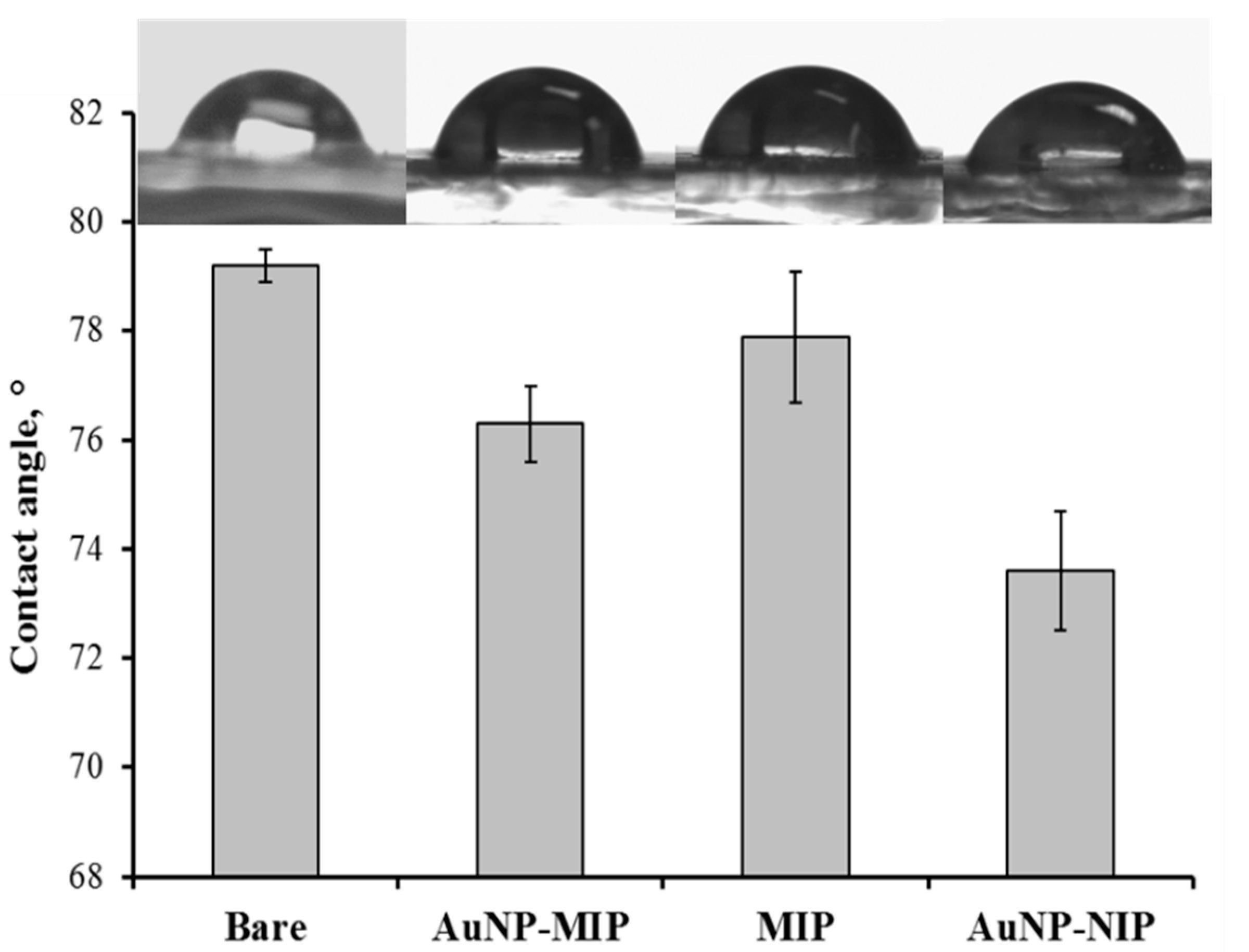
3.2. Characterization of Plasmonic Sensor Surfaces
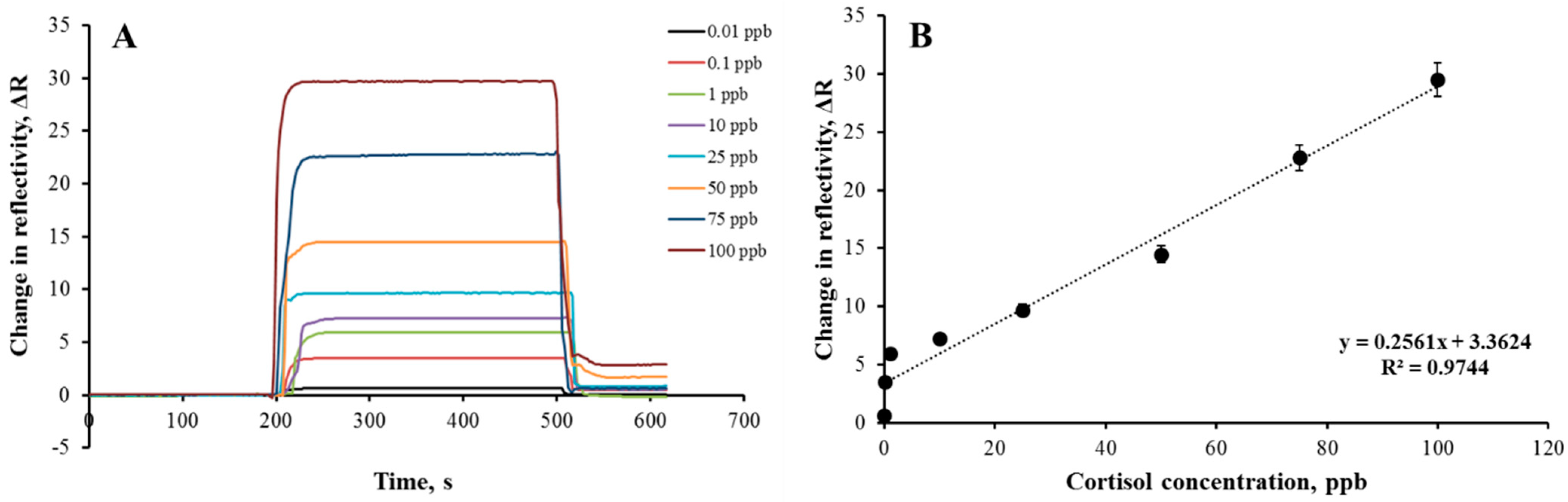
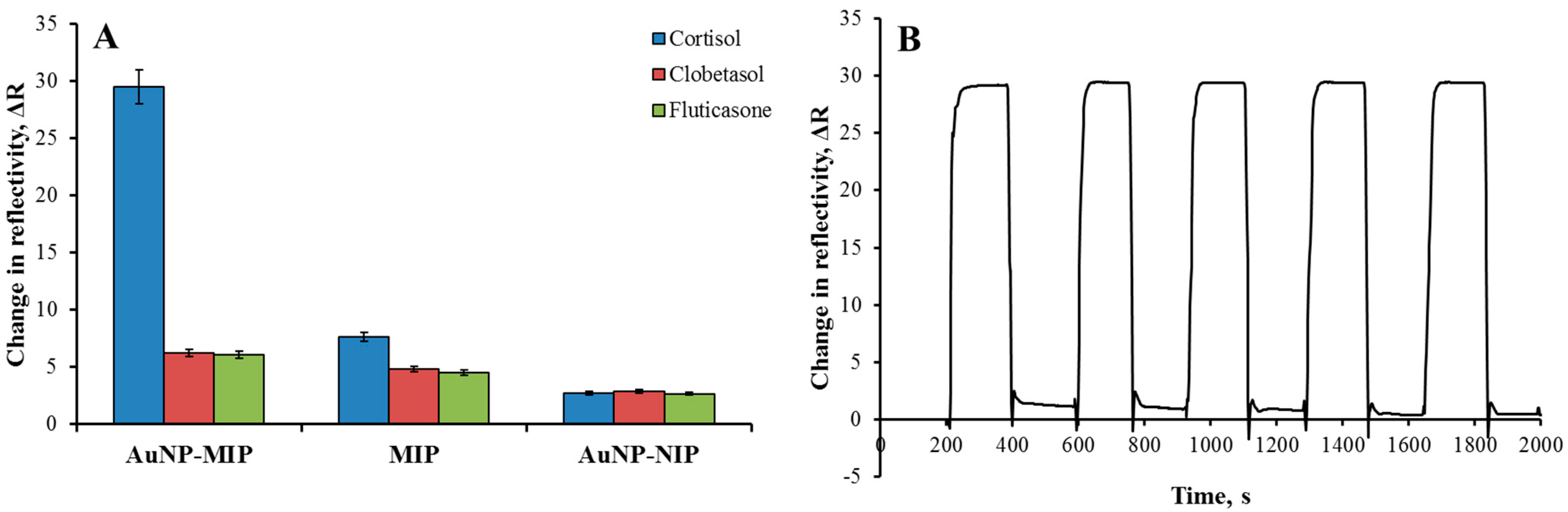
3.3. Kinetic Analyses with Plasmonic Sensors
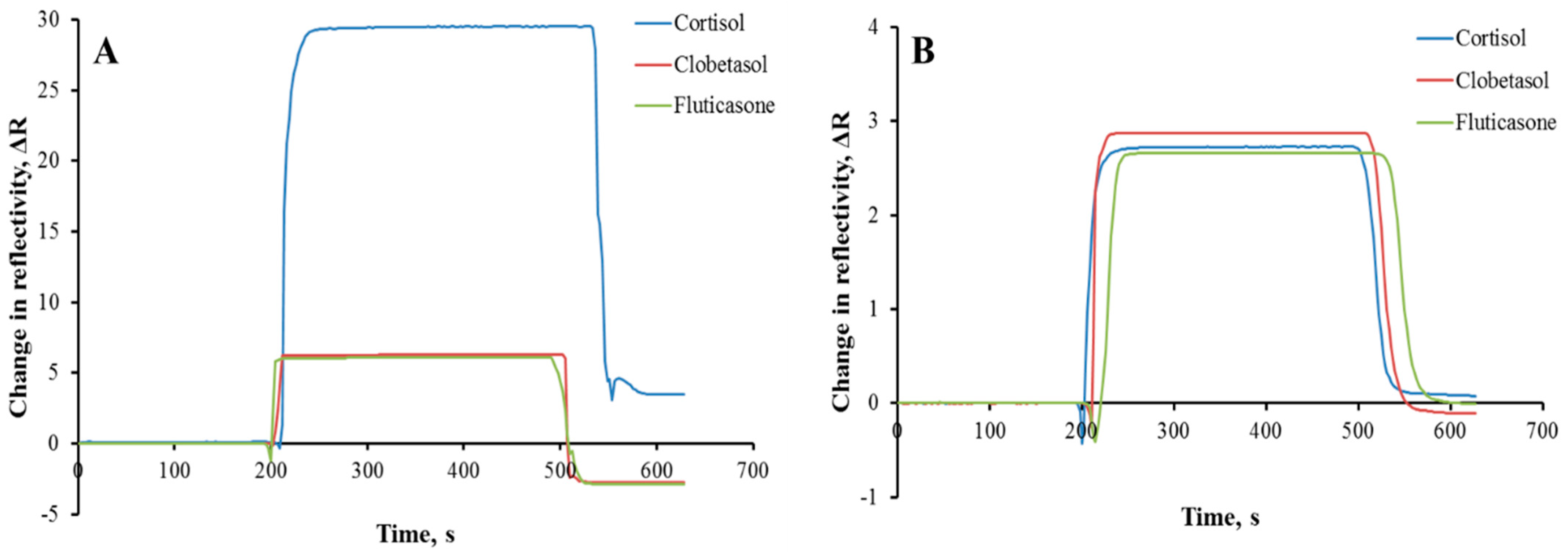
3.4. Selectivity and Reusability Analyses
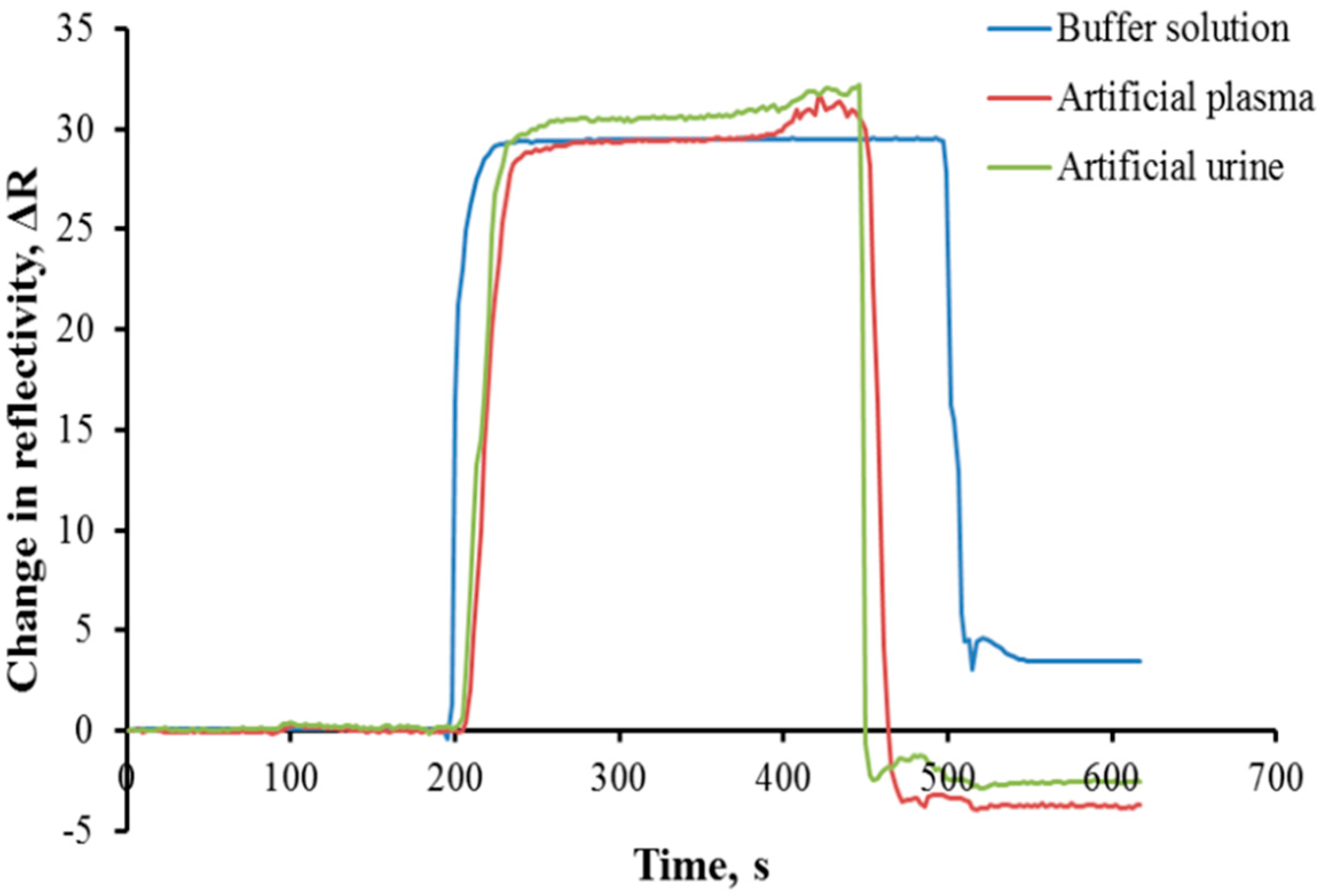
3.5. Complex Environment Analyses
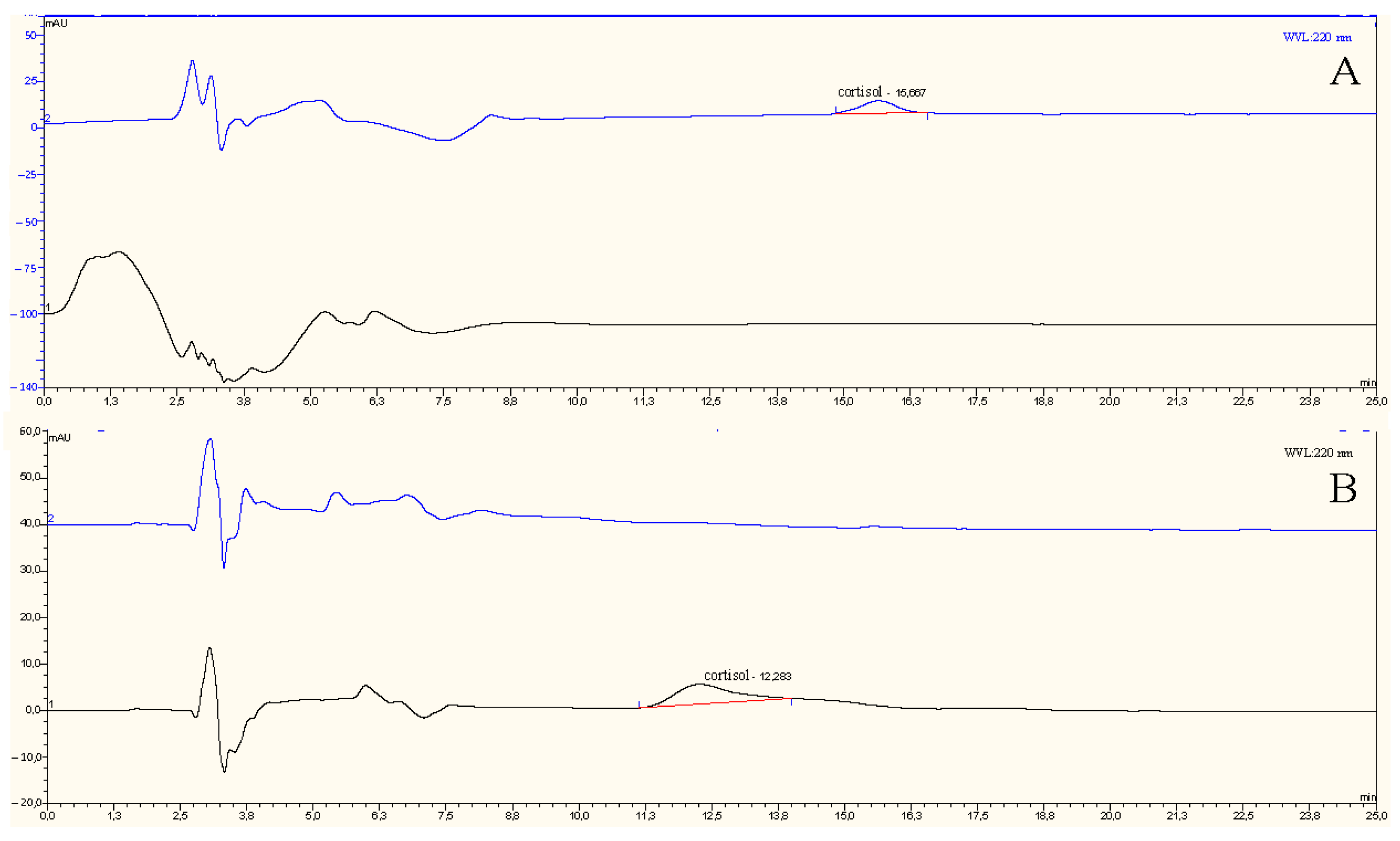
3.6. Validation Analyses
4. Conclusions and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Levine, A.; Zagoory-Sharon, O.; Feldman, R.; Lewis, J.G.; Weller, A. Measuring cortisol in human psychobiological studies. Physiol. Behav. 2007, 90, 43–53. [Google Scholar]
- Dhull, N.; Kaur, G.; Gupta, V.; Tomar, M. Highly sensitive and non-invasive electrochemical immunosensor for salivary cortisol detection. Sens. Actuators B Chem. 2019, 293, 281–288. [Google Scholar]
- Yamaguchi, N.; Matsuda, Y.; Sasaki, S.; Sasaki, M.; Kadoma, Y.; Imai, Y.; Niwa, D.; Shetty, V. Immunosensor with fluid control mechanism for salivary cortisol analysis. Biosens. Bioelectron. 2013, 41, 186–191. [Google Scholar]
- Boonen, E.; Vervenne, H.; Meersseman, P.; Andrew, R.; Mortier, L.; Declercq, P.E.; Vanwijngaerden, Y.M.; Spriet, I.; Wouters, P.J.; Vander Perre, S.; et al. Reduced cortisol metabolism during critical illness. NEJM 2013, 368, 1477–1488. [Google Scholar]
- Tan, T.; Khoo, B.; Mills, E.G.; Phylactou, M.; Patel, B.; Eng, P.C.; Thurston, L.; Muzi, B.; Meeran, K.; Prevost, A.T.; et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 659–660. [Google Scholar]
- Amiri-Dashatan, N.; Koushki, M.; Parsamanesh, N.; Chiti, H. Serum cortisol concentration and COVID-19 severity: A systematic review and meta-analysis. J. Investig. Med. 2022, 70, 766–772. [Google Scholar]
- Hansen, Å.M.; Garde, A.H.; Christensen, J.M.; Eller, N.H.; Netterstrøm, B. Evaluation of a radioimmunoassay and establishment of a reference interval for salivary cortisol in healthy subjects in Denmark. Scand. J. Clin. Lab. Investig. 2003, 63, 303–310. [Google Scholar]
- Di Nardo, F.; Cavalera, S.; Baggiani, C.; Giovannoli, C.; Anfossi, L. Direct vs. mediated coupling of antibodies to gold nanoparticles: The case of salivary cortisol detection by lateral flow immunoassay. ACS Appl. Mater. Interfaces 2019, 11, 32758–32768. [Google Scholar]
- Albar, W.F.; Russell, E.W.; Koren, G.; Rieder, M.J.; Van Umm, S.H. Human hair cortisol analysis: Comparison of the internationally-reported ELISA methods. Clin. Investig. Med. 2013, 36, E312–E316. [Google Scholar]
- Jo, S.; Lee, W.; Park, J.; Kim, W.; Kim, W.; Lee, G.; Lee, H.J.; Hong, J.; Park, J. Localized surface plasmon resonance aptasensor for the highly sensitive direct detection of cortisol in human saliva. Sens. Actuators B Chem. 2020, 304, 127424. [Google Scholar]
- Das, A.P.; Kumar, P.S.; Swain, S. Recent advances in biosensor based endotoxin detection. Biosens. Bioelectron. 2014, 51, 62–75. [Google Scholar]
- Saylan, Y.; Akgönüllü, S.; Denizli, A. Plasmonic sensors for monitoring biological and chemical threat agents. Biosensors 2020, 10, 142. [Google Scholar]
- Saylan, Y.; Denizli, A. Molecular fingerprints of hemoglobin on a nanofilm chip. Sensors 2018, 18, 3016. [Google Scholar]
- Inci, F.; Saylan, Y.; Kojouri, A.M.; Ogut, M.G.; Denizli, A.; Demirci, U. A disposable microfluidic-integrated hand-held plasmonic platform for protein detection. Appl. Mater. Today 2020, 18, 100478. [Google Scholar]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar]
- Bakhshpour, M.; Göktürk, I.; Bereli, N.; Yılmaz, F.; Denizli, A. Selective detection of penicillin G antibiotic in milk by molecularly imprinted polymer-based plasmonic SPR sensor. Biomimetics 2021, 6, 72. [Google Scholar]
- Pandey, A.K.; Sharma, A.K.; Marques, C. On the application of SiO2/SiC grating on Ag for high-performance fiber optic plasmonic sensing of cortisol concentration. Materials 2020, 13, 1623. [Google Scholar]
- Stevens, R.C.; Soelberg, S.C.; Near, C.; Furlong, C.E. Detection of cortisol in saliva with a flow-filtered, portable surface plasmon resonance biosensor system. Anal. Chem. 2008, 80, 6747–6751. [Google Scholar]
- Safran, V.; Göktürk, I.; Bakhshpour, M.; Yılmaz, F.; Denizli, A. Development of molecularly imprinted polymer-based optical sensor for the sensitive penicillin G detection in milk. Chem. Sel. 2021, 6, 11865–11875. [Google Scholar]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar]
- Silva, F.; Cabral Campello, M.P.; Paulo, A. Radiolabeled gold nanoparticles for imaging and therapy of cancer. Materials 2020, 14, 4. [Google Scholar]
- Jung, H.; Jung, J.; Kim, Y.-H.; Kwon, D.; Kim, B.G.; Bin Na, H.; Lee, H.H. Surface plasmon resonance characteristics of Au nanoparticles layered sensor chip for direct detection of stress hormone conjugated by nanoparticles. Biochip J. 2018, 12, 249–256. [Google Scholar]
- Usha, S.P.; Shrivastav, A.M.; Gupta, B.D. A contemporary approach for design and characterization of fiber-optic-cortisol sensor tailoring LMR and ZnO/PPY molecularly imprinted film. Biosens. Bioelectron. 2017, 87, 178–186. [Google Scholar]
- Kämäräinen, S.; Mäki, M.; Tolonen, T.; Palleschi, G.; Virtanen, V.; Micheli, L.; Sesay, A.M. Disposable electrochemical immunosensor for cortisol determination in human saliva. Talanta 2018, 188, 50–57. [Google Scholar]
- Dalirirad, S.; Steckl, A.J. Aptamer-based lateral flow assay for point of care cortisol detection in sweat. Sens. Actuators B Chem. 2019, 283, 79–86. [Google Scholar]
- Erdem, Ö.; Cihangir, N.; Saylan, Y.; Denizli, A. Comparison of molecularly imprinted plasmonic nanosensor performances for bacteriophage detection. New J. Chem. 2020, 44, 17654–17663. [Google Scholar]
- Gerdan, Z.; Saylan, Y.; Uğur, M.; Denizli, A. Ion-imprinted polymer-on-a-sensor for copper detection. Biosensors 2022, 12, 91. [Google Scholar]
- Saylan, Y.; Akgönüllü, S.; Denizli, A. Preparation of magnetic nanoparticles-assisted plasmonic biosensors with metal affinity for interferon-α detection. Mater. Sci. Eng. B 2022, 280, 115687. [Google Scholar]
- Garipcan, B.; Denizli, A. A novel affinity support material for the separation of immunoglobulin G from human plasma. Macromol. Biosci. 2002, 2, 135–144. [Google Scholar]
- Enustun, B.V.; Turkevich, J. Coagulation of colloidal gold. J. Am. Chem. Soc. 1963, 85, 3317–3328. [Google Scholar]
- Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf. B 2007, 58, 3–7. [Google Scholar]
- Gupta, S.; Tiwari, M.; Prakash, R. Gold nanoparticles incorporated 3-(trimethoxysilyl)propyl methacrylate modified electrode for non-enzymatic electro-sensing of urea. J. Nanosci. Nanotechnol. 2014, 14, 2786–2791. [Google Scholar]
- Gallego, J.L.; Arroyo, J.P. Comparison of HPLC and multivariate regression methods for hydrocortisone and lidocaine analysis of pharmaceutical preparations. Anal. Bioanal. Chem. 2002, 374, 282–288. [Google Scholar]
- Manivannan, P.; Kumar, R.T.; Periyanayagasamy, V. Spectral analysis of hydrocortisone. J. Chem. Pharm. Sci. 2009, 2, 1–5. [Google Scholar]
- Špringer, T.; Ermini, M.L.; Špačková, B.; Jabloňků, J.; Homola, J. Enhancing sensitivity of surface plasmon resonance biosensors by functionalized gold nanoparticles: Size matters. Anal. Chem. 2014, 86, 10350–10356. [Google Scholar]
- Wu, C.; Zhou, X.; Wei, J. Localized surface plasmon resonance of silver nanotriangles synthesized by a versatile solution, reaction. Nanoscale Res. Lett. 2015, 10, 354. [Google Scholar]
- Hong, X.; Hall, E.A.H. Contribution of gold nanoparticles to the signal amplification in surface plasmon resonance. Analyst 2012, 137, 4712. [Google Scholar]
- Frasconi, M.; Mazzarino, M.; Botrè, F.; Mazzei, F. Surface plasmon resonance immunosensor for cortisol and cortisone determination. Anal. Bioanal. Chem. 2009, 394, 2151–2159. [Google Scholar]
- Khan, M.S.; Dighe, K.; Wang, Z.; Srivastava, I.; Schwartz-Duval, A.S.; Misra, S.K.; Pan, D. Electrochemical-digital immunosensor with enhanced sensitivity for detecting human salivary glucocorticoid hormone. Analyst 2019, 144, 1448–1457. [Google Scholar]
- Munje, R.; Muthukumar, S.; Panneer Selvam, A.; Prasad, S. Flexible nanoporous tunable electrical double layer biosensors for sweat diagnostics. Sci. Rep. 2015, 5, 14586. [Google Scholar]
- Manickam, P.; Edwin Fernandez, R.; Umasankar, Y.; Gurusamy, M.; Arizaleta, F.; Urizar, G.; Bhansali, S. Salivary cortisol analysis using metalloporphyrins and multi-walled carbon nanotubes nanocomposite functionalized electrodes. Sens. Actuators B Chem. 2018, 274, 47–53. [Google Scholar]
- Parlak, O.; Keene, S.T.; Marais, A.; Curto, V.F.; Salleo, A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 2018, 4, 2904. [Google Scholar]
- Kinnamon, D.; Ghanta, R.; Lin, K.C.; Muthukumar, S.; Prasad, S. Portable biosensor for monitoring cortisol in low-volume perspired human sweat. Sci. Rep. 2017, 7, 13312. [Google Scholar]
- Vasudev, A.; Kaushik, A.; Tomizawa, Y.; Norena, N.; Bhansali, S. An LTCC-based microfluidic system for label-free, electrochemical detection of cortisol. Sens. Actuators B Chem. 2013, 182, 139–146. [Google Scholar]
- Cruz, A.F.D.; Norena, N.; Kaushik, A.; Bhansali, S. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens. Bioelectron. 2014, 62, 249–254. [Google Scholar]
- Manickam, P.; Pasha, S.K.; Snipes, S.A.; Bhansali, S. A reusable electrochemical biosensor for monitoring of small molecules (cortisol) using molecularly imprinted polymers. J. Electrochem. Soc. 2016, 164, B54–B59. [Google Scholar]
- Nandhakumar, P.; Haque, A.M.J.; Lee, N.S.; Yoon, Y.H.; Yang, H. Washing-free displacement immunosensor for cortisol in human serum containing numerous interfering species. Anal. Chem. 2018, 90, 10982–10989. [Google Scholar]
- Lee, M.H.; Thomas, J.L.; Liu, W.C.; Zhang, Z.X.; Liu, B.D.; Yang, C.H.; Lin, H.Y. A multichannel system integrating molecularly imprinted conductive polymers for ultrasensitive voltammetric determination of four steroid hormones in urine. Microchim. Acta 2019, 186, 695. [Google Scholar]
- Abdulsattar, J.O.; Greenway, G.M.; Wadhawan, J.D. Electrochemical immunoassay for the detection of stress biomarkers. Heliyon 2020, 6, e03558. [Google Scholar]
- Sun, B.; Gou, Y.; Ma, Y.; Zheng, X.; Bai, R.; Ahmed Abdelmoaty, A.A.; Hu, F. Investigate electrochemical immunosensor of cortisol based on gold nanoparticles/magnetic functionalized reduced graphene oxide. Biosens. Bioelectron. 2017, 88, 55–62. [Google Scholar]
- Liu, J.; Xu, N.; Men, H.; Li, S.; Lu, Y.; Low, S.S.; Li, X.; Zhu, L.; Cheng, C.; Xu, G.; et al. Salivary cortisol determination on smartphone-based differential pulse voltammetry system. Sensors 2020, 20, 1422. [Google Scholar]
- Kumar, A.; Aravamudhan, S.; Gordic, M.; Bhansali, S.; Mohapatra, S.S. Ultrasensitive detection of cortisol with enzyme fragment complementation technology using functionalized nanowire. Biosens. Bioelectron. 2007, 22, 2138–2144. [Google Scholar]
| AuNP-MIP | AuNP-NIP | ||||
|---|---|---|---|---|---|
| ΔR | k | ΔR | k | k′ | |
| Cortisol | 29.52 | - | 2.73 | - | - |
| Clobetasol | 6.26 | 4.72 | 2.87 | 0.95 | 4.96 |
| Fluticasone | 6.11 | 4.83 | 2.66 | 1.03 | 4.71 |
| Reference | Sensor Type | Detection Range | Detection Limit | Real Sample |
|---|---|---|---|---|
| [3] | Immunosensor | 0.1–10 ng/mL | - | Saliva |
| [18] | Optical | 1.5–10 ng/mL | 1.0 ng/mL | Saliva |
| [38] | Optical | 9–132 μg/L | 3 μg/L | Saliva |
| [39] | Electrochemical | 1 pg/mL–10 ng/mL | 0.87 pg/ mL | Saliva |
| [40] | Electrochemical | 10–200 ng/mL | 1 pg/mL | Human and Artificial Sweat |
| [41] | Electrochemical | 1.81–36.2 ng/mL | 18 fg/mL | Saliva |
| [42] | Electrochemical | 3.6–3624 ng/mL | - | Sweat |
| [43] | Electrochemical | 0.5–500 ng/mL | 0.5 ng/mL | Sweat |
| [44] | Electrochemical | 0.003–36 ng/mL | 10 pg/mL | - |
| [45] | Electrochemical | 0.003–181 ng/mL | 0.36 pg/mL | - |
| [46] | Electrochemical | 0.36 pg/mL–3624 ng/mL | 3624 ng/mL | - |
| [47] | Immunosensor | 10.8 pg/mL–0.36 μg/mL | 10.8 pg/mL | Serum |
| [48] | Electrochemical | 1–100 ag/mL | 2 ag/mL | Urine |
| [49] | Electrochemical | 0.001–50 ng/mL | 1.03 pg/mL | Artificial Salivia |
| [50] | Electrochemical | 0.1–1000 ng/mL | 0.05 ng/mL | Serum |
| [51] | Electrochemical | 0.18–72 ng/mL | 40 pg/mL | Saliva |
| [52] | Electrochemical | 3.6–28.9 μg/mL | 7.4 μg/mL | Serum |
| This work | Optical | 0.01–100 ppb (ng/mL) | 0.0082 ppb (ng/mL) | Artificial Plasma and Urine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yılmaz, G.E.; Saylan, Y.; Göktürk, I.; Yılmaz, F.; Denizli, A. Selective Amplification of Plasmonic Sensor Signal for Cortisol Detection Using Gold Nanoparticles. Biosensors 2022, 12, 482. https://doi.org/10.3390/bios12070482
Yılmaz GE, Saylan Y, Göktürk I, Yılmaz F, Denizli A. Selective Amplification of Plasmonic Sensor Signal for Cortisol Detection Using Gold Nanoparticles. Biosensors. 2022; 12(7):482. https://doi.org/10.3390/bios12070482
Chicago/Turabian StyleYılmaz, Gaye Ezgi, Yeşeren Saylan, Ilgım Göktürk, Fatma Yılmaz, and Adil Denizli. 2022. "Selective Amplification of Plasmonic Sensor Signal for Cortisol Detection Using Gold Nanoparticles" Biosensors 12, no. 7: 482. https://doi.org/10.3390/bios12070482
APA StyleYılmaz, G. E., Saylan, Y., Göktürk, I., Yılmaz, F., & Denizli, A. (2022). Selective Amplification of Plasmonic Sensor Signal for Cortisol Detection Using Gold Nanoparticles. Biosensors, 12(7), 482. https://doi.org/10.3390/bios12070482









