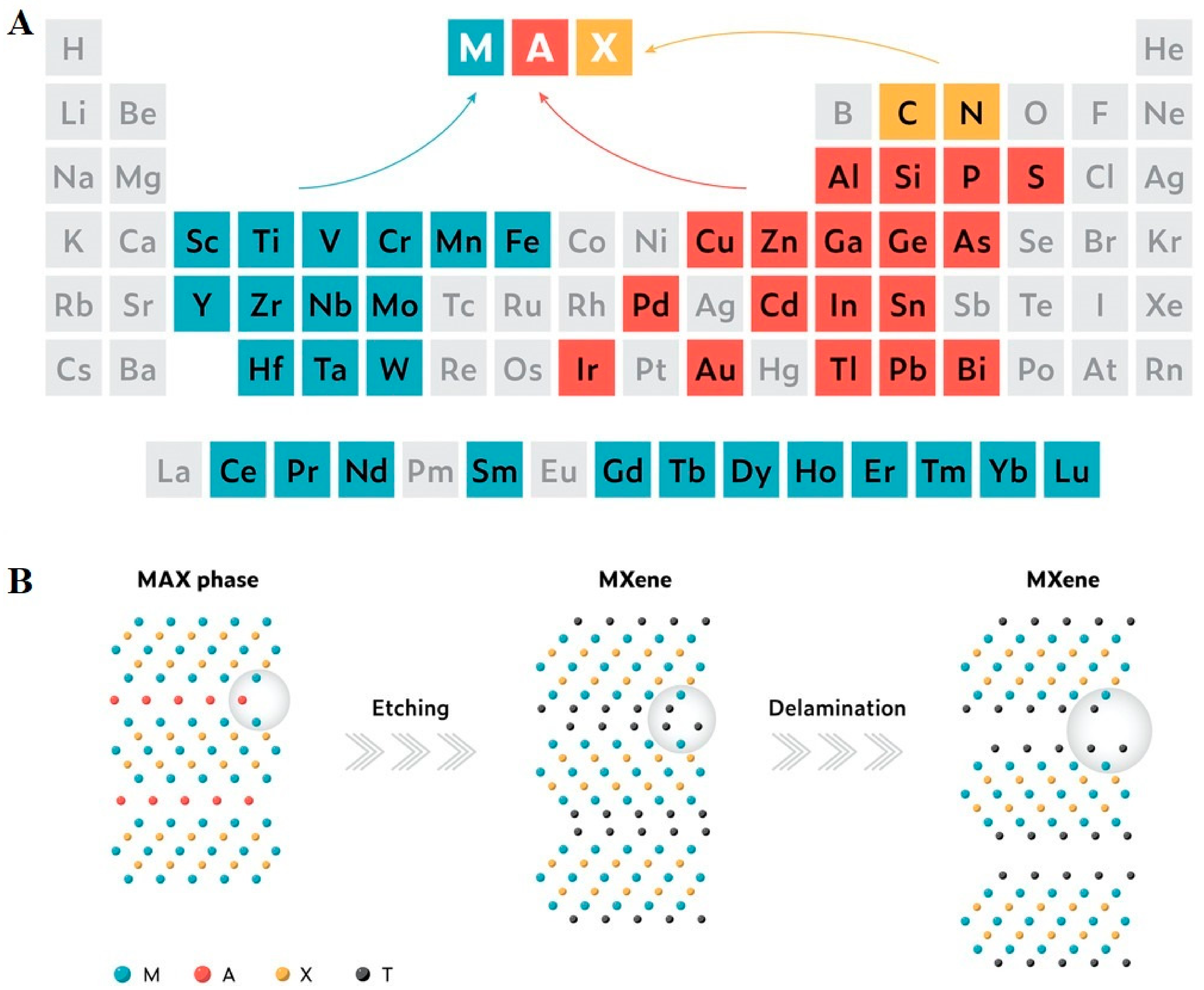
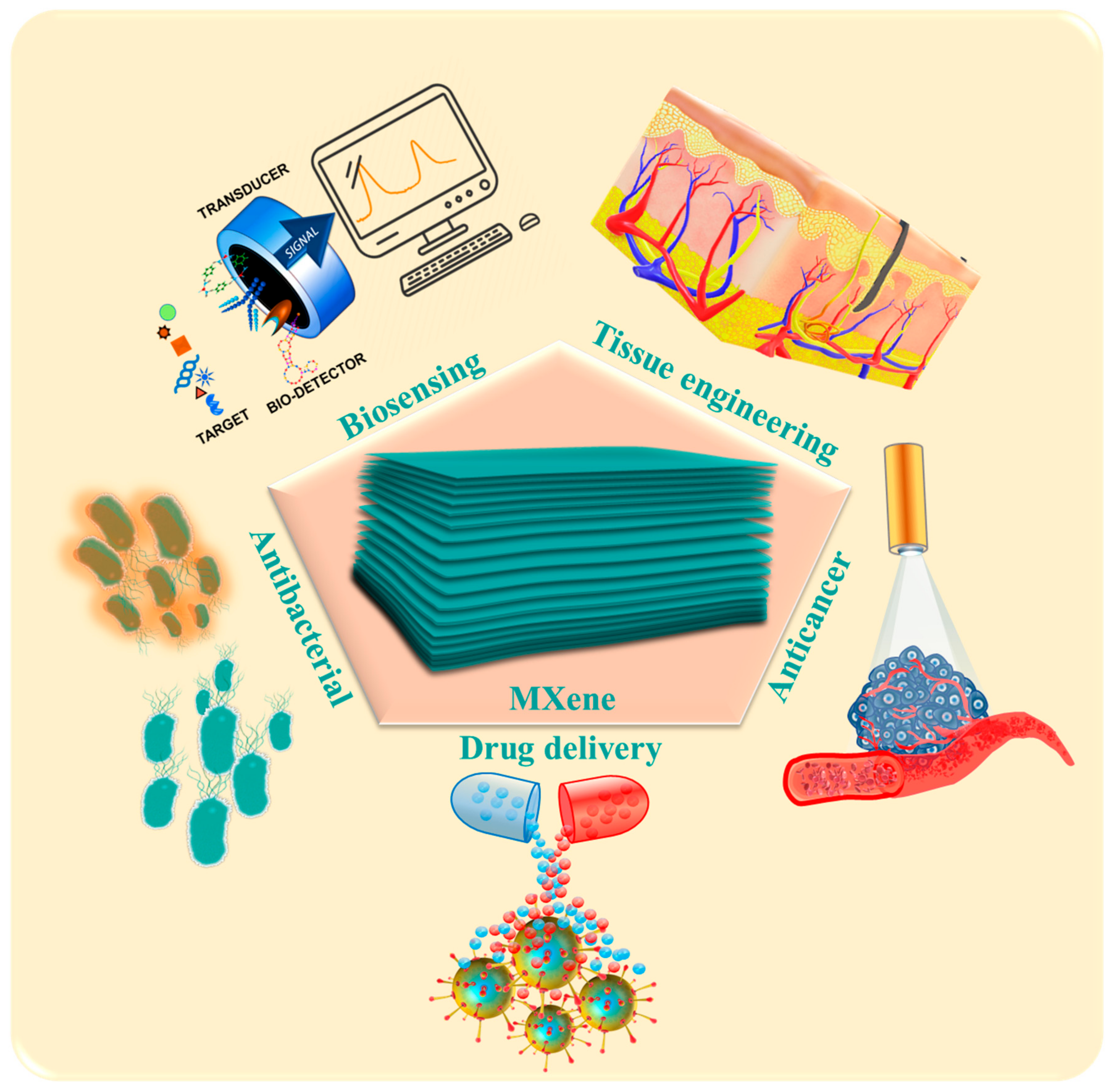
Advances of MXenes; Perspectives on Biomedical Research
Abstract
:1. Introduction
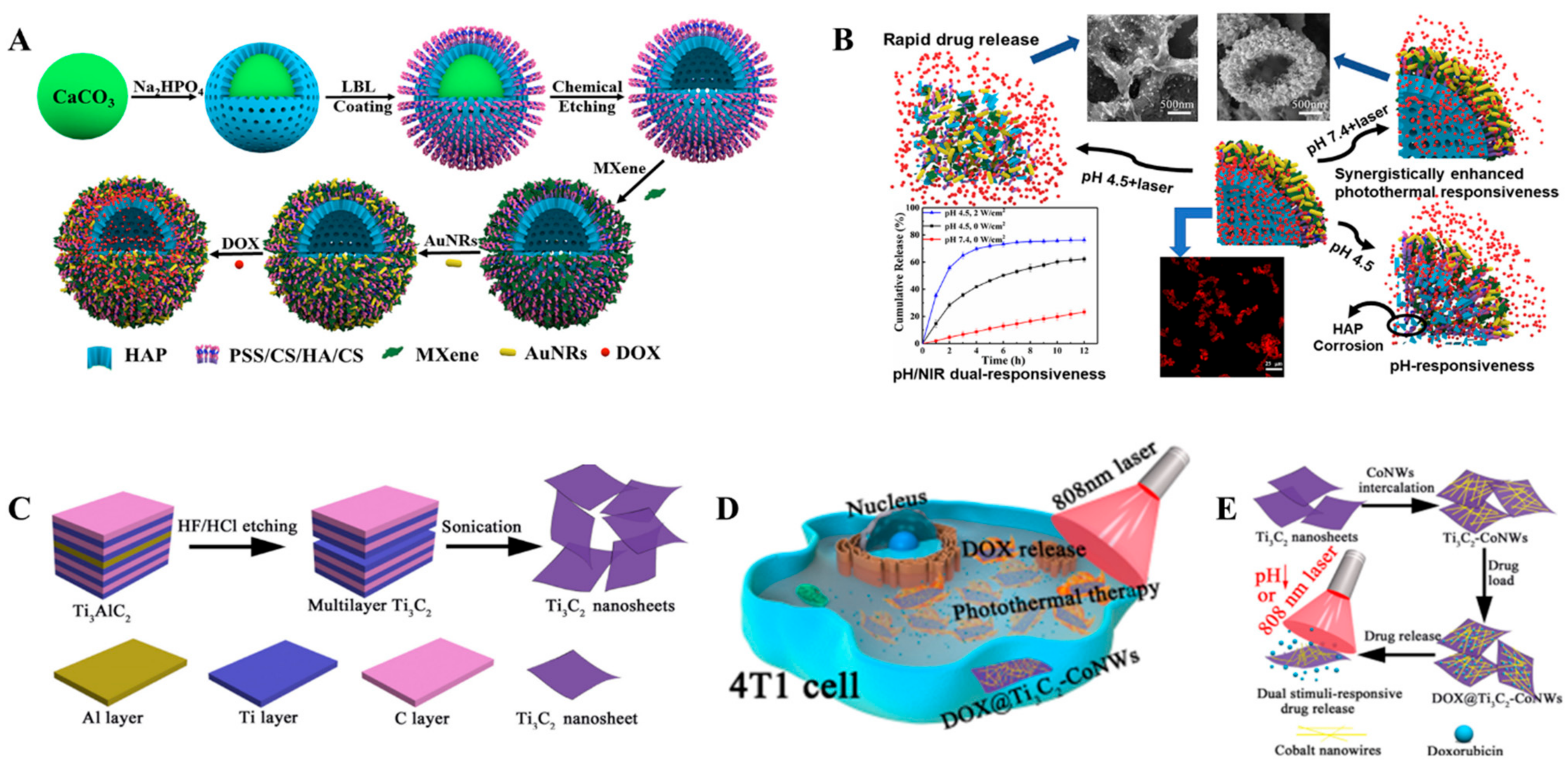
2. Drug Delivery Applications
3. Anticancer Therapy
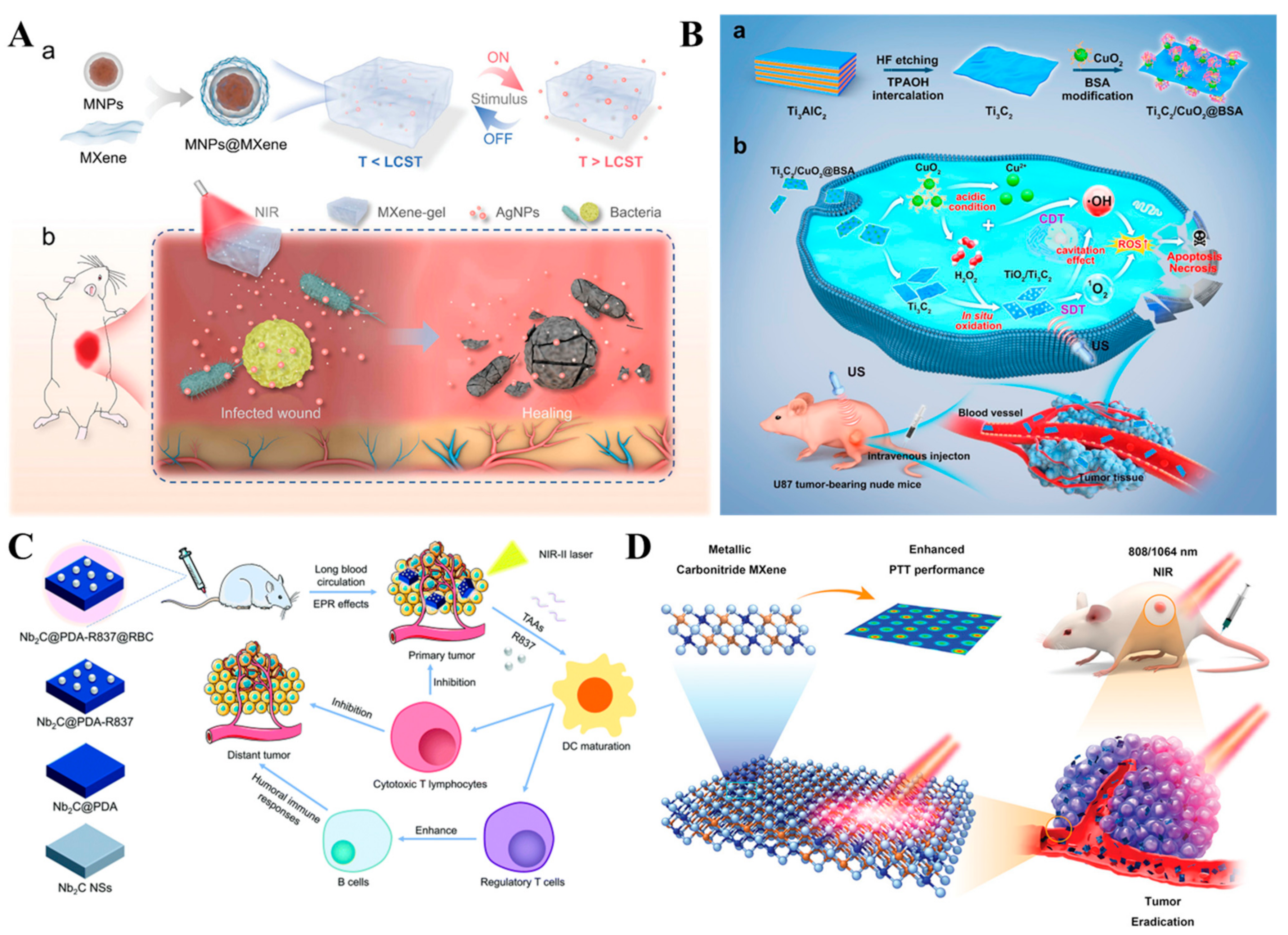
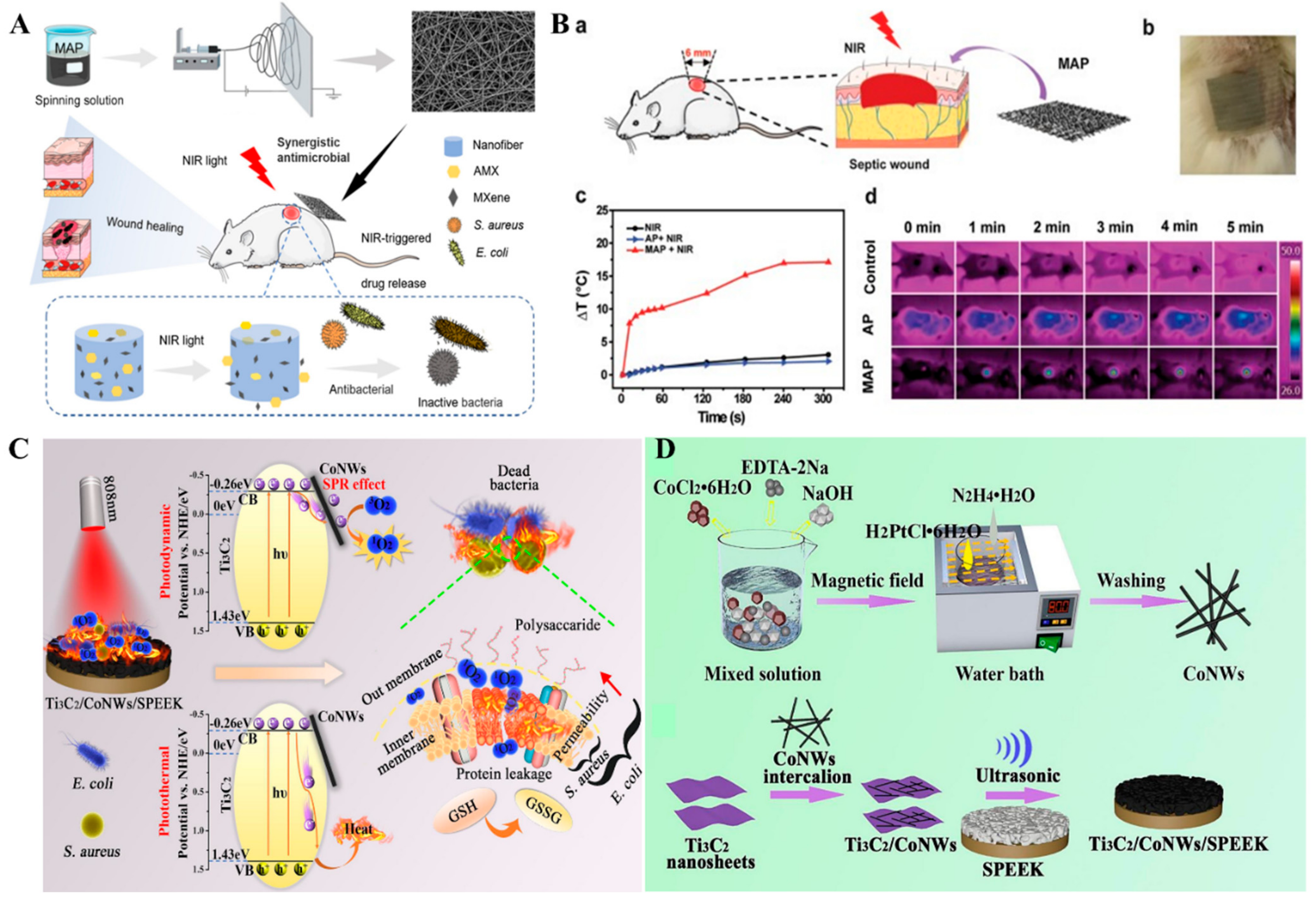
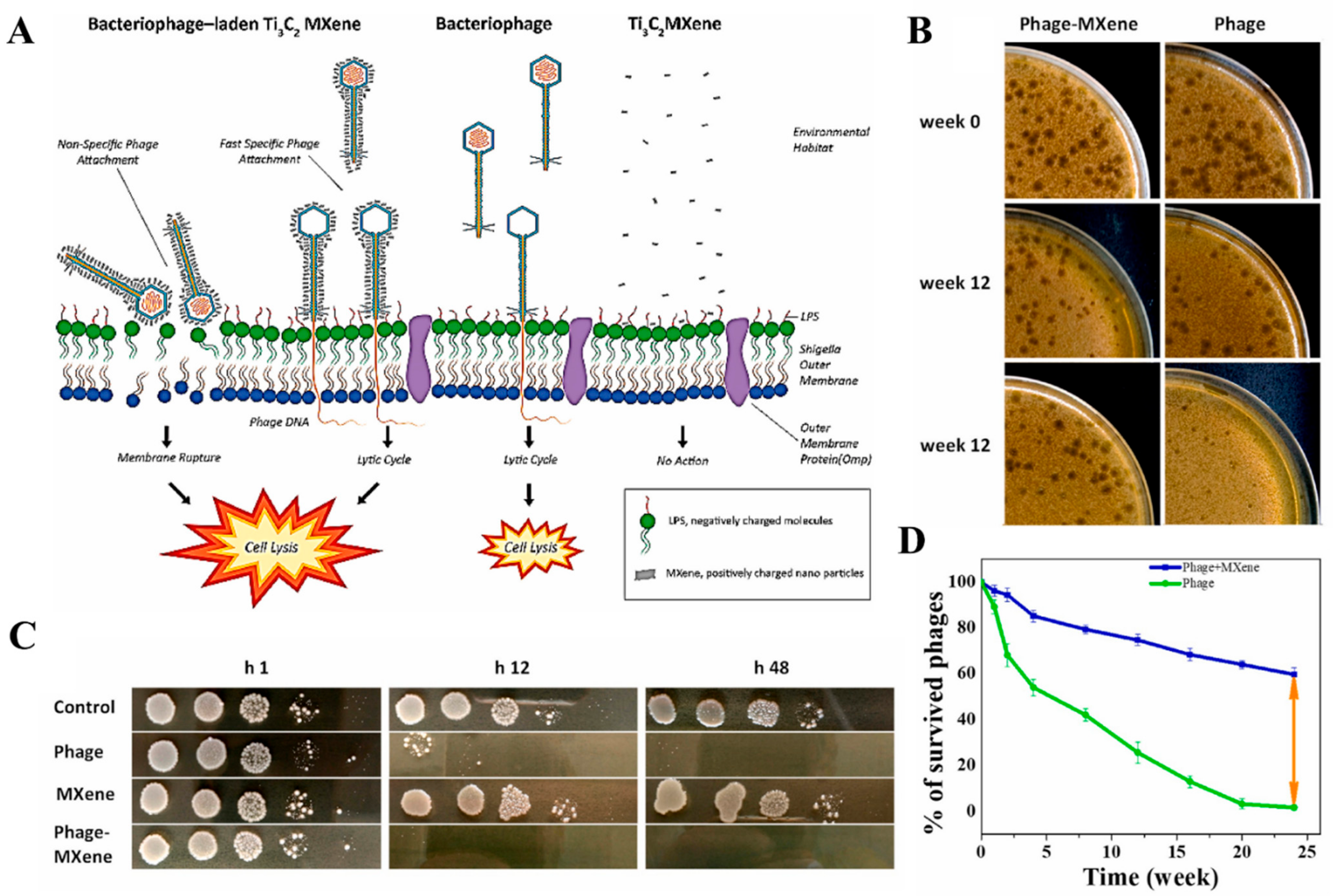
4. Antimicrobial Applications
5. Biosensor and Smart Sensor Applications
5.1. MXene-Based Smart Sensors
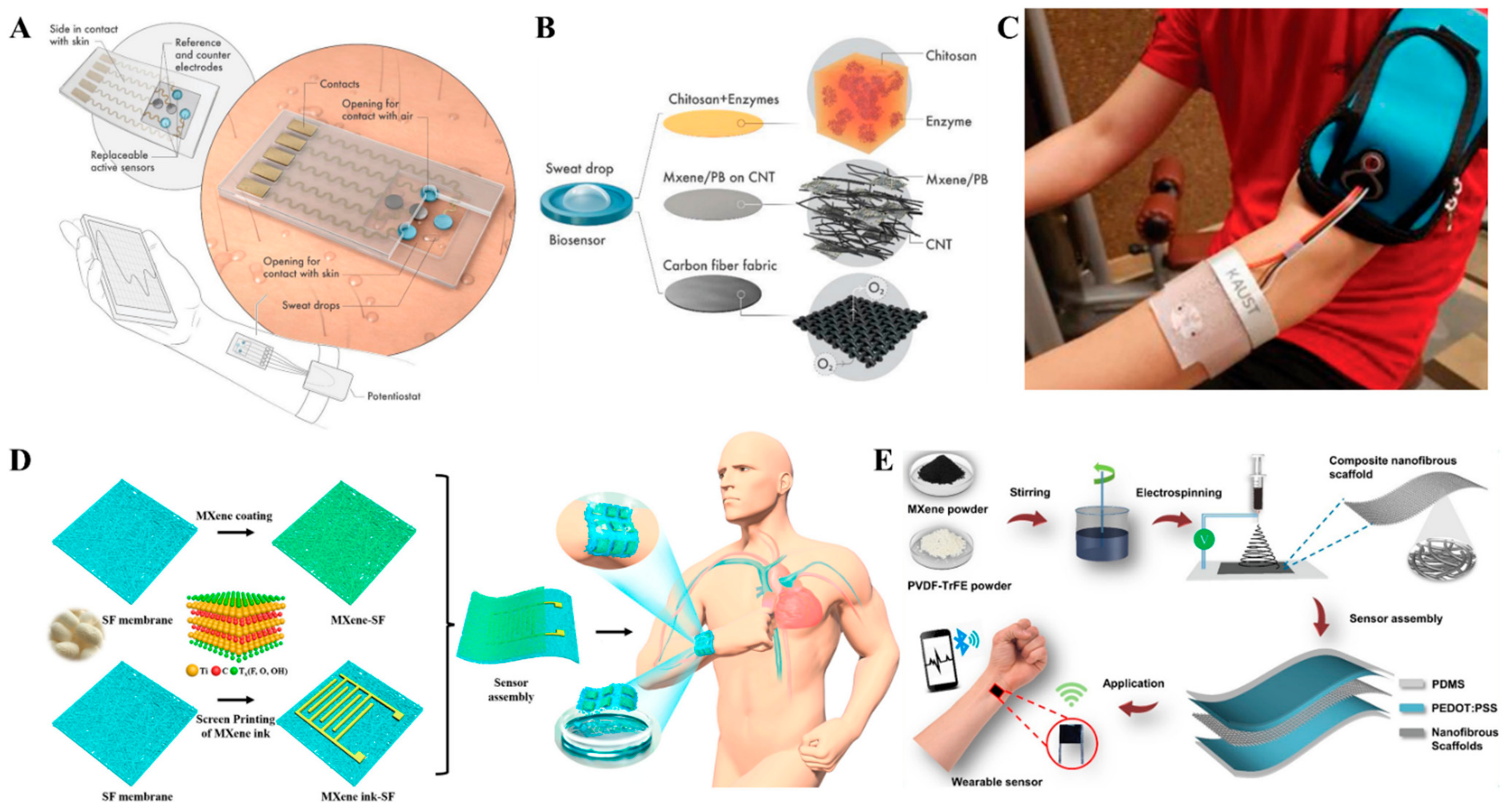
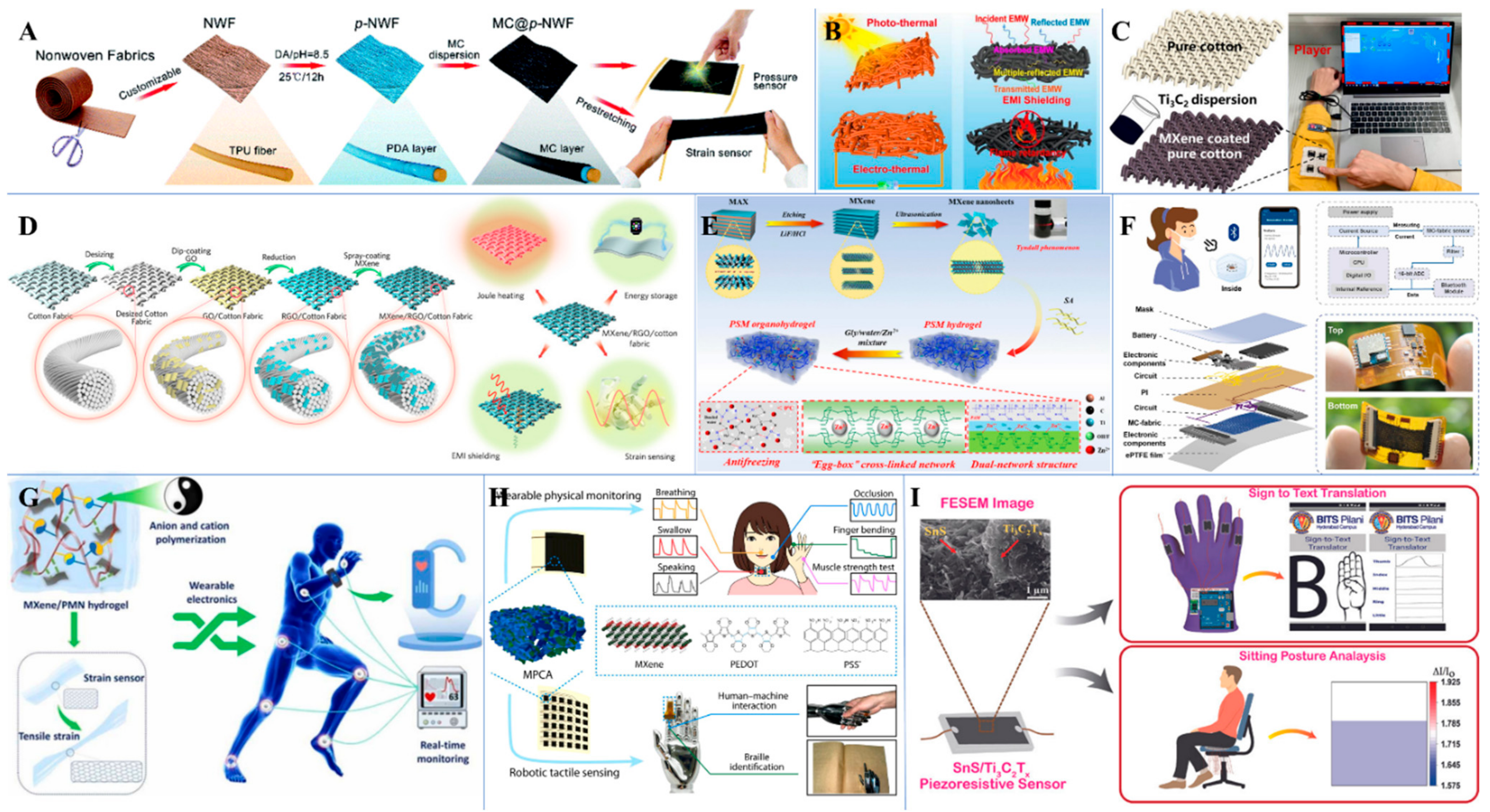
5.2. Biomarker Detection
5.3. Enzymatic Sensors
6. Tissue Engineering
| MXene/Composite | Applications | Description | Ref. |
|---|---|---|---|
| Ti3C2Tx-enhanced poly (lactic acid) nanocomposite | Guided bone regeneration | Ti3C2Tx-Poly (lactic acid) composite addition to MC3T3-E1 mouse preosteoblasts enhanced the in vitro adhesion, proliferation, and osteogenic differentiation. | [184] |
| Electrospun MXene/PLLA-PHA nanofibers | Cell culture | MXene composite nanofibers enhanced the differentiation of BMSCs to osteoblasts. | [187] |
| Ti3C2Tx-PEG composite | Cardiac tissue engineering | 3D-printed Ti3C2Tx-PEG hydrogel aligned the iCMs with an increase in MYH7, TNNT2, and SERCA2 expressions. | [188] |
| Ti3C2Tx-Bioactive glass scaffold | Tissue reconstruction | MXene-bioactive glass scaffold demonstrated accelerated in vivo growth of newborn bone tissue. | [189] |
| Multilayered Ti3C2Tx | Guided bone regeneration | Evaluated the guided bone regeneration ability of multilayered Ti3C2Tx in vitro and in vivo. | [190] |
| Ti3C2Tx Quantum Dots-Chitosan hydrogel | Tissue repair | MXene Quantum dot-chitosan hydrogel enhanced the physicochemical properties for tissue repair and stem cell delivery. | [191] |
| Mesoporous Silica@ Nb2C-Scaffolds | Nitric oxide-Augmented bone regeneration | NIR-triggered hyperthermia on the Nb2C MXene wrapped with S-Nitroso thiol-mesoporous silica with 3D-printing bioactive glass scaffolds and precisely released controlled nitric oxide. | [192] |
| Reduced graphene oxide-Ti3C2Tx hydrogel | 3D cellular network formation | rGO-MXene hydrogel enhanced the formation of a 3D cellular network of human cell lines HeLa, SH-SY5Y, and MSU 1.1. | [193] |
| MXene-Hydroxyapatite nanoparticle composite | Osteogenic properties | MXene-Hydroxyapatite nanocomposite promoted the growth and osteogenic differentiation of BMSCs. | [194] |
| Ti3C2Tx-CSH scaffold | Maxillofacial tissue regeneration | MXene-CSH scaffold stimulated the in vivo formation of maxillofacial bone, and induced the osteogenic protein expression of MC3T3-E1 in vitro. | [195] |
| Nb2C@Titanium plate | Tissue regeneration | The scavenging of excessive ROS from the infectious tissue environment by the Nb2C@Titanium plate alleviated the proinflammatory responses, thereby benefiting angiogenesis and tissue regeneration. | [196] |
| Ultrathin Ti3C2Tx nanoflakes | Periodontal regeneration | Human PDLCs pretreated with Ti3C2Tx displayed excellent in vivo new bone formation and enhanced osteoclast inhibition. | [197] |
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ab1 | Monoclonal anti-CEA antibody |
| AChE | Acetylcholinesterase |
| AFBPB | 4-amino-1-(4-formyl-benzyl) pyridinium bromide |
| AgNPs | Silver nanoparticles |
| AgNRs | Silver nanorods |
| AMF | Alternating magnetic field |
| anti-ASGPR | Anti-asialoglycoprotein receptor |
| anti-CEA | Anti-Carcinoembryonic antigen |
| Apo A1 | Apolipoprotein A1 |
| Apt | Aptamer |
| ATCl | Acetylthiocholine chloride |
| AuNPs | Gold nanoparticles |
| Au-PCB | Gold printed circuit board |
| BC | Bacterial cellulose |
| BMSCs | Bone marrow derived mesenchymal stem cells |
| BSA | Bovine serum albumin |
| CA | Chronoamperometry |
| cDNA | Complementary DNA |
| CGDSTC NSs | Chlorin e6/GOx/Dopamine/Sodium ascorbate/Ti3C2Tx nanosheets |
| Chi | Chitosan |
| ChOx | Cholesterol Oxidase |
| CNC | Cellulose nanocrystal |
| CNTs | Carbon nanotubes |
| CP | Compound polysaccharide |
| CPO | Chloroperoxidase |
| Cre | Creatinine |
| CSH | Collagen/Silk/Hydroxyapatite |
| CuP | Copper-organophosphate |
| CV | Cyclic Voltammetry |
| CVD | Chemical Vapor Deposition |
| DA | Dopamine |
| DIDμE | Dual interdigitated microelectrode |
| DMM | Digital multimeter |
| DNA | Deoxyribonucleic acid |
| DPV | Differential Pulse Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| EMG | Electromyography |
| FA | Folic acid |
| Fc | Ferrocene |
| FePcQDs | Phthalocyanine quantum dots |
| FRET | Fluorescence Resonance Energy Transfer |
| GA | Glutaraldehyde |
| GCE | Glassy carbon electrode |
| GCPE | Graphite Carbon Paste Electrode |
| GDH | Glutamate dehydrogenase |
| GO | Graphene oxide |
| GONR | Graphene oxide nanoribbon |
| GSH | Glutathione |
| H&E | Hematoxylin and eosin |
| H2O2 | Hydrogen peroxide |
| Hb | Hemoglobin |
| HF | Hydrofluoric acid |
| HGF | Hepatic growth factor |
| HP1 | Hairpin DNA |
| HRP | Horseradish peroxidase |
| HT | Hexane thiol |
| IC | Ion chromatography |
| LOD | Limit of detection |
| MB | Methylene blue |
| miRNA-21 | micro-Ribonucleic acid-21 |
| miRNA-10b | micro-Ribonucleic acid-10b |
| miRNA-141 | micro-Ribonucleic acid-141 |
| miRNA-155 | micro-Ribonucleic acid-155 |
| MQDs | MXene quantum dots |
| MUC1 | Mucin 1 |
| MWCNT | Multi-walled carbon nanotubes |
| MXNSs | 2D MXene-Ti3C2Tx nanosheets |
| NAD | Nicotinamide adenine dinucleotide |
| NIR | Near Infrared |
| NMP22 | Nuclear Matrix Protein22 |
| NSE | Neuron specific enolase |
| NWF | Non-woven fabric |
| OPN | Osteo5pontin |
| P(DPA) | Poly (di picolinic acid) |
| PAYR | Poly alizarine yellow R |
| PB | Prussian blue |
| Pd | Palladium |
| PDA | Polydopamine |
| PDLCs | Periodontal ligament cells |
| PDMS | Polydimethylsiloxane |
| PEC | Photoelectrochemical |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEG | Polyethylene glycol |
| PEPLD | Plasma-enhanced pulsed laser deposition |
| PHA | Poly hydroxy alkanoate |
| PLLA | Poly (L-lactic acid) |
| PMo12 | Phosphomolybdic acid |
| POC | Point-of-care |
| PPy | Polypyrrole |
| PSA | Prostate specific antigen |
| PtNPs | Platinum nanoparticles |
| PU | Polyurethane |
| PVA | Polyvinyl alcohol |
| PVDF | Polyvinylidene fluoride |
| QDs | Quantum dots |
| rGO | Reduced graphene oxide |
| ROS | Reactive oxygen species |
| Ru | Ruthenium |
| SED | Soft electronic devices |
| SEM | Scanning electron microscopy |
| SOx | Sarcosine oxidase |
| SPA | Staphylococcal protein A |
| SPE | Screen printed electrode |
| SPEEK | Sulfonated PEEK substrates |
| SPGE | Screen-printed gold electrode |
| SPR | Surface plasmon resonance |
| ssDNAs | Single stranded DNAs |
| SWV | Square wave voltammetry |
| TBA | Tetrabutylammonium |
| TGA | Thio glycolic acid |
| TPU | Thermoplastic polyurethane |
| TPZ | Tirapazamine |
| TrFE | Trifluoro ethylene |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| Tyr | Tyrosine |
| UA | Uric acid |
| UHAPNWs | Ultralong hydroxyapatite nanowires |
| VEGF165 | Vascular endothelial growth factor 165 |
| β-HBD | β-hydroxybutyrate dehydrogenase |
References
- The Collected Works of Irving Langmuir: Surface Phenomena; Published with the Editorial Assistance of the General Electric Company by Pergamon Press; Pergamon Press: Oxford, UK, 1961; Volume 9.
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology; Macmillan Publishers Ltd.: London, UK, 2009; pp. 11–19. [Google Scholar]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Akinwande, D.; Brennan, C.J.; Bunch, J.S.; Egberts, P.; Felts, J.R.; Gao, H.; Huang, R.; Kim, J.-S.; Li, T.; Li, Y.; et al. A review on mechanics and mechanical properties of 2D materials—Graphene and beyond. Extrem. Mech. Lett. 2017, 13, 42–77. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihsanullah, I. MXenes as next-generation materials for the photocatalytic degradation of pharmaceuticals in water. J. Environ. Chem. Eng. 2022, 10, 107381. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Munir, S.; Rasheed, A.; Rasheed, T.; Ayman, I.; Ajmal, S.; Rehman, A.; Shakir, I.; Agboola, P.O.; Warsi, M.F. Exploring the Influence of Critical Parameters for the Effective Synthesis of High-Quality 2D MXene. ACS Omega 2020, 5, 26845–26854. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.-Å.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef]
- Wang, X.; Garnero, C.; Rochard, G.; Magne, D.; Morisset, S.; Hurand, S.; Chartier, P.; Rousseau, J.; Cabioc’h, T.; Coutanceau, C. A new etching environment (FeF3/HCl) for the synthesis of two-dimensional titanium carbide MXenes: A route towards selective reactivity vs. water. J. Mater. Chem. A 2017, 5, 22012–22023. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, B.; Hu, Q.; Wang, L.; Zhou, A. The Synthesis Process and Thermal Stability of V2C MXene. Materials 2018, 11, 2112. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, D.; Lian, W.; Hu, Q.; Liu, X.; Zhou, A. The preparation of V2CTx by facile hydrothermal-assisted etching processing and its performance in lithium-ion battery. J. Mater. Res. Technol. 2020, 9, 984–993. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, A.; Chen, J.; Jia, J.; Zhou, W.; Wang, L.; Hu, Q. Preparation of Ti3C2 and Ti2C MXenes by fluoride salts etching and methane adsorptive properties. Appl. Surf. Sci. 2017, 416, 781–789. [Google Scholar] [CrossRef]
- Kvashina, T.S.; Uvarov, N.F.; Korchagin, M.A.; Krutskiy, Y.L.; Ukhina, A.V. Synthesis of MXene Ti3C2 by selective etching of MAX-phase Ti3AlC2. Mater. Today Proc. 2020, 31, 592–594. [Google Scholar] [CrossRef]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical etching of Ti2AlC to Ti2CTx (MXene) in low-concentration hydrochloric acid solution. J. Mater. Chem. A 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, Q.; Liang, G.; Huang, Z.; Ma, L.; Wang, D.; Mo, F.; Dong, B.; Huang, Q. In situ electrochemical synthesis of MXenes without Acid/Alkali usage in/for an aqueous zinc ion battery. Adv. Energy Mater. 2020, 10, 2001791. [Google Scholar] [CrossRef]
- Pang, S.-Y.; Wong, Y.-T.; Yuan, S.; Liu, Y.; Tsang, M.-K.; Yang, Z.; Huang, H.; Wong, W.-T.; Hao, J. Universal strategy for HF-free facile and rapid synthesis of two-dimensional MXenes as multifunctional energy materials. J. Am. Chem. Soc. 2019, 141, 9610–9616. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B. Fluorine-free synthesis of high-purity Ti3C2Tx (T = OH, O) via alkali treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Li, G.; Tan, L.; Zhang, Y.; Wu, B.; Li, L. Highly efficiently delaminated single-layered MXene nanosheets with large lateral size. Langmuir 2017, 33, 9000–9006. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbankowski, P.; Anasori, B.; Hantanasirisakul, K.; Yang, L.; Zhang, L.; Haines, B.; May, S.J.; Billinge, S.J.L.; Gogotsi, Y. 2D molybdenum and vanadium nitrides synthesized by ammoniation of 2D transition metal carbides (MXenes). Nanoscale 2017, 9, 17722–17730. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.-L.; Cheng, H.-M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Chen, L.; Liu, Z.; Cheng, H.-M.; Ren, W. Bottom-up synthesis of 2D transition metal carbides and nitrides. In 2D Metal Carbides and Nitrides (MXenes); Springer: Cham, Switzerland, 2019; pp. 89–109. [Google Scholar]
- Xu, Y.; Zhang, K.; Chen, S.; Zhang, X.; Chen, Y.; Li, D.; Xu, F. Two-dimensional lamellar MXene/three-dimensional network bacterial nanocellulose nanofiber composite Janus membranes as nanofluidic osmotic power generators. Electrochim. Acta 2022, 412, 140162. [Google Scholar] [CrossRef]
- Dwivedi, N.; Dhand, C.; Kumar, P.; Srivastava, A.K. Emergent 2D materials for combating infectious diseases: The potential of MXenes and MXene–graphene composites to fight against pandemics. Mater. Adv. 2021, 2, 2892–2905. [Google Scholar] [CrossRef]
- Wang, X.; Luo, D.; Wang, J.; Sun, Z.; Cui, G.; Chen, Y.; Wang, T.; Zheng, L.; Zhao, Y.; Shui, L. Strain engineering of a MXene/CNT hierarchical porous hollow microsphere electrocatalyst for a high-efficiency lithium polysulfide conversion process. Angew. Chem. Int. Ed. 2021, 60, 2371–2378. [Google Scholar] [CrossRef]
- Cao, B.; Liu, H.; Zhang, X.; Zhang, P.; Zhu, Q.; Du, H.; Wang, L.; Zhang, R.; Xu, B. MOF-Derived ZnS Nanodots/Ti3C2Tx MXene Hybrids Boosting Superior Lithium Storage Performance. Nano-Micro Lett. 2021, 13, 202. [Google Scholar] [CrossRef]
- Wang, B.; Lai, X.; Li, H.; Jiang, C.; Gao, J.; Zeng, X. Multifunctional MXene/chitosan-coated cotton fabric for intelligent fire protection. ACS Appl. Mater. Interfaces 2021, 13, 23020–23029. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Gong, Z.; Xie, J.; Lu, T.; Pan, L. Nitrogen and sulfur co-doped vanadium carbide MXene for highly reversible lithium-ion storage. J. Colloid Interface Sci. 2021, 587, 489–498. [Google Scholar] [CrossRef]
- Yoon, Y.; Tiwari, A.P.; Choi, M.; Novak, T.G.; Song, W.; Chang, H.; Zyung, T.; Lee, S.S.; Jeon, S.; An, K.S. Precious-Metal-Free Electrocatalysts for Activation of Hydrogen Evolution with Nonmetallic Electron Donor: Chemical Composition Controllable Phosphorous Doped Vanadium Carbide MXene. Adv. Funct. Mater. 2019, 29, 1903443. [Google Scholar] [CrossRef]
- Liu, R.; Cao, W.; Han, D.; Mo, Y.; Zeng, H.; Yang, H.; Li, W. Nitrogen-doped Nb2CTx MXene as anode materials for lithium ion batteries. J. Alloys Compd. 2019, 793, 505–511. [Google Scholar] [CrossRef]
- Zheng, S.; Li, S.; Mei, Z.; Hu, Z.; Chu, M.; Liu, J.; Chen, X.; Pan, F. Electrochemical nitrogen reduction reaction performance of single-boron catalysts tuned by MXene substrates. J. Phys. Chem. Lett. 2019, 10, 6984–6989. [Google Scholar] [CrossRef] [PubMed]
- Kan, D.; Wang, D.; Zhang, X.; Lian, R.; Xu, J.; Chen, G.; Wei, Y. Rational design of bifunctional ORR/OER catalysts based on Pt/Pd-doped Nb2CT2 MXene by first-principles calculations. J. Mater. Chem. A 2020, 8, 3097–3108. [Google Scholar] [CrossRef]
- Fatima, M.; Fatheema, J.; Monir, N.B.; Siddique, A.H.; Khan, B.; Islam, A.; Akinwande, D.; Rizwan, S. Nb-doped MXene with enhanced energy storage capacity and stability. Front. Chem. 2020, 8, 168. [Google Scholar] [CrossRef]
- Balcı, E.; Akkuş, Ü.Ö.; Berber, S. Doped Sc2C(OH)2 MXene: New type s-pd band inversion topological insulator. J. Phys. Condens. Matter 2018, 30, 155501. [Google Scholar] [CrossRef]
- Gao, Z.W.; Zheng, W.; Lee, L.Y.S. Highly Enhanced Pseudocapacitive Performance of Vanadium-Doped MXenes in Neutral Electrolytes. Small 2019, 15, 1902649. [Google Scholar] [CrossRef]
- Huang, W.; Hu, L.; Tang, Y.; Xie, Z.; Zhang, H. Recent Advances in Functional 2D MXene-Based Nanostructures for Next-Generation Devices. Adv. Funct. Mater. 2020, 30, 2005223. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Thomas, S. Inert ceramics. In Fundamental Biomaterials: Ceramics; Elsevier: Amsterdam, The Netherlands, 2018; p. 117. [Google Scholar]
- George, S.M.; Kandasubramanian, B. Advancements in MXene-Polymer composites for various biomedical applications. Ceram. Int. 2020, 46, 8522–8535. [Google Scholar] [CrossRef]
- Chen, K.; Qiu, N.; Deng, Q.; Kang, M.-H.; Yang, H.; Baek, J.-U.; Koh, Y.-H.; Du, S.; Huang, Q.; Kim, H.-E. Cytocompatibility of Ti3AlC2, Ti3SiC2, and Ti2AlN: In Vitro Tests and First-Principles Calculations. ACS Biomater. Sci. Eng. 2017, 3, 2293–2301. [Google Scholar] [CrossRef]
- Lin, H.; Gao, S.; Dai, C.; Chen, Y.; Shi, J. A Two-Dimensional Biodegradable Niobium Carbide (MXene) for Photothermal Tumor Eradication in NIR-I and NIR-II Biowindows. J. Am. Chem. Soc. 2017, 139, 16235–16247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, T.; Tian, Z.; Bibi, K.; Zhang, Y.-Z.; Alshareef, H.N. MXenes for Energy Harvesting. Adv. Mater. 2022, 34, 2108560. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthc. Mater. 2018, 7, 1701394. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, Q.; Yang, W.; Gan, X.; Yang, Y.; Xie, K.; Xie, L.; Deng, Y. Two-dimensional MXene/cobalt nanowire heterojunction for controlled drug delivery and chemo-photothermal therapy. Mater. Sci. Eng. C 2020, 116, 111212. [Google Scholar] [CrossRef]
- Zhu, B.; Shi, J.; Liu, C.; Li, J.; Cao, S. In-situ self-assembly of sandwich-like Ti3C2 MXene/gold nanorods nanosheets for synergistically enhanced near-infrared responsive drug delivery. Ceram. Int. 2021, 47, 24252–24261. [Google Scholar] [CrossRef]
- Xu, B.; Zhi, C.; Shi, P. Latest advances in MXene biosensors. J. Phys. Mater. 2020, 3, 031001. [Google Scholar] [CrossRef]
- Iqbal, A.; Hong, J.; Ko, T.Y.; Koo, C.M. Improving oxidation stability of 2D MXenes: Synthesis, storage media, and conditions. Nano Converg. 2021, 8, 9. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.-J.; Li, P.; Zhao, Y.; Niu, Q.J. Fabrication of novel MXene(Ti3C2)/polyacrylamide nanocomposite hydrogels with enhanced mechanical and drug release properties. Soft Matter 2020, 16, 162–169. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, J.; Song, P.; Li, J.; Cao, S. Chitosan/hyaluronic acid based hollow microcapsules equipped with MXene/gold nanorods for synergistically enhanced near infrared responsive drug delivery. Int. J. Biol. Macromol. 2021, 183, 870–879. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple Stimuli-Responsive MXene-Based Hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing. Small 2022, 18, 2104368. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Guo, X.; Gao, D.; Wu, C.; Hu, B.; Tan, G.; Du, N.; Cai, X.; Yang, Z.; Zhang, X. NIR-responsive MXene nanobelts for wound healing. NPG Asia Mater. 2021, 13, 24. [Google Scholar] [CrossRef]
- Dong, Y.; Li, S.; Li, X.; Wang, X. Smart MXene/agarose hydrogel with photothermal property for controlled drug release. Int. J. Biol. Macromol. 2021, 190, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Wei, S.; He, F.; Li, Z.; Wang, H.-H.; Huang, Y.; Nie, Z. Near-infrared light-controllable MXene hydrogel for tunable on-demand release of therapeutic proteins. Acta Biomater. 2021, 130, 138–148. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, L.; Yan, J.; Liu, P.; Wen, H.; Liu, H. Folic Acid-Targeted MXene Nanoparticles for Doxorubicin Loaded Drug Delivery. Aust. J. Chem. 2021, 74, 847–855. [Google Scholar] [CrossRef]
- Bai, L.; Yi, W.; Sun, T.; Tian, Y.; Zhang, P.; Si, J.; Hou, X.; Hou, J. Surface modification engineering of two-dimensional titanium carbide for efficient synergistic multitherapy of breast cancer. J. Mater. Chem. B 2020, 8, 6402–6417. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y.; Qian, Z.; Yang, Z.; Zong, S.; Wang, Z.; Cui, Y. A Ti2N MXene-based nanosystem with ultrahigh drug loading for dual-strategy synergistic oncotherapy. Nanoscale 2021, 13, 18546–18557. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Jouyandeh, M.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M.; Shokouhimehr, M.; Varma, R.S. Natural Polymers Decorated MOF-MXene Nanocarriers for Co-delivery of Doxorubicin/pCRISPR. ACS Appl. Bio Mater. 2021, 4, 5106–5121. [Google Scholar] [CrossRef]
- Jain, V.; Jain, S.; Mahajan, S.C. Nanomedicines Based Drug Delivery Systems for Anti-Cancer Targeting and Treatment. Curr. Drug Deliv. 2015, 12, 177–191. [Google Scholar] [CrossRef]
- Kumar, P.; Srivastava, R. Nanomedicine for Cancer Therapy: From Chemotherapeutic to Hyperthermia-Based Therapy; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Havel, H.A. Where Are the Nanodrugs? An Industry Perspective on Development of Drug Products Containing Nanomaterials. AAPS J. 2016, 18, 1351–1353. [Google Scholar] [CrossRef]
- Caster, J.M.; Patel, A.N.; Zhang, T.; Wang, A. Investigational nanomedicines in 2016: A review of nanotherapeutics currently undergoing clinical trials. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1416. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. MXenes for Cancer Therapy and Diagnosis: Recent Advances and Current Challenges. ACS Biomater. Sci. Eng. 2021, 7, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Szuplewska, A.; Kulpińska, D.; Dybko, A.; Jastrzębska, A.M.; Wojciechowski, T.; Rozmysłowska, A.; Chudy, M.; Grabowska-Jadach, I.; Ziemkowska, W.; Brzózka, Z.; et al. 2D Ti2C(MXene) as a novel highly efficient and selective agent for photothermal therapy. Mater. Sci. Eng. C 2019, 98, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, R.; Zhou, Y.; Ding, L.; Gao, X.; Zhou, B.; Hu, P.; Chen, Y. Ultrathin Molybdenum Carbide MXene with Fast Biodegradability for Highly Efficient Theory-Oriented Photonic Tumor Hyperthermia. Adv. Funct. Mater. 2019, 29, 1901942. [Google Scholar] [CrossRef]
- Dai, C.; Chen, Y.; Jing, X.; Xiang, L.; Yang, D.; Lin, H.; Liu, Z.; Han, X.; Wu, R. Two-Dimensional Tantalum Carbide (MXenes) Composite Nanosheets for Multiple Imaging-Guided Photothermal Tumor Ablation. ACS Nano 2017, 11, 12696–12712. [Google Scholar] [CrossRef]
- Yin, H.; Guan, X.; Lin, H.; Pu, Y.; Fang, Y.; Yue, W.; Zhou, B.; Wang, Q.; Chen, Y.; Xu, H. Nanomedicine-Enabled Photonic Thermogaseous Cancer Therapy. Adv. Sci. 2020, 7, 1901954. [Google Scholar] [CrossRef]
- Kong, W.; Niu, Y.; Liu, M.; Zhang, K.; Xu, G.; Wang, Y.; Wang, X.; Xu, Y.; Li, J. One-step hydrothermal synthesis of fluorescent MXene-like titanium carbonitride quantum dots. Inorg. Chem. Commun. 2019, 105, 151–157. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, T.; Zhang, K.; Meng, X.; Dai, W.; Wang, D.; Dong, H.; Zhang, X. Engineered Exosome-Mediated Near-Infrared-II Region V2C Quantum Dot Delivery for Nucleus-Target Low-Temperature Photothermal Therapy. ACS Nano 2019, 13, 1499–1510. [Google Scholar] [CrossRef]
- Korupalli, C.; You, K.-L.; Getachew, G.; Rasal, A.S.; Dirersa, W.B.; Zakki Fahmi, M.; Chang, J.-Y. Engineering the surface of Ti3C2 MXene nanosheets for high stability and multimodal anticancer therapy. Pharmaceutics 2022, 14, 304. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, D.; Dong, C.; Huang, H.; Feng, G.; Chen, Q.; Zheng, Y.; Tang, H.; Chen, Y.; Jing, X. Two-Dimensional MXene-Originated In Situ Nanosonosensitizer Generation for Augmented and Synergistic Sonodynamic Tumor Nanotherapy. ACS Nano 2022. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; An, J.; Sedgwick, A.C.; Li, M.; Xie, J.; Hu, W.; Kang, J.; Sen, S.; Steinbrueck, A.; et al. 2D-ultrathin MXene/DOXjade platform for iron chelation chemo-photothermal therapy. Bioact. Mater. 2022, 14, 76–85. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.; Hou, X.; Feng, M.; Cao, Z.; Liu, J. Functionalized 2D Nb2C nanosheets for primary and recurrent cancer photothermal/immune-therapy in the NIR-II biowindow. Nanoscale 2021, 13, 17822–17836. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tang, X.; Liu, Q.; Xia, Y.; Zhai, X.; Zhang, H.; Duan, D.; Wang, H.; Zhan, W.; Wu, L.; et al. Metallic Carbonitride MXene Based Photonic Hyperthermia for Tumor Therapy. Small 2022, 18, 2200646. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, X.; Xu, W.; Sun, K.-y.; Wang, Z.; Lv, Z.; Wang, Y.; Wang, Y.; Zhong, W.; Wei, J.; et al. NIR-Activated Multimodal Photothermal/Chemodynamic/Magnetic Resonance Imaging Nanoplatform for Anticancer Therapy by Fe(II) Ions Doped MXenes (Fe-Ti3C2). Small 2021, 17, 2101705. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, L.; Lu, Y.; Tang, J.; Lv, Q.; Chen, X.; Chen, Y.; Liu, J. A MXene-Based Bionic Cascaded-Enzyme Nanoreactor for Tumor Phototherapy/Enzyme Dynamic Therapy and Hypoxia-Activated Chemotherapy. Nano-Micro Lett. 2021, 14, 22. [Google Scholar] [CrossRef]
- Shurbaji, S.; Manaph, N.P.A.; Ltaief, S.M.; Al-Shammari, A.R.; Elzatahry, A.; Yalcin, H.C. Characterization of MXene as a Cancer Photothermal Agent Under Physiological Conditions. Front. Nanosci. 2021, 3, 689718. [Google Scholar] [CrossRef]
- Han, X.; Jing, X.; Yang, D.; Lin, H.; Wang, Z.; Ran, H.; Li, P.; Chen, Y. Therapeutic mesopore construction on 2D Nb2C MXenes for targeted and enhanced chemo-photothermal cancer therapy in NIR-II biowindow. Theranostics 2018, 8, 4491–4508. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, W.; Yang, C.; Wang, F.; Song, C.; Gao, Y.; Qiu, Y.; Yan, M.; Yang, B.; Guo, C. The theranostic nanoagent Mo2C for multi-modal imaging-guided cancer synergistic phototherapy. Biomater. Sci. 2019, 7, 2729–2739. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [Green Version]
- Arabi Shamsabadi, A.; Sharifian Gh, M.; Anasori, B.; Soroush, M. Antimicrobial Mode-of-Action of Colloidal Ti3C2Tx MXene Nanosheets. ACS Sustain. Chem. Eng. 2018, 6, 16586–16596. [Google Scholar] [CrossRef]
- Khatami, M.; Iravani, P.; Jamalipour Soufi, G.; Iravani, S. MXenes for antimicrobial and antiviral applications: Recent advances. Mater. Technol. 2021, 1–16. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Karwowska, E.; Wojciechowski, T.; Ziemkowska, W.; Rozmysłowska, A.; Chlubny, L.; Olszyna, A. The Atomic Structure of Ti2C and Ti3C2 MXenes is Responsible for Their Antibacterial Activity toward E. coli Bacteria. J. Mater. Eng. Perform. 2019, 28, 1272–1277. [Google Scholar] [CrossRef]
- Peng, C.; Wang, H.; Yu, H.; Peng, F. (111) TiO2−x/Ti3C2: Synergy of active facets, interfacial charge transfer and Ti3+ doping for enhance photocatalytic activity. Mater. Res. Bull. 2017, 89, 16–25. [Google Scholar] [CrossRef]
- Huang, K.C.; Mukhopadhyay, R.; Wen, B.; Gitai, Z.; Wingreen, N.S. Cell shape and cell-wall organization in Gram-negative bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 19282–19287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tocheva, E.I.; López-Garrido, J.; Hughes, H.V.; Fredlund, J.; Kuru, E.; Vannieuwenhze, M.S.; Brun, Y.V.; Pogliano, K.; Jensen, G.J. Peptidoglycan transformations during Bacillus subtilis sporulation. Mol. Microbiol. 2013, 88, 673–686. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wang, S.; Wu, H.; Liu, Y.; Xu, F.; Zhao, J. A multimodal antimicrobial platform based on MXene for treatment of wound infection. Colloids Surf. B Biointerfaces 2021, 207, 111979. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Han, Q.; Yin, J.; Zhang, J.; Yu, Y.; Yang, W.; Deng, Y. Synergism of 2D/1D MXene/cobalt nanowire heterojunctions for boosted photo-activated antibacterial application. Chem. Eng. J. 2021, 410, 128209. [Google Scholar] [CrossRef]
- Lu, S.; Meng, G.; Wang, C.; Chen, H. Photocatalytic inactivation of airborne bacteria in a polyurethane foam reactor loaded with a hybrid of MXene and anatase TiO2 exposing {001} facets. Chem. Eng. J. 2021, 404, 126526. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Liu, X.; Li, C.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Zhu, S.; Hu, W.; et al. Interfacial engineering of Bi2S3/Ti3C2Tx MXene based on work function for rapid photo-excited bacteria-killing. Nat. Commun. 2021, 12, 1224. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Street, R.M.; McDaniel, R.M.; Barsoum, M.W.; Schauer, C.L. Antibacterial properties of electrospun Ti3C2Tz(MXene)/chitosan nanofibers. RSC Adv. 2018, 8, 35386–35394. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.P.; Rasheed, P.A.; Gomez, T.; Rasool, K.; Ponraj, J.; Prenger, K.; Naguib, M.; Mahmoud, K.A. Effect of Sheet Size and Atomic Structure on the Antibacterial Activity of Nb-MXene Nanosheets. ACS Appl. Nano Mater. 2020, 3, 11372–11382. [Google Scholar] [CrossRef]
- Wang, W.; Feng, H.; Liu, J.; Zhang, M.; Liu, S.; Feng, C.; Chen, S. A photo catalyst of cuprous oxide anchored MXene nanosheet for dramatic enhancement of synergistic antibacterial ability. Chem. Eng. J. 2020, 386, 124116. [Google Scholar] [CrossRef]
- Zheng, K.; Li, S.; Jing, L.; Chen, P.-Y.; Xie, J. Synergistic Antimicrobial Titanium Carbide (MXene) Conjugated with Gold Nanoclusters. Adv. Healthc. Mater. 2020, 9, 2001007. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, F.; Sharifian, M.; Attanayake, N.H.; Thenuwara, A.C.; Gogotsi, Y.; Anasori, B.; Strongin, D.R. Antimicrobial Properties of 2D MnO2 and MoS2 Nanomaterials Vertically Aligned on Graphene Materials and Ti3C2 MXene. Langmuir 2018, 34, 7192–7200. [Google Scholar] [CrossRef] [PubMed]
- Mansoorianfar, M.; Shahin, K.; Hojjati-Najafabadi, A.; Pei, R. MXene–laden bacteriophage: A new antibacterial candidate to control bacterial contamination in water. Chemosphere 2022, 290, 133383. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Jia, K.; Abraha, B.S.; Li, Y.; Peng, W.; Zhang, F.; Fan, X.; Zhang, L. A near-infrared light-mediated antimicrobial based on Ag/Ti3C2Tx for effective synergetic antibacterial applications. Nanoscale 2020, 12, 19129–19141. [Google Scholar] [CrossRef]
- He, Q.; Hu, H.; Han, J.; Zhao, Z. Double transition-metal TiVCTX MXene with dual-functional antibacterial capability. Mater. Lett. 2022, 308, 131100. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, M.; Ma, Z.; Xu, X.; Mohesen Seraji, S.; Yu, B.; Sun, Z.; Wang, H.; Song, P. A reactive copper-organophosphate-MXene heterostructure enabled antibacterial, self-extinguishing and mechanically robust polymer nanocomposites. Chem. Eng. J. 2022, 430, 132712. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, Y.; Yang, S.; Mo, A.; Zeng, X.; Chen, Q.; Peng, Q. Size-dependent photothermal antibacterial activity of Ti3C2Tx MXene nanosheets against methicillin-resistant Staphylococcus aureus. J. Colloid Interface Sci. 2022, 617, 533–541. [Google Scholar] [CrossRef]
- Li, Y.; Han, M.; Cai, Y.; Jiang, B.; Zhang, Y.; Yuan, B.; Zhou, F.; Cao, C. Muscle-inspired MXene/PVA hydrogel with high toughness and photothermal therapy for promoting bacteria-infected wound healing. Biomater. Sci. 2022, 10, 1068–1082. [Google Scholar] [CrossRef]
- Zada, S.; Lu, H.; Yang, F.; Zhang, Y.; Cheng, Y.; Tang, S.; Wei, W.; Qiao, Y.; Fu, P.; Dong, H.; et al. V2C Nanosheets as Dual-Functional Antibacterial Agents. ACS Appl. Bio Mater. 2021, 4, 4215–4223. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cheng, Y.; Wei, Q.; Zhao, Y.; Zhang, W.; Yang, Y.; Li, D. Multifunctional Biomass Composite Aerogel Co-Modified by MXene and Ag Nanowires for Health Monitoring and Synergistic Antibacterial Applications. Appl. Surf. Sci. 2022, 598, 153783. [Google Scholar] [CrossRef]
- Rasool, K.; Mahmoud, K.A.; Johnson, D.J.; Helal, M.; Berdiyorov, G.R.; Gogotsi, Y. Efficient Antibacterial Membrane based on Two-Dimensional Ti3C2Tx(MXene) Nanosheets. Sci. Rep. 2017, 7, 1598. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Aneesh, K.; Vusa, C.S.R.; Berchmans, S. Dual enzyme mimicry exhibited by ITO nanocubes and their application in spectrophotometric and electrochemical sensing. Analyst 2016, 141, 4024–4028. [Google Scholar] [CrossRef]
- Koyappayil, A.; Yeon, S.-H.; Chavan, S.G.; Jin, L.; Go, A.; Lee, M.-H. Efficient and rapid synthesis of ultrathin nickel-metal organic framework nanosheets for the sensitive determination of glucose. Microchem. J. 2022, 179, 107462. [Google Scholar] [CrossRef]
- Ganpat Chavan, S.; Kumar Yagati, A.; Koyappayil, A.; Go, A.; Yeon, S.; Lee, M.-H. Recombinant Histidine-Tagged Nano-protein-based Highly Sensitive Electro-Sensing Device for Salivary Cortisol. Bioelectrochemistry 2022, 144, 108046. [Google Scholar] [CrossRef]
- Koyappayil, A.; Kim, H.T.; Lee, M.-H. An efficient and rapid synthesis route to highly fluorescent copper microspheres for the selective and sensitive excitation wavelength-dependent dual-mode sensing of NADH. Sens. Actuators B Chem. 2021, 327, 128887. [Google Scholar] [CrossRef]
- Koyappayil, A.; Berchmans, S.; Lee, M.-H. Dual enzyme-like properties of silver nanoparticles decorated Ag2WO4 nanorods and its application for H2O2 and glucose sensing. Colloids Surf. B Biointerfaces 2020, 189, 110840. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, N.; Xu, Q.; Dai, Y.; Wang, Z. Recent Advances in Flexible Tactile Sensors for Intelligent Systems. Sensors 2021, 21, 5392. [Google Scholar] [CrossRef]
- Shen, G. Recent advances of flexible sensors for biomedical applications. Prog. Nat. Sci. Mater. Int. 2021, 31, 872–882. [Google Scholar] [CrossRef]
- Koyappayil, A.; Lee, M.-H. Ultrasensitive Materials for Electrochemical Biosensor Labels. Sensors 2021, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lou, Z.; Jiang, K.; Shen, G. Bio-Multifunctional Smart Wearable Sensors for Medical Devices. Adv. Intell. Syst. 2019, 1, 1900040. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Feng, C.; Ponnamma, D.; Yi, Z.; Parameswaranpillai, J.; Thomas, S.; Salim, N.V. Review on exploration of graphene in the design and engineering of smart sensors, actuators and soft robotics. Chem. Eng. J. Adv. 2020, 4, 100034. [Google Scholar] [CrossRef]
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-Enabled Wearable Sensors for Healthcare. Adv. Healthc. Mater. 2018, 7, 1700889. [Google Scholar] [CrossRef]
- Davis, F.; Shimizu, F.M.; Altintas, Z. Smart Nanomaterials. In Biosensors and Nanotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 219–276. [Google Scholar]
- Oliveira, O.N.; Iost, R.M.; Siqueira, J.R.; Crespilho, F.N.; Caseli, L. Nanomaterials for Diagnosis: Challenges and Applications in Smart Devices Based on Molecular Recognition. ACS Appl. Mater. Interfaces 2014, 6, 14745–14766. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Quan, X. Interface Engineering Catalytic Graphene for Smart Colorimetric Biosensing. ACS Nano 2012, 6, 3142–3151. [Google Scholar] [CrossRef]
- Wang, D.; Li, D.; Zhao, M.; Xu, Y.; Wei, Q. Multifunctional wearable smart device based on conductive reduced graphene oxide/polyester fabric. Appl. Surf. Sci. 2018, 454, 218–226. [Google Scholar] [CrossRef]
- Luo, J.; Gao, S.; Luo, H.; Wang, L.; Huang, X.; Guo, Z.; Lai, X.; Lin, L.; Li, R.K.Y.; Gao, J. Superhydrophobic and breathable smart MXene-based textile for multifunctional wearable sensing electronics. Chem. Eng. J. 2021, 406, 126898. [Google Scholar] [CrossRef]
- Yuen, A.C.Y.; Chen, T.B.Y.; Lin, B.; Yang, W.; Kabir, I.I.; De Cachinho Cordeiro, I.M.; Whitten, A.E.; Mata, J.; Yu, B.; Lu, H.-D.; et al. Study of structure morphology and layer thickness of Ti3C2 MXene with Small-Angle Neutron Scattering (SANS). JCOMC Compos. Part C Open Access 2021, 5, 100155. [Google Scholar] [CrossRef]
- Kumar, S.; Kang, D.; Nguyen, V.H.; Nasir, N.; Hong, H.; Kim, M.; Nguyen, D.C.; Lee, Y.-J.; Lee, N.; Seo, Y. Application of Titanium-Carbide MXene-Based Transparent Conducting Electrodes in Flexible Smart Windows. ACS Appl. Mater. Interfaces 2021, 13, 40976–40985. [Google Scholar] [CrossRef] [PubMed]
- Firestein, K.L.; von Treifeldt, J.E.; Kvashnin, D.G.; Fernando, J.F.S.; Zhang, C.; Kvashnin, A.G.; Podryabinkin, E.V.; Shapeev, A.V.; Siriwardena, D.P.; Sorokin, P.B.; et al. Young’s Modulus and Tensile Strength of Ti3C2 MXene Nanosheets As Revealed by In Situ TEM Probing, AFM Nanomechanical Mapping, and Theoretical Calculations. Nano Lett. 2020, 20, 5900–5908. [Google Scholar] [CrossRef]
- Jin, C.; Bai, Z. MXene-Based Textile Sensors for Wearable Applications. ACS Sens. 2022, 7, 929–950. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.-H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-Based Wearable Biosensor System for High-Performance In Vitro Perspiration Analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, H.; Li, X.; Lu, Y.; Wu, Y.; He, Y.; Chen, Q.; Liu, Q.; Han, R.P.S. MXene/MWCNT electronic fabric with enhanced mechanical robustness on humidity sensing for real-time respiration monitoring. Sens. Actuators B Chem. 2022, 361, 131704. [Google Scholar] [CrossRef]
- Chao, M.; He, L.; Gong, M.; Li, N.; Li, X.; Peng, L.; Shi, F.; Zhang, L.; Wan, P. Breathable Ti3C2Tx MXene/Protein Nanocomposites for Ultrasensitive Medical Pressure Sensor with Degradability in Solvents. ACS Nano 2021, 15, 9746–9758. [Google Scholar] [CrossRef]
- Sharma, S.; Chhetry, A.; Sharifuzzaman, M.; Yoon, H.; Park, J.Y. Wearable Capacitive Pressure Sensor Based on MXene Composite Nanofibrous Scaffolds for Reliable Human Physiological Signal Acquisition. ACS Appl. Mater. Interfaces 2020, 12, 22212–22224. [Google Scholar] [CrossRef]
- Li, Q.; Yin, R.; Zhang, D.; Liu, H.; Chen, X.; Zheng, Y.; Guo, Z.; Liu, C.; Shen, C. Flexible conductive MXene/cellulose nanocrystal coated nonwoven fabrics for tunable wearable strain/pressure sensors. J. Mater. Chem. A 2020, 8, 21131–21141. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Z.; Ma, X.; He, G.; Xu, T.; Tan, J.; Wang, L.; Zhang, X.; Qu, L.; Zhang, X. A lightweight MXene-Coated nonwoven fabric with excellent flame Retardancy, EMI Shielding, and Electrothermal/Photothermal conversion for wearable heater. Chem. Eng. J. 2022, 430, 132605. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Li, M.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. MXene-Coated Air-Permeable Pressure-Sensing Fabric for Smart Wear. ACS Appl. Mater. Interfaces 2020, 12, 46446–46454. [Google Scholar] [CrossRef]
- Zheng, X.; Nie, W.; Hu, Q.; Wang, X.; Wang, Z.; Zou, L.; Hong, X.; Yang, H.; Shen, J.; Li, C. Multifunctional RGO/Ti3C2Tx MXene fabrics for electrochemical energy storage, electromagnetic interference shielding, electrothermal and human motion detection. Mater. Des. 2021, 200, 109442. [Google Scholar] [CrossRef]
- Adepu, V.; Kunchur, A.; Tathacharya, M.; Mattela, V.; Sahatiya, P. SnS/Ti3C2Tx(MXene) Nanohybrid-Based Wearable Electromechanical Sensors for Sign-to-Text Translation and Sitting Posture Analysis. ACS Appl. Electron. Mater. 2022, 4, 1756–1768. [Google Scholar] [CrossRef]
- Wang, L.; Tian, M.; Zhang, Y.; Sun, F.; Qi, X.; Liu, Y.; Qu, L. Helical core-sheath elastic yarn-based dual strain/humidity sensors with MXene sensing layer. J. Mater. Sci. 2020, 55, 6187–6194. [Google Scholar] [CrossRef]
- Uzun, S.; Seyedin, S.; Stoltzfus, A.L.; Levitt, A.S.; Alhabeb, M.; Anayee, M.; Strobel, C.J.; Razal, J.M.; Dion, G.; Gogotsi, Y. Knittable and Washable Multifunctional MXene-Coated Cellulose Yarns. Adv. Funct. Mater. 2019, 29, 1905015. [Google Scholar] [CrossRef]
- Li, H.; Du, Z. Preparation of a Highly Sensitive and Stretchable Strain Sensor of MXene/Silver Nanocomposite-Based Yarn and Wearable Applications. ACS Appl. Mater. Interfaces 2019, 11, 45930–45938. [Google Scholar] [CrossRef]
- Sha, B.; Zhao, S.; Gu, M.; Zhao, G.; Wang, L.; Bi, G.-Q.; Du, Z. Multi-Functionalized Self-Bonding MXene for Minimal-invasive Jet-injected Neural Interface and Tissue Healing. bioRxiv 2021. [Google Scholar] [CrossRef]
- Seyedin, S.; Uzun, S.; Levitt, A.; Anasori, B.; Dion, G.; Gogotsi, Y.; Razal, J.M. MXene Composite and Coaxial Fibers with High Stretchability and Conductivity for Wearable Strain Sensing Textiles. Adv. Funct. Mater. 2020, 30, 1910504. [Google Scholar] [CrossRef]
- Wang, H.; Zou, Y.; Ji, Y.; Zhong, K.; Du, X.; Du, Z.; Cheng, X.; Wang, S. Tough and extremely temperature-tolerance nanocomposite organohydrogels as ultrasensitive wearable sensors for wireless human motion monitoring. Compos.-A Appl. Sci. Manuf. 2022, 157, 106905. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Y.; Wang, F.; Liu, M.; Liu, P.; Li, C.; Yu, Y.; Xiao, X.; Feng, Q. Ultra-stretchable, adhesive, and self-healing MXene/polyampholytes hydrogel as flexible and wearable epidermal sensors. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128897. [Google Scholar] [CrossRef]
- Zhang, S.; Tu, T.; Li, T.; Cai, Y.; Wang, Z.; Zhou, Y.; Wang, D.; Fang, L.; Ye, X.; Liang, B. 3D MXene/PEDOT:PSS Composite Aerogel with a Controllable Patterning Property for Highly Sensitive Wearable Physical Monitoring and Robotic Tactile Sensing. ACS Appl. Mater. Interfaces 2022, 14, 23877–23887. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R. Biomarkers: Potential uses and limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Alwarappan, S.; Nesakumar, N.; Sun, D.; Hu, T.Y.; Li, C.-Z. 2D metal carbides and nitrides (MXenes) for sensors and biosensors. Biosens. Bioelectron. 2022, 205, 113943. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, J.; Zhang, S.; Dai, H.; Yan, J.; Lv, L. A portable multi-signal readout sensing platform based on plasmonic MXene induced signal amplification for point of care biomarker detection. Sens. Actuators B Chem. 2022, 352, 131059. [Google Scholar] [CrossRef]
- Kumar, S.; Lei, Y.; Alshareef, N.H.; Quevedo-Lopez, M.A.; Salama, K.N. Biofunctionalized two-dimensional Ti3C2 MXenes for ultrasensitive detection of cancer biomarker. Biosens. Bioelectron. 2018, 121, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Kalkal, A.; Kadian, S.; Kumar, S.; Manik, G.; Sen, P.; Kumar, S.; Packirisamy, G. Ti3C2-MXene decorated with nanostructured silver as a dual-energy acceptor for the fluorometric neuron specific enolase detection. Biosens. Bioelectron. 2022, 195, 113620. [Google Scholar] [CrossRef]
- Xia, T.; Liu, G.; Wang, J.; Hou, S.; Hou, S. MXene-based enzymatic sensor for highly sensitive and selective detection of cholesterol. Biosens. Bioelectron. 2021, 183, 113243. [Google Scholar] [CrossRef]
- Sharifuzzaman, M.; Barman, S.C.; Zahed, M.A.; Sharma, S.; Yoon, H.; Nah, J.S.; Kim, H.; Park, J.Y. An Electrodeposited MXene-Ti3C2Tx Nanosheets Functionalized by Task-Specific Ionic Liquid for Simultaneous and Multiplexed Detection of Bladder Cancer Biomarkers. Small 2020, 16, 2002517. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Q.; Ren, S.-N.; Liu, Z.; Chen, F.-F. Ti3C2-MXene-assisted signal amplification for sensitive and selective surface plasmon resonance biosensing of biomarker. Chin. J. Anal. Chem. 2022, 50, 13–18. [Google Scholar] [CrossRef]
- Chen, F.; Lu, Q.; Zhang, Y.; Yao, S. Strand displacement dual amplification miRNAs strategy with FRET between NaYF4: Yb, Tm/Er upconversion nanoparticles and Ti3C2 nanosheets. Sens. Actuators B Chem. 2019, 297, 126751. [Google Scholar] [CrossRef]
- Zhou, S.; Gu, C.; Li, Z.; Yang, L.; He, L.; Wang, M.; Huang, X.; Zhou, N.; Zhang, Z. Ti3C2Tx MXene and polyoxometalate nanohybrid embedded with polypyrrole: Ultra-sensitive platform for the detection of osteopontin. Appl. Surf. Sci. 2019, 498, 143889. [Google Scholar] [CrossRef]
- Wu, Q.; Li, N.; Wang, Y.; Xu, Y.; Wei, S.; Wu, J.; Jia, G.; Fang, X.; Chen, F.; Cui, X. A 2D transition metal carbide MXene-based SPR biosensor for ultrasensitive carcinoembryonic antigen detection. Biosens. Bioelectron. 2019, 144, 111697. [Google Scholar] [CrossRef] [PubMed]
- Kashefi-Kheyrabadi, L.; Koyappayil, A.; Kim, T.; Cheon, Y.-P.; Lee, M.-H. A MoS2@Ti3C2Tx MXene hybrid-based electrochemical aptasensor (MEA) for sensitive and rapid detection of Thyroxine. Bioelectrochemistry 2021, 137, 107674. [Google Scholar] [CrossRef] [PubMed]
- Mohammadniaei, M.; Koyappayil, A.; Sun, Y.; Min, J.; Lee, M.-H. Gold nanoparticle/MXene for multiple and sensitive detection of oncomiRs based on synergetic signal amplification. Biosens. Bioelectron. 2020, 159, 112208. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, J.; Lu, L.; Yang, X.; Xia, J.; Zhang, F.; Wang, Z. Competitive electrochemical aptasensor based on a cDNA-ferrocene/MXene probe for detection of breast cancer marker Mucin. Anal. Chim. Acta 2020, 1094, 18–25. [Google Scholar] [CrossRef]
- Duan, F.; Guo, C.; Hu, M.; Song, Y.; Wang, M.; He, L.; Zhang, Z.; Pettinari, R.; Zhou, L. Construction of the 0D/2D heterojunction of Ti3C2Tx MXene nanosheets and iron phthalocyanine quantum dots for the impedimetric aptasensing of microRNA-155. Sens. Actuators B Chem. 2020, 310, 127844. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, H.; Chai, Y.; Yuan, R.; Liu, H. Ti3C2/BiVO4 Schottky junction as a signal indicator for ultrasensitive photoelectrochemical detection of VEGF165. Chem. Commun. 2019, 55, 13729–13732. [Google Scholar] [CrossRef]
- Liu, M.; He, Y.; Zhou, J.; Ge, Y.; Zhou, J.; Song, G. A “naked-eye” colorimetric and ratiometric fluorescence probe for uric acid based on Ti3C2 MXene quantum dots. Anal. Chim. Acta 2020, 1103, 134–142. [Google Scholar] [CrossRef]
- Hroncekova, S.; Bertok, T.; Hires, M.; Jane, E.; Lorencova, L.; Vikartovska, A.; Tanvir, A.; Kasak, P.; Tkac, J. Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples. Processes 2020, 8, 580. [Google Scholar] [CrossRef]
- Zheng, J.; Diao, J.; Jin, Y.; Ding, A.; Wang, B.; Wu, L.; Weng, B.; Chen, J. An Inkjet Printed Ti3C2-GO Electrode for the Electrochemical Sensing of Hydrogen Peroxide. J. Electrochem. Soc. 2018, 165, B227–B231. [Google Scholar] [CrossRef]
- Shankar, S.S.; Shereema, R.M.; Rakhi, R.B. Electrochemical Determination of Adrenaline Using MXene/Graphite Composite Paste Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 43343–43351. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.L.; Mayorga-Martinez, C.C.; Antonatos, N.; Sofer, Z.; Gonzalez-Julian, J.J.; Webster, R.D.; Pumera, M. MXene Titanium Carbide-based Biosensor: Strong Dependence of Exfoliation Method on Performance. Anal. Chem. 2020, 92, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, X.; Zhang, R.; Zhang, Y.; Wu, L.; Lu, W.; Li, J.; Li, Y.; Zhang, H. MXene-Enabled Electrochemical Microfluidic Biosensor: Applications toward Multicomponent Continuous Monitoring in Whole Blood. Adv. Funct. Mater. 2019, 29, 1807326. [Google Scholar] [CrossRef]
- Laochai, T.; Yukird, J.; Promphet, N.; Qin, J.; Chailapakul, O.; Rodthongkum, N. Non-invasive electrochemical immunosensor for sweat cortisol based on L-cys/AuNPs/MXene modified thread electrode. Biosens. Bioelectron. 2022, 203, 114039. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gao, H.; Yuan, R.; Xiang, Y. Trimetallic nanoparticle-decorated MXene nanosheets for catalytic electrochemical detection of carcinoembryonic antigen via Exo III-aided dual recycling amplifications. Sens. Actuators B Chem. 2022, 359, 131617. [Google Scholar] [CrossRef]
- Koyappayil, A.; Chavan, S.G.; Mohammadniaei, M.; Go, A.; Hwang, S.Y.; Lee, M.-H. β-Hydroxybutyrate dehydrogenase decorated MXene nanosheets for the amperometric determination of β-hydroxybutyrate. Microchim. Acta 2020, 187, 277. [Google Scholar] [CrossRef] [PubMed]
- Rakhi, R.B.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef] [Green Version]
- Bao-Kai, M.A.; Mian, L.I.; Ling-Zhi, C.; Xin-Chu, W.; Cai, S.; Qing, H. Enzyme-MXene Nanosheets: Fabrication and Application in Electrochemical Detection of H2O2. J. Inorg. Mater. 2019, 35, 131. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhang, X.; Pei, L.; Yue, S.; Ma, L.; Zhou, L.; Huang, Z.; He, Y.; Gao, J. Silver nanoparticles modified two-dimensional transition metal carbides as nanocarriers to fabricate acetycholinesterase-based electrochemical biosensor. Chem. Eng. J. 2018, 339, 547–556. [Google Scholar] [CrossRef]
- Wu, L.; Lu, X.; Dhanjai; Wu, Z.-S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef]
- Wang, G.; Sun, J.; Yao, Y.; An, X.; Zhang, H.; Chu, G.; Jiang, S.; Guo, Y.; Sun, X.; Liu, Y. Detection of Inosine Monophosphate (IMP) in Meat Using Double-Enzyme Sensor. Food Anal. Methods 2020, 13, 420–432. [Google Scholar] [CrossRef]
- Ding, C.; Liang, J.; Zhou, Z.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Photothermal enhanced enzymatic activity of lipase covalently immobilized on functionalized Ti3C2TX nanosheets. Chem. Eng. J. 2019, 378, 122205. [Google Scholar] [CrossRef]
- Gu, M.; Liu, Y.; Chen, T.; Du, F.; Zhao, X.; Xiong, C.; Zhou, Y. Is graphene a promising nano-material for promoting surface modification of implants or scaffold materials in bone tissue engineering? Tissue Eng. Part B Rev. 2014, 20, 477–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Controlled Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Han, S.S. Molybdenum disulfide (MoS2)-based nanostructures for tissue engineering applications: Prospects and challenges. J. Mater. Chem. B 2022, 10, 2761–2780. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. MXenes and MXene-based materials for tissue engineering and regenerative medicine: Recent advances. Mater. Adv. 2021, 2, 2906–2917. [Google Scholar] [CrossRef]
- Rafieerad, A.; Sequiera, G.L.; Yan, W.; Kaur, P.; Amiri, A.; Dhingra, S. Sweet-MXene hydrogel with mixed-dimensional components for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 101, 103440. [Google Scholar] [CrossRef]
- Chen, K.; Chen, Y.; Deng, Q.; Jeong, S.-H.; Jang, T.-S.; Du, S.; Kim, H.-E.; Huang, Q.; Han, C.-M. Strong and biocompatible poly(lactic acid) membrane enhanced by Ti3C2Tz(MXene) nanosheets for Guided bone regeneration. Mater. Lett. 2018, 229, 114–117. [Google Scholar] [CrossRef]
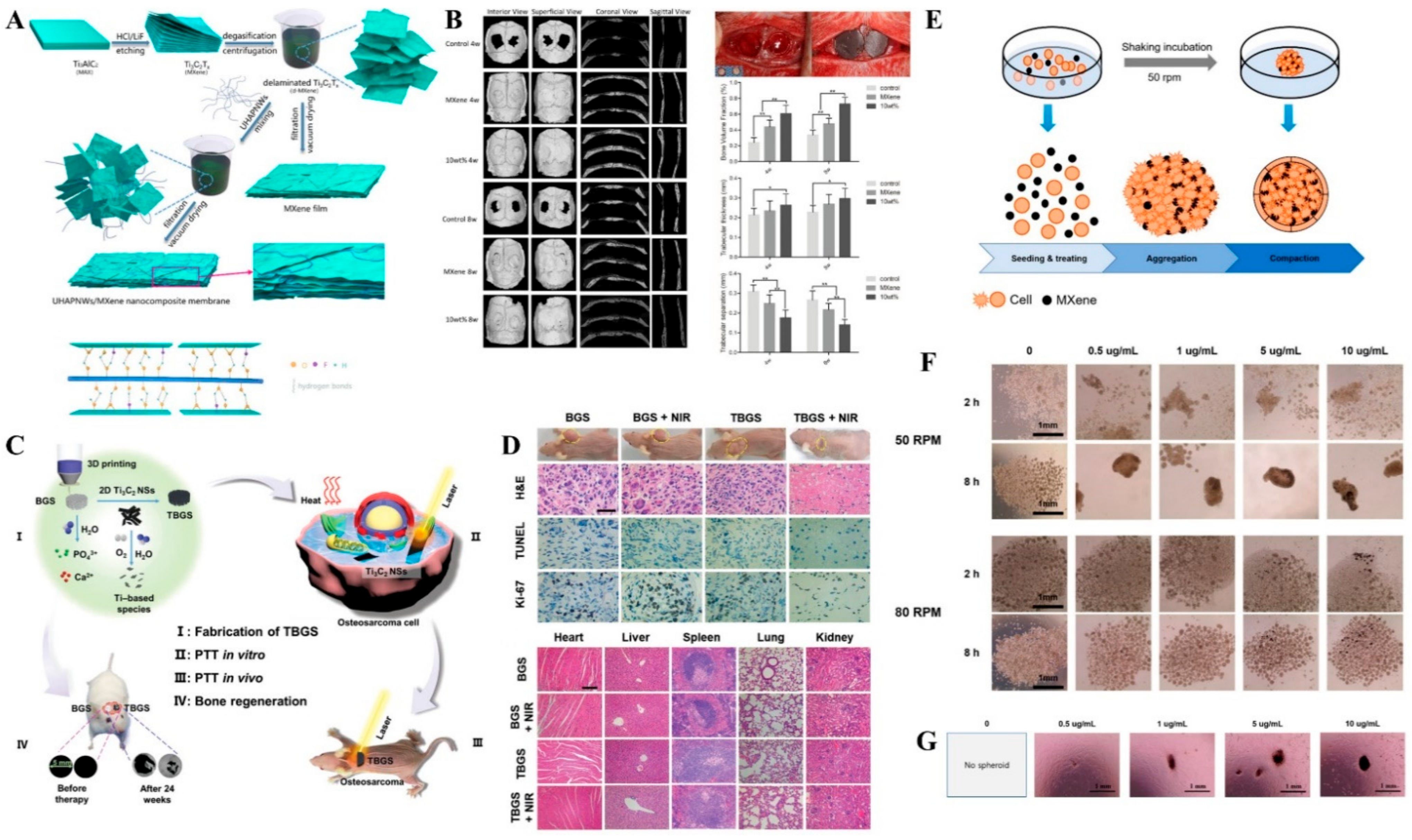
- Jang, J.; Lee, E.-J. Rapid Formation of Stem Cell Spheroids Using Two-Dimensional MXene Particles. Processes 2021, 9, 957. [Google Scholar] [CrossRef]
- Awasthi, G.P.; Maharjan, B.; Shrestha, S.; Bhattarai, D.P.; Yoon, D.; Park, C.H.; Kim, C.S. Synthesis, characterizations, and biocompatibility evaluation of polycaprolactone–MXene electrospun fibers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124282. [Google Scholar] [CrossRef]
- Huang, R.; Chen, X.; Dong, Y.; Zhang, X.; Wei, Y.; Yang, Z.; Li, W.; Guo, Y.; Liu, J.; Yang, Z.; et al. MXene Composite Nanofibers for Cell Culture and Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Basara, G.; Saeidi-Javash, M.; Ren, X.; Bahcecioglu, G.; Wyatt, B.C.; Anasori, B.; Zhang, Y.; Zorlutuna, P. Electrically conductive 3D printed Ti3C2Tx MXene-PEG composite constructs for cardiac tissue engineering. Acta Biomater. 2022, 139, 179–189. [Google Scholar] [CrossRef]
- Pan, S.; Yin, J.; Yu, L.; Zhang, C.; Zhu, Y.; Gao, Y.; Chen, Y. 2D MXene-Integrated 3D-Printing Scaffolds for Augmented Osteosarcoma Phototherapy and Accelerated Tissue Reconstruction. Adv. Sci. 2020, 7, 1901511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Fu, Y.; Mo, A. Multilayered titanium carbide MXene film for guided bone regeneration. Int. J. Nanomed. 2019, 14, 10091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafieerad, A.; Yan, W.; Sequiera, G.L.; Sareen, N.; Abu-El-Rub, E.; Moudgil, M.; Dhingra, S. Application of Ti3C2 MXene Quantum Dots for Immunomodulation and Regenerative Medicine. Adv. Healthc. Mater. 2019, 8, 1900569. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, H.; Xu, T.; Zhu, D.; Yin, J.; Chen, Y.; Yu, X.; Gao, J.; Zhang, C.; Chen, Y.; et al. Engineering 2D Mesoporous Silica@MXene-Integrated 3D-Printing Scaffolds for Combinatory Osteosarcoma Therapy and NO-Augmented Bone Regeneration. Small 2020, 16, 1906814. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Litowczenko, J.; Tadyszak, K.; Natu, V.; Aparicio, C.; Peplińska, B.; Barsoum, M.W.; Otyepka, M.; Scheibe, B. Unique cellular network formation guided by heterostructures based on reduced graphene oxide-Ti3C2Tx MXene hydrogels. Acta Biomater. 2020, 115, 104–115. [Google Scholar] [CrossRef]
- Li, C.; Chu, D.; Jin, L.; Tan, G.; Li, Z. Synergistic Effect of the Photothermal Performance and Osteogenic Properties of MXene and Hydroxyapatite Nanoparticle Composite Nanofibers for Osteogenic Application. J. Biomed. Nanotechnol. 2021, 17, 2014–2020. [Google Scholar] [CrossRef]
- Li, F.; Yan, Y.; Wang, Y.; Fan, Y.; Zou, H.; Liu, H.; Luo, R.; Li, R.; Liu, H. A bifunctional MXene-modified scaffold for photothermal therapy and maxillofacial tissue regeneration. Regen. Biomater. 2021, 8, rbab057. [Google Scholar] [CrossRef]
- Yang, C.; Luo, Y.; Lin, H.; Ge, M.; Shi, J.; Zhang, X. Niobium Carbide MXene Augmented Medical Implant Elicits Bacterial Infection Elimination and Tissue Regeneration. ACS Nano 2021, 15, 1086–1099. [Google Scholar] [CrossRef]
- Cui, D.; Kong, N.; Ding, L.; Guo, Y.; Yang, W.; Yan, F. Ultrathin 2D Titanium Carbide MXene(Ti3C2Tx) Nanoflakes Activate WNT/HIF-1α-Mediated Metabolism Reprogramming for Periodontal Regeneration. Adv. Healthc. Mater. 2021, 10, 2101215. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, J.; Lin, H.; Mo, A. 2D titanium carbide(MXene) nanosheets and 1D hydroxyapatite nanowires into free standing nanocomposite membrane: In vitro and in vivo evaluations for bone regeneration. Mater. Sci. Eng. C 2021, 118, 111367. [Google Scholar] [CrossRef] [PubMed]
| MXene-Based Drug Carrier | Stimuli for Drug Release | Drug | Advantages | Ref. |
|---|---|---|---|---|
| Ti3C2Tx-SP | pH, NIR | Doxorubicin | High drug-loading capability of 211.8%. | [47] |
| Ti3C2Tx-CoNWs | pH, NIR | Doxorubicin | High drug-loading capacity of 225.05%. | [49] |
| Ti3C2Tx@GNRs/PDA/Ti3C2Tx | NIR | Doxorubicin | 95.88% drug-loading ability. | [50] |
| Ti3C2Tx/Polyacrylamide | pH | Chloramphenicol | Ti3C2Tx/Polyacrylamide hydrogels exhibited a high drug-loading of 97.5–127.7 mg/g and drug release percentages of 62.1–81.4%. | [53] |
| HAP/CS/HA/MXene/AuNRs | pH, NIR | Doxorubicin | Drug encapsulation efficiency of 83.9% | [54] |
| Polymer-coated MXene nanobelt fibers | NIR | Vitamin E | NIR-induced relaxation of the interface by the polymeric coating layer to dissolve and release Vitamin E. | [56] |
| Ti3C2Tx@Agarose hydrogel | NIR | Doxorubicin | The DOX-loaded MXene-hydrogel exhibited rapid DOX release under NIR the irradiation, while almost no DOX release when NIR was turned off, proving an NIR switch for controlled drug release. | [57] |
| MXene@Agarose | NIR | HGF | Flexible and controllable release of the protein drugs with high precision. | [58] |
| MXenes-FA-SP | pH | Doxorubicin | Drug-loading capacity of 69.9% and 48 h long drug release time. | [59] |
| Ti3C2Tx@Met@CP | pH, NIR | Metformin | The functionalized Ti3C2Tx nanosheets in the composite exhibited effective singlet oxygen generation, strong NIR absorption, and high photothermal conversion efficiency of ~59.6%. | [60] |
| Ti2N@oSi | NIR | Doxorubicin | Ultrahigh drug-loading capacity of 796.3%. | [61] |
| MXene@MOF-5@DOX | pH | Doxorubicin/pCRISPR | Achieved a drug payload of 35.7%. | [62] |
| MXene/Composite | Anticancer Strategy | Advantages | Ref. |
|---|---|---|---|
| Nb2C nanosheets | Photothermal therapy | The surface-engineered Nb2C nanosheets feature low phototoxicity, high biocompatibility, biodegradability, and efficient in vivo photothermal ablation. | [45] |
| V2C Quantum dots | Photothermal therapy | V2C QDs photothermal agent combined with the low-temperature nucleus-targeted photothermal therapy mediated by engineered exosome vector for effective tumor eradication. | [73] |
| CGDSTC NSs | Photodynamic therapy | Sodium ascorbate and dopamine-modified Ti3C2Tx nanosheets conjugated with glucose oxidase and chlorin e6 photosensitizer for the efficient killing of cancer cells through cooperative effect. | [74] |
| Ti3C2Tx-PVP@DOXjade | Chemo-photothermal therapy | The photoactivated DOXjade at the Ti3C2Tx-PVP results in iron chelation and chemotherapeutic functions at the tumor sites. The MXene platform achieved a photothermal conversion efficiency of 40%. | [76] |
| Delaminated Ti3CN | Photothermal therapy | The photonic hyperthermia resulted in highly efficient tumor-killing both in vitro and in vivo. | [78] |
| Fe-Ti3C2Tx | Chemodynamic, MIR, and photothermal therapy | Effective against MKN45 tumor in Balb/c nude mice. | [79] |
| Ti3C2Tx-GOX-CPO/TPZ | Macrophage-mediated phagocytosis | A combination of enzyme dynamic therapy, tumor phototherapy, and hypoxia-activated chemotherapy for efficient tumor eradication. | [80] |
| 2D ultra-thin Ti3C2Tx | Photothermal therapy | Efficient photothermal therapy against MDA-231 breast cancer cells. | [81] |
| 2D Nb2C MXenes | Chemo-photothermal therapy | A “therapeutic mesopore” layer is constructed on the surface of 2D Nb2C MXene, thereby supplementing the photothermal therapy with chemotherapy for enhanced ablation of U87 cancer cell line. | [82] |
| Mo2C nanospheres | Photodynamic-photothermal therapy | Biocompatible and multifunctional theranostic platform with minimal tissue toxicity for effective in vivo tumor depiction. | [83] |
| MXene/Composite | Antimicrobial Applications | Ref. |
|---|---|---|
| Ti3C2Tx | Antibacterial activity against E. coli and B. subtilis with 98% viability loss within 4 h. | [84] |
| Colloidal Ti3C2Tx | Antibacterial activity against B. subtilis and E. coli. | [85] |
| Ti3C2Tx | Antibacterial activity against E. coli. | [87] |
| Ti3C2Tx | Photocatalytic inactivation of airborne E. coli. | [93] |
| Bi2S3/Ti3C2Tx | Photoexcited antimicrobial effects on S. aureus and E. coli. | [94] |
| Ti3C2Tz/Chitosan | Antibacterial activity against E. coli and S. aureus. | [95] |
| Nb2CTx and Nb4C3Tx | Bactericidal property against E. coli and S. aureus. | [96] |
| Cu2O/Ti3C2Tx | Antibacterial activity against S. aureus and Pseudomonas aeruginosa. | [97] |
| Ti3C2Tx-AuNCs | Antibacterial performance on S. aureus and E. coli. | [98] |
| MoS2/Ti3C2Tx | Antibacterial activity against E. coli and B. subtilis. | [99] |
| Ti3C2Tx-Laden bacteriophage | Antibacterial activity against Shigella. | [100] |
| Ag/Ti3C2Tx | Inhibitory activity against E. coli and S. aureus. | [101] |
| TiVCTX | Antibacterial activities against E. coli, photothermal sterilization effect on E. coli and B. subtilis. | [102] |
| CuP-sTi3C2Tx | Antibacterial activity against E. coli and S. aureus. | [103] |
| Ti3C2Tx | Size-dependent photothermal antibacterial activity against S. aureus. | [104] |
| Ti3C2Tx/PVA hydrogel | Antibacterial activity against E. coli and S. aureus. | [105] |
| V2C NSs | Antibacterial activity against E. coli, and B. subtilis. | [106] |
| BC/Chi/Ti3C2Tx/AgNWs aerogel | Antibacterial activity against E. coli and S. aureus. | [107] |
| Sensing Platform | Device Type | Applications | Ref. |
|---|---|---|---|
| PDA-MXene-PDMS | Wrist band | Body motion monitoring | [125] |
| MXene/Prussian blue | Wrist band | Perspiration analysis | [130] |
| MXene/MWCNT electronic fabric | Mask | Respiration analysis | [131] |
| Ti3C2Tx/PVDF-TrFE | Patch | Acquisition of physiological signal | [133] |
| MXene/CNC coated TPU NWF | Patch | Strain/Pressure sensor | [134] |
| MXene coated NWF | Fabric | EMI Shielding, Wearable heater | [135] |
| MXene coated cotton | Fabric | Pressure sensor | [136] |
| rGO/Ti3C2Tx | Fabric | Human motion monitoring | [137] |
| SnS/Ti3C2Tx nanohybrid | Patch | Sitting posture analysis and sign-to-text translation | [138] |
| MXene-based core-sheath yarns | Knitted band | Strain/Humidity sensor | [139] |
| MXene coated cellulose | Knitted wrist band | Pressure sensor | [140] |
| Ag/MXene nanocomposite | Patch | Strain Sensor | [141] |
| MXene-NSD-PEDOT | Patch | EMG signal analysis | [142] |
| MXene/PU composite fibers | Knitted band | Strain sensor | [143] |
| MXene-Based Sensor Platform | Sensing Technique | Biomarker | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|
| Ti3C2Tx@CuNCs/MB | Photothermal | Human anti-ASGPR | 10−8 U/mL | 1.76 × 10−9 U/mL | [150] |
| - | |||||
| 10−2 U/mL | |||||
| BSA/anti-CEA/f-Ti3C2Tx-MXene/GC | CV | CEA | 0.0001 | 0.000018 ng/mL | [151] |
| - | |||||
| 2000 ng/mL | |||||
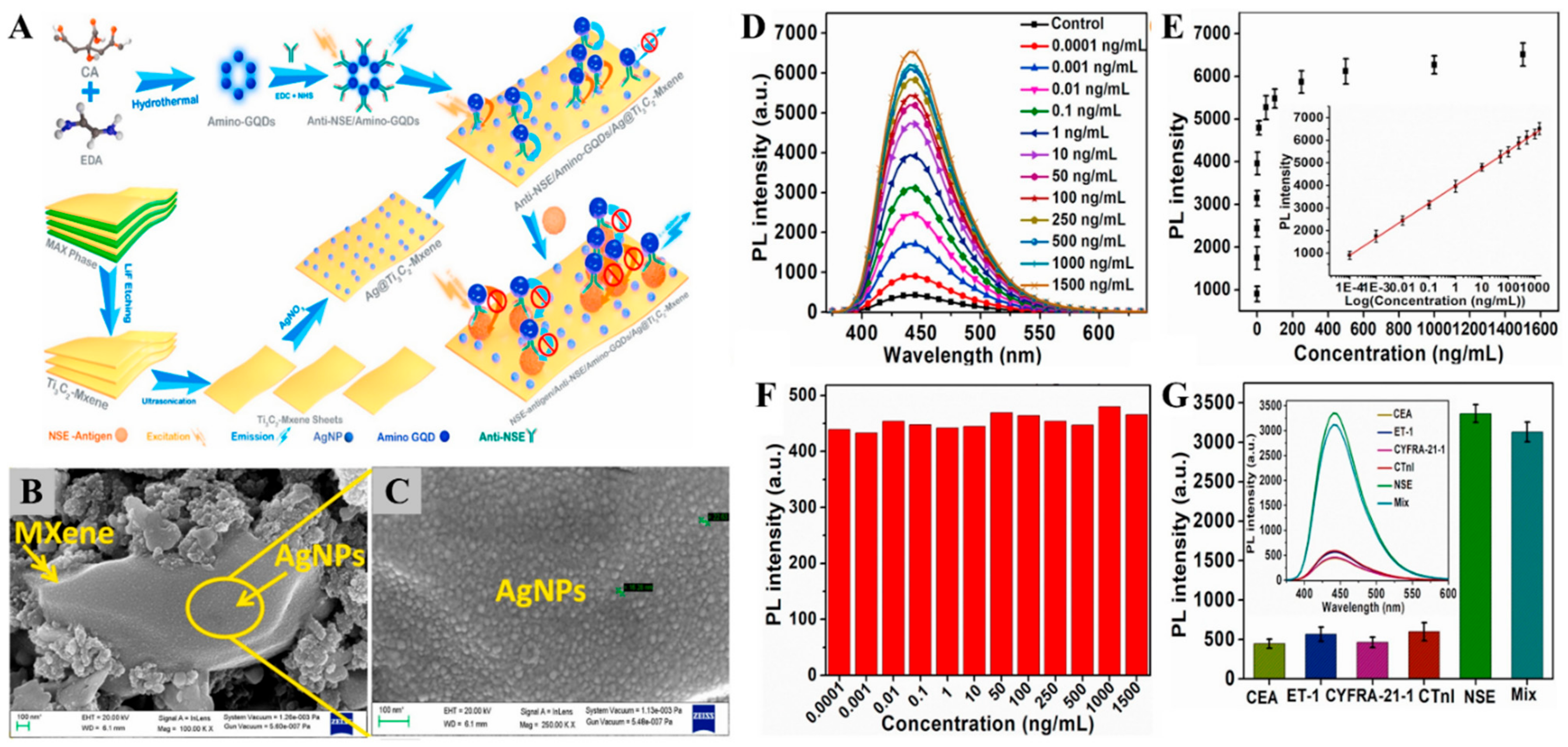
| Ag@Ti3C2Tx | Fluorometric | NSE | 0.0001 | 0.05 pg/mL | [152] |
| - | |||||
| 1500 ng/mL | |||||
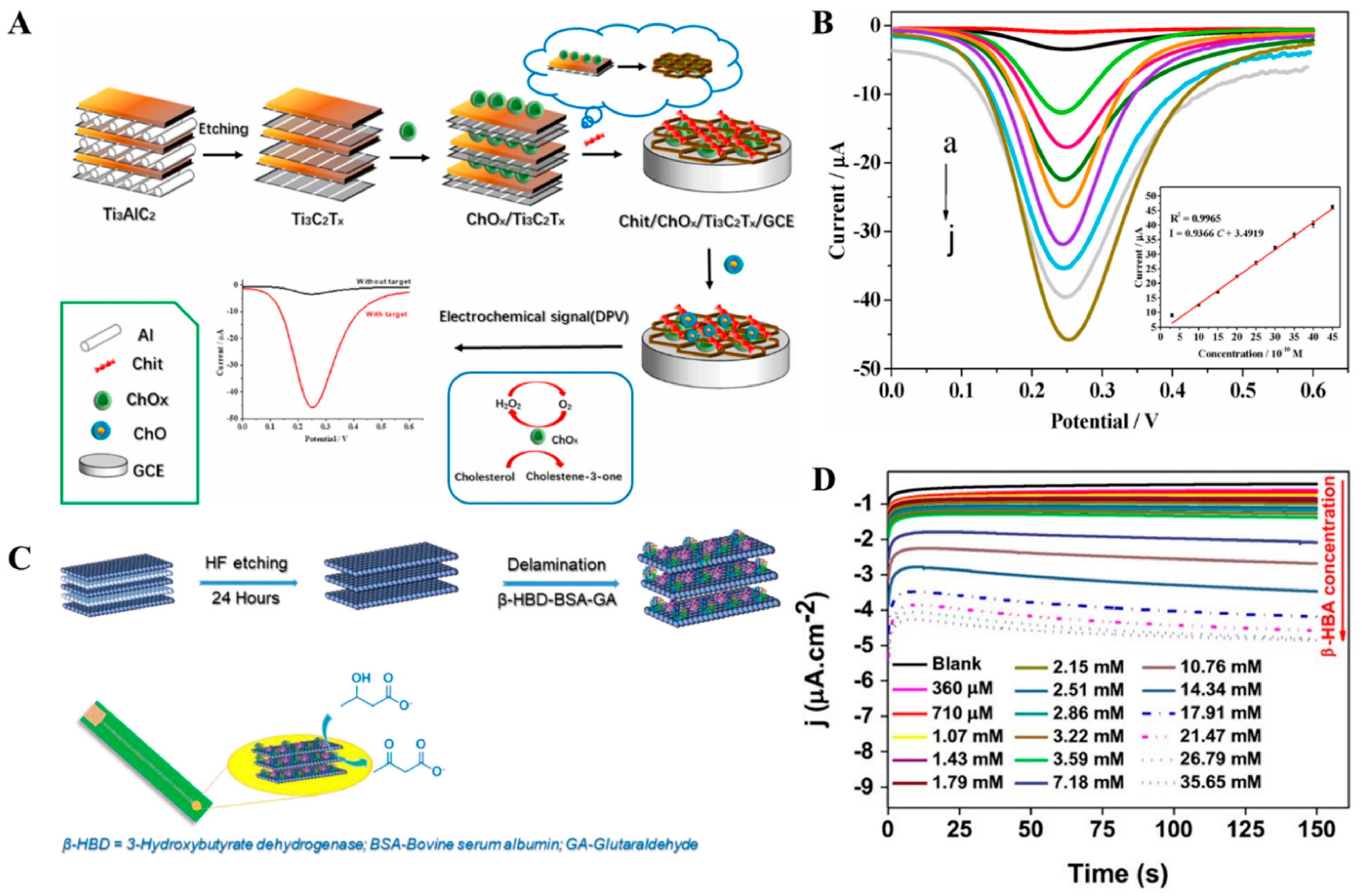
| Chi/ChOx/Ti3C2Tx | DPV | Cholesterol | 0.3–4.5 nM | 0.11 nM | [153] |
| Ti3C2Tx-AFBPB-film modified DIDμE | DPV | Apo A1 | 0.1 pg/mL | 0.3 pg/mL | [154] |
| - | |||||
| 50 ng/mL | |||||
| NMP22 | 0.1 pg/mL | 0.7 pg/mL | |||
| - | |||||
| 50 ng/mL | |||||
| N-Ti3C2Tx-MXene | SPR | CEA | 10−11–10−6 g/mL | 1.7 pg/mL | [155] |
| DNA modified Ti3C2Tx nanosheets | FRET | miRNA-21 a | 5 fM–100 pM | 0.62 fM | [156] |
| miRNA-10 b | 5 fM–100 pM | 0.85 fM | |||
| PPy@Ti3C2Tx/PMo12 | EIS | OPN | 0.05–10,000 pg/mL | 0.98 fg/mL | [157] |
| Ti3C2Tx/AuNPs/SPA/Ab1 | SPR | CEA | 2 × 10−16 | 0.07 fM | [158] |
| - | |||||
| 2 × 10−8 M | |||||
| MoS2@Ti3C2Tx | DPV | Thyroxine | 0.78 | 0.39 pg/mL | [159] |
| - | |||||
| 7.8 × 106 pg/mL | |||||
| ssDNAs/AuNP@Ti3C2Tx/SPGE | DPV | miRNA-21 | 500 aM | 204 aM | [160] |
| - | |||||
| 50 nM | |||||
| miRNA-141 | 500 aM | 138 aM | |||
| - | |||||
| 50 nM | |||||
| cDNA-Fc/Ti3C2Tx/Apt/Au/GCE | DPV | MUC1 | 1 pM–10 µM | 0.33 pM | [161] |
| cDNA/Ti3C2Tx@FePcQDs/AE | EIS | miRNA-155 | 0.01 fM | 4.3 aM | [162] |
| - | |||||
| 10 pM | |||||
| MB/DNA/HT/HP1/AuNPs/Ti3C2Tx/BiVO4/GCE | PEC | VEGF165 | 10 fM | 3.3 fM | [163] |
| - | |||||
| 100 nM | |||||
| GSH-Ti3C2Tx MQDs | FRET | UA | - | 125 nM | [164] |
| SOx/Ti3C2Tx-Chi/GCE | CA | Sarcosine | 36–7800 nM | 18 nM | [165] |
| Hb-Ti3C2Tx-GO/Au | DPV | H2O2 | 2 μM–1 mM | 1.95 μM | [166] |
| Ti3C2Tx/GCPE | CA | Adrenaline | 0.02–10 μM, 10–100 μM | 9.5 nM | [167] |
| Ti3C2Tx-HF/TBA | CA | Glucose | 50–250 μM | 23 μM | [168] |
| Urease-MB/Ti3C2Tx/SPE | SWV | UA | 30–500 μM | 5 μM | [169] |
| Urea | 0.1–3 μM | 0.2 μM | |||
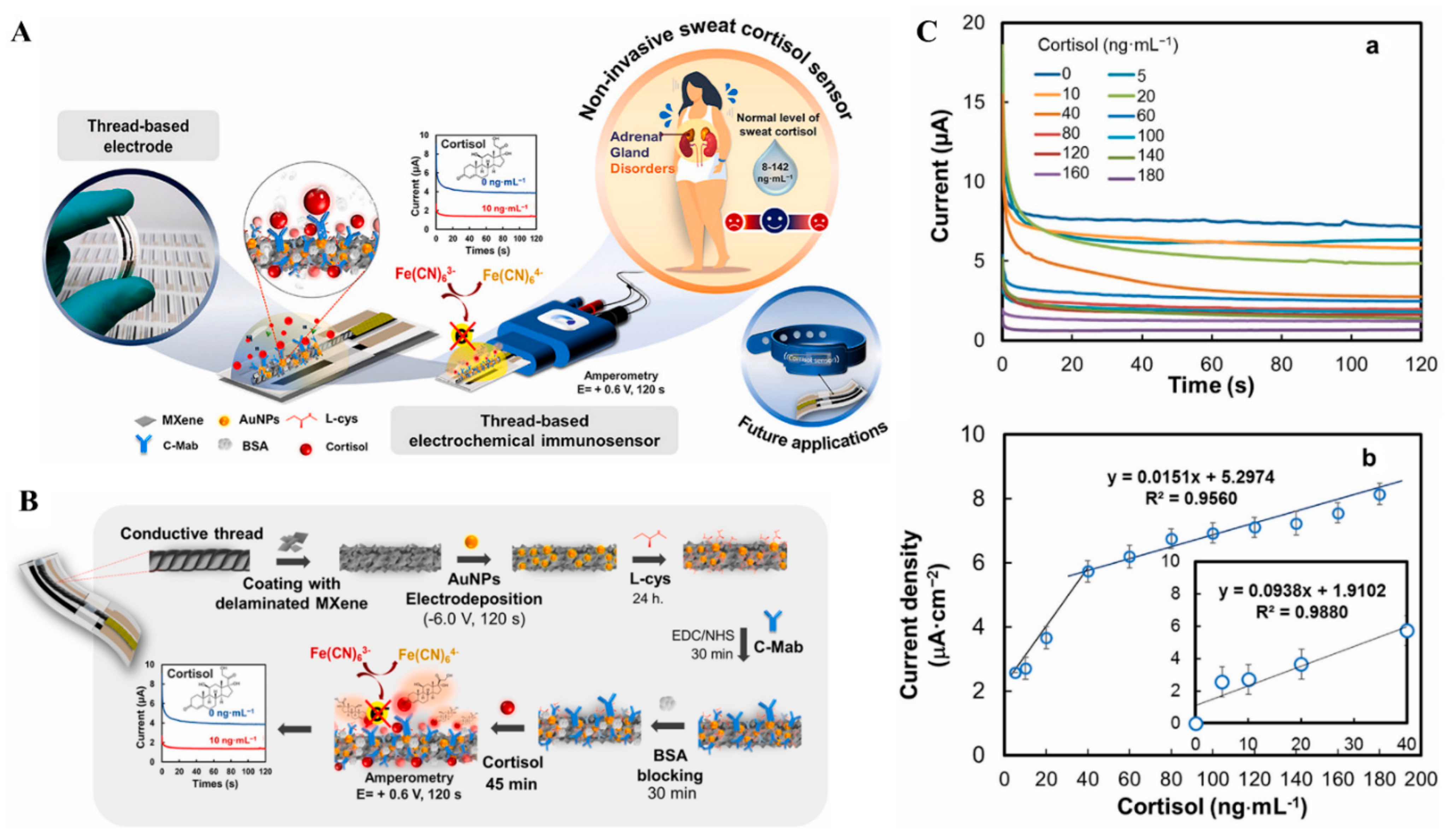
| L-cys/AuNPs/Ti3C2Tx | CA | Sweat cortisol | 5–40 ng/mL | 0.54 ng/mL | [170] |
| 40–180 ng/mL | |||||
| Au-Pd-Pt/Ti3C2Tx | DPV | CEA | 1 fg/mL | 0.32 fg/mL | [171] |
| - | |||||
| 1 ng/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyappayil, A.; Chavan, S.G.; Roh, Y.-G.; Lee, M.-H. Advances of MXenes; Perspectives on Biomedical Research. Biosensors 2022, 12, 454. https://doi.org/10.3390/bios12070454
Koyappayil A, Chavan SG, Roh Y-G, Lee M-H. Advances of MXenes; Perspectives on Biomedical Research. Biosensors. 2022; 12(7):454. https://doi.org/10.3390/bios12070454
Chicago/Turabian StyleKoyappayil, Aneesh, Sachin Ganpat Chavan, Yun-Gil Roh, and Min-Ho Lee. 2022. "Advances of MXenes; Perspectives on Biomedical Research" Biosensors 12, no. 7: 454. https://doi.org/10.3390/bios12070454
APA StyleKoyappayil, A., Chavan, S. G., Roh, Y.-G., & Lee, M.-H. (2022). Advances of MXenes; Perspectives on Biomedical Research. Biosensors, 12(7), 454. https://doi.org/10.3390/bios12070454






