Potentiometric Performance of Ion-Selective Electrodes Based on Polyaniline and Chelating Agents: Detection of Fe2+ or Fe3+ Ions
Abstract
:1. Introduction
2. Materials and Methods
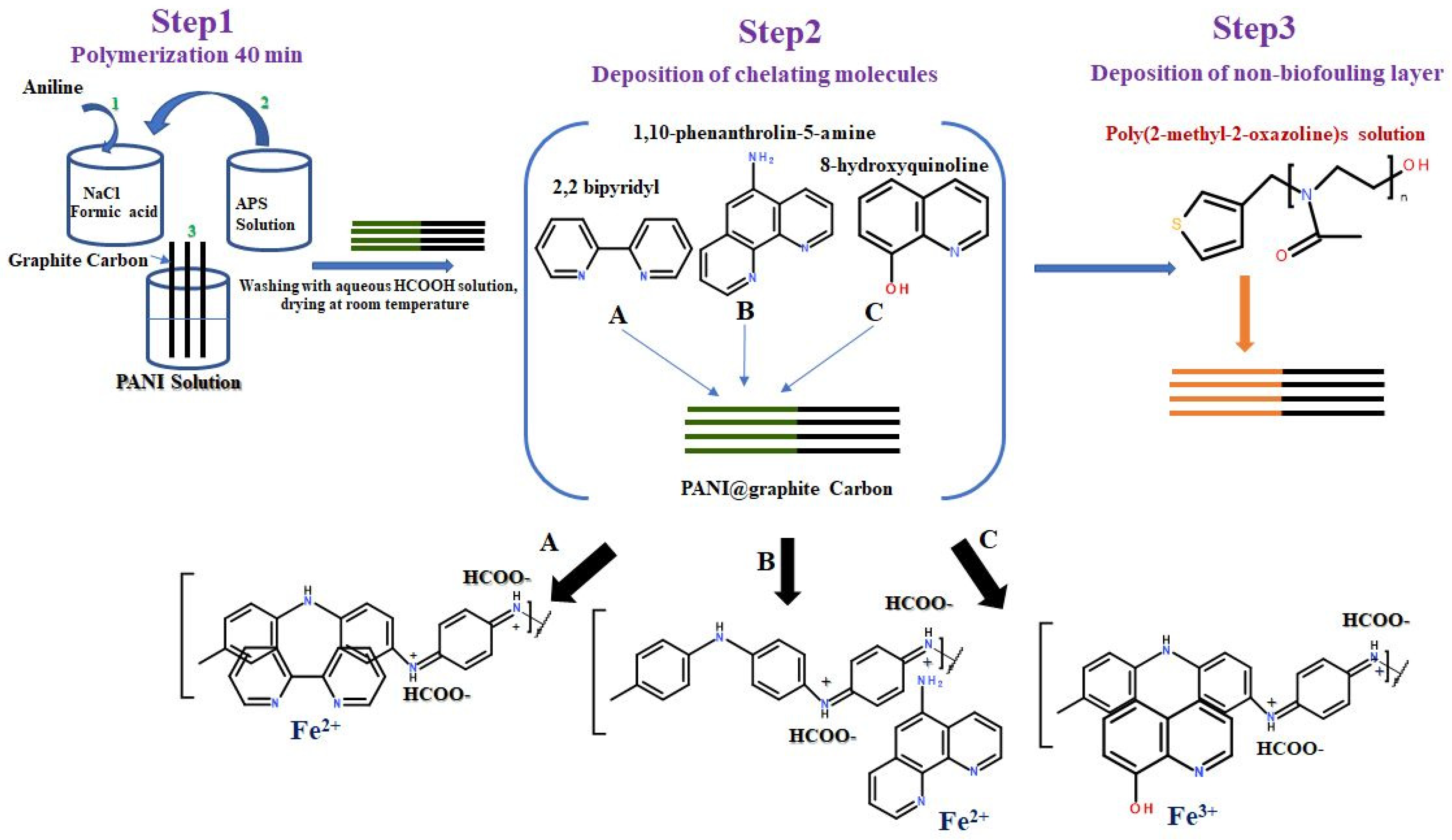
2.1. Synthesis of Polyaniline/Graphite Carbon Rod (PANI@G.C)
2.2. Deposition of Chelating Molecules on PANI@G.C Electrodes
2.3. Deposition of POX Layer on PANI@G.C Electrodes
2.4. Characterization Methods
3. Results and Discussion
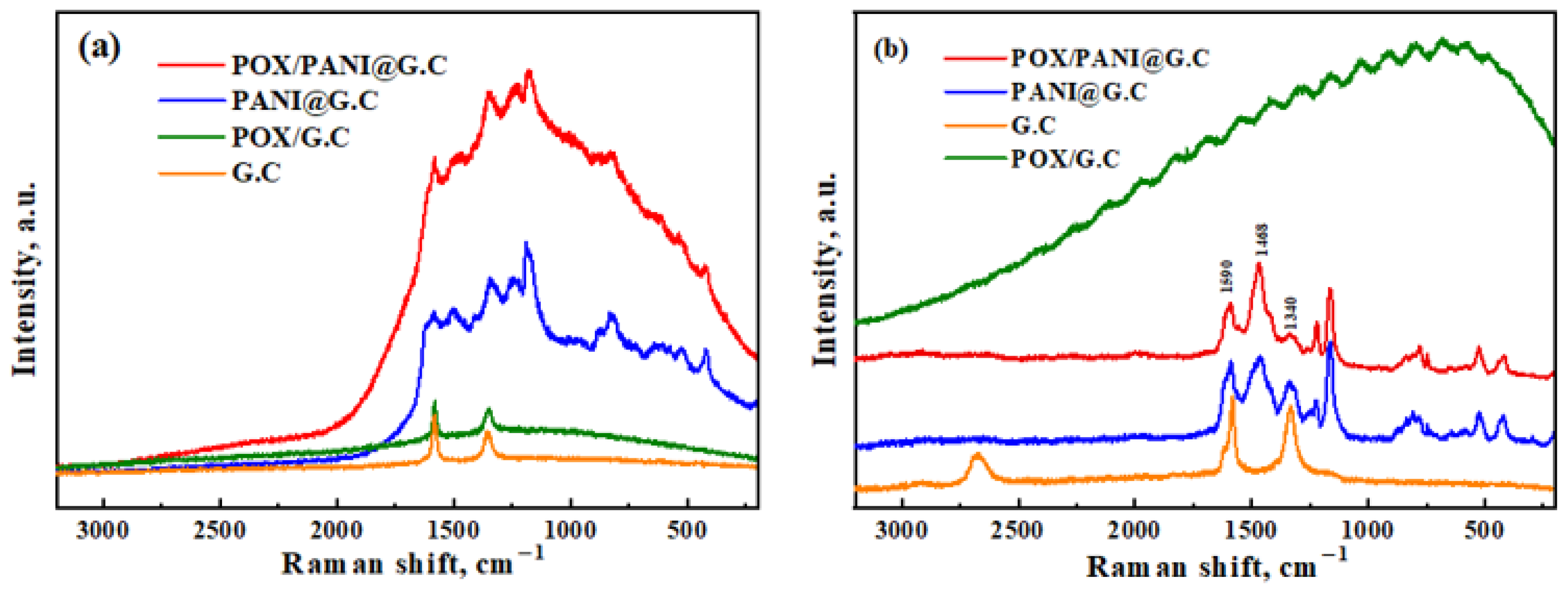
3.1. Raman Spectroscopy
3.2. Electrochemical Characterization
3.2.1. Cyclic Voltammetry
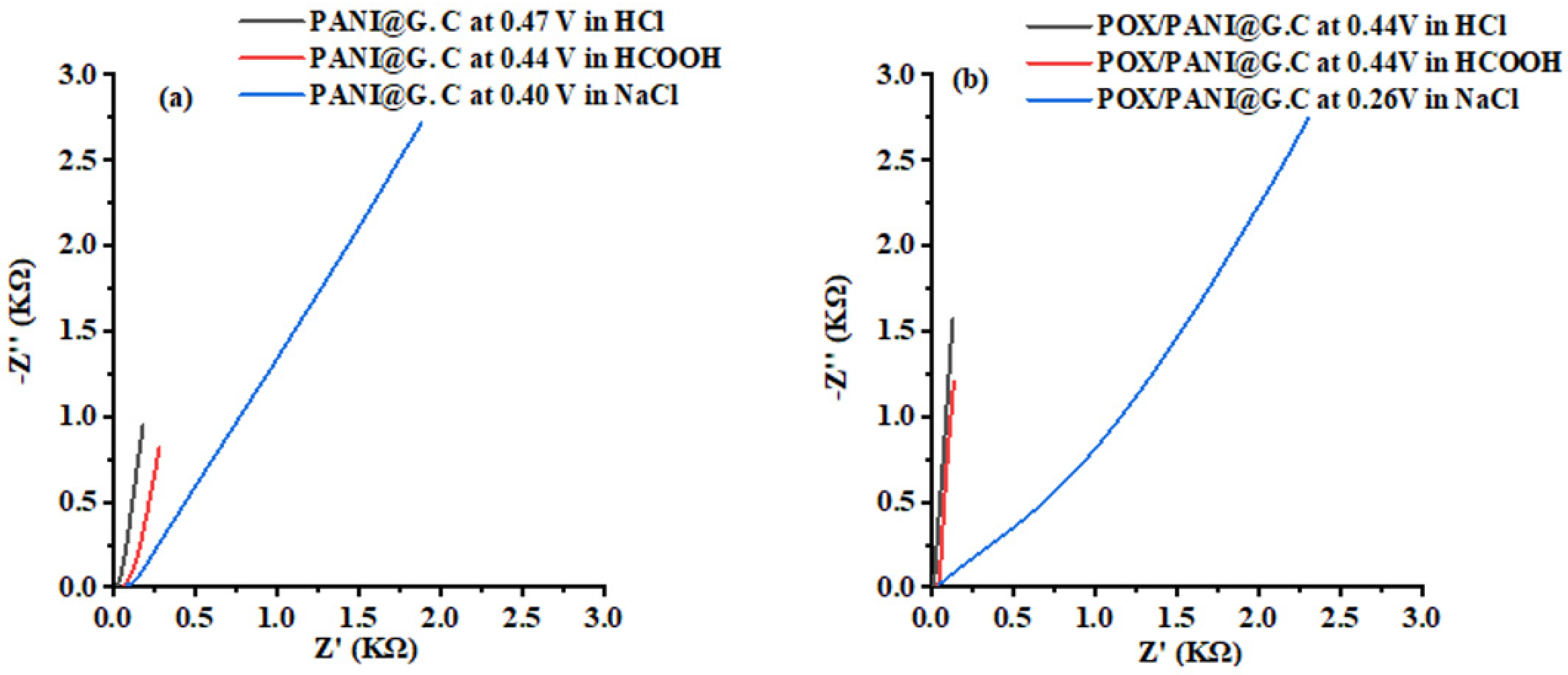
3.2.2. Electrochemical Impedance Spectroscopy
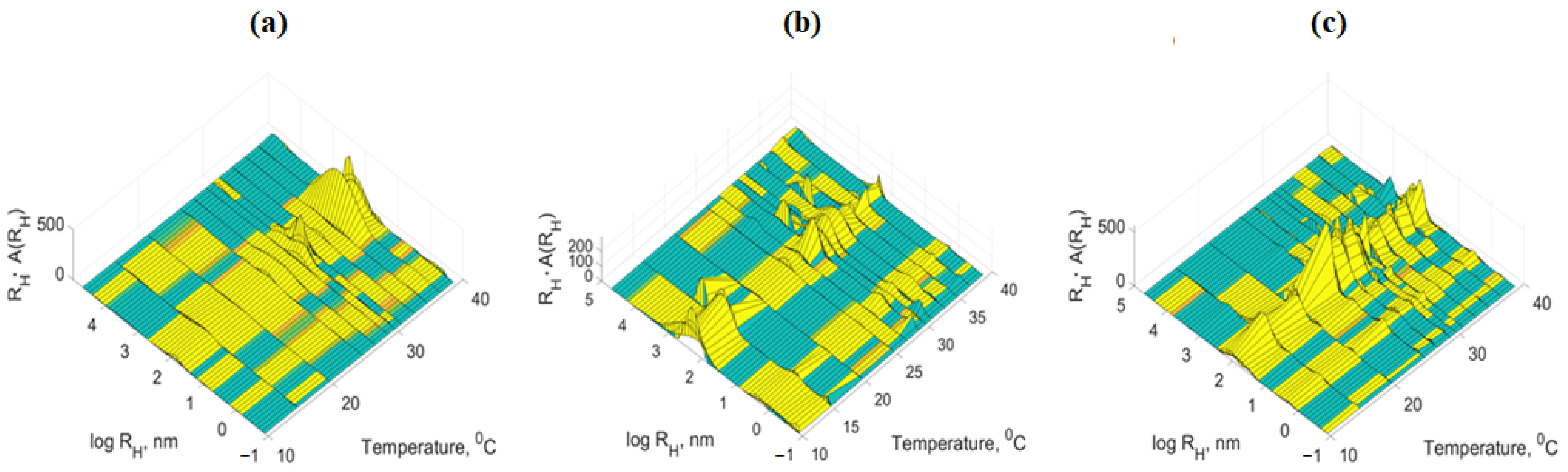
3.3. Dynamic Light Scattering Measurement (DLS)
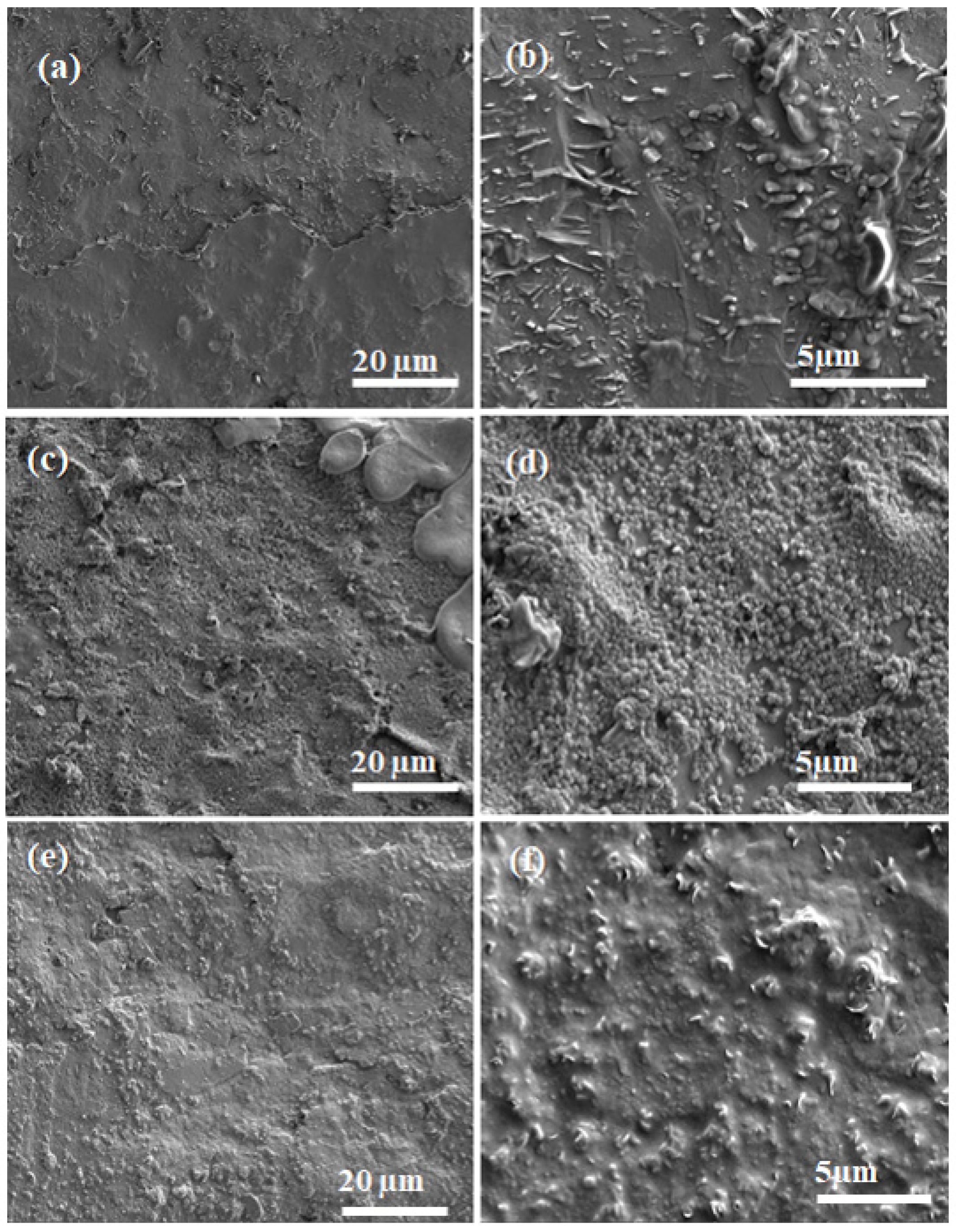
3.4. Scanning Electron Microscopy (SEM)
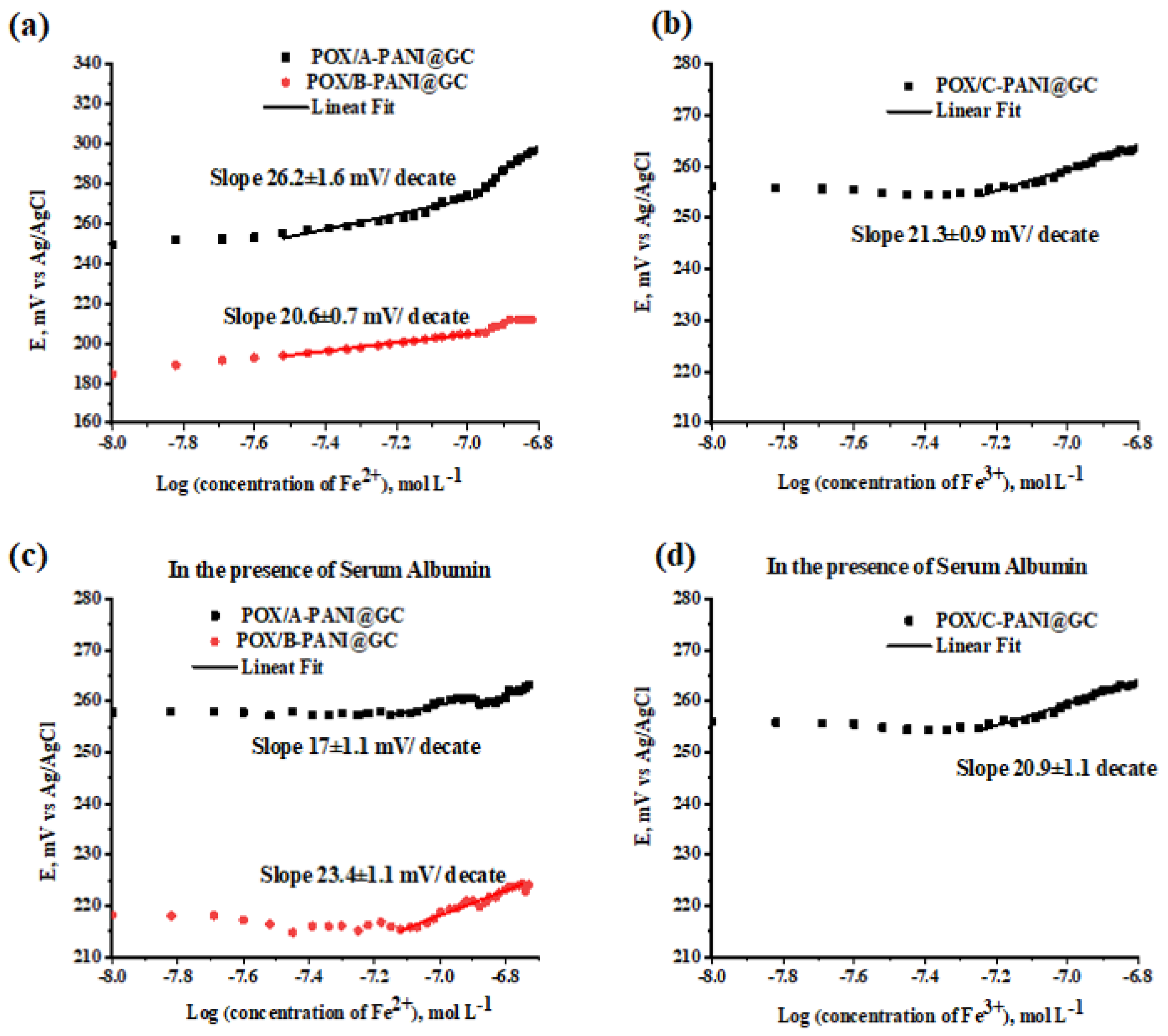
4. Potentiometric Testing of Fe2+ and Fe3+ Ions
Selectivity Testing in the Presence of Cu2+
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karimi-Maleh, H.; Karimi, F.; Malekmohammadi, S.; Zakariae, N.; Esmaeili, R.; Rostamnia, S.; Yola, M.L.; Atar, N.; Movagharnezhad, S.; Rajendran, S. An amplified voltammetric sensor based on platinum nanoparticle/polyoxometalate/two-dimensional hexagonal boron nitride nanosheets composite and ionic liquid for determination of N-hydroxysuccinimide in water samples. J. Mol. Liq. 2020, 310, 113185. [Google Scholar] [CrossRef]
- Le, V.T.; Vasseghian, Y.; Dragoi, E.N.; Moradi, M.; Mousavi Khaneghah, A. A review on graphene-based electrochemical sensor for mycotoxins detection. Food Chem. Toxicol. 2021, 148, 111931. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Naghib, S.M.; Seyfoori, A.; Maleki, A.; Rahmanian, M. Ultrasonic-Assisted Synthesis and In Vitro Biological Assessments of a Novel Herceptin-Stabilized Graphene using Three Dimensional Cell Spheroid. Ultrason. Sonochem. 2019, 58, 104615. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, E.; Naghib, S.M. A Comparative Study on Biological Properties of Novel Nanostructured Monticellite-Based Composites with Hydroxyapatite Bioceramic. Mater. Sci. Eng. C 2019, 98, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Komathi, S.; Gopalan, A.; Muthuchamy, N.; Lee, K. Polyaniline nanoflowers grafted onto nanodiamonds via a soft template-guided secondary nucleation process for high-performance glucose sensing. RSC Adv. 2017, 7, 15342–15351. [Google Scholar] [CrossRef] [Green Version]
- Muthuchamy, N.; Gopalan, A.; Lee, K.-P. Highly selective non-enzymatic electrochemical sensor based on a titanium dioxide nanowire–poly (3-aminophenyl boronic acid)–gold nanoparticle ternary nanocomposite. RSC Adv. 2018, 8, 2138–2147. [Google Scholar] [CrossRef] [Green Version]
- Muthuchamy, N.; Lee, K.; Gopalan, A. Enhanced photoelectrochemical biosensing performances for graphene (2D)–Titanium dioxide nanowire (1D) heterojunction polymer conductive nanosponges. Biosens. Bioelectron. 2017, 89, 390–399. [Google Scholar] [CrossRef]
- Itoi, H.; Hayashi, S.; Matsufusa, H.; Ohzawa, Y. Electrochemical synthesis of polyaniline in the micropores of activated carbon for high-performance electrochemical capacitors. Chem. Commun. 2017, 53, 3201–3204. [Google Scholar] [CrossRef] [Green Version]
- Li, G.R.; Feng, Z.P.; Zhong, J.H.; Wang, Z.L.; Tong, Y.X. Electrochemical Synthesis of Polyaniline Nanobelts with Predominant Electrochemical Performances. Macromolecules 2010, 43, 2178–2183. [Google Scholar] [CrossRef]
- Simotwo, S.K.; DelRe, C.; Kalra, V. Supercapacitor Electrodes Based on High-Purity Electrospun Polyaniline and Polyaniline−Carbon Nanotube Nanofibers. CS Appl. Mater. Interfaces 2016, 8, 21261–21269. [Google Scholar] [CrossRef]
- Lakhdari, D.; Guittoum, A.; Benbrahim, N.; Belgherbi, O.; Berkani, M.; Vasseghian, Y.; Lakhdari, N. A nvel non-enzymatic glucose sensor based on NiFe(NPs)-polyaniline hybrid materials. Food Chem. Toxicol. 2021, 151, 112099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Wang, D.; Niu, J.; Du, P.; Liu, J.; Liu, P. Enhanced electrochemical performance of polyaniline-based electrode for supercapacitors in mixed aqueous electrolyte. Electrochim. Acta 2020, 349, 136348. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R.; Farid, S.; Afzal, A.M. Co-MOF/polyanilinebased electrode material for high performance asymmetric supercapacitor devices. Electrochim. Acta 2020, 346, 136039. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, Y.; Xiao, J.; Fan, H. Fabrication and characterisation of magnetic graphene oxide Incorporated Fe3O4 @polyaniline for the removal of Bisphenol A, t-octyl-phenol and α-naphthol from water. Sci. Rep. 2017, 7, 11316. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, A.; Prasankumar, T.; Sivaprakash, S.; Wiston, B.R.; Biradar, S.; Jose, S. Removol of elevated level of chromium in Groundwater by the fabricated PANI/Fe3O4 nanocomposites. Environ. Sci. Pollut. Res. Int. 2017, 24, 7490–7498. [Google Scholar] [CrossRef]
- Pal, R.; Goyal, S.L.; Rawal, I.; Sharma, S. Efcient room temperature methanol sensors based on polyaniline/ graphene micro/nanocomposites. Iran. Polym. J. 2020, 29, 591–603. [Google Scholar] [CrossRef]
- Sriramprabha, R.; Sekar, M.; Revathi, R.; Viswanathan, C.; Wilson, J. Fe2O3/polyaniline supramolecular nanocomposite: A receptor free sensor platform for the quantitative determination of serum creatinine. Anal. Chim. Acta 2020, 1137, 103–114. [Google Scholar] [CrossRef]
- Tomšík, E.; Dallas, P.; Šeděnková, I.; Svoboda, J.; Hrubý, M. Electrochemical deposition of highly hydrophobic perfluorinated polyaniline film for biosensor applications. RSC Adv. 2021, 11, 18852–18859. [Google Scholar] [CrossRef]
- Chen, S.; Song, N.; Mu, M.; Wang, C.; Lu, X.F. Sacrificial template synthesis of ultrathin polyaniline nanosheets and their application in highly sensitive electrochemical dopamine detection. Mater. Today Chem. 2021, 20, 100479. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Shoaei, I.S.; Monadi, N. A novel ion selective membrane potentiometric sensor for direct determination of Fe(III) in the presence of Fe(II). Talanta 2004, 64, 1048–1052. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Raheedayat, F. Chromium(III) Ion Selective Electrode Based on Oxalic AcidBis(Cyclohexylidene Hydrazide. Electroanalysis 2004, 16, 1330–1335. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Sharif, F. Chromium(III) ion selective electrode based on glyoxal bis(2-hydroxyanil). Talanta 2003, 60, 707–713. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Gupta, A.V.K.; Jain, S. PVC-Based 2,2,2-Cryptand Sensor for Zinc Ions. Anal. Chem. 1996, 68, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Zamani, H.A.; Imani, A.; Arvinfar, A.; Rahimi, F.; Ganjali, M.R.; Faridbod, F.; Meghdadi, S. Neodymium(III)–PVC membrane sensor based on a new four dentate ionophore. Mater. Sci. Eng. C 2011, 31, 588–592. [Google Scholar] [CrossRef]
- Zamani, H.A.; Faridbod, F.; Ganjali, M.R. Dysprosium selective potentiometric membrane sensor. Mater. Sci. Eng. C 2013, 33, 608–612. [Google Scholar] [CrossRef]
- Shamsipur, M.; Mizani, F.; Saboury, A.A.; Sharghi, H.; Khalifeh, R. Highly Selective and Sensitive Membrane Sensors for Copper(II)Ion Based on a New Benzo-Substituted Macrocyclic Diamide 6,7,8,9,10-Hexahydro-2H-1,13,4,7,10-benzodioxatriazacyclopenta-decine-3,11(4H,12H)-dione. Electroanalysis 2007, 19, 587–596. [Google Scholar] [CrossRef]
- Abdallah, N.A. Novel Potentiometric Solid-contact Electrode for the Determination of Fe2+ Ions via MWCNTs-Gemifloxacin Composite. Electroanalysis 2021, 33, 1283–1289. [Google Scholar] [CrossRef]
- Ali, T.A.; Farag, A.A.; Mohamed, G.G. Potentiometric determination of iron in polluted water samples using new modified Fe(III)-screen printed ion selective electrode. J. Ind. Eng. Chem. 2014, 20, 2394–2400. [Google Scholar] [CrossRef]
- Mizani, F.; Ganjali, M.R.; Faridbod, F.; Esmaeilnia, S. A Novel Iron(III) Selective Potentiometric Sensor Based on 9- Ethylacenaphtho [1, 2-B] Quinoxaline. Int. J. Electrochem. Sci. 2013, 8, 10473–10486. [Google Scholar]
- Lorson, T.; Lubtow, M.M.; Wegener, E.; Haider, M.S.; Borova, S.; Nahm, D.; Jordan, R.; Sokolski-Papkov, M.; Kabanov, A.V.; Luxenhofer, R. Poly(2-oxazoline)s based biomaterials: A comprehensive and critical update. Biomaterials 2018, 178, 204–280. [Google Scholar] [CrossRef]
- Trachsel, L.; Zenobi-Wong, M.; Benetti, E.M. The role of poly(2-alkyl-2-oxazoline)s in hydrogels and biofabrication. Biomater. Sci. 2021, 9, 2874–2886. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.; Schubert, S.U. Poly(2-oxazolines) in biological and biomedical application contexts. Adv. Drug Deliv. Rev. 2007, 59, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Pelegri-O’Day, E.M.; Lin, E.W.; Maynard, H.D. Therapeutic protein-polymer conjugates: Advancing beyond PEGylation. J. Am. Chem. Soc. 2014, 136, 14323–14332. [Google Scholar] [CrossRef] [PubMed]
- Urbánek, T.; Ivanko, I.; Svoboda, J.; Tomšík, E.; Hrubý, M. Selective potentiometric detection of reactive oxygen species (ROS) in biologically relevant concentrations by a modified metalized polyporphyrine sensing layer coated with nonbiofouling poly(2-alkyl-2oxazoline)s. Sens. Actuators B Chem. 2022, 363, 131827. [Google Scholar] [CrossRef]
- Hruby, M.; Martinez, I.V.S.; Stephan, H.; Pouckova, P.; Benes, J.; Stepanek, P. Chelators for Treatment of Iron and Copper Overload: Shift from Low-Molecular-Weight Compounds to Polymers. Polymer 2021, 13, 3969. [Google Scholar] [CrossRef] [PubMed]
- Jirak, D.; Svoboda, J.; Filipova, M.; Pop-Georgievski, O.; Sedlacek, O. Antifouling fluoropolymer-coated nanomaterials for 19F MRI. Chem. Comm. 2021, 57, 4718–4723. [Google Scholar] [CrossRef]
- Pop-Georgievski, O.; Štěpán, P.; Houska, M.; Chvostoá, D.; Proks, V.; Rypáček, F. Poly(ethylene oxide) Layers Grafted to Dopamine-melanin Anchoring Layer: Stability and Resistance to Protein Adsorption. Biomacromolecules 2011, 12, 3232–3242. [Google Scholar] [CrossRef]
- Al-Attar, H.A.; Al-Alawina, Q.H.; Monkman, A.P. Spectroscopic ellipsometry of electrochemically prepared thin filmpolyaniline. Thin Solid Film. 2003, 429, 286–294. [Google Scholar] [CrossRef]
- Tomsik, E.; Kohut, O.; Ivanko, I.; Pikarek, M.; Bieloshapka, I.; Dallas, P. Assembly and Interaction of Polyaniline Chains: Impact on Electro- and Physical–Chemical Behavior. J. Phys. Chem. C 2018, 122, 8022–8030. [Google Scholar] [CrossRef]
- Trung, T.; Trung, T.H.; Ha, C.S. Preparation and cyclic voltammetry studies on nickel-nanoclusters containing polyaniline composites having layer-by-layer structures. Electrochim. Acta 2005, 51, 984–990. [Google Scholar] [CrossRef]
- Masuoka, J.; Saltman, P. Zinc (II) and Copper (II) Binding to Serum Albumin. J. Biol. Chem. 1994, 269, 25557–25561. [Google Scholar] [CrossRef]
- Ueno, H.M.; Urazono, H.; Kobayashi, T. Serum albumin forms a lactoferrin-like soluble iron-binding complex in presence of hydrogen carbonate ions. Food Chem. 2014, 145, 90–94. [Google Scholar] [CrossRef] [PubMed]


| Deposited Chelating Molecules and POX | Names | |
|---|---|---|
| 2,2-Bipyridyl |  | POX/A-PANI@G.C |
| 1,10-Phenanthroline-5-amine |  | POX/B-PANI@G.C |
| 8-Hydroxyquinoline | 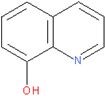 | POX/C-PANI@G.C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, R.; Šeděnková, I.; Černochová, Z.; Romanenko, I.; Pop-Georgievski, O.; Hrubý, M.; Tomšík, E. Potentiometric Performance of Ion-Selective Electrodes Based on Polyaniline and Chelating Agents: Detection of Fe2+ or Fe3+ Ions. Biosensors 2022, 12, 446. https://doi.org/10.3390/bios12070446
Ismail R, Šeděnková I, Černochová Z, Romanenko I, Pop-Georgievski O, Hrubý M, Tomšík E. Potentiometric Performance of Ion-Selective Electrodes Based on Polyaniline and Chelating Agents: Detection of Fe2+ or Fe3+ Ions. Biosensors. 2022; 12(7):446. https://doi.org/10.3390/bios12070446
Chicago/Turabian StyleIsmail, Rimeh, Ivana Šeděnková, Zulfiya Černochová, Iryna Romanenko, Ognen Pop-Georgievski, Martin Hrubý, and Elena Tomšík. 2022. "Potentiometric Performance of Ion-Selective Electrodes Based on Polyaniline and Chelating Agents: Detection of Fe2+ or Fe3+ Ions" Biosensors 12, no. 7: 446. https://doi.org/10.3390/bios12070446
APA StyleIsmail, R., Šeděnková, I., Černochová, Z., Romanenko, I., Pop-Georgievski, O., Hrubý, M., & Tomšík, E. (2022). Potentiometric Performance of Ion-Selective Electrodes Based on Polyaniline and Chelating Agents: Detection of Fe2+ or Fe3+ Ions. Biosensors, 12(7), 446. https://doi.org/10.3390/bios12070446






