Tracking Arachis hypogaea Allergen in Pre-Packaged Foodstuff: A Nanodiamond-Based Electrochemical Biosensing Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation and Reagents
2.2. Sample Preparation
2.3. Immunosensor Construction and Electrochemical Detection
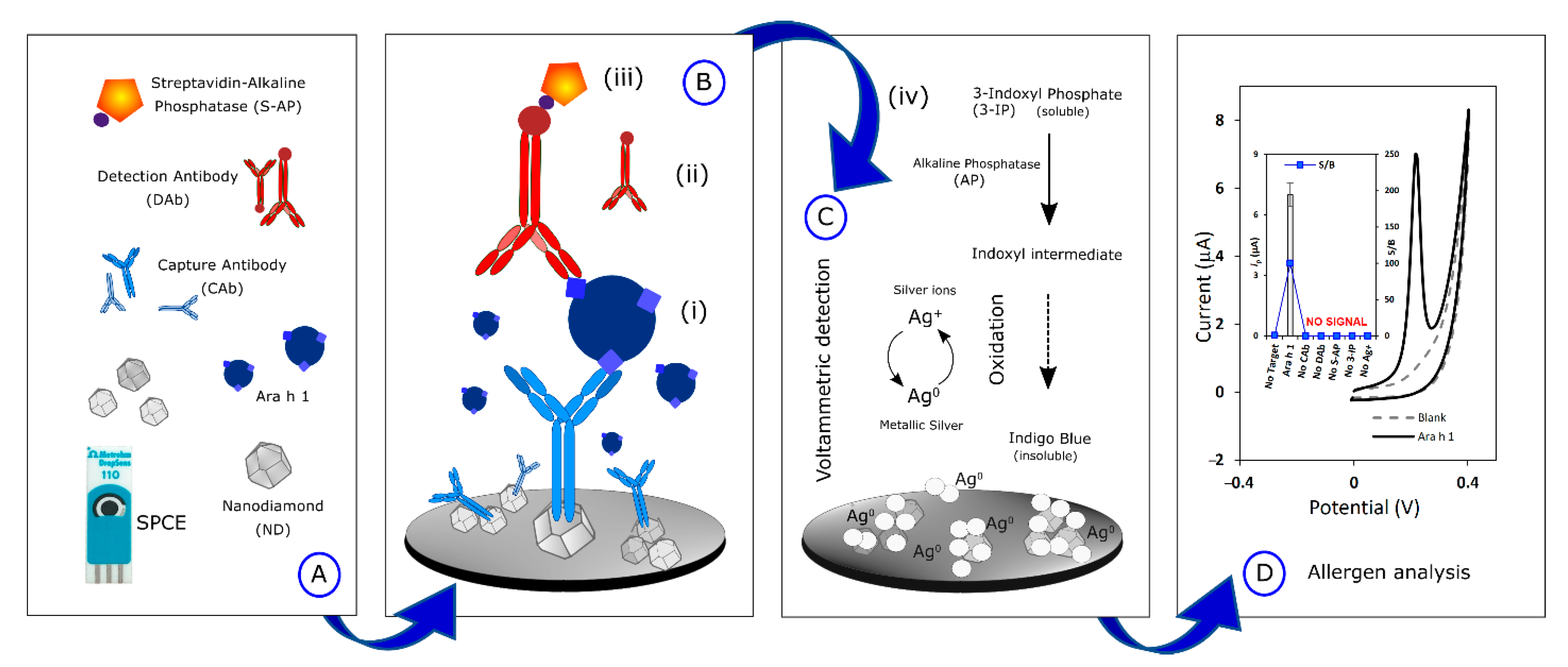
- (A)
- The SPCEs were nanostructured by drop-casting a 15-µL aliquot of NDs (100 µg·mL−1, previously dispersed in H2O and ultrasonicated for 1h to improve the nucleation density). Then, 10 µL of CAb (10 µg·mL−1) was placed on the SPCE and incubated overnight, at 2–8 °C, in a humidity chamber (immobilization through physisorption).
- (B)
- The sandwich-type electrochemical immunoassay consisted of sequential incubation steps: (i) Ara h 1 standard allergen/food sample extract (40 µL, 30 min), (ii) DAb (40 µL, 250×, 60 min), (iii) S-AP (40 µL, 20,000×, 30 min).
- (C)
- The enzymatic reaction took place by adding (iv) a 40 µL-aliquot of a mixture containing (iv) 3-IP (1.0 × 10−3 M) and AgNO3 (4.0 × 10−4 M), that reacted for 20 min. Silver ions were firstly reduced to metallic silver and subsequently, the silver particles were co-deposited with an insoluble component—indigo blue. Washing steps were performed using T1 (before steps i, ii, and iii) and T3 (before step iv).
- (D)
- The electrochemical (oxidation) analysis of the deposited silver was carried out by LSV (potential range from −0.03 V to +0.4 V, scan rate: 50 mV·s−1).
3. Results and Discussion
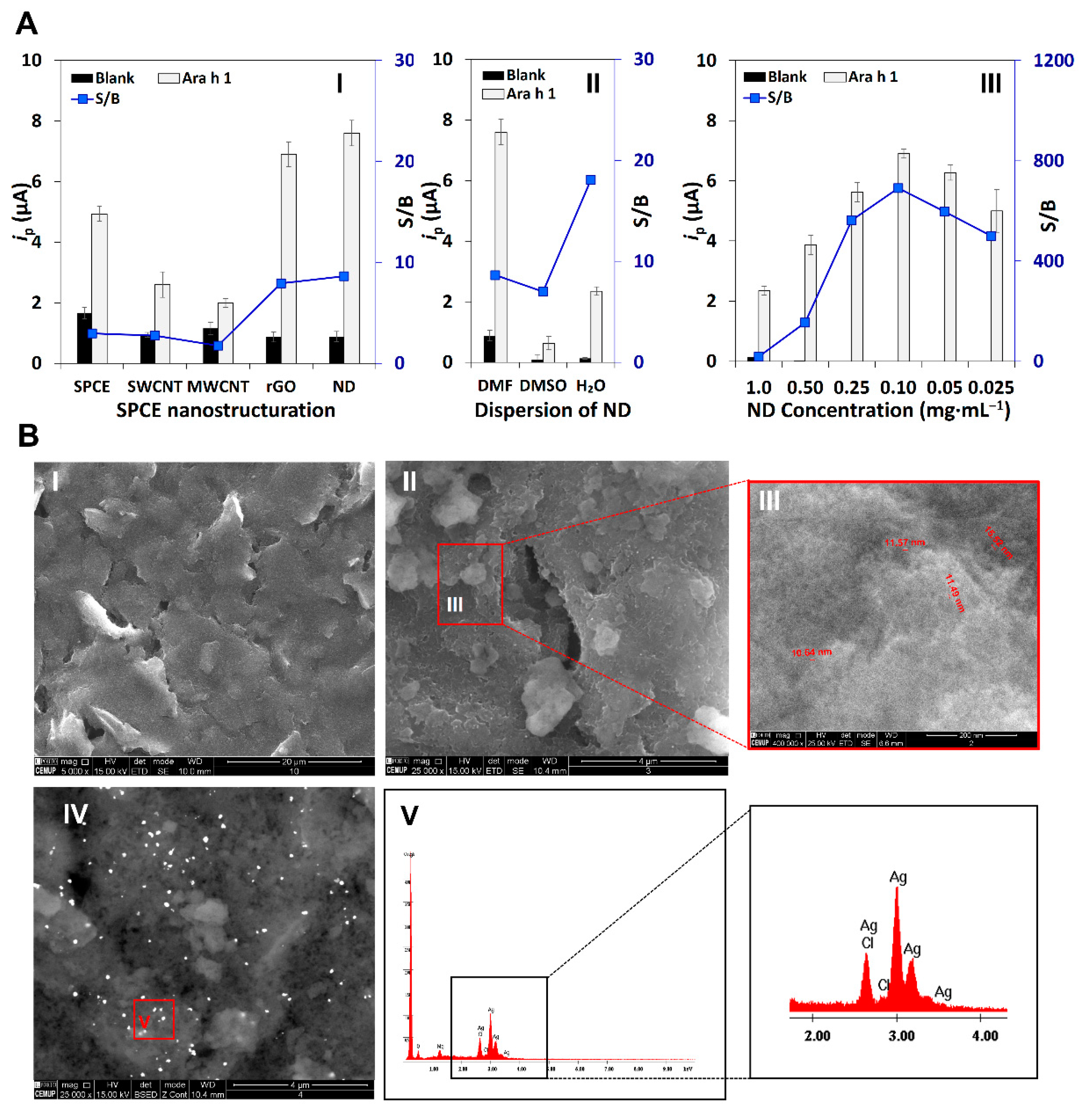
3.1. Electrode Surface Nanostructuration and Characterization
3.2. Evaluation of the Immunoassay’s Performance
3.3. Optimization of Experimental Variables
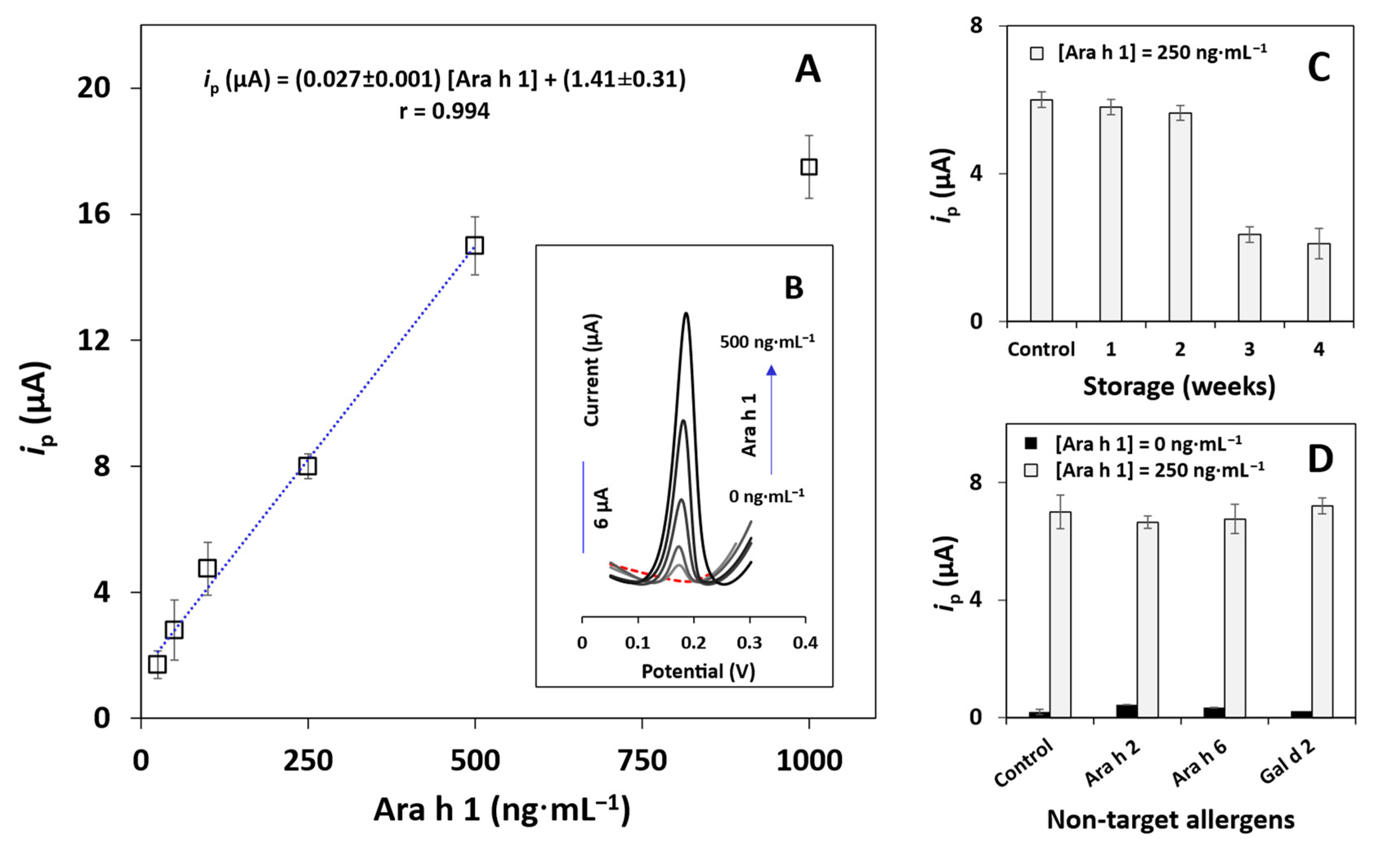
3.4. Analytical Performance, Storage Stability, and Selectivity of the Immunosensor
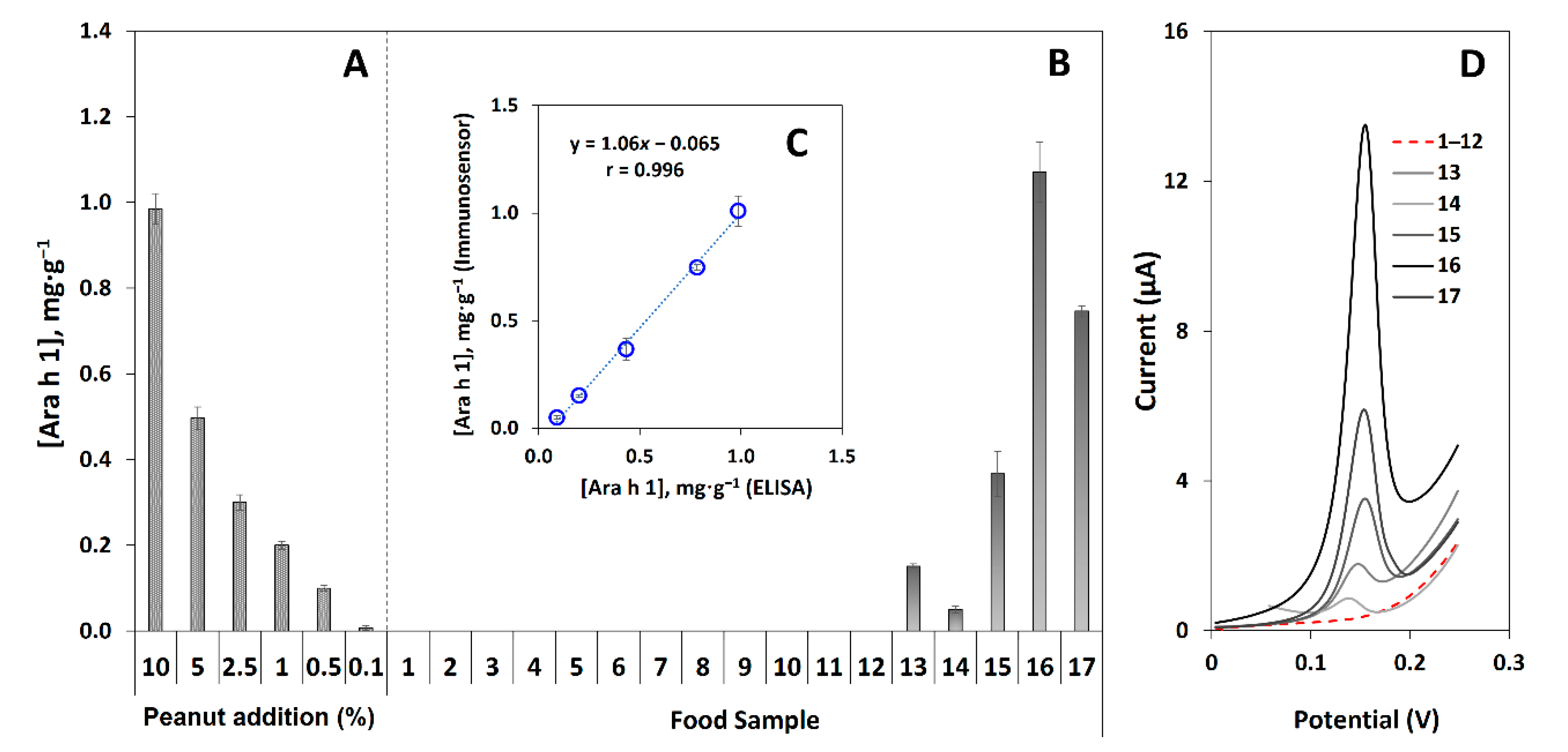
3.5. Quantification of Ara h 1 in Raw Peanuts and Analysis of Commercial Pre-Packed Food Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EC. Regulation (EU) No 1169/2011 on the Provision of Food Information to Consumers; European Commission: Luxembourg, 2011. [Google Scholar]
- Food and Drug Administration (FDA). Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA); FDA: Silver Spring, MD, USA, 2004. [Google Scholar]
- Madsen, C.B.; van den Dungen, M.W.; Cochrane, S.; Houben, G.F.; Knibb, R.C.; Knulst, A.C.; Ronsmans, S.; Yarham, R.A.R.; Schnadt, S.; Turner, P.J.; et al. Can we define a level of protection for allergic consumers that everyone can accept? Regul. Toxicol. Pharmacol. 2020, 117, 104751. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New Perspectives in Food Allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, W.J.; Taylor, S.L.; Phipatanakul, W.; Brough, H.A. Environmental Food Exposure: What Is the Risk of Clinical Reactivity from Cross-Contact and What Is the Risk of Sensitization. J. Allergy Clin. Immunol. Pract. 2018, 6, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Hass, S.L.; Donelson, S.M.; Robison, D.; Cameron, A.; Etschmaier, M.; Duhig, A.; McCann, W.A. The Peanut Allergy Burden Study: Impact on the quality of life of patients and caregivers. World Allergy Organ. J. 2021, 14, 100512. [Google Scholar] [CrossRef] [PubMed]
- WHO/IUIS. Allergen Nomenclature Sub-Committee Allergen Nomenclature. Available online: http://allergen.org/search.php?allergenname=Ara+h+&allergensource=&TaxSource=&TaxOrder=&foodallerg=all&bioname= (accessed on 1 February 2022).
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Correr, W.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of the peanut allergen Ara h 6 in foodstuffs using a voltammetric biosensing approach. Anal. Bioanal. Chem. 2015, 407, 7157–7163. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Reese, G.; Randow, S.; Zennaro, D.; Quaratino, D.; Palazzo, P.; Ciardiello, M.A.; Petersen, A.; Becker, W.-M.; Mari, A. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J. Allergy Clin. Immunol. 2009, 124, 771–778.e5. [Google Scholar] [CrossRef]
- Mueller, G.A.; Maleki, S.J.; Pedersen, L.C. The Molecular Basis of Peanut Allergy. Curr. Allergy Asthma Rep. 2014, 14, 429. [Google Scholar] [CrossRef] [Green Version]
- Sena-Torralba, A.; Pallás-Tamarit, Y.; Morais, S.; Maquieira, Á. Recent advances and challenges in food-borne allergen detection. TrAC Trends Anal. Chem. 2020, 132, 116050. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef]
- Alves, R.C.; Barroso, M.F.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. New Trends in Food Allergens Detection: Toward Biosensing Strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 2304–2319. [Google Scholar] [CrossRef]
- Freitas, M.; Neves, M.M.P.S.; Nouws, H.P.A.; Delerue-Matos, C. Electrochemical Immunosensor for the Simultaneous Determination of Two Main Peanut Allergenic Proteins (Ara h 1 and Ara h 6) in Food Matrices. Foods 2021, 10, 1718. [Google Scholar] [CrossRef]
- Huang, Y.; Bell, M.C.; Suni, I.I. Impedance Biosensor for Peanut Protein Ara h 1. Anal. Chem. 2008, 80, 9157–9161. [Google Scholar] [CrossRef]
- Sobhan, A.; Oh, J.-H.; Park, M.-K.; Kim, S.W.; Park, C.; Lee, J. Single walled carbon nanotube based biosensor for detection of peanut allergy-inducing protein ara h1. Korean J. Chem. Eng. 2018, 35, 172–178. [Google Scholar] [CrossRef]
- Montiel, V.R.-V.; Campuzano, S.; Pellicanò, A.; Torrente-Rodríguez, R.M.; Reviejo, A.J.; Cosio, M.S.; Pingarrón, J.M. Sensitive and selective magnetoimmunosensing platform for determination of the food allergen Ara h 1. Anal. Chim. Acta 2015, 880, 52–59. [Google Scholar] [CrossRef]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.A.; Marques, R.C.B.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. Detection of Ara h 1 (a major peanut allergen) in food using an electrochemical gold nanoparticle-coated screen-printed immunosensor. Biosens. Bioelectron. 2015, 64, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Voltammetric Immunosensor to Track a Major Peanut Allergen (Ara h 1) in Food Products Employing Quantum Dot Labels. Biosensors 2021, 11, 426. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Schrand, A.M.; Hens, S.A.C.; Shenderova, O.A. Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Power, A.C.; Gorey, B.; Chandra, S.; Chapman, J. Carbon nanomaterials and their application to electrochemical sensors: A review. Nanotechnol. Rev. 2018, 7, 19–41. [Google Scholar] [CrossRef]
- Bilal, M.; Cheng, H.; González-González, R.B.; Parra-Saldívar, R.; Iqbal, H.M.N. Bio-applications and biotechnological applications of nanodiamonds. J. Mater. Res. Technol. 2021, 15, 6175–6189. [Google Scholar] [CrossRef]
- Qin, J.-X.; Yang, X.-G.; Lv, C.-F.; Li, Y.-Z.; Liu, K.-K.; Zang, J.-H.; Yang, X.; Dong, L.; Shan, C.-X. Nanodiamonds: Synthesis, properties, and applications in nanomedicine. Mater. Des. 2021, 210, 110091. [Google Scholar] [CrossRef]
- Zhang, W.; Patel, K.; Schexnider, A.; Banu, S.; Radadia, A.D. Nanostructuring of Biosensing Electrodes with Nanodiamonds for Antibody Immobilization. ACS Nano 2014, 8, 1419–1428. [Google Scholar] [CrossRef]
- Simioni, N.B.; Silva, T.A.; Oliveira, G.G.; Fatibello-Filho, O. A nanodiamond-based electrochemical sensor for the determination of pyrazinamide antibiotic. Sens. Actuators B Chem. 2017, 250, 315–323. [Google Scholar] [CrossRef]
- Fernandes-Junior, W.S.; Zaccarin, L.F.; Oliveira, G.G.; de Oliveira, P.R.; Kalinke, C.; Bonacin, J.A.; Prakash, J.; Janegitz, B.C. Electrochemical Sensor Based on Nanodiamonds and Manioc Starch for Detection of Tetracycline. J. Sens. 2021, 2021, 6622612. [Google Scholar] [CrossRef]
- Huang, T.S.; Tzeng, Y.; Liu, Y.K.; Chen, Y.C.; Walker, K.R.; Guntupalli, R.; Liu, C. Immobilization of antibodies and bacterial binding on nanodiamond and carbon nanotubes for biosensor applications. Diam. Relat. Mater. 2004, 13, 1098–1102. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Approaches to Establish Thresholds for Major Food Allergens and for Gluten in Food. Available online: https://www.fda.gov/files/food/published/Approaches-to-EstablishThresholds-for-Major-Food-Allergens-and-for-Gluten-in-Food.pdf (accessed on 1 February 2022).
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Hernández-Santos, D.; González-García, M.B.; Costa-García, A. Alkaline Phosphatase-Catalyzed Silver Deposition for Electrochemical Detection. Anal. Chem. 2007, 79, 5272–5277. [Google Scholar] [CrossRef]
| Experimental Variable | Selected Parameter/Value | |
|---|---|---|
| Nanomaterial (SWCNT, MWCNT, rGO, ND) |  | ND |
| [ND] (mg·mL−1) (1.0, 0.50, 0.25, 0.10, 0.05, 0.025) | 0.10 | |
| [CAb] (µg·mL−1) (5.0, 10, 25) |  | 10 |
| DAb (Dilution) (1000×, 500×, 250×) |  | 250× |
| S-AP (Dilution) (100,000×, 150,000, 200,000×, 250,000×) |  | 200,000× |
| Assay Format (Step-by-step, Ara h 1 + DAb, DAb + S-AP) |  | Step-by-step (Format 1) |
| Assay time |  | 2 h 20min |
| Food/Ingredient | Ara h 1 (mg·g−1) | Relative Deviation (%) | Sample Dilution | |
|---|---|---|---|---|
| Immunosensor | ELISA | |||
| Wheat flour | ND | ND | ND | 100× |
| Oat | ND | ND | ND | |
| Lupine | ND | ND | ND | |
| Pea | ND | ND | ND | |
| Soybean | ND | ND | ND | |
| Almond | ND | ND | ND | |
| Hazelnut | ND | ND | ND | |
| Energy Bar (No peanut) | ND | ND | ND | |
| Biscuit (No peanut) | ND | ND | ND | |
| Water Cracker (May contain peanut) | ND | ND | ND | |
| Oatmeal cookie (May contain peanut) | ND | ND | ND | |
| Whole-grain cereal (May contain peanut) | ND | ND | ND | |
| Granola (May contain peanut) | 0.18 ± 0.01 | 0.20 ± 0.01 | −11.1 | |
| Muesli (May contain peanut) | 0.08 ± 0.02 | 0.09 ± 0.01 | −8.60 | |
| Protein Bar (5% peanut) | 0.37 ± 0.05 | 0.40 ± 0.04 | −7.50 | 1000× |
| Protein Bar (12% peanut) | 1.07 ± 0.07 | 0.95 ± 0.05 | 12.6 | |
| Peanut and Pineapple Cookie (8% peanut) | 0.75 ± 0.01 | 0.78 ± 0.03 | −2.60 | |
| Raw peanut | 4.29 ± 0.16 | 4.33 ± 0.31 | −0.92 | |
| Peanut Butter | 4.85 ± 0.26 | 4.70 ± 0.41 | 3.19 | |
| Biosensor Construction and Detection Technique | Nanomaterial | LOD | Sample | Ref |
|---|---|---|---|---|
| SPCE/NDs with CAb immobilized by physical adsorption. Sandwich-type assay, AP used as label. Detection through LSV. | NDs | 0.78 ng·mL−1 | Biscuits, crackers, cookies, cereals, energetic/protein bars | This work |
| AuE/11-MUA with CAb immobilized through covalent binding (EDC/NHS). Label-free assay, [Fe(CN)6 ]3−/4− used for detection. EIS employed as electrochemical technique. | — | 0.3 nM | n.d. | [15] |
| Silicon wafer/SWCNT with CAb covalently immobilized (1-PBSE). Label-free assay. Analysis performed by LSV. | SWCNT | 1.0 ng·mL−1 | n.d. | [16] |
| SPCE/MBs with CAb immobilized through covalent binding (EDC/NHS). Sandwich-type assay, HRP used as label. Amperometry was elected for detection. | MBs | 6.3 ng·mL−1 | Food extracts, saliva | [17] |
| SPCE/AuNP with CAb immobilized by chemisorption. Sandwich-type assay, AP used as label. Detection through LSV. | AuNP | 3.8 ng·mL−1 | Cookies, chocolate | [18] |
| Bare SPCE with CAb immobilized through physical adsorption. Sandwich-type assay, QDs used as label. DPV used for detection. | QDs | 3.5 ng·mL−1 | Cookies, cereal, protein bars | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, M.; Carvalho, A.; Nouws, H.P.A.; Delerue-Matos, C. Tracking Arachis hypogaea Allergen in Pre-Packaged Foodstuff: A Nanodiamond-Based Electrochemical Biosensing Approach. Biosensors 2022, 12, 429. https://doi.org/10.3390/bios12060429
Freitas M, Carvalho A, Nouws HPA, Delerue-Matos C. Tracking Arachis hypogaea Allergen in Pre-Packaged Foodstuff: A Nanodiamond-Based Electrochemical Biosensing Approach. Biosensors. 2022; 12(6):429. https://doi.org/10.3390/bios12060429
Chicago/Turabian StyleFreitas, Maria, André Carvalho, Henri P. A. Nouws, and Cristina Delerue-Matos. 2022. "Tracking Arachis hypogaea Allergen in Pre-Packaged Foodstuff: A Nanodiamond-Based Electrochemical Biosensing Approach" Biosensors 12, no. 6: 429. https://doi.org/10.3390/bios12060429
APA StyleFreitas, M., Carvalho, A., Nouws, H. P. A., & Delerue-Matos, C. (2022). Tracking Arachis hypogaea Allergen in Pre-Packaged Foodstuff: A Nanodiamond-Based Electrochemical Biosensing Approach. Biosensors, 12(6), 429. https://doi.org/10.3390/bios12060429







