Abstract
The presence of pyrethroids in food and the environment due to their excessive use and extensive application in the agriculture industry represents a significant threat to public health. Therefore, the determination of the presence of pyrethroids in foods by simple, rapid, and sensitive methods is warranted. Herein, recognition methods for pyrethroids based on electrochemical and optical biosensors from the last five years are reviewed, including surface-enhanced Raman scattering (SERS), surface plasmon resonance (SPR), chemiluminescence, biochemical, fluorescence, and colorimetric methods. In addition, recognition elements used for pyrethroid detection, including enzymes, antigens/antibodies, aptamers, and molecular-imprinted polymers, are classified and discussed based on the bioreceptor types. The current research status, the advantages and disadvantages of existing methods, and future development trends are discussed. The research progress of rapid pyrethroid detection in our laboratory is also presented.
1. Introduction
The withdrawal of highly hazardous organophosphate pesticides from the world market over the last ten years has resulted in pyrethroid insecticides becoming the preferred alternative pesticides due to their effectiveness in pest control [1,2]. Pyrethroids have the broadest application, highest efficiency and lowest residue in addition to their moderate toxicity and biodegradability in plants. To date, more than 70 pyrethroid pesticides have been used in agriculture [3]. Type I pyrethroid pesticides lack α-cyanogen and are represented by permethrin, bifenthrin, and others, while type II structures contain α-cyanogen and are represented by fenpropathrin, cyfluthrin, deltamethrin, and fenvalerate [4,5]. Type II pyrethroid pesticides have good efficacy and stability and are widely used to control pests during crop production.
Pyrethroid pesticides show developmental and neurotoxic effects on mammals and aquatic organisms, which can delay embryo development, increase mortality and the risk of cancer, and even lead to the extinction of aquatic species [6,7,8]. They are also potentially toxic to plants, soil, and aquatic ecology. For example, they are phytotoxic to cucumber seed germination rate, root elongation, branch length, and leaf length [9]. They also disturb soil microbial communities and reduce natural biodegradation [10] and are widely used in household hygiene due to their effective treatment of household pests such as mosquitoes; however, direct contact with pyrethroid pesticides increases health risks [11,12]. Pyrethroid pesticides have strong absorbability and are directly and indirectly transmitted into the food chain, which eventually poses a threat to human health and life [13]. Pyrethroid pesticides are endocrine-disrupting compounds (EDCs) that indirectly interfere with upstream endocrine signal transduction signaling pathways through direct receptor interactions by mimicking and cooperating with endogenous hormones [14]. The pyrethroid metabolite 3-phenoxybenzaldehyde (3-PBA) bioaccumulates in human breast milk, which negatively impacts babies relying on breast milk [15,16]. Although pyrethroid pesticides have no acute toxic effects on humans, long-term exposure may damage male sperm quality and reduce sperm count in F1 offspring during pregnancy and lactation [17,18].
The wide use of pyrethroid insecticides has prompted food safety research to focus on pyrethroid residue detection in crops. Therefore, many countries and organizations have employed strict residue limits for pyrethroid pesticides. For example, the maximum residue level (MRL) of pyrethroid pesticides is in the range of 0.01–4 mg/kg (up to 31 mg/kg for tea) for crops in the European Union; 0.01–20 mg/kg in the USA; 0.01–20 mg/kg (up to 50 mg/kg for hops) in Japan; and 0.01–10 mg/kg (up to 20 mg/kg for tea) in China. Therefore, rapid, sensitive, and effective detection methods should be established to monitor pyrethroid residues in crops and reduce human exposure.
Pyrethroid detection technology includes instrument-based methods [19,20,21,22,23,24] and sensor-based methods. Instrument-based confirmation methods can utilize separation by chromatography [25] combined with strong selectivity and mass spectrometry. This has the advantage of more structural information and high-throughput rapid detection for the accurate analysis of pyrethroid pesticides is possible [25]. The instrument-based method is time-consuming and expensive and requires professional technicians for operation. Therefore, sensor-based methods of agricultural residues have quickly developed. Currently, many recognition elements used to detect pyrethroid pesticides are being used in conjunction with detection techniques. However, few reports have summarized the identification elements and sensor-based methods of pyrethroid pesticides. This review introduces the recognition elements (enzymes, antigens/antibodies, aptamers, and molecular-imprinted polymers) for pyrethroid pesticides and the corresponding determination sensor (SERS or surface-enhanced Raman scattering, SPR or surface plasmon resonance, chemiluminescence sensor, biochemical sensor, fluorescence sensor, and colorimetric sensor) used for detection. Furthermore, the pros and cons associated with sensor-based methods for pyrethroid pesticide detection were analyzed (Table 1).

Table 1.
Representative examples of pyrethroid residue detection based on sensors.
2. Recognition Elements for Pyrethroid Pesticide Detection
Pesticide sensors use biorecognition elements to directly contact the conduction system in space and convert biochemical information into electrical, thermal, optical, and other output forms [38]. These chemical sensors are called biosensors (or biomimic recognition sensors) and can be classified as enzymes, antigens/antibodies, and artificial receptors such as molecularly imprinted polymers (MIPs) and aptamers. They have the advantages of simplicity, rapidity, specificity, high sensitivity, and low cost [39]. The mertis and demertis of the recognition elements for the determination of pyrethroids are summarized in Table 2.

Table 2.
The advantages and disadvantages of the recognition elements for the determination of pyrethroids.
2.1. Enzyme-Based Biosensors
Enzyme-based sensors have been studied since 1962 [40] and their sensitivity and universality allow for widespread use in pesticide detection. These are divided into two types of biosensors for pesticide detection: inhibitory and catalytic. Pyrethroids are degraded by the oxidation of P450 monooxygenase, coupling with glutathione S-transferase, and the hydrolysis of phosphotriesterase or carboxyesterase [41]. The hydrolysis of pyrethroids by carboxyl esterase is the primary means of pyrethroid microbial biodegradation. Dongqing et al. described the ester bond catalytic mechanism of carboxylesterase PytH (pyrethroid-degrading carboxylesterase) for pyrethroid pesticides. Carboxylesterases from Sphingobium faniae JZ-2 have α/β hydrolase folding proteins that typically catalyze Ser–His–Asp triples and can effectively hydrolyze many pyrethroid pesticides. PytH has no isomer selectivity compared to other reported pyrethroid-hydrolyzing carboxylesterases, making it a good candidate for pyrethroid residue elimination. Since the hydrolysis efficiency of PytH is relatively low, it can be improved by direct evolution or rational protein design [42]. However, enzymes have strict preservation conditions, poor stability and selectivity, and are inactivated at high organic solvent concentrations [43]. Therefore, to improve their detection performance for pesticides, enzymes are used in conjunction with electrochemical sensors [44].
2.2. Antigen/Antibody-Based Biosensors
Immunoassays generally require two steps: hapten construction and antibody preparation. Hapten construction is a prerequisite to obtain the complete antigen of pesticides [45]. Pyrethroid pesticides are hydrophobic molecules and there are therefore great challenges involved in constructing effective haptens for immunoassay methods. Cui et al. [46] synthesized β-cyhalothrin haptens using a one-step method by selectively hydrolyzing the -CN group with a low-toxicity reagent. The haptens were coupled with succinic anhydride-activated carrier protein and the complete antigens were used to prepare polyclonal antibodies (Figure 1). This method replaced the trimethylchlorosilane catalyst with trimethylsilyl trifluoromethane sulfonate to increase the acidity and yield of the preparation to 69%. The method has higher structural fidelity and maintains the integrity of most of the functional groups while modifying the reaction groups. Fruhmann et al. [27] selected and synthesized the immune deltamethrin hapten D133 and cypermethrin hapten C134, established antisera As358-363, and encapsulated the antigens C134-AD, D133-AD, F1-BSA, F2-BSA, F3-BSA, and 3-PBA-BSA. The best combination is As360 (C134-HCH), and the homologous competitor C134-AD can be used for the direct determination of deltamethrin. The IC50 was 21.4 ± 0.3 μg/L, and the detection limit was 1.21 ± 0.04 μg/L. The IC50 of cypermethrin was 78.6 ± 0.7 μg/L and the limit of detection was 4.56 ± 0.05 μg/L. The antiserum induced by chlorinated compounds was better able to recognize brominated derivatives. This may be because bromine atoms are larger than chlorine atoms and are more suitable for antibody binding sites, which facilitates the antibody recognition of deltamethrin. Alternatively, the difference in electron distribution between chloride and bromide may facilitate recognition. The antibodies prepared by this study are specific to deltamethrin and do not interfere with the determination of other contaminants commonly found in environmental samples by cross-reactivity.
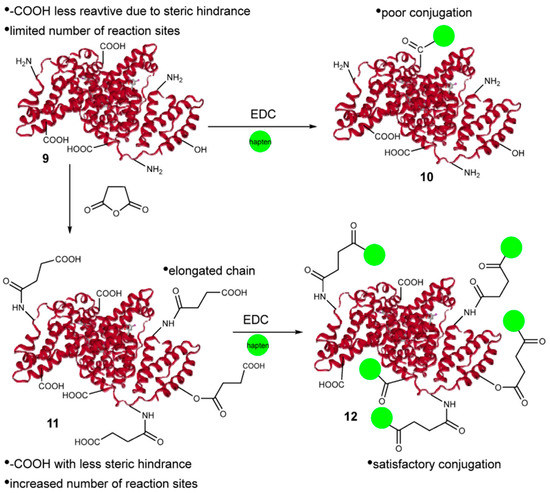
Figure 1.
Illustrative figure of the succinic acid treatment and conjugation process ([46]).
2.3. Aptamer-Based Biosensors
Aptamers are single-stranded DNA (ssDNA) or RNA molecules that are synthesized by in vitro chemical methods with high affinity, selectivity, and stability [47]. They consist of 25–30 bases and are systematically optimized for aptamer selection by exponential enrichment using the systematic evolution of ligands by the exponential enrichment (SELEX) technique. Aptamers are more environmentally stable than antibodies and are easier to synthesize in large quantities. Trace targets of ng/kg or even pg/kg can be detected when used in combination with optical and electrochemical techniques [48,49]. However, aptamer development is time-consuming, and complex computational methods can reduce the time and cost by minimizing experimentation [50]. Aptamers are used for pesticide detection by folding ssDNA or RNA into tertiary structures. However, false positive and nonspecific signals may occur due to unpredictable structures and ineffective folding in complex matrices [44]. Yang et al. [48] solved this problem by using a modified capture-SELEX strategy for the selection of λ-cyhalothrin pesticide adaptors. The ssDNA library was fixed to the magnetic bead, and the binding affinity sequence was competitively captured in the magnetic bead after target addition. The recognition mechanism and action site of the aptamer and target were studied using molecular docking technology (Figure 2). A new λ-cyhalothrin colorimetric detection method was established using the aptamer as the recognition molecule and colloidal gold-controlled aggregation mediated by a cationic polymer as the sensing signal. This study provides a reference method for small molecule detection in food. However, aptamers specific to whole-cell SELEX showed significant non-specificity, which may be due to antigen-binding sites on membrane proteins that are ubiquitous in cells [51].
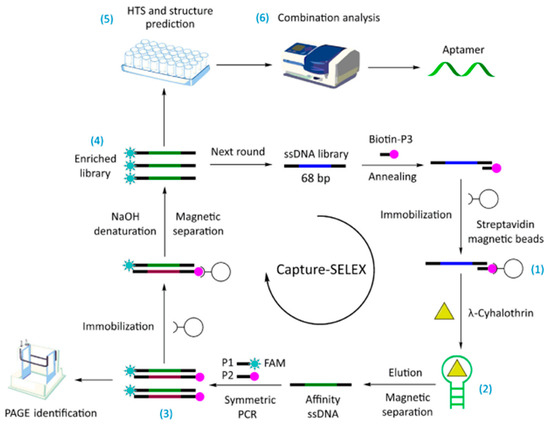
Figure 2.
The capture-SELEX technology roadmap for screening aptamers bound to l-cyhalothrin ([48]). (1 Immobilization of random ssDNA library; 2 Elution of affinity ssDNA sequences; 3 Symmetric PCR amplification and electrophoresis identification; 4 Preparation of ssDNA enrichment library; 5 High-Throughput Sequencing (HTS) and secondary structure prediction; 6 Binding characteristics of affinity sequences).
2.4. MIP-Based Sensors
MIPs take target molecules [52] or structural analogs [42,43] as templates and functional monomers to synthesize highly cross-linked three-dimensional network structures through covalent or noncovalent crosslinking agents. Template molecules washed with organic solvents leave specific recognition sites in the polymer network that are complementary to template molecules in shape, size, and function [53]. MIPs can be used as a bionic adhesive for biosensors and for grabbing samples during pretreatment. It is superior to antibodies in terms of stability and shelf life, and its target binding performance is equal to or superior to that of natural antibodies [54]. Therefore, MIPs are often used as substitutes for natural antibodies, receptors, and enzymes in biosensors due to their predetermination, recognition and practicability, simple preparation, low cost, and good chemical stability [55] and wide use in environmental monitoring. For instance, Heravizadeh et al. [56] synthesized an MIP with highly specific adsorption for permethrin by precipitation polymerization. The adsorption mass of cis-permethrin and trans-permethrin was up to 7.71 mg, and the method was reliable and effective for the detection of permethrin isomers in biological and environmental samples. Chen et al. [57] used a cyhalothrin template to prepare magnetic MIPs for the rapid high-affinity detection of cyhalothrin in honeysuckle by the precipitation polymerization with an adsorption capacity of 4.9 mg/g. This provides a method to increase MIP binding sites and improve their selectivity. However, using pesticide as the template prevents its complete washing, resulting in template leakage and causing false positive results during detection [58]. Materials with structures and properties similar to those of the target molecule can be used as templates in the synthesis of MIPs to overcome these challenges and improve the application range of MIPs [59]. Cai et al. [60] used phenyl ether as a virtual template to replace pyrethroid pesticides to synthesize molecularly imprinted microspheres (MIMs) and fluorescent tracers (Figure 3). An optimized multiple fluorescence method was then used to simultaneously determine 10 pyrethroid pesticides from 60 real beef and mutton samples with standard recovery rates of 67.77–109%. The advantage of this experiment lies in the virtual template, but its low specificity cannot accurately screen specific pesticides. This problem may be caused by the large MIP surface area, which binds to nonspecific sites such as the sample matrix [61].
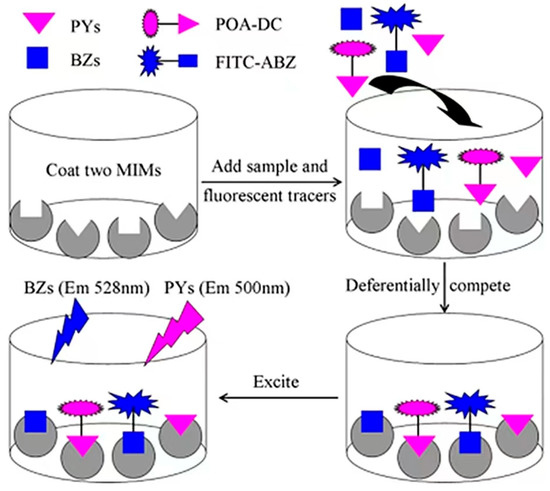
Figure 3.
Assay principles of the MIM-based multiplexed fluorescence method ([60]).
3. Sensor-Based Methods for Pyrethroid Pesticide Determination
3.1. Detection of Pyrethroids Based on Electrochemical Sensors
Electrochemical sensors are a change in current or impedance caused by the combination of a target on an electrode. Changes in the chemical signal modify the electrical signal to quantify pesticides [44]. Esquivel-Blanco et al. [62] developed an electrochemical method for the determination of the pyrethroid metabolite 3-PDB (3-phenoxybenzaldehyde) using laccase as an alternative recognition element. The enzyme is immobilized onto a gold electrode layered with alkanethiol through amide bond formation between the lysine residue of the enzyme and the activated carboxyl group of alkanethiol. The enzyme directly oxidizes the substrate onto the gold electrode with a detection limit of 0.061 μM. In this context, Silva et al. [26] used a polished silver solid alloy electrode (P-AGSAE) related to square wave cathode adsorption stripping voltammetry (SWCASV) as an electrical element for the determination of β-cyhalothrin in water and tea. There was no significant difference compared with gas chromatography–mass spectrometry at the 95% confidence level, proving that the electrochemical method is robust with good stability and sensitivity. Furthermore, Ribeiro et al. [63] used Molegro Virtual Docker-MVD to simulate the docking of four permethrin pesticides at the active site of GST. The results showed that the compounds they selected had high affinity for the catalytic site of the GST enzyme. Therefore, a GST-based screen-printed electrochemical biosensor was developed with LODs of 0.9, 1.6, 3.6, and 9.5 μg/L, respectively, with good accuracy, reproducibility, and stability. Moreover, Borah et al. [64] demonstrated that glutathione S-transferase (GST) immobilized in graphene oxide can be used as an electrocurrent bioelectrochemical sensor for the determination of β-cypermethrin containing 25% methanol, showing that biosensors can be used in relatively high organic solvent concentrations. Additionally, Fruhmann et al. [27] developed an immunosensor based on antigen–antibody measurements and amperometric electrochemical readings that can detect deltamethrin in different water environments by changing electrical parameters, and the limit of detection was 4.7 mug/L in water. MIPs make important contributions to the selectivity and sensitivity of traditional electrochemical methods. If nanomaterials are reintroduced into molecular imprinting, electrochemical sensors will have improved catalytic performance and enhanced conductivity through a rough conductive sensing interface surface [65]. Chansi et al. [66] established a novel immune sensing platform, BSA/Chi-AuNP-rIgG-BSA/MOF/ITO, which used MOF and IgG polyclonal antibody dual screening to detect a variety of pesticides including pyrethroids (Figure 4). The theoretical analysis of rIgG binding was consistent with its functional affinity for a variety of pesticides. Sample detection may be performed with a portable device for simplifying pesticide analysis with minimal heavy metal ion interference, short analysis time, and good stability. Two-dimensional hexagonal boron nitride nanosheets (2D-hBN or white graphene) were used as binding nanomaterials for electrochemical sensors due to their high temperature stability, large surface area, high mechanical strength, and terminal conductivity [67,68]. For instance, Atar and Yola prepared layered nanosheets of an amine-functionalized Fe@AuNPs/2D-hBN nanocomposite electrochemical sensor, which improved the sensitivity of β-cypermethrin detection in wastewater samples based on the synergistic effect of MIPs and nanocomposites [28].
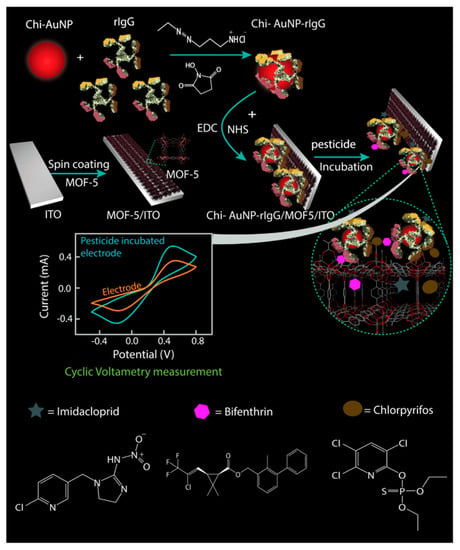
Figure 4.
Schematic representation of the mechanism of fabrication and detection of pesticides by immunoelectrode ([66]).
3.2. Detection of Pyrethroids Based on Optical Sensors
3.2.1. Surface-Enhanced Raman Scattering Method
SERS is an ultrasensitive vibrational spectroscopy technique used to detect molecules on or near the surface of plasma nanostructures [69]. It has the characteristics of supersensitive, quantitative, real-time detection and multiplexing, and has a wide range of applications in biochemistry and life sciences [70]. The instability of biological components and their insensitivity in identifying small molecule analytes limits their application. When the analyte is adsorbed on the surface of heavy metals, the Raman signal is enhanced, whereas the service life of the SERS substrate is shortened since it does not eliminate the molecules adsorbed on the surface. However, MIP shows high mechanical and chemical stability for small molecule detection, and its molecular selectivity combined with spectroscopy provides a synergistic effect for the fingerprint identification of complex samples [71]. In addition, MIP prevents SERS substrate oxidation and protects the core material [29]. SiO2@TiO2@Ag@MIPs were designed by Li et al. [29] for the detection of fenvalerate in river water (Figure 5). The use of SiO2 prevents the agglomeration of TiO2 and Ag and ensures good optical transparency. The composite structure material improves the functionality and selectivity of the SERS substrate, enhances the SERS performance of Ag particles, and degrades the templates adsorbed on its surface. Wang et al. [72] synthesized Fe3O4/GO/Ag-MIPs (FGA-MIPs) and combined SERS technology to form an FGA-MIP/SERS-imprinted sensor that shows selectivity, good magnetic separation, and the sensitive detection of λ-cyhalothrin in water. This provides a new method for the determination of pyrethroid pesticides in aquatic environments.
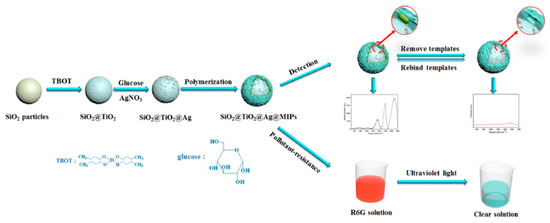
Figure 5.
Illustration of the preparation of SiO2@TiO2@Ag@MIPs and SERS detection of FE upon specific recognition ([29]).
3.2.2. Surface Plasmon Resonance Method
SPR is a kind of charge density oscillation with the resonance oscillation of the conduction electron generated by incident light irradiating the interface of a material—such as a dielectric metal film—and the corresponding quantum called a surface plasmon [73]. SPR works by measuring the refractive index near a metal surface, although it cannot distinguish between solutions with the same refractive index. However, modifying the SPR system or modifying the metal film by the active layer or sensing element rectifies this issue (Figure 6) [74].
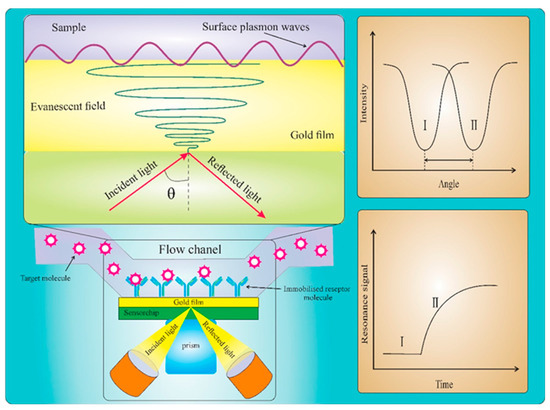
Figure 6.
A schematic illustration of the conventional Kretschmann optical configuration for SPR biosensing and the associated angle shift and sensorgram plot of the resonance signal change with time ([67]).
The combination of small molecule pesticides on the surface of traditional SPR sensors results in a small refractive index with the introduction of nanomaterials enhancing the change in refractive index [75,76]. For example, Liu et al. [30] combined an SPR sensor with Fe3O4 magnetic nanoparticles (MNPs) by coupling Fe3O4 and an antibody with MNPs to deliver the carrier of the target analyte to the surface of the sensor. The favorable characteristics of MNPs (large surface area, good magnetism, high refractive index, and high molecular weight) increase the SPR signal, improve sensitivity, and reduce the background interference. This method can simplify sample pretreatment and improve the determination accuracy. The sensitivity of this method for deltamethrin in soybean increases by four orders of magnitude compared with the direct SPR method. In addition, the SPR phase measurement based on the topological properties of the system can replace the amplitude measurement [77].
3.2.3. Chemiluminescence Method
Chemiluminescence sensors can selectively respond to receptor molecules and extract information about specific analytes in complex samples. The optical changes of receptor molecules are of great concern [78]. However, chemiluminescence sensitivity is low and the highly specific recognition of MIPs can play a synergistic role [79]. For example, Zang et al. [80] developed a highly selective chemiluminescence system for fenvalerate by synthesizing fenvalerate MIP using in situ polymerization and applying the quenching mechanism of the luminol–H2O2–NaOH chemiluminescence system. A chemiluminescence method for fenpropathrin detection was developed in a similar way by Zhao et al. [81] in the same laboratory. This method improved the adsorption performance of MIP and enhanced the enrichment performance of fenpropathrin compared with the chemiluminescence method by synthesizing double-sided hollow MIP microspheres. However, the special Y-shaped tubes used in these two articles meant that only fenvalerate and fenprothrin were determined instead of multiple simultaneous detections. Huang et al. [31] prepared an MIP that identifies 10 pyrethroid pesticides using double virtual templates and designed a chemiluminescence sensor for the determination of chicken pyrethroid pesticides. This method was repeatable four times, had a detection time of 12 min, and the limits of detection were in the range of 0.3–6.0 pg/mL in the 10 analytes.
3.2.4. Fluorescence Method
A fluorescent biosensor measures analytes by fluorescence enhancement or quenching caused by direct interaction between the fluorescent probe and analyte [82]. Current fluorescent substances used for analysis and determination include quantum dots, carbon dots, rare earth elements, and fluorescent dyes [83].
The fluorescence detection method has high efficiency, simplicity, and sensitivity and may be combined with an MIP for specific recognition and enrichment [33]. Samples are detected by fluorescence quenching after the target substance binds with MIP. Wang et al. [33] showed that the 5(6)-isothiocyanate (FITC) and 3- aminopropyltriethoxysilane (APTS)/SiO2 composite fluorescent MIP selectively recognizes and detects λ-cyhalothrin. This method eliminates the interfering substances in the sample and improves the detection limit.
Quantum dots. QDs are a new type of semiconductor fluorescent nanocrystal with a high quantum yield and narrow emission spectrum. Narrow photoluminescence bands caused by quantum dots provide bright light even for individual molecules [65]. Most quantum dots are synthesized by toxic, unstable heavy metals such as cadmium, which potentially harm the environment and organisms [84]. Therefore, it is necessary to passivate the shell to reduce heavy metal leakage. Quantum dots can be protected by coupling them with enzyme-, antibody-, and MIP-based nanomaterials on their surface [85]. For example, MIP-QDs obtained after the surface functionalization of quantum dots show high selectivity and fluorescence characteristics for the target and may be used for pesticide detection [86,87,88]. Li et al. prepared a novel eco-friendly MIP-QD sensitive fluorescence nanosensor for the selective quenching fluorescence of cyfluthrin based on FeSe-QDs using an optimized reverse microemulsion method (Figure 7). The specific recognition of cyfluthrin is due to ionic interactions, molecular structure selection and hydrogen bond interactions to prevent charge transfer from FeSe-QDs to cyfluthrin, resulting in the phenomenon of fluorescence quenching. This method has excellent linearity, selectivity, and sensitivity, and was used for detecting cyfluthrin in fish samples [34].
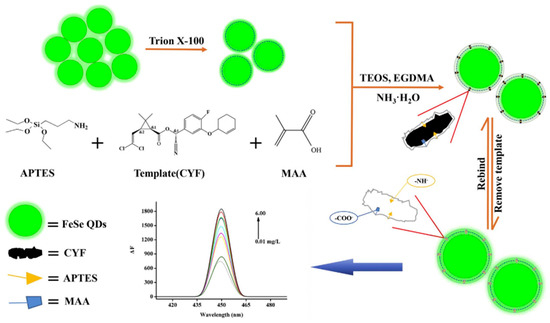
Figure 7.
Schematic representation of a fluorescent nanosensor based on MIP-FeSe-QD ([34]).
Carbon dots. Fluorescent carbon dots (CDs), also known as carbon quantum dots, are novel, zero dimensional (0D; diameter below 10 nm), nontoxic photoluminescent carbon nanomaterials [89]. They are used as a fluorescence response signal due to their strong luminescence and controllable performance [90,91]. They are widely studied because of their chemical stability, good electrical conductivity, biocompatibility, luminescence, and wide absorption wavelength range [92,93,94]. In recent years, many studies have applied CDs to provide technical guidance for pollutant detection in the environment [95]. In most cases, the fluorescence quenching of solid CDs occurs [96] but their combination with titanium, nickel, and cadmium enhances light absorption and the visible light response, which improves their photocatalytic performance [97]. Although fluorescent CDs can be directly used to detect analytes, their sensitivity and anti-interference ability are low. However, MIPs can compensate for this defect, and combination with CDs to prepare fluorescent nanomaterials for pesticide detection is possible [98,99,100].
Zhu et al. [35] developed a simple and effective two-channel specific fluorescence method for the determination of λ-cyhalothrin based on dual-emission blue–green CD functionalized core–shell nanospheres. The blue and green CDs were taken as the reference signal and the detection signal, respectively. The use of ionic liquids with a wide viscosity range and good stability can improve the detection sensitivity and selection range of core–shell nanospheres. Combination with smartphone integrated optics enables the real-time detection of λ-cyhalothrin by monitoring the fluorescence changes from green to blue. The interference of shortwave backgrounds was overcome by the introduction of functional groups resulting in the development of a red-emitting carbon point (RCD) with stable emission characteristics wherein the CD moves in the direction of red wavelengths [101]. Zhu et al. prepared an on-site visualization and rapid detection RCD MIP sensor for the quantitative detection of λ-cyhalothrin using a smartphone (Figure 8) [102].
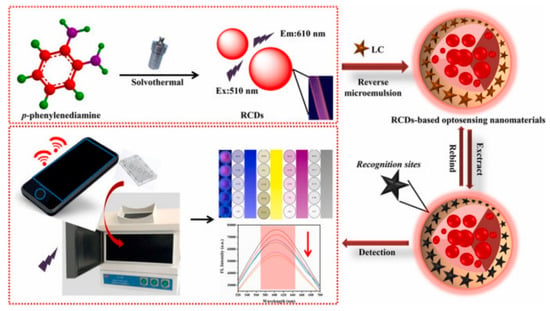
Figure 8.
Schematic illustration of the platform comprising RCD-based optosensing nanomaterials for the detection of LC ([102]).
Time-resolved fluorescence microsphere. Ordinary fluorescent groups are easy to quench during detection, and the detection time is greatly reduced (Stokes shift is 1–100 nm) due to high photobleaching and chemical degradation efficiency [103]. Trivalent rare earth ions such as Eu(III), Tb(III), and Sm(III) were used as labels in time-resolved fluorescence analysis and may be used as a fluorescence quantitative immunoassay. The Stokes shift and fluorescence lifetime were above 150 nm and up to 1 millisecond, respectively [104]. Lanthanide chelates produce high-intensity fluorescence, strongly resist photobleaching, and have a long decay time (1250 μs). This delays the measurement time and eliminates the interference of natural fluorescence, which greatly improves method sensitivity [105,106,107]. Rare earth-based nanomaterials are stable and bright fluorescent probes, so they are often the best nanoprobes for hypersensitive biological detection [108]. They are typically used to detect agricultural residues through the preparation of an immunochromatographic strip [109,110,111,112]. However, we did not find relevant literature in the Web of Science containing time-resolved fluorescence and pyrethroid pesticides as keywords.
3.3. Detection of Pyrethroids Based on Biosensors
3.3.1. Biochemical Method
The use of antibodies and enzymes as recognition elements has the advantage of high throughput, convenience, good sensitivity, and simplicity [113]. Typically, enzyme-linked immunoassays (ELISAs) are employed. The enzyme has very high sensitivity and can be used for the trace detection of pesticides with a detection limit of 10−10 M. However, the short lifetime of the enzyme and the interference of impurities such as metal ions in the matrix result in weaker specificity and limit its application [46,114]. Although ELISA has good detection sensitivity, there may be technical difficulties during pesticide labeling [115]. López Dávila et al. [116] used the Abraxis pyrethroid assay kit to determine permethrin in water (control group), cucumber, tomato, and bell pepper and determined a minimum detectable concentration of 10 μg/L using the Log10 value of B/B0% as the Y axis and the permethrin concentration as the X axis. The cross-reaction test of 12 pesticides showed consistent results with gas chromatography–electron capture detection (GC-EDC) and ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS). This study became the basis for the Cuban pesticide residue detection program ELISA kit. Huo et al. [117] developed a fast and sensitive direct competitive fluorescein immunoassay (DC-FEIA) to detect the pyrethroid metabolite 3-PBA based on a nanobody (Nb)-alkaline phosphatase (AP) fusion protein. The IC50 of this method is nearly ten times higher than that of direct ELISA and can detect 3-PBA in urine. Xiao et al. [32] established an ELISA method to detect 0.05–620 mg/kg cypermethrin fish based on MIP-QDs (MIP-quantum dots) (Figure 9). The method shows linear fluorescence quenching and combines the advantages of rapid, sensitive, and efficient ELISA with the high specificity and sensitivity of MIP-QDs.
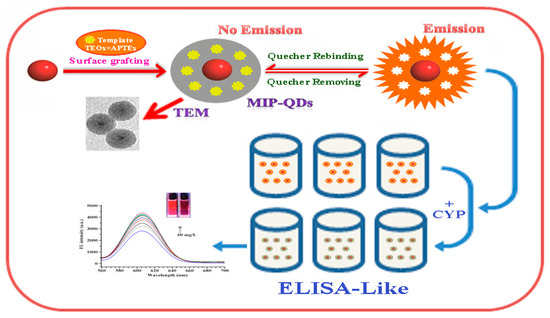
Figure 9.
An ELISA-like method based on the MIP-QDs to monomer cypermethrin in the samples ([32]).
3.3.2. Colorimetric Method
Some colorimetric signals can be observed with the naked eye or read with a smartphone. Although colorimetric methods are easy to prepare and enable rapid detection, most food extracts are colored, which interferes with detection [118]. Colorimetric reaction methods are mostly based on membranes and paper or microfluidic chips [119]. Immunochromatography is a colorimetric analysis method that combines immunoassays with chromatography. It is widely used for monitoring agricultural products because of its fast detection, strong specificity, and lack of requirement for professional instruments or specialized staff for operation, in contrast to ELISA [120]. Therefore, market regulators and ordinary consumers can instantly detect pesticide residues in agricultural products. In addition, immunochromatography can detect pyrethroids within 10 min, which is much faster than ELISA.
Most experiments involving small molecule antigens with single epitopes—such as pesticides and veterinary drugs—are designed and explored by the competition method. Meanwhile, large molecule antigens with multiple epitopes, such as proteins and toxins, are determined by the sandwich method. A traditional immunochromatographic technique involves labeling colloidal gold [121] or fluorescent substances [122] on the monoclonal antibody to conjugated antigen at the test line. By reading the values of the test line and control line, the results can be qualitatively and quantitatively judged. However, antibody labeling with colloidal gold results in low sensitivity compared with labeling using fluorescent substances [123]. Costa et al. [124] developed a silicon dioxide-coated mesoporous material to selectively identify type I pyrethroids based on lateral-flow strips. The analyte can be detected in 2 min with a limit of detection of 1 ppb using signal readings from smartphones. Although this method can quickly detect permethrin with high sensitivity, the experimental design is complicated and unsuitable for large-scale use. Li et al. [36] established an immunochromatographic method for the determination of cypermethrin and fenvalerate using two test lines(Figure 10). Competitive interference between the different pesticides was prevented by coating the two test lines with two types of haptens with qualitative analysis observed from the two color changes. The method determined dual pesticides in tap water, river water, and milk with the data analyzed by the 2plex-speclysis application. SERS technology was used for the quantitative analysis of the tested pesticides with the following limits of detection for cypermethrin and fenvalerate: 2.3 × 10−4 and 2.6 × 10−5 ng/mL, respectively. In addition, the data analysis method is customized and can be used by nonprofessionals.
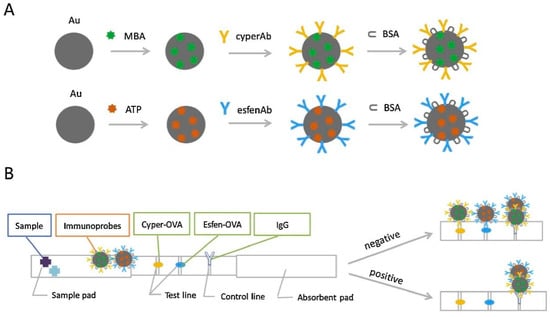
Figure 10.
(A) Schematic illustration showing the preparation of two types of immunoprobes Au-MBA-cyperAb and Au-ATP-esfenAb; and (B) assembly of the ICA-SERS strip and schematic diagram of the mechanism for multiplex detection ([36]).
A typical successful case involving the combination of a colorimetric biosensor and a fluorescent biosensor is fluorescence immunochromatography. This enables sensors on antibodies to fluoresce under certain excitation wavelengths. Fluorescein is commonly used in immunoassay methods, including fluorochrome [125], carbon dots [126], and lanthanide series [127]. For example, Zhao et al. [37] developed smartphone-based dual-channel immunochromatographic strips (ICTS) to synthesize carbon point PCD with ultrahigh fluorescence brightness as a signal amplifier to simultaneously detect cypermethrin and its 3-PBA metabolite. Images were analyzed and recorded on smartphone devices according to the red fluorescence obtained. This has potential in the direct detection of pyrethroid insecticides. However, it is difficult to produce antibodies against pesticides in animal bodies due to their small molecular structure, long antibody preparation period, harsh preservation conditions, and the need for sacrificing animals. Therefore, it is necessary to develop a highly specific bionic recognition material to replace animal antibodies and combine immunological methods for the rapid determination of pyrethroid pesticides.
MIPs can be used as a biomimetic material for the rapid and specific identification of pyrethroid pesticides when combined with immunofluorescence technology. However, if the lateral flow chromatography strip is designed according to the preliminary study of our laboratory, the free MIP is directly fixed on the nitrocellulose membrane of the strip, and the polymer will elute with the chromatography liquid, resulting in inaccurate results. Therefore, a substance that can be used as a carrier to fix the polymer on the nitrocellulose membrane as a material for antigen recognition is required for immunochromatographic analysis. The combination of molecularly imprinted electrospinning identification material can replace the use of monoclonal antibodies in immunoassays. For example, He et al. [128] established a method to determine triazolin in water based on a molecularly imprinted biomimetic immunofluorescence strip from our laboratory (Figure 11). In this method, triazolin MIP was fixed on the strip by an electrospinning membrane to replace antibodies. All water samples were negative, which was consistent with the LC–MS/MS results. This experiment provides a worthy idea for studying immunological methods by combining molecularly imprinted electrostatic bionic materials with fluorescence immunochromatography.
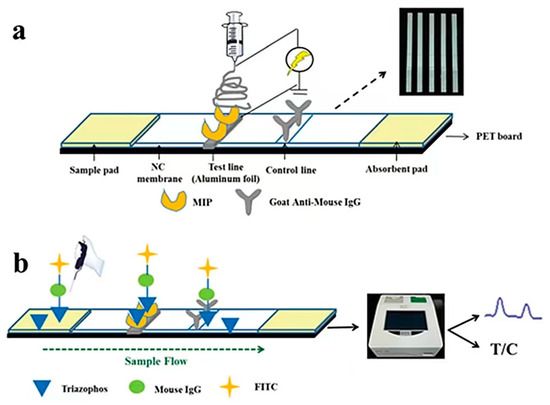
Figure 11.
(a) Structure diagram of the molecularly imprinted electrospun test strip and (b) direct competitive fluorescence detection process ([128]).
Our laboratory improved the sensitivity of the method while reducing background interference by using time-resolved fluorescent latex microspheres labeled with fenvalerate hapten-IgG as a fluorescent probe in immunochromatography for the detection of pyrethroid pesticide residues. This study is based on a competitive experimental design. When fenvalerate is present in the sample, it competes with the fluorescent probe for the specific binding site of MIP and causes fluorescence signal changes. These ideas were used to construct a new bionic fluorescent immunochromatographic strip for the detection of pyrethroid pesticides.
4. Conclusions and Future Perspectives
The use of pyrethroids in agriculture and health is increasing; therefore, rapid detection has become the mainstream research direction. Sensors that specifically identify pyrethroids are becoming more popular. Most pyrethroids are optical isomers containing chiral carbon atoms with similar structures. Therefore, the immunoanalysis cross-reaction rate is high, and the pesticide is a small molecule that results in the time-consuming preparation of traditional monoclonal antibodies. In comparison, bionic identification materials can solve the technical problems encountered during monoclonal antibody preparation and may replace animal antibodies in the future. However, low sensitivity is a key problem that urgently needs an breakthrough. The use of fluorescent material for labeling can improve the detection sensitivity. The combination of a variety of technologies may further improve the rapid detection of pyrethroid pesticides, which is of great significance for agricultural planting and public health.
Author Contributions
L.Z., M.Z., M.X., H.S. and Y.S. discussed the contents of as well as wrote and reviewed the manuscript. M.-H.I. and A.M.A. edited, reviewed, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Point Research and Invention Program (No. 2021YFD1600105), Central Public-interest Scientific Institution Basal Research Fund (No. Y2021XK21), National Natural Science Foundation of China (Nos. 31772071).
Data Availability Statement
This article has not been submitted to other journals, and the cited materials are labeled references.
Acknowledgments
We appreciate the financial support from the National Key Point Research and Invention Program (No. 2021YFD1600105), Central Public-interest Scientific Institution Basal Research Fund (No. Y2021XK21), and the National Natural Science Foundation of China (No. 31772071).
Conflicts of Interest
The authors report there are no competing interest to declare.
References
- Asrorov, A.M.; Matušíková, I.; Ziyavitdinov, J.F.; Gregorová, Z.; Majerčíková, V.; Mamadrakhimov, A.A. Changes in Soluble Protein Profile in Cotton Leaves Indicate Rubisco Damage after Treatment with Sumi-Alpha Insecticide. Agriculture (Poľnohospodárstvo) 2020, 66, 40–44. [Google Scholar] [CrossRef]
- Hua, N. Progress and trend of pyrethroid pesticides. Pestic. Mark. News 2015, 3, 26–29. [Google Scholar] [CrossRef]
- Chen, Y.; Lai, J.H.; Zhang, M.M.; Zhao, T.Y.; Wang, S.; Li, J.L.; Liu, S.L. Status of pyrethroid pesticide pollution in agricultural products and its removal technology: A review. Food Sci. 2021, 43, 285–292. [Google Scholar] [CrossRef]
- Soderlund, D.M. Molecular mechanisms of pyrethroid insecticide neurotoxicity: Recent advances. Arch. Toxicol. 2011, 86, 165–181. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.F.; Miller, G.W.; Harvey, D.J.; Brander, S.M.; Geist, J.; Connon, R.E.; Lein, P.J. Bifenthrin causes transcriptomic alterations in mTOR and ryanodine receptor-dependent signaling and delayed hyperactivity in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 200, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Q.; Li, S.; Mi, P.; Chen, D.; Zhao, X.; Feng, X. Developmental toxicity and neurotoxicity of synthetic organic insecticides in zebrafish (Danio rerio): A comparative study of deltamethrin, acephate, and thiamethoxam. Chemosphere 2018, 199, 16–25. [Google Scholar] [CrossRef]
- Özdemir, S.; Altun, S.; Özkaraca, M.; Ghosi, A.; Toraman, E.; Arslan, H. Cypermethrin, chlorpyrifos, deltamethrin, and imidacloprid exposure up-regulates the mRNA and protein levels of bdnf and c-fos in the brain of adult zebrafish (Danio rerio). Chemosphere 2018, 203, 318–326. [Google Scholar] [CrossRef]
- Hua, J.; Relyea, R. The effect of a common pyrethroid insecticide on wetland communities. Environ. Res. Commun. 2019, 1, 015003. [Google Scholar] [CrossRef]
- Bragança, I.; Lemos, P.C.; Barros, P.; Delerue-Matos, C.; Domingues, V.F. Phytotoxicity of pyrethroid pesticides and its metabolite towards Cucumis sativus. Sci. Total Environ. 2018, 619–620, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Bragança, I.; Mucha, A.P.; Tomasino, M.P.; Santos, F.; Lemos, P.C.; Delerue-Matos, C.; Domingues, V.F. Deltamethrin impact in a cabbage planted soil: Degradation and effect on microbial community structure. Chemosphere 2019, 220, 1179–1186. [Google Scholar] [CrossRef]
- Vorselaars, A.D.; van den Berg, P.M.; Drent, M. Severe pulmonary toxicity associated with inhalation of pyrethroid-based domestic insecticides (Bop/Sapolio): A case series and literature review. Curr. Opin. Pulm. Med. 2021, 27, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Mimura, M.; Sakon, N. Estimating household exposure to pyrethroids and the relative contribution of inhalation pathway in a sample of Japanese children. Environ. Sci. Pollut. Res. Int. 2021, 28, 19310–19324. [Google Scholar] [CrossRef] [PubMed]
- Işıldar, G.Y.; Günal, A.; Şahin, D.; Memmi, B.K.; Dinçel, A.S. How potential endocrine disruptor deltamethrin effects antioxidant enzyme levels and total antioxidant status on model organisms. Turk. J. Biochem. 2020, 45, 415–421. [Google Scholar] [CrossRef]
- Sheikh, I.; Beg, M. Structural Aspects of Potential Endocrine-Disrupting Activity of Stereoisomers for a Common Pesticide Permethrin against Androgen Receptor. Biology 2021, 10, 143. [Google Scholar] [CrossRef]
- Corcellas, C.; Feo, M.L.; Torres, J.P.; Malm, O.; Ocampo-Duque, W.; Eljarrat, E.; Barceló, D. Pyrethroids in human breast milk: Occurrence and nursing daily intake estimation. Environ. Int. 2012, 47, 17–22. [Google Scholar] [CrossRef]
- Wu, C.; Feng, C.; Qi, X.; Wang, G.; Zheng, M.; Chang, X.; Zhou, Z. Urinary metabolite levels of pyrethroid insecticides in infants living in an agricultural area of the Province of Jiangsu in China. Chemosphere 2013, 90, 2705–2713. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Ren, X.; Chen, X.; Wang, S.; Qin, C. Pyrethroids Toxicity to Male Reproductive System and Offspring as a Function of Oxidative Stress Induction: Rodent Studies. Front. Endocrinol. 2021, 12, 656106. [Google Scholar] [CrossRef]
- Knapke, E.T.; Magalhaes, D.d.P.; Dalvie, M.A.; Mandrioli, D.; Perry, M.J. Environmental and occupational pesticide exposure and human sperm parameters: A Navigation Guide review. Toxicology 2021, 465, 153017. [Google Scholar] [CrossRef]
- Shaikh, H.; Andaç, M.; Memon, N.; Bhanger, M.I.; Nizamani, S.M.; Denizli, A. Synthesis and characterization of molecularly imprinted polymer embedded composite cryogel discs: Application for the selective extraction of cypermethrins from aqueous samples prior to GC-MS analysis. RSC Adv. 2015, 5, 26604–26615. [Google Scholar] [CrossRef]
- Lin, X.-Y.; Mou, R.-X.; Cao, Z.-Y.; Cao, Z.-Z.; Chen, M.-X. Analysis of pyrethroid pesticides in Chinese vegetables and fruits by GC–MS/MS. Chem. Pap. 2018, 72, 1953–1962. [Google Scholar] [CrossRef]
- Tuck, S.; Furey, A.; Crooks, S.; Danaher, M. A review of methodology for the analysis of pyrethrin and pyrethroid residues in food of animal origin. Food Addit. Contam. Part A 2018, 35, 911–940. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Hoque, S.; Bhowmik, S.; Ferdousi, S.; Kabiraz, M.P.; van Brakel, M.L. Monitoring of pesticide residues from fish feed, fish and vegetables in Bangladesh by GC-MS using the QuEChERS method. Heliyon 2021, 7, e06390. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Feng, X.; Wang, J.; Pan, L.; Jing, J.; Zhou, Y.; Xin, J.; Pan, C.; Zhang, H. Method Development and Validation of Seven Pyrethroid Insecticides in Tea and Vegetable by Modified QuEChERS and HPLC–MS/MS. Bull. Environ. Contam. Toxicol. 2022, 108, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Fitsev, I.M.; Rakhmetova, E.R.; Mukhammetshina, A.G.; Burkin, K.E.; Shlyamina, O.V. Gas Chromatography–Mass Spectrometry Determination of Deltamethrin in Food. Uchenye Zap. Kazan. Univ. Seriya Estestv. Nauk. 2021, 163, 569–580. [Google Scholar] [CrossRef]
- Petrarca, M.H.; Ccanccapa-Cartagena, A.; Masiá, A.; Godoy, H.T.; Picó, Y. Comparison of green sample preparation techniques in the analysis of pyrethrins and pyrethroids in baby food by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2017, 1497, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.G.; Silva, L.M.; e Silva, B.C.; Garrido, S.S.; Boldrin, M.V.; De Souza, D. Cathodic stripping voltammetric determination of β-cyfluthrin, a pyrethroid insecticide, using polished silver solid amalgam electrode. J. Solid State Electrochem. 2020, 24, 1819–1826. [Google Scholar] [CrossRef]
- Fruhmann, P.; Sanchis, A.; Mayerhuber, L.; Vanka, T.; Kleber, C.; Salvador, J.-P.; Marco, M.-P. Immunoassay and amperometric biosensor approaches for the detection of deltamethrin in seawater. Anal. Bioanal. Chem. 2018, 410, 5923–5930. [Google Scholar] [CrossRef]
- Atar, N.; Yola, M.L. Core-Shell Nanoparticles/Two-Dimensional (2D) Hexagonal Boron Nitride Nanosheets with Molecularly Imprinted Polymer for Electrochemical Sensing of Cypermethrin. J. Electrochem. Soc. 2018, 165, H255–H262. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, Y.; Zhang, J.; Qiao, Y.; Wang, Q.; Che, G. Fabrication of pollutant-resistance SERS imprinted sensors based on SiO2@TiO2@Ag composites for selective detection of pyrethroids in water. J. Phys. Chem. Solids 2019, 138, 109254. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Liu, Y.-Q.; Shi, X.-B.; Li, W.-J.; Yang, Y.; Mao, L.-G. Ultrasensitive detection of deltamethrin by immune magnetic nanoparticles separation coupled with surface plasmon resonance sensor. Biosens. Bioelectron. 2014, 59, 328–334. [Google Scholar] [CrossRef]
- Huang, J.J.; Liu, J.; Liu, J.X.; Wang, J.P. A microtitre chemiluminescence sensor for detection of pyrethroids based on dual-dummy-template molecularly imprinted polymer and computational simulation. Luminescence 2019, 35, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.-T.; Shi, X.-Z.; Jiao, H.-F.; Sun, A.-L.; Ding, H.; Zhang, R.-R.; Pan, D.-D.; Li, D.-X.; Chen, J. Selective and sensitive determination of cypermethrin in fish via enzyme-linked immunosorbent assay-like method based on molecularly imprinted artificial antibody-quantum dot optosensing materials. Biosens. Bioelectron. 2016, 75, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, L.; Han, D.; Pan, J.; Qiu, H.; Li, H.; Wei, X.; Dai, J.; Yang, J.; Yao, H.; et al. Optical Detection of λ-Cyhalothrin by Core–Shell Fluorescent Molecularly Imprinted Polymers in Chinese Spirits. J. Agric. Food Chem. 2015, 63, 2392–2399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiao, H.-F.; Shi, X.-Z.; Sun, A.; Wang, X.; Chai, J.; Li, D.-X.; Chen, J. Development and application of a novel fluorescent nanosensor based on FeSe quantum dots embedded silica molecularly imprinted polymer for the rapid optosensing of cyfluthrin. Biosens. Bioelectron. 2018, 99, 268–273. [Google Scholar] [CrossRef]
- Zhu, X.; Han, L.; Liu, H.; Sun, B. A smartphone-based ratiometric fluorescent sensing system for on-site detection of pyrethroids by using blue-green dual-emission carbon dots. Food Chem. 2022, 379, 132154. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Song, Y.; Zhu, J.; Wang, D.; Li, W. Surface-enhanced Raman spectroscopy (SERS)-based immunochromatographic assay (ICA) for the simultaneous detection of two pyrethroid pesticides. Sens. Actuators B Chem. 2018, 283, 230–238. [Google Scholar] [CrossRef]
- Zhao, Y.; Ruan, X.; Song, Y.; Smith, J.N.; Vasylieva, N.; Hammock, B.D.; Lin, Y.; Du, D. Smartphone-Based Dual-Channel Immunochromatographic Test Strip with Polymer Quantum Dot Labels for Simultaneous Detection of Cypermethrin and 3-Phenoxybenzoic Acid. Anal. Chem. 2021, 93, 13658–13666. [Google Scholar] [CrossRef]
- Hashwan, S.S.B.; Bin Khir, M.H.; Al-Douri, Y.; Ahmed, A.Y. Recent Progress in the Development of Biosensors for Chemicals and Pesticides Detection. IEEE Access 2020, 8, 82514–82527. [Google Scholar] [CrossRef]
- Pundir, C.S.; Chauhan, N. Acetylcholinesterase inhibition-based biosensors for pesticide determination: A review. Anal. Biochem. 2012, 429, 19–31. [Google Scholar] [CrossRef]
- Guilbault, G.G.; Kramer, D.N.; Cannon, P.L., Jr. Electrochemical Determination of Organophosphorous Compounds. Anal. Chem. 1962, 34, 1437–1439. [Google Scholar] [CrossRef]
- Cycoń, M.; Piotrowska-Seget, Z. Pyrethroid-Degrading Microorganisms and Their Potential for the Bioremediation of Contaminated Soils: A Review. Front. Microbiol. 2016, 7, 1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Gao, Y.; Sun, B.; Ran, T.; Zeng, L.; He, J.; He, J.; Wang, W. Pyrethroid Carboxylesterase PytH from Sphingobium faniae JZ-2: Structure and Catalytic Mechanism. Appl. Environ. Microbiol. 2020, 86, e02971-19. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Ramírez, G.; Cortina, M.; Ramírez-Silva, M.T.; Marty, J.-L. Acetylcholinesterase-based biosensors for quantification of carbofuran, carbaryl, methylparaoxon, and dichlorvos in 5% acetonitrile. Anal. Bioanal. Chem. 2008, 392, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Cheng, N.; Luo, Y.; Lin, Y.; Xu, W.; Du, D. Recent advances in nanomaterials-based electrochemical (bio)sensors for pesticides detection. TrAC Trends Anal. Chem. 2020, 132, 116041. [Google Scholar] [CrossRef]
- Jin, M.; Chen, G.; Du, P.; Zhang, C.; Cui, X.; Gee, S.J.; She, Y.; Zheng, L.; Wang, S.; Shao, H.; et al. Developments on Immunoassays for Pyrethroid Chemicals. Curr. Org. Chem. 2018, 21, 2653–2661. [Google Scholar] [CrossRef]
- Cui, N.; Cao, L.; Sui, J.; Lin, H.; Han, X.; Chen, X.; Xie, H.; Sun, X. Quick and convenient construction of lambda-cyhalothrin antigen for the generation of specific antibody. Anal. Biochem. 2020, 597, 113669. [Google Scholar] [CrossRef]
- Blind, M.; Blank, M. Aptamer Selection Technology and Recent Advances. Mol. Ther.-Nucleic Acids 2015, 4, e223. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Wang, C.; Liu, B.; Wu, Y. Selection and identification of a DNA aptamer for ultrasensitive and selective detection of λ-cyhalothrin residue in food. Anal. Chim. Acta 2021, 1179, 338837. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, Q.; Zhou, M.; Cui, H. A novel homogeneous label-free aptasensor for 2,4,6-trinitrotoluene detection based on an assembly strategy of electrochemiluminescent graphene oxide with gold nanoparticles and aptamer. Biosens. Bioelectron. 2013, 43, 137–142. [Google Scholar] [CrossRef]
- Hamada, M. In silico approaches to RNA aptamer design. Biochimie 2018, 145, 8–14. [Google Scholar] [CrossRef]
- Kaur, H. Recent developments in cell-SELEX technology for aptamer selection. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Nezhadali, A.; Feizy, J.; Beheshti, H.R. A Molecularly Imprinted Polymer for the Selective Extraction and Determination of Fenvalerate from Food Samples Using High-Performance Liquid Chromatography. Food Anal. Methods 2014, 8, 1225–1237. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Zhao, M.; Li, H.; Shao, H.; She, Y.; Jin, F.; Wang, J. Advances in the Preparation of Molecularly Imprinted Polymers of Mycotoxins and their Rapid Detection Techniques. Cereal Feed. Ind. 2021, 3, 45–51. [Google Scholar] [CrossRef]
- Naseri, M.; Mohammadniaei, M.; Sun, Y.; Ashley, J. The Use of Aptamers and Molecularly Imprinted Polymers in Biosensors for Environmental Monitoring: A Tale of Two Receptors. Chemosensors 2020, 8, 32. [Google Scholar] [CrossRef]
- Sergeyeva, T.; Yarynka, D.; Piletska, E.; Linnik, R.; Zaporozhets, O.; Brovko, O.; Piletsky, S.; El’Skaya, A. Fluorescent sensor systems based on nanostructured polymeric membranes for selective recognition of Aflatoxin B1. Talanta 2017, 175, 101–107. [Google Scholar] [CrossRef]
- Heravizadeh, O.R.; Khadem, M.; Nabizadeh, R.; Shahtaheri, S.J. Synthesis of molecularly imprinted nanoparticles for selective exposure assessment of permethrin: Optimization by response surface methodology. J. Environ. Health Sci. Eng. 2019, 17, 393–406. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Z.; Zhang, L.; Hu, X. Effective preparation of magnetic molecularly imprinted polymer nanoparticle for the rapid and selective extraction of cyfluthrin from honeysuckle. J. Biomater. Sci. Polym. Ed. 2020, 31, 954–968. [Google Scholar] [CrossRef]
- Hanginaka, K.N.J. Preparation and evaluation of molecularly imprinted polymers for promazine and chlorpromazine by multi-step swelling and polymerization: The application for the determination of promazine in rat serum ny column-switching LC. Anal. Sci. 2019, 35, 659–664. [Google Scholar]
- Madikizela, L.M.; Tavengwa, N.T.; Tutu, H.; Chimuka, L. Green aspects in molecular imprinting technology: From design to environmental applications. Trends Environ. Anal. Chem. 2018, 17, 14–22. [Google Scholar] [CrossRef]
- Cai, Y.; He, X.; Cui, P.L.; Yuan, W.Z.; Wang, J.P.; Liu, J. Molecularly imprinted microspheres based multiplexed fluorescence method for simultaneous detection of benzimidazoles and pyrethroids in meat samples. Food Chem. 2020, 319, 126539. [Google Scholar] [CrossRef]
- Gast, M.; Sobek, H.; Mizaikoff, B. Advances in imprinting strategies for selective virus recognition a review. TrAC Trends Anal. Chem. 2019, 114, 218–232. [Google Scholar] [CrossRef]
- Esquivel-Blanco, V.; Quintanilla-Villanueva, G.; Villarreal-Chiu, J.; Rodríguez-Delgado, J.; Rodríguez-Delgado, M. The Potential Use of a Thin Film Gold Electrode Modified with Laccases for the Electrochemical Detection of Pyrethroid Metabolite 3-Phenoxybenzaldehyde. Materials 2021, 14, 1992. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.B.; Ribeiro, D.B.; dos Santos Soares, A.M.; Marques, P.R.B.; Badea, M.; Targa, M.; Granato, J.A.; Nunes, G.S. A novel glutathione-S-transferase-based biosensor for pyrethroid insecticides: From inhibition study to detection. Sens. Actuators Rep. 2022, 4, 100093. [Google Scholar] [CrossRef]
- Borah, H.; Gogoi, S.; Kalita, S.; Puzari, P. A broad spectrum amperometric pesticide biosensor based on glutathione S-transferase immobilized on graphene oxide-gelatin matrix. J. Electroanal. Chem. 2018, 828, 116–123. [Google Scholar] [CrossRef]
- Maria, A.C.G.; Varghese, A.; Nidhin, M. Recent Advances in Nanomaterials Based Molecularly Imprinted Electrochemical Sensors. Crit. Rev. Anal. Chem. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Chansi; Bhardwaj, R.; Rao, R.P.; Mukherjee, I.; Agrawal, P.K.; Basu, T.; Bharadwaj, L.M. Layered construction of nano immuno-hybrid embedded MOF as an electrochemical sensor for rapid quantification of total pesticides load in vegetable extract. J. Electroanal. Chem. 2020, 873, 114386. [Google Scholar] [CrossRef]
- Ferrari, A.G.-M.; Rowley-Neale, S.J.; Banks, C.E. Recent advances in 2D hexagonal boron nitride (2D-hBN) applied as the basis of electrochemical sensing platforms. Anal. Bioanal. Chem. 2020, 413, 663–672. [Google Scholar] [CrossRef]
- Maestre, C.; Toury, B.; Steyer, P.; Garnier, V.; Journet, C. Hexagonal boron nitride: A review on selfstanding crystals synthesis towards 2D nanosheets. J. Phys. Mater. 2021, 4, 044018. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, B.; Chen, L. SERS Tags: Novel Optical Nanoprobes for Bioanalysis. Chem. Rev. 2012, 113, 1391–1428. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Liu, P. Biocompatible Triplex Ag@SiO2@mTiO2 Core-Shell Nanoparticles for Simultaneous Fluorescence-SERS Bimodal Imaging and Drug Delivery. Chem.–A Eur. J. 2012, 18, 5935–5943. [Google Scholar] [CrossRef]
- Kamra, T.; Zhou, T.; Montelius, L.; Schnadt, J.; Ye, L. Implementation of Molecularly Imprinted Polymer Beads for Surface Enhanced Raman Detection. Anal. Chem. 2015, 87, 5056–5061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Wang, X.; Wang, Z.; Wang, M.; Li, Y.; Wang, Q. Preparation of a high-performance magnetic molecularly imprinted sensor for SERS detection of cyfluthrin in river. J. Raman Spectrosc. 2019, 50, 926–935. [Google Scholar] [CrossRef]
- Wang, D.; Loo, J.F.C.; Chen, J.; Yam, Y.; Chen, S.-C.; He, H.; Kong, S.K.; Ho, H.P. Recent Advances in Surface Plasmon Resonance Imaging Sensors. Sensors 2019, 19, 1266. [Google Scholar] [CrossRef] [Green Version]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Fauzi, N.I.M.; Hashim, H.S.; Ramdzan, N.S.M.; Omar, N.A.S. Recent Advances in Surface Plasmon Resonance Optical Sensors for Potential Application in Environmental Monitoring. Sens. Mater. 2020, 32, 4191. [Google Scholar] [CrossRef]
- Fang, L.; Liao, X.; Jia, B.; Shi, L.; Kang, L.; Zhou, L.; Kong, W. Recent progress in immunosensors for pesticides. Biosens. Bioelectron. 2020, 164, 112255. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudpour, M.; Dolatabadi, J.E.N.; Torbati, M.; Homayouni-Rad, A. Nanomaterials based surface plasmon resonance signal enhancement for detection of environmental pollutions. Biosens. Bioelectron. 2018, 127, 72–84. [Google Scholar] [CrossRef]
- Mohammadzadeh-Asl, S.; Keshtkar, A.; Dolatabadi, J.E.N.; de la Guardia, M. Nanomaterials and phase sensitive based signal enhancment in surface plasmon resonance. Biosens. Bioelectron. 2018, 110, 118–131. [Google Scholar] [CrossRef]
- Thangadurai, T.D.; Manjubaashini, N. Progressions in chemical and biological analytes sensing technology based on nanostructured materials: A comprehensive review. Mater. Sci. Eng. B 2021, 271, 115307. [Google Scholar] [CrossRef]
- Suzuki, Y.; Katagi, T. Novel Fluorescence Detection of Free Radicals Generated in Photolysis of Fenvalerate. J. Agric. Food Chem. 2008, 56, 10811–10816. [Google Scholar] [CrossRef]
- Zang, D.; Yan, M.; Zhao, P.; Ge, L.; Liu, S.; Yu, J. A novel high selectivity chemiluminescence sensor for fenvalerate based on double-sided hollow molecularly imprinted materials. Analyst 2012, 137, 4247–4253. [Google Scholar] [CrossRef]
- Zhao, P.; Yu, J.; Liu, S.; Yan, M.; Zang, D.; Gao, L. One novel chemiluminescence sensor for determination of fenpropathrin based on molecularly imprinted porous hollow microspheres. Sens. Actuators B Chem. 2012, 162, 166–172. [Google Scholar] [CrossRef]
- Al Yahyai, I.; Hassanzadeh, J.; Al-Lawati, H.A. A novel and selective multi-emission chemiluminescence system for the quantification of deltamethrin in food samples. Sens. Actuators B Chem. 2020, 327, 128927. [Google Scholar] [CrossRef]
- Kalyani, N.; Goel, S.; Jaiswal, S. On-site sensing of pesticides using point-of-care biosensors: A review. Environ. Chem. Lett. 2020, 19, 345–354. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Zou, X.-M.; Song, S.-H.; Chen, G.-H. Quantum Dots Applied to Methodology on Detection of Pesticide and Veterinary Drug Residues. J. Agric. Food Chem. 2018, 66, 1307–1319. [Google Scholar] [CrossRef] [PubMed]
- Nsibande, S.A.; Forbes, P.B.C. Fluorescence detection of pesticides using quantum dot materials—A review. Anal. Chim. Acta 2016, 945, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Meng, M.; Song, Z.; Gao, L.; Li, H.; Dai, J.; Zhou, Z.; Li, C.; Pan, J.; Yu, P.; et al. Synthesis of molecularly imprinted silica nanospheres embedded mercaptosuccinic acid-coated CdTe quantum dots for selective recognition of λ-cyhalothrin. J. Lumin. 2014, 153, 326–332. [Google Scholar] [CrossRef]
- Wei, X.; Hao, T.; Xu, Y.; Lu, K.; Li, H.; Yan, Y.; Zhou, Z. Facile polymerizable surfactant inspired synthesis of fluorescent molecularly imprinted composite sensor via aqueous CdTe quantum dots for highly selective detection of λ-cyhalothrin. Sens. Actuators B Chem. 2016, 224, 315–324. [Google Scholar] [CrossRef]
- Qiu, H.; Gao, L.; Wang, J.; Pan, J.; Yan, Y.; Zhang, X. A precise and efficient detection of Beta-Cyfluthrin via fluorescent molecularly imprinted polymers with ally fluorescein as functional monomer in agricultural products. Food Chem. 2017, 217, 620–627. [Google Scholar] [CrossRef]
- Yulong, Y.; Xinsheng, P. Recent advances in carbon-based dots for electroanalysis. Analyst 2016, 141, 2619–2628. [Google Scholar] [CrossRef]
- Zhou, Y.; Benetti, D.; Tong, X.; Jin, L.; Wang, Z.M.; Ma, D.; Zhao, H.; Rosei, F. Colloidal carbon dots based highly stable luminescent solar concentrators. Nano Energy 2017, 44, 378–387. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Kirthi, A.V.; Akksadha, M.; Indu, S.; Dharshini, U.D.; Pushpamalar, J.; Karthik, L.; Arivarasan, V.K.; Janarthanan, P. Recent advancements in the applications of carbon nanodots: Exploring the rising star of nanotechnology. Nanoscale Adv. 2020, 2, 1760–1773. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Devi, P.; Rajput, P.; Thakur, A.; Kim, K.-H.; Kumar, P. Recent advances in carbon quantum dot-based sensing of heavy metals in water. TrAC Trends Anal. Chem. 2019, 114, 171–195. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2020, 406, 126848. [Google Scholar] [CrossRef]
- Xu, J.; Miao, Y.; Zheng, J.; Wang, H.; Yang, Y.; Liu, X. Carbon dot-based white and yellow electroluminescent light emitting diodes with a record-breaking brightness. Nanoscale 2018, 10, 11211–11221. [Google Scholar] [CrossRef]
- Liu, C.; Fu, Y.; Xia, Y.; Zhu, C.; Hu, L.; Zhang, K.; Wu, H.; Huang, H.; Liu, Y.; Xie, T.; et al. Cascaded photo-potential in a carbon dot-hematite system driving overall water splitting under visible light. Nanoscale 2017, 10, 2454–2460. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Nasr-Esfahani, P.; Rezaei, B. Synthesis of molecularly imprinted polymer on carbon quantum dots as an optical sensor for selective fluorescent determination of promethazine hydrochloride. Sens. Actuators B Chem. 2018, 257, 889–896. [Google Scholar] [CrossRef]
- Shirani, M.P.; Rezaei, B.; Ensafi, A.A. A novel optical sensor based on carbon dots embedded molecularly imprinted silica for selective acetamiprid detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 210, 36–43. [Google Scholar] [CrossRef]
- Jalili, R.; Khataee, A.; Rashidi, M.-R.; Razmjou, A. Detection of penicillin G residues in milk based on dual-emission carbon dots and molecularly imprinted polymers. Food Chem. 2020, 314, 126172. [Google Scholar] [CrossRef]
- Jia, R.; Jin, K.; Zhang, J.; Zheng, X.; Wang, S.; Zhang, J. Colorimetric and fluorescent detection of glutathione over cysteine and homocysteine with red-emitting N-doped carbon dots. Sens. Actuators B Chem. 2020, 321, 128506. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, X.; Han, L.; Liu, H.; Sun, B. A smartphone-integrated optosensing platform based on red-emission carbon dots for real-time detection of pyrethroids. Biosens. Bioelectron. 2021, 191, 113460. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, J.; Li, Z.; Lv, X.; Liang, L.; Yuan, Q. Recent Progress in Time-Resolved Biosensing and Bioimaging Based on Lanthanide-Doped Nanoparticles. Small 2019, 15, e1804969. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, Z.; Duan, C.; Dou, L.; Wen, K.; Wang, Z.; Yu, X.; Shen, J. Comparison of two fluorescence quantitative immunochromatographic assays for the detection of amantadine in chicken muscle. Food Chem. 2022, 377, 131931. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, D.; Huang, Z.; Xing, K.; Chen, Y.; Peng, J.; Lai, W. Ultra-sensitive method based on time-resolved fluorescence immunoassay for detection of sulfamethazine in raw milk. Food Agric. Immunol. 2018, 29, 1137–1149. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Gulzar, A.; Yang, P.; Bi, H.; Yang, D.; Gai, S.; He, F.; Lin, J.; Xing, B.; Jin, D. Recent advances in near-infrared emitting lanthanide-doped nanoconstructs: Mechanism, design and application for bioimaging. Coord. Chem. Rev. 2018, 381, 104–134. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Wang, Q.; Gao, S.; Pei, X.; Jiang, H.; Wen, K. Multi-residue fluorescent microspheres immunochromatographic assay for simultaneous determination of macrolides in raw milk. Anal. Bioanal. Chem. 2015, 407, 9125–9133. [Google Scholar] [CrossRef]
- Xue, Z.; Zeng, S.; Hao, J. Non-invasive through-skull brain vascular imaging and small tumor diagnosis based on NIR-II emissive lanthanide nanoprobes beyond 1500 nm. Biomaterials 2018, 171, 153–163. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Li, P.; Zhang, Q.; Zhang, W. Time-Resolved Fluorescent Immunochromatography of Aflatoxin B1 in Soybean Sauce: A Rapid and Sensitive Quantitative Analysis. Sensors 2016, 16, 1094. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Li, P.; Zhang, Q.; Zhang, Z.; Zhang, W.; Jiang, J. Time-Resolved Fluorescence Immunochromatographic Assay Developed Using Two Idiotypic Nanobodies for Rapid, Quantitative, and Simultaneous Detection of Aflatoxin and Zearalenone in Maize and Its Products. Anal. Chem. 2017, 89, 11520–11528. [Google Scholar] [CrossRef]
- Chen, B.; Shen, X.; Li, Z.; Wang, J.; Li, X.; Xu, Z.; Shen, Y.; Lei, Y.; Huang, X.; Wang, X.; et al. Antibody Generation and Rapid Immunochromatography Using Time-Resolved Fluorescence Microspheres for Propiconazole: Fungicide Abused as Growth Regulator in Vegetable. Foods 2022, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Li, P.; Zhang, Q.; Li, X.; Tang, X.; Li, J. A reliable and sensitive time-resolved fluorescent immunochromatographic assay (TRFICA) for ochratoxin A in agro-products. Food Control 2015, 47, 126–134. [Google Scholar] [CrossRef]
- Capoferri, D.; Della Pelle, F.; Del Carlo, M.; Compagnone, D. Affinity Sensing Strategies for the Detection of Pesticides in Food. Foods 2018, 7, 148. [Google Scholar] [CrossRef] [Green Version]
- Amine, A.; Arduini, F.; Moscone, D.; Palleschi, G. Recent advances in biosensors based on enzyme inhibition. Biosens. Bioelectron. 2015, 76, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Songa, E.; Okonkwo, J.O. Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: A review. Talanta 2016, 155, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Dávila, E.L.; Houbraken, M.; Gil Unday, Z.; Romero, O.R.; Du Laing, G.; Spanoghe, P. ELISA, a feasible technique to monitor organophosphate, carbamate, and pyrethroid residues in local vegetables. Cuban case study. SN Appl. Sci. 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Huo, J.; Li, Z.; Wan, D.; Li, D.; Qi, M.; Barnych, B.; Vasylieva, N.; Zhang, J.; Hammock, B.D. Development of a Highly Sensitive Direct Competitive Fluorescence Enzyme Immunoassay Based on a Nanobody–Alkaline Phosphatase Fusion Protein for Detection of 3-Phenoxybenzoic Acid in Urine. J. Agric. Food Chem. 2018, 66, 11284–11290. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Zhou, X.; Yu, Y.; Liu, J.; Hu, N.; Wang, H.; Li, G.; Wu, Y. Recent progress in the construction of nanozyme-based biosensors and their applications to food safety assay. TrAC Trends Anal. Chem. 2019, 121, 115668. [Google Scholar] [CrossRef]
- Castellarnau, M.; Ramón-Azcón, J.; Gonzalez-Quinteiro, Y.; López, J.; Grimalt, J.O.; Marco, M.-P.; Nieuwenhuijsen, M.; Picado, A. Assessment of analytical methods to determine pyrethroids content of bednets. Trop. Med. Int. Health 2016, 22, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Kranthi, K.; Davis, M.; Mayee, C.; Russell, D.; Shukla, R.; Satija, U.; Kshirsagar, M.; Shiware, D.; Kranthi, S. Development of a colloidal-gold based lateral-flow immunoassay kit for ‘quality-control’ assessment of pyrethroid and endosulfan formulations in a novel single strip format. Crop Prot. 2009, 28, 428–434. [Google Scholar] [CrossRef]
- Shi, H.; Han, F.; Wang, X.; Ren, X.; Lei, R.; Huang, L.; Zhao, S.; Xu, S. Highly precise FIR thermometer based on the thermally enhanced upconversion luminescence for temperature feedback photothermal therapy. RSC Adv. 2022, 12, 8274–8282. [Google Scholar] [CrossRef] [PubMed]
- Pyo, D. Comparison of Fluorescence Immunochromatographic Assay Strip and Gold Colloidal Immunochromatographic Assay Strip for Detection of Microcystin. Anal. Lett. 2007, 40, 907–919. [Google Scholar] [CrossRef]
- Costa, E.; Climent, E.; Ast, S.; Weller, M.G.; Canning, J.; Rurack, K. Development of a lateral flow test for rapid pyrethroid detection using antibody-gated indicator-releasing hybrid materials. Analyst 2020, 145, 3490–3494. [Google Scholar] [CrossRef]
- Shen, Y.; Yan, F.; Huang, X.; Zhang, X.; Zhang, Y.; Zhang, C.; Jin, J.; Li, H.; Yao, S. A new water-soluble and colorimetric fluorescent probe for highly sensitive detection of organophosphorus pesticides. RSC Adv. 2016, 6, 88096–88103. [Google Scholar] [CrossRef]
- Molaei, M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta 2018, 196, 456–478. [Google Scholar] [CrossRef]
- Liao, T.; Yuan, F.; Shi, C.; He, C.-X.; Li, Z. Lanthanide chelate-encapsulated polystyrene nanoparticles for rapid and quantitative immunochromatographic assay of procalcitonin. RSC Adv. 2016, 6, 103463–103470. [Google Scholar] [CrossRef]
- He, Y.; Hong, S.; Wang, M.; Wang, J.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Khan, M.; She, Y. Development of fluorescent lateral flow test strips based on an electrospun molecularly imprinted membrane for detection of triazophos residues in tap water. New J. Chem. 2020, 44, 6026–6036. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).