Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors
Abstract
:1. Introduction
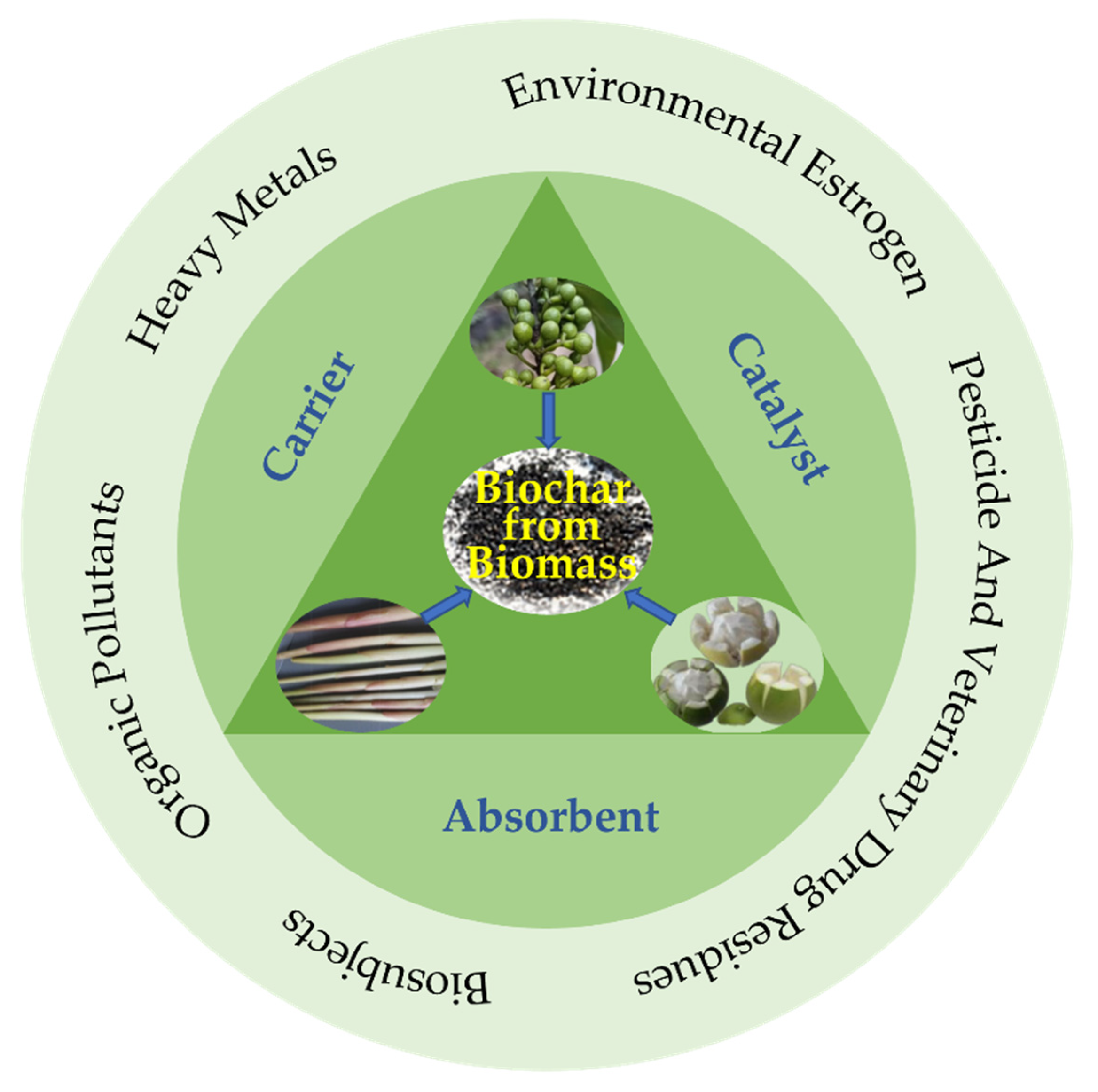
2. The Role of Biochar in the Fabrication of Electrochemical Sensors and Biosensors
2.1. Absorbent
2.2. Catalyst
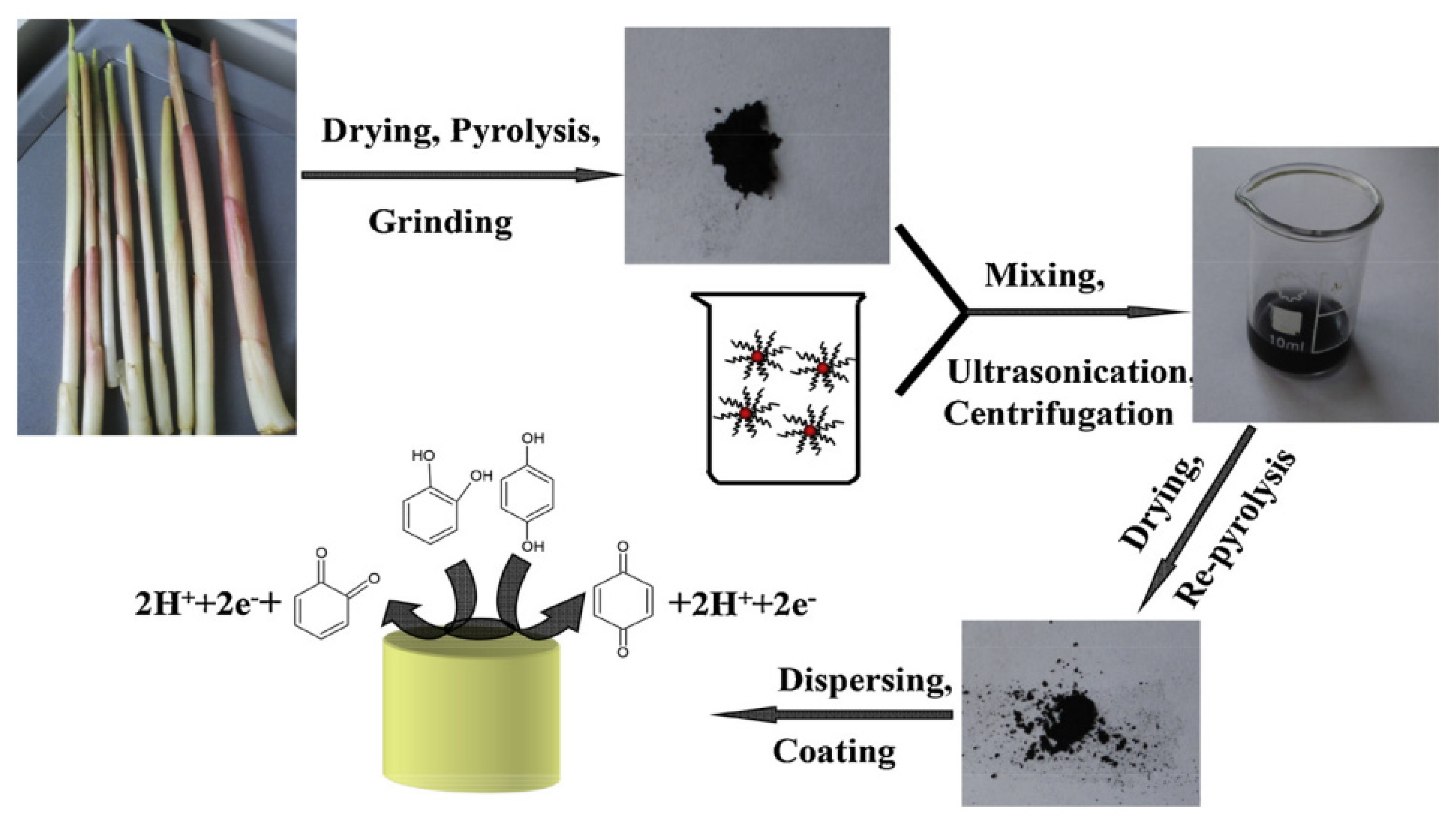
2.3. Carrier
3. The Application of Electrochemical Sensors and Biosensors Based on Biochar
3.1. Application in Detecting Heavy Metals
3.2. Application in Detecting Pesticide and Veterinary Drug Residues
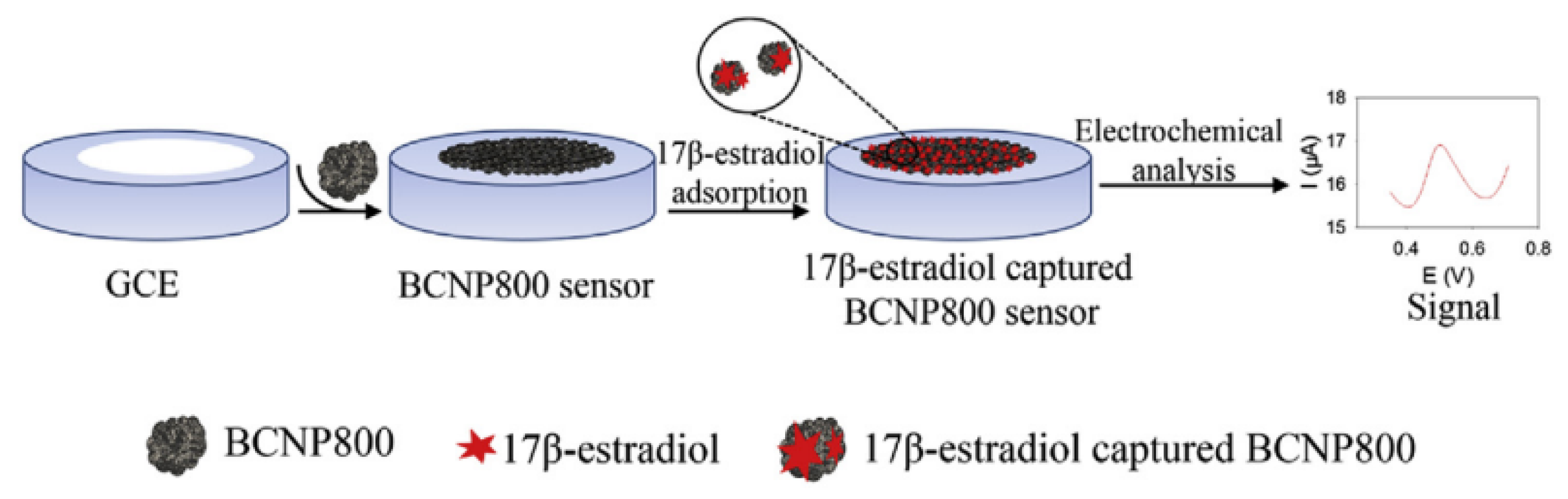
3.3. Application in Detecting Environmental Estrogen
3.4. Application in Detecting Organic Pollutants
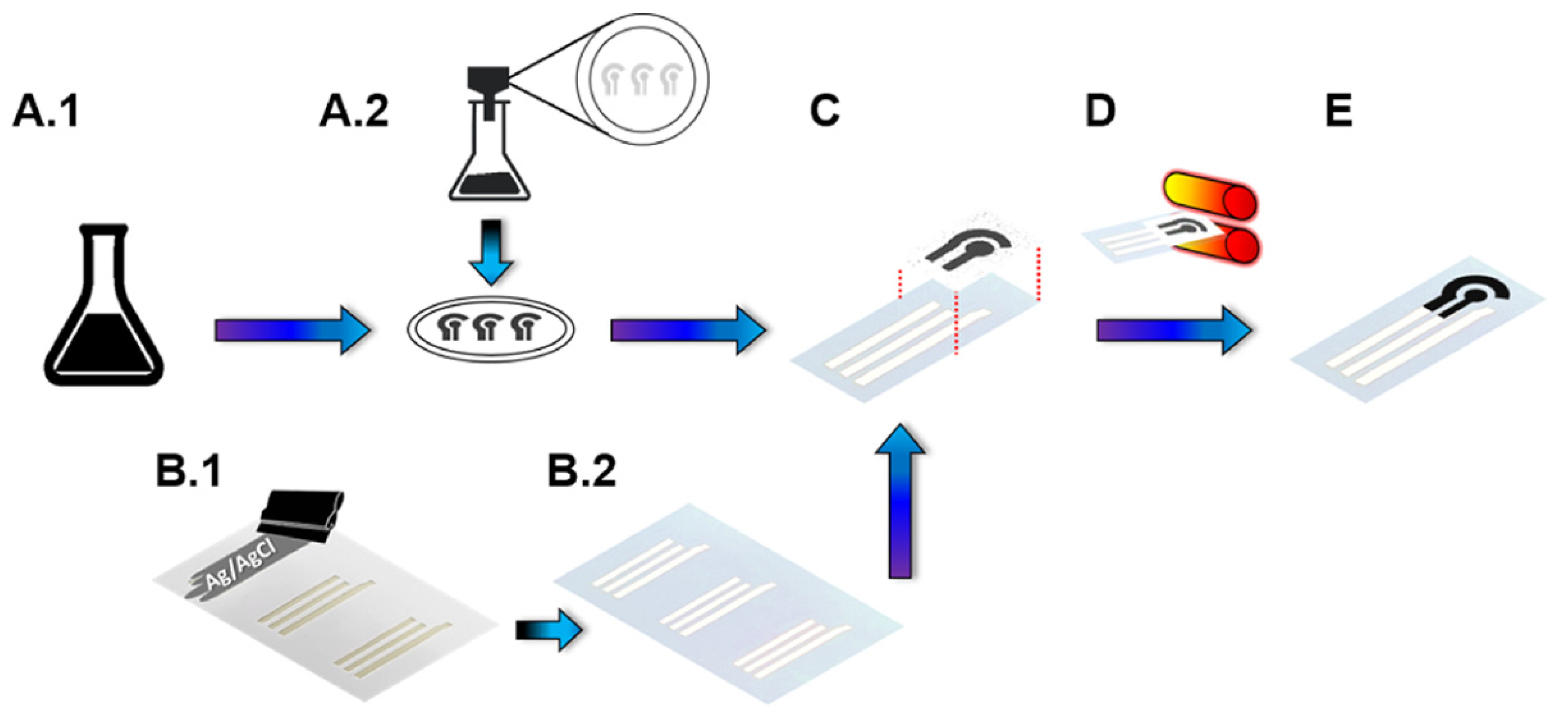
3.5. Application in Biosensors
3.6. Applications in Detecting Other Subjects
4. Challenges and Prospects for the Study of Electrochemical Sensors and Biosensors Based on Biochar
- (1)
- From the above works and other reported works, the mesopores/macropores structure of biochar is very beneficial for sensing surface construction and can provide biochar with excellent properties. Generally, the surface area and pore size of biochar increases with rising pyrolysis temperatures [68]. However, higher temperatures could result in the porous structure being destroyed and blocked [69] and, more importantly, higher pyrolysis temperatures would decrease the number of functional groups [70]. Therefore, how to improve the biochar sorption capacity and thus improve the electrochemical response signal need to be addressed.
- (2)
- It is worth noting that various redox couples of metal ions have been used as the redox probe in the fabrication of electrodes. Although the metal ions exhibit excellent redox properties, the dispersibility of metal ions in biochar still need to be improved because of the great influence of the redox property in dispersibility. Therefore, how to carry, absorb, or wrap redox metal ions and thus improve the dispersibility of metal ions in the biochar need to be considered further.
- (3)
- The application of biochar is increasingly popular because one of its advantages is low-cost. However, the current technique of preparing biochar always needs a high-temperature treatment. Therefore, it is still a significant challenge to optimize the synthesis process for a mild and low-energy-consuming condition. Additionally, further studies are needed to prepare activated biochar that has a special structure and abundant functional groups under low temperatures.
- (4)
- Whether used as an absorbent, a catalyst, or a carrier, the possible interference produced from the original biomass (e.g., from heavy metals) must be considered, because such interference might contaminate the analytes or affect the repeatability of the sensors, which is an important technical indicator for the evaluation of sensors and biosensors [71,72]. Therefore, how to realize the controlling preparation of biochar with uniform size and composition still needs wider and more thorough study.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, F.; Xia, Y.; Lu, Q.; Xu, Q.; Shu, Y.; Hu, X. Recent advances in inorganic functional nanomaterials based flexible electrochemical sensors. Talanta 2022, 123419. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, J.; Yan, F.; Ju, H. A facile strategy for quantitative sensing of glycans on cell surface using organic electrochemical transistors. Biosens. Bioelectron. 2020, 175, 112878. [Google Scholar] [CrossRef] [PubMed]
- Zwieten, L.V.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.; Chiou, C.T.; Xing, B. Compositions and Sorptive Properties of Crop Residue-Derived Chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S.G. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Qin, F.; Yu, J.; Tang, L.; Pang, Y.; Zhang, C.; Wang, J.; Deng, L. Tailoring biochar for persulfate-based environmental catalysis: Impact of biomass feedstocks. J. Hazard. Mater. 2021, 424, 127663. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Paula, A.J.; Ferreira, O.P.; Filho, A.G.S.; Filho, F.N.; Andrade, C.E.; Faria, A.F. Machine Learning and Natural Language Processing Enable a Data-Oriented Experimental Design Approach for Producing Biochar and Hydrochar from Biomass. Chem. Mater. 2022, 34, 979–990. [Google Scholar] [CrossRef]
- Wong, A.; de Lima, D.G.; Ferreira, P.A.; Khan, S.; da Silva, R.A.B.; de Faria, J.L.B.; Sotomayor, M.D.P.T. Voltammetric sensing of glyphosate in different samples using carbon paste electrode modified with biochar and copper(II) hexadecafluoro-29H,31 phtalocyanine complex. J. Appl. Electrochem. 2021, 51, 761–768. [Google Scholar] [CrossRef]
- Ferreira, P.A.; Backes, R.; Martins, C.A.; Carvalho, C.T.; Silva, R.A.B. Biochar: A low-cost electrode modifier for electrocatalytic, sensitive and selective detection of similar organic compounds. Electroanalysis 2018, 30, 2233–2236. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, H.; Yang, J.; Shi, Z.; Tan, Y.; Jin, J.; Wang, R.; Zhang, S.; Wang, J. Biochar decorated with gold nanoparticles for electrochemical sensing application. Electrochim. Acta 2018, 261, 464–473. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: Science, technology and implementation. Sci. Technol. Earthscan. 2015, 25, 15801–15811. [Google Scholar]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 1–25. [Google Scholar] [CrossRef]
- Sharma, S.; Sahu, B.K.; Cao, L.; Bindra, P.; Kaur, K.; Chandel, M.; Koratkar, N.; Huang, Q.; Shanmugham, V. Porous nanomaterials: Main vein of agricultural nanotechnology. Prog. Mater. Sci. 2021, 121, 100812. [Google Scholar] [CrossRef]
- Torrinha, A.; Oliveira, T.M.B.F.; Ribeiro, F.W.P.; Correia, A.N.; Lima-Neto, P.; Morais, S. Application of nanostructured carbon-based electrochemical (bio)sensors for screening of emerging pharmaceutical pollutants in waters and aquatic species: A Review. Nanomaterials 2020, 10, 1268. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Scognamiglio, V.; Mazzaracchio, V.; Moscone, D. Sustainable materials for the design of forefront printed (bio)sensors applied in agrifood sector. TrAC Trends Anal. Chem. 2020, 128, 115909. [Google Scholar] [CrossRef]
- Spanu, D.; Binda, G.; Dossi, C.; Monticelli, D. Biochar as an alternative sustainable platform for sensing applications: A review. Microchem. J. 2020, 159, 105506. [Google Scholar] [CrossRef]
- Kalinke, C.; de Oliveira, P.R.; Bonacin, J.A.; Janegitz, B.C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. State-of-the-art and perspectives in the use of biochar for electrochemical and electroanalytical applications. Green Chem. 2021, 23, 5272–5301. [Google Scholar] [CrossRef]
- de Almeida, L.S.; Oreste, E.Q.; Maciel, J.V.; Heinemann, M.G.; Dias, D. Electrochemical devices obtained from biochar: Advances in renewable and environmentally-friendly technologies applied to analytical chemistry. Trends Environ. Anal. Chem. 2020, 26, e00089. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Review on applications of carbon nanomaterials for simultaneous electrochemical sensing of environmental contaminant dihydroxybenzene isomers. Arab. J. Chem. 2020, 13, 6092–6105. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X. Nanotechnology and nanomaterial-based no-wash electrochemical biosensors: From design to application. Nanoscale 2019, 11, 19105–19118. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Zhang, K.; Wang, X.; Chang, J.; Sheng, Y.; Bai, L.; Wen, Y. Facile preparation of water-processable biochar based on pitch pine and its electrochemical application for cadmium ion sensing. Int. J. Electrochem. Sci. 2016, 11, 1041–1054. [Google Scholar]
- Silva, M.K.L.; Leão, A.L.; Sain, M.; Cesarino, I. A functionalized renewable carbon-based surface for sensor development. J. Solid State Electrochem. 2021, 25, 1093–1099. [Google Scholar] [CrossRef]
- Dong, X.; He, L.; Liu, Y.; Piao, Y. Preparation of highly conductive biochar nanoparticles for rapid and sensitive detection of 17 beta-estradiol in water. Electrochim. Acta 2018, 292, 55–62. [Google Scholar] [CrossRef]
- Cao, L.; Ding, Q.; Liu, M.; Lin, H.; Yang, D.P. Biochar-Supported Cu2+/Cu+ Composite as an Electrochemical Ultrasensitive Interface for Ractopamine Detection. ACS Appl. Bio Mater. 2021, 4, 1424–1431. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; He, S.; Liu, X.; Chen, Q.; Chen, J.; Cao, H. Waste eggshell membrane-templated synthesis of functional Cu2+-Cu+/biochar for an ultrasensitive electrochemical enzyme-free glucose sensor. RSC Adv. 2021, 11, 18994–18999. [Google Scholar] [CrossRef]
- Cao, L.; Kang, Z.W.; Ding, Q.; Zhang, X.; Lin, H.; Lin, M.; Yang, D.P. Rapid pyrolysis of Cu2+-polluted eggshell membrane into a functional Cu2+-Cu+/biochar for ultrasensitive electrochemical detection of nitrite in water. Sci. Total Environ. 2020, 723, 138008. [Google Scholar] [CrossRef]
- De Oliveira, P.R.; Kalinke, C.; Gogola, J.L.; Mangrich, A.S.; Junior, L.H.M.; Bergamini, M.F. The use of activated biochar for development of a sensitive electrochemical sensor for determination of methyl parathion. J. Electroanal. Chem. 2017, 799, 602–608. [Google Scholar] [CrossRef]
- Bukhari, Q.U.A.; Silveri, F.; Della Pelle, F.; Scroccarello, A.; Zappi, D.; Cozzoni, E.; Compagnone, D. Water-phase exfoliated biochar nanofibers from eucalyptus scraps for electrode modification and conductive film fabrication. ACS Sustain. Chem. Eng. 2021, 9, 13988–13998. [Google Scholar] [CrossRef]
- Kalinke, C.; de Oliveira, P.R.; Bonet San Emeterio, M.; González-Calabuig, A.; del Valle, M.; Salvio Mangrich, A.; Bergamini, M.F. Voltammetric electronic tongue based on carbon paste electrodes modified with biochar for phenolic compounds stripping detection. Electroanalysis 2019, 31, 2238–2245. [Google Scholar] [CrossRef]
- Kalinke, C.; Moscardi, A.P.Z.; de Oliveira, P.R.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Simple and low-cost sensor based on activated biochar for the stripping voltammetric detection of caffeic acid. Microchem. J. 2020, 159, 105380. [Google Scholar] [CrossRef]
- Luo, S.; Yang, M.; Wu, Y.; Li, J.; Qin, J.; Feng, F. A low cost Fe3O4-activated biochar electrode sensor by resource utilization of excess sludge for detecting tetrabromobisphenol A. Micromachines 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Lu, X.; Xu, L.; Zhu, Y.; Xue, T.; Ge, Y.; Duan, Z.; Duan, X.; Wen, Y.; Xu, J. Green synthesis of kudzu vine biochar decorated graphene-like MoSe2 with the oxidase-like activity as intelligent nanozyme sensing platform for hesperetin. Chemosphere 2021, 289, 133116. [Google Scholar] [CrossRef]
- Kalinke, C.; Wosgrau, V.; Oliveira, P.R.; Oliveira, G.A.; Martins, G.; Mangrich, A.S.; Bergamini, M.F.; Marcolino-Junior, L.H. Green method for glucose determination using microfluidic device with a non-enzymatic sensor based on nickel oxyhydroxide supported at activated biochar. Talanta 2019, 200, 518–525. [Google Scholar] [CrossRef]
- Zou, J.; Yu, Q.; Gao, Y.; Chen, S.; Huang, X.; Hu, D.; Liu, S.; Lu, L.-M. Bismuth nanoclusters/porous carbon composite: A facile ratiometric electrochemical sensing platform for Pb2+ detection with high sensitivity and selectivity. ACS Omega 2022, 7, 1132–1138. [Google Scholar] [CrossRef]
- De Oliveira, P.R.; Lamy-Mendes, A.C.; Gogola, J.L.; Mangrich, A.S.; Junior, L.H.M.; Bergamini, M.F. Mercury nanodroplets supported at biochar for electrochemical determination of zinc ions using a carbon paste electrode. Electrochim. Acta 2015, 151, 525–530. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.S.; Yang, J.H.; Chun, Y.; Yoo, H.Y.; Han, S.O.; Kim, S.W. Enhanced electron transfer mediator based on biochar from microalgal sludge for application to bioelectrochemical systems. Bioresour. Technol. 2018, 264, 387–390. [Google Scholar] [CrossRef]
- Sant’Anna, M.V.; Carvalho, S.W.; Gevaerd, A.; Silva, J.O.; Santos, E.; Carregosa, I.S.; Wisniewski, A.; Marcolino-Junior, L.H.; Bergamini, M.F.; Sussuchi, E.M. Electrochemical sensor based on biochar and reduced graphene oxide nanocomposite for carbendazim determination. Talanta 2020, 220, 121334. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, J.; Xu, P.; Liu, H.; Zhang, L.; Zhang, S.; Tian, L. Gold nanoparticles decorated biochar modified electrode for the high-performance simultaneous determination of hydroquinone and catechol. Sens. Actuators B Chem. 2019, 306, 127590. [Google Scholar] [CrossRef]
- He, L.; Yang, Y.; Kim, J.; Yao, L.; Dong, X.; Li, T.; Piao, Y. Multi-layered enzyme coating on highly conductive magnetic biochar nanoparticles for bisphenol A sensing in water. Chem. Eng. J. 2019, 384, 123276. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-Pour, A.; Verma, M.; Surampalli, R. Immobilized laccase on oxygen functionalized nanobiochars through mineral acids treatment for removal of carbamazepine. Sci. Total Environ. 2017, 584-585, 393–401. [Google Scholar] [CrossRef]
- Zhang, Y.; Piao, M.; He, L.; Yao, L.; Piao, T.; Liu, Z.; Piao, Y. Immobilization of laccase on magnetically separable biochar for highly efficient removal of bisphenol A in water. RSC Adv. 2020, 10, 4795–4804. [Google Scholar] [CrossRef]
- Martins, G.; Gogola, J.L.; Caetano, F.R.; Kalinke, C.; Jorge, T.R.; Santos, C.N.; Bergamini, M.F.; Marcolino-Junior, L.H. Quick electrochemical immunoassay for hantavirus detection based on biochar platform. Talanta 2019, 204, 163–171. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, N.; Huang, X.; Wang, Q.; Wang, X.; Wang, S. Fabrication of a novel nanocomposite electrode with ZnO-MoO3 and biochar derived from mushroom biomaterials for the detection of acetaminophen in the presence of DA. Microchem. J. 2021, 161, 105709. [Google Scholar] [CrossRef]
- Rao, L.; Zhou, P.; Liu, P.; Lu, X.; Duan, X.; Wen, Y.; Xu, J. Green preparation of amorphous molybdenum sulfide nanocomposite with biochar microsphere and its voltametric sensing platform for smart analysis of baicalin. J. Electroanal. Chem. 2021, 898, 115591. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, L.; He, L.; Liu, N.; Piao, Y. Electrochemical enzyme biosensor bearing biochar nanoparticle as signal enhancer for bisphenol A detection in water. Sensors 2019, 19, 1619. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Du, S.; Cao, J.; Huang, W.; Zhang, X.; Qi, B.; Zhang, S. Simultaneous Determination of Hydroquinone and Catechol by N-doped Porous Biochar-modified Electrode. Bull. Korean Chem. Soc. 2020, 41, 261–265. [Google Scholar] [CrossRef]
- Zhang, K. Development of New Electrochemical Sensor based on Kudzu Vine Biochar Modified Flexible Carbon Electrode for Portable Wireless Intelligent Analysis of Clenbuterol. Int. J. Electrochem. Sci. 2020, 7326–7336. [Google Scholar] [CrossRef]
- Oliveira, P.R.; Lamy-Mendes, A.C.; Rezende, E.I.P.; Mangrich, A.S.; Junior, L.H.M.; Bergamini, M.F. Electrochemical determination of copper ions in spirit drinks using carbon paste electrode modified with biochar. Food Chem. 2015, 171, 426–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Guo, M.; Wu, S.; Fornara, D.; Sarkar, B.; Sun, L.; Wang, H. Electrochemical sensor based on corncob biochar layer supported chitosan-MIPs for determination of dibutyl phthalate (DBP). J. Electroanal. Chem. 2021, 897, 115549. [Google Scholar] [CrossRef]
- Ateş, A.; Oskay, K.O. Preparation and characterization of nanosized Fe3O4-biochar electrocatalysts with large surface area for H2O2 sensing. Surf. Interfaces 2022, 29, 101733. [Google Scholar] [CrossRef]
- Oliveira, P.R.; Kalinke, C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Copper hexacyanoferrate nanoparticles supported on biochar for amperometric determination of isoniazid. Electrochim. Acta 2018, 285, 373–380. [Google Scholar] [CrossRef]
- Yao, L.; He, L.; Yang, Y.; Zhang, Y.; Liu, Z.; Liang, L.; Piao, Y. Nanobiochar paper based electrochemical immunosensor for fast and ultrasensitive detection of microcystin-LR. Sci. Total Environ. 2020, 750, 141692. [Google Scholar] [CrossRef]
- Sobhan, A.; Muthukumarappan, K.; Wei, L.; Qiao, Q.; Rahman, T.; Ghimire, N. Development and characterization of a novel activated biochar-based polymer composite for biosensors. Int. J. Polym. Anal. Charact. 2021, 1–17. [Google Scholar] [CrossRef]
- Sant’Anna, M.V.; Silva, J.D.O.S.; Gevaerd, A.; Lima, L.S.; Monteiro, M.D.; Carregosa, I.S.; Wisniewski, A.; Marcolino-Junior, L.H.; Bergamini, M.F.; Sussuchi, E.M. Selective carbonaceous-based (nano)composite sensors for electrochemical determination of paraquat in food samples. Food Chem. 2021, 373, 131521. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Gevaerd, A.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Biochar obtained from spent coffee grounds: Evaluation of adsorption properties and its application in a voltammetric sensor for lead (II) ions. Microchem. J. 2021, 165, 106114. [Google Scholar] [CrossRef]
- Chen, X.; Lu, K.; Lin, D.; Li, Y.; Yin, S.; Zhang, Z.; Tang, M.; Chen, G. Hierarchical Porous Tubular Biochar Based Sensor for Detection of Trace Lead (II). Electroanalysis 2020, 33, 473–482. [Google Scholar] [CrossRef]
- Zou, J.; Qian, W.; Li, Y.; Yu, Q.; Yu, Y.; Chen, S.; Qu, F.; Gao, Y.; Lu, L. Multilayer activated biochar/UiO-66-NH2 film as intelligent sensing platform for ultra-sensitive electrochemical detection of Pb2+ and Hg2+. Appl. Surf. Sci. 2021, 569, 151006. [Google Scholar] [CrossRef]
- Wong, A.; Ferreira, P.A.; Santos, A.M.; Cincotto, F.H.; da Silva, R.A.B.; Sotomayor, M.D. A new electrochemical sensor based on eco-friendly chemistry for the simultaneous determination of toxic trace elements. Microchem. J. 2020, 158, 105292. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Wu, D.; Li, Y.; Du, B.; Wei, Q. Label-free immunosensor based on Au@Ag2S nanoparticles/magnetic chitosan matrix for sensitive determination of ractopamine. J. Electroanal. Chem. 2015, 741, 14–19. [Google Scholar] [CrossRef]
- Cheng, R.; Ding, Y.; Wang, Y.; Wang, H.; Zhang, Y.; Wei, Q. A novel molecularly imprinted electrochemiluminescence sensor based on cobalt nitride nanoarray electrode for the sensitive detection of bisphenol S. RSC Adv. 2021, 11, 11011–11019. [Google Scholar] [CrossRef] [PubMed]
- Inroga, F.A.; Rocha, M.O.; Lavayen, V.; Arguello, J. Development of a tyrosinase-based biosensor for Bisphenol A detection using gold leaf–like microstructures. J. Solid State Electrochem. 2019, 23, 1659–1666. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Li, B.; Ren, X.; Ma, H.; Wei, Q. Novel ratiometric electrochemical sensor for no-wash detection of fluorene-9-bisphenol based on combining CoN nanoarrays with molecularly imprinted polymers. Analyst 2020, 145, 3320–3328. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wang, Y.; Kuang, X.; Ma, H.; Wei, Q. Directly assembled electrochemical sensor by combining self-supported CoN nanoarray platform grown on carbon cloth with molecularly imprinted polymers for the detection of Tylosin. J. Hazard. Mater. 2020, 398, 122778. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Mowbray, S.E.; Amiri, A.M. A Brief Overview of Medical Fiber Optic Biosensors and Techniques in the Modification for Enhanced Sensing Ability. Diagnostics 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, B.M.; Viphavakit, C.; Chitaree, R.; Ghosh, S.; Pathak, A.K.; Verma, S.; Sakda, N. Optical fiber, nanomaterial, and thz-metasurface-mediated nano-biosensors: A Review. Biosensors 2022, 12, 42. [Google Scholar] [CrossRef] [PubMed]


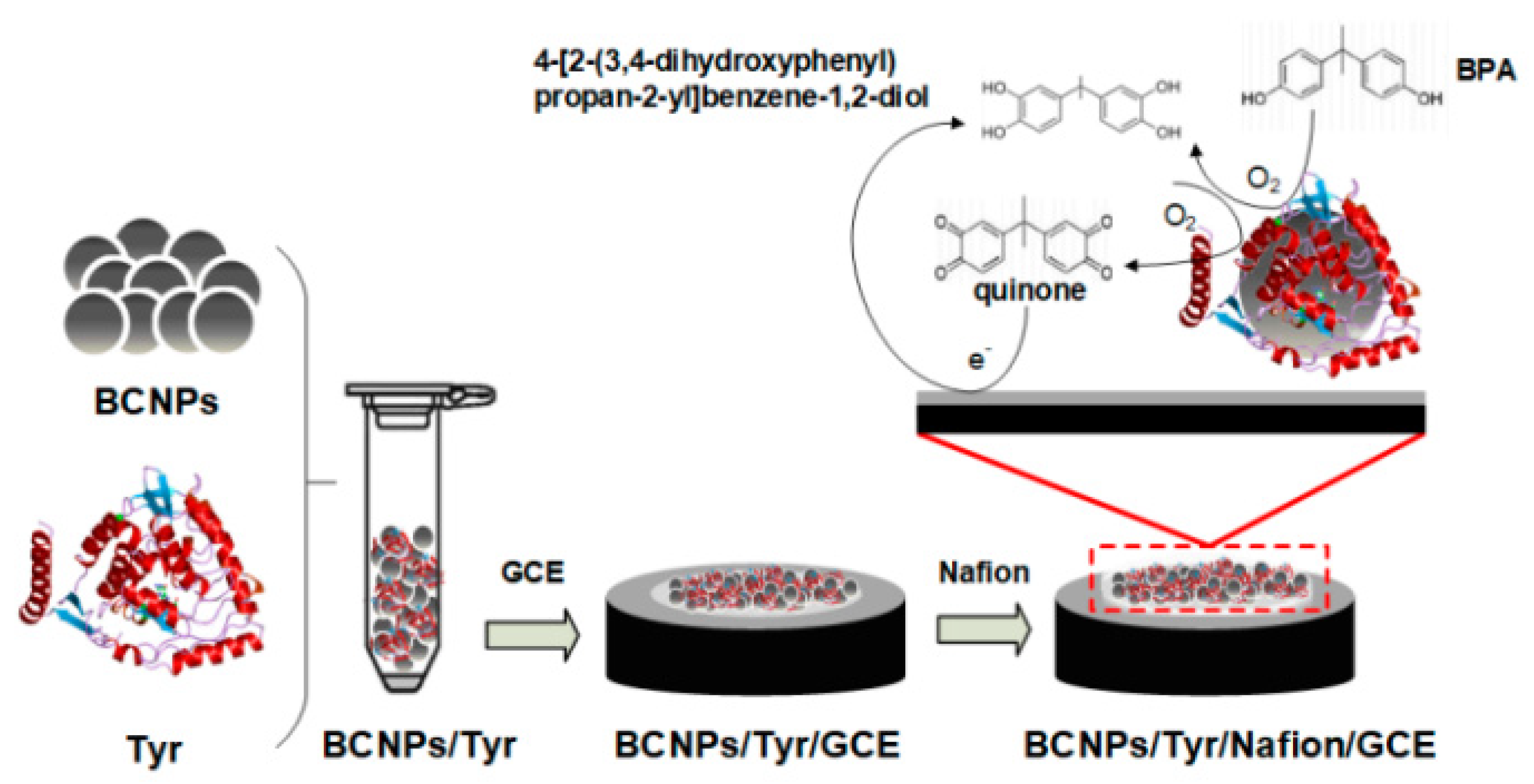

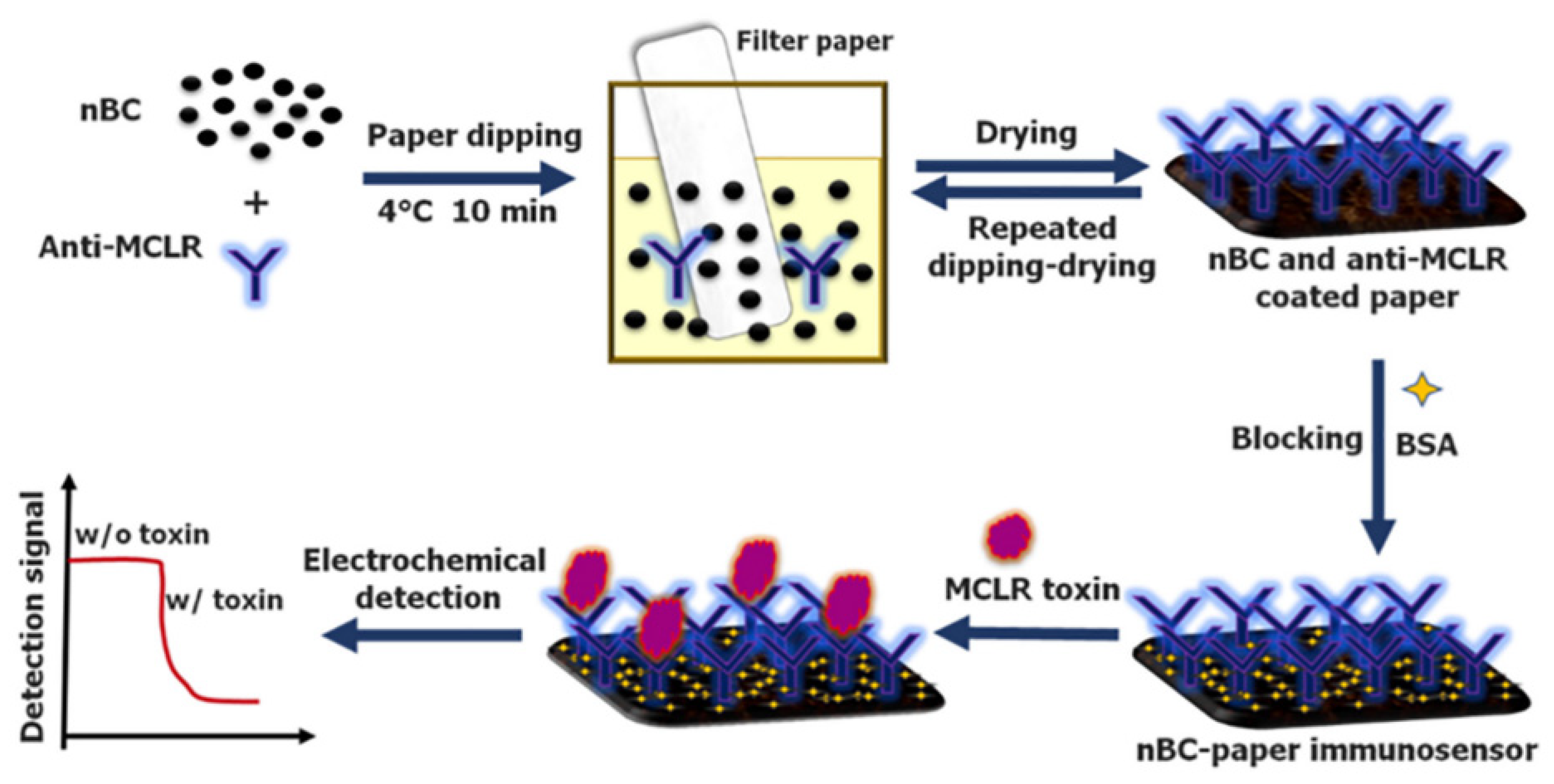

| Target Analyte | Biomass Resource | Preparation Method | Types of Biochar for Electrode Modification | Analysis Technique | Linear Range (μM) | LOD (nM) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| 17β-estradiol | Bagasse | Pyrolysis | Biochar | DPV | 0.05–20 | 11.30 | Ground water | [27] |
| Acetaminophen | Mushroom | Pyrolysis | Zno-moo3/biochar | DPV | 2.5–2000 | 1140 | Blood serum and Tablet | [47] |
| Baicalin | Pomelo peel | Hydrothermal synthesis & Pyrolysis | A-mosx/biochar | DPV | 0.01–5 | 2 | Shuang-Huang-Lian oral liquid | [48] |
| Bisphenol A | Bagasse | Pyrolysis | Tyrosinase/biochar | I-t | 0.02–10 | 3.18 | Ground water | [49] |
| Bisphenol A | Bagasse | Pyrolysis | Magnetic nanoscale biochar/tyrosinase | I-t | 0.01–1.01 | 2.78 | Environmental water | [43] |
| Carbendazim | Eichhornia crassipes | Pyrolysis | Reduced graphene oxide/biochar | DPV | 0.03–0.9 | 2.3 | Orange juice, lettuce leaves, drinking water, and wastewater | [41] |
| Catechol and hydroquinone Simultaneously | Rice flour; Urea; Sodium citrate | Hydrothermal synthesis | N-doped porous biochar | DPV | 0.4–15 and 0.4–20 | 37 and 47 | - | [50] |
| Catechol and hydroquinone; levofloxacin and norfloxacin; tert-butylhydroquinone and butylated hydroxy-anisole. | Babassu petiole | Pyrolysis | Ferrocyanide/biochar | SWV | - | - | - | [10] |
| Clenbuterol | Kudzu vine | Pyrolysis | Zn/biochar | DPV | 0.95–14.31 | 750 | Bovine serum | [51] |
| Cu2+ | Castor oil cake | Pyrolysis | Biochar | DPASV | 1.5– 31 | 400 | Spirit drinks | [52] |
| Dibutyl phthalate | Corncob | Pyrolysis | MIP/biochar | DPV | 0–1.8 | 2.6 | Rice wine | [53] |
| Glucose | Eggshell membrane | Pyrolysis, | Cu2+–Cu+/biochar | I-t | 12.5–70 | 1040 | Human serum | [29] |
| Glucose | Castor oil cake | Pyrolysis | Ni/biochar | I-t | 5.0–100.0 | 137 | Human saliva and blood serum | [37] |
| Glucose | Waste microalgal sludge | Pyrolysis | Co/chitosan/biochar | I-t | - | - | - | [40] |
| Glyphosate herbicide | Babassu petiole | Pyrolysis | Copper hexadecafuoro-29H/biochar | SWV | 0.3–4 | 20 | Lake water and River water | [9] |
| H2O2 | Hazelnut shell | Microwave assisted pyrolysis | Fe3O4/biochar | I-t | 600–10,000 | 503 × 103 | Milk | [54] |
| Hantavirus nucleoprotein | Castor bean | Pyrolysis | Biochar | CV | 5.0 ng·mL−1–1.0 μg·mL−1 | 0.14 ng·mL−1 | Human serum | [46] |
| Hesperetin | Kudzu vine | Hydrothermal synthesis | MoSe2/biochar | DPV | 0.01–9.5 | 2 | Oranges | [36] |
| Hydroquinone Catechol | White myoga ginger | Pyrolysis | Au/biochar | DPV | 0.008–1.0 and 1.0–7.0; 0.01–1.0 and 1.0–7.0 | 2; 4 | Tap water | [42] |
| Hydroquinone Catechol | Dracaena sanderiana | Co-pyrolysis | Au/biochar | DPV | 0.01–0.2 and 0.2–10; 0.04–0.4 and 0.4–15 | 3.4; 9.0 | Local tap water | [11] |
| Isoniazid | Castor oil cake | Pyrolysis | Copper hexacyanoferrate/biochar | I-t | 1.0–10 | 63 | Human urine | [55] |
| Methyl parathion | Castor oil cake | Pyrolysis | Biochar | DPV | 0.1–70 | 39 | Tap water | [31] |
| Microcystin-LR | Sugarcane waste | Pyrolysis | Antibody/biochar | I-t | 0.1 × 10−3–0.1 | 0.017 | Lake water and River water | [56] |
| NH3(g) | Corn stover | Pyrolysis & Solvent casting method | Polylactic acid/biochar | LSV | 80–170 ppm | 80 ppm | [57] | |
| Paraquat | Water hyacinth | Pyrolysis | Rgo/biochar | DPV | 0.74–9.82 | 20 | Coconut water, wastewater, honey, lettuce and lemon | [58] |
| Pb2+ | Spent coffee grounds | Pyrolysis | Biochar | DPASV | 0.128–2.44 | 4.5 | Gunshot residues and hair dye | [59] |
| Pb2+ | Peach wood | Pyrolysis, | Biochar | SWASV | 0.5–120 μg·L−1 | 0.02 μg·L−1 | Tap water | [60] |
| Pb2+ | Litsea cubeba shell | Pyrolysis & solvothermal method | Bismuth nanocluster/biochar | DPASV | 0.014 × 10−3–4.83 | 0.005 | Paddy water | [38] |
| Pb2+; Hg2+ | Magnolia grandiflora fruit | Pyrolysis | Biochar/uio-66-NH2/biochar | DPASV | 0.001–1000 μg·L−1 | 0.3 ng·L−1 | Lake water and paddy water | [61] |
| Pb2+; Cd2+ | Babassu petiole | Pyrolysis | Nanodiamonds/Biochar/Chitosan | SWASV | 1.0–75.0; 0.25 –6.00 | 110; 56 | River water | [62] |
| RAC | Eggshell membrane | Pyrolysis, | Cu2+–Cu+/biochar | DPV | 0.1–1.75 | 41 | The pork sausage | [28] |
| Tetrabromo-bisphenol A | Excess sludge | Pyrolysis | Fe3O4/biochar | DPV | 0.005–1 | 3.2 | River water | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xu, R.; Wang, H.; Xu, W.; Tian, L.; Huang, J.; Liang, C.; Zhang, Y. Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors. Biosensors 2022, 12, 377. https://doi.org/10.3390/bios12060377
Li Y, Xu R, Wang H, Xu W, Tian L, Huang J, Liang C, Zhang Y. Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors. Biosensors. 2022; 12(6):377. https://doi.org/10.3390/bios12060377
Chicago/Turabian StyleLi, Yunxiao, Rui Xu, Huabin Wang, Wumei Xu, Liyan Tian, Jingxin Huang, Chengyue Liang, and Yong Zhang. 2022. "Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors" Biosensors 12, no. 6: 377. https://doi.org/10.3390/bios12060377
APA StyleLi, Y., Xu, R., Wang, H., Xu, W., Tian, L., Huang, J., Liang, C., & Zhang, Y. (2022). Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors. Biosensors, 12(6), 377. https://doi.org/10.3390/bios12060377








