Bioluminescent-Inhibition-Based Biosensor for Full-Profile Soil Contamination Assessment
Abstract
:1. Introduction


2. Materials and Methods
2.1. Soil Collection and Characterization
2.2. Bioluminescent Enzymatic Assay
2.3. Data Processing
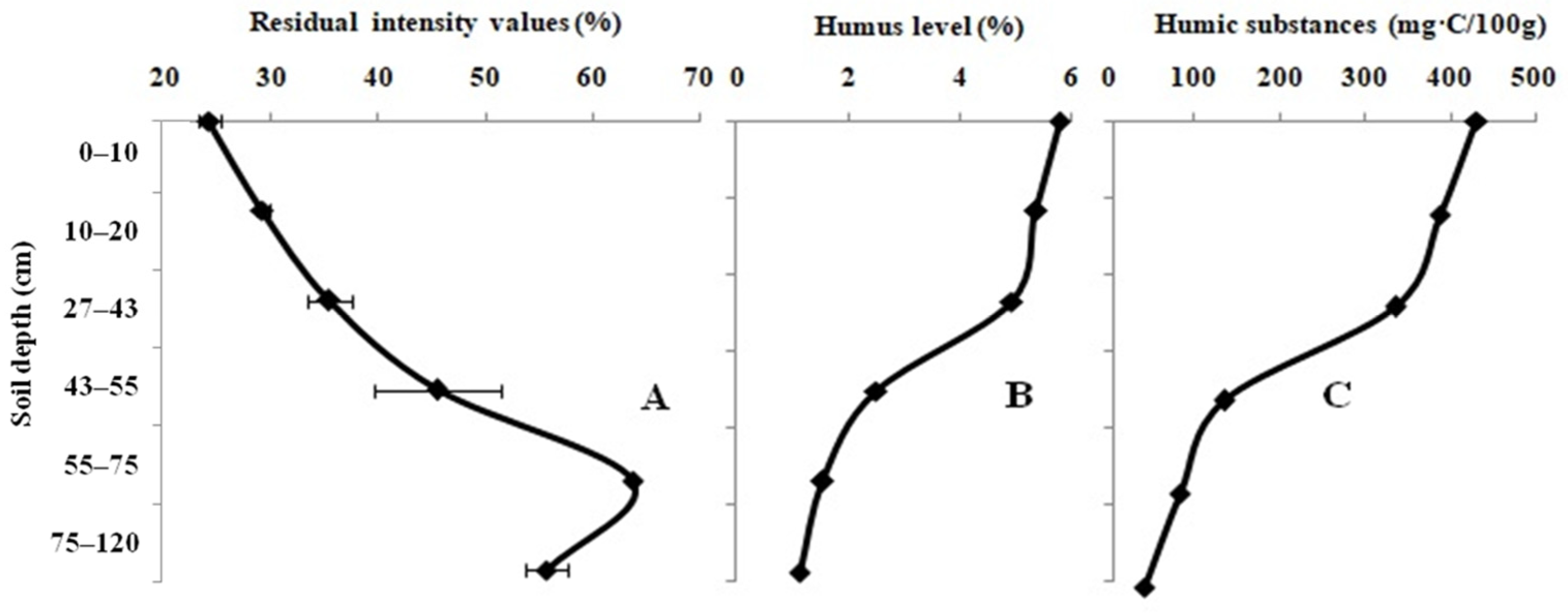
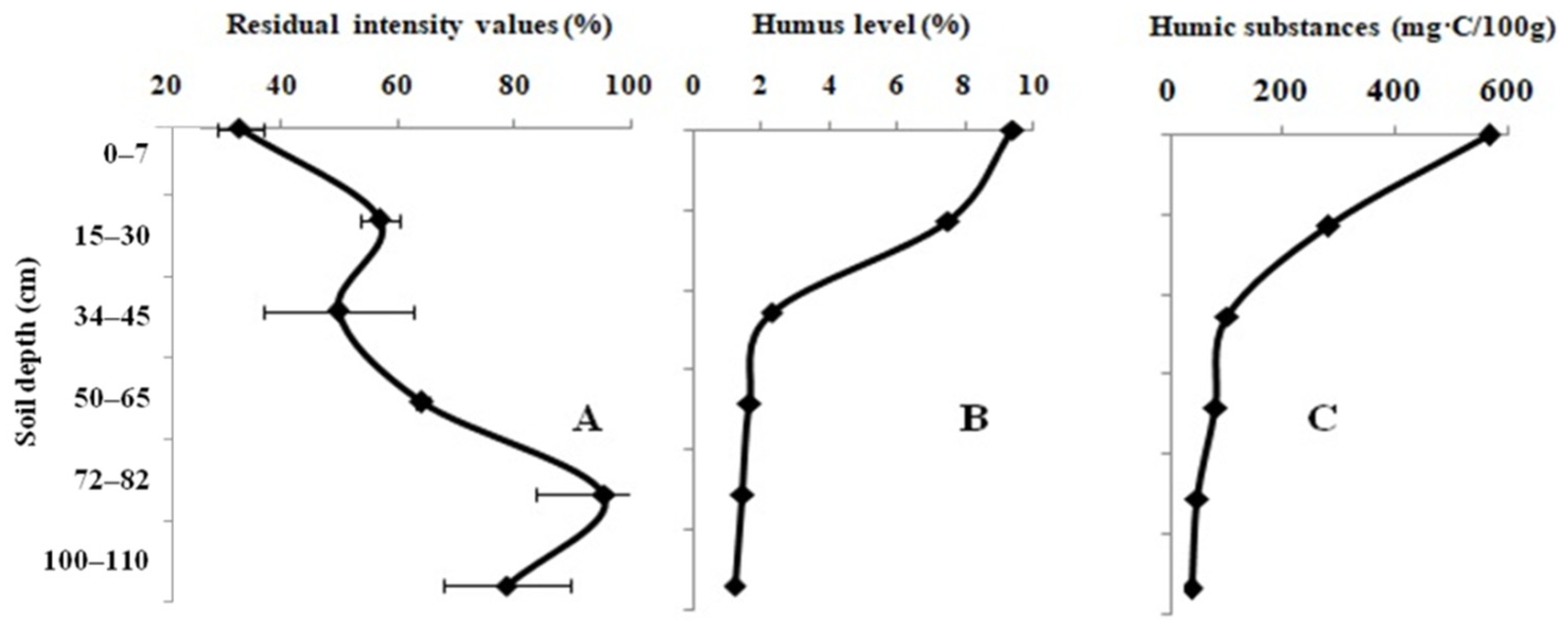
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Compendium of Chemical Terminology. Available online: https://goldbook.iupac.org/ (accessed on 25 April 2022).
- Esimbekova, E.N.; Kalyabina, V.P.; Kratasyuk, V.A. Application of Bioluminescent Enzymatic Tests. Ecotoxicol. J. Int. Sci. Publ. Ecol. Saf. 2018, 12, 135–146. [Google Scholar]
- Esimbekova, E.N.; Torgashina, I.G.; Kalyabina, V.P.; Kratasyuk, V.A. Enzymatic Biotesting: Scientific Basis and Application. Contemp. Probl. Ecol. 2021, 14, 290–304. [Google Scholar] [CrossRef]
- Jager, T.; van der Wal, L.; Fleuren, R.H.; Barendregt, A.; Hermens, J.L. Bioaccumulation of organic chemicals in contaminated soils: Evaluation of bioassays with earthworms. Environ. Sci. Technol. 2005, 39, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mädera, P.; et al. Soil quality–A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Karlen, D.L.; Ditzler, C.A.; Andrews, S.S. Soil quality: Why and how? Geoderma 2003, 114, 145–156. [Google Scholar] [CrossRef]
- Verbeeck, M.; Hiemstra, T.; Thiry, Y.; Smolders, E. Soil organic matter reduces the sorption of arsenate and phosphate: A soil profile study and geochemical modelling. Eur. J. Soil Sci. 2017, 68, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Seo, B.H.; Kim, H.S.; Kwon, S.I.; Owens, G.; Kim, K.R. Heavy metal accumulation and mobility in a soil profile depend on the organic waste type applied. J. Soils Sediments 2019, 19, 822–829. [Google Scholar] [CrossRef]
- Xi, B.; Yu, H.; Li, Y.; Dang, Q.; Tan, W.; Wang, Y.; Cui, D. Insights into the effects of heavy metal pressure driven by long-term treated wastewater irrigation on bacterial communities and nitrogen-transforming genes along vertical soil profiles. J. Hazard. Mater. 2020, 403, 123853. [Google Scholar] [CrossRef]
- Varvel, G.E.; Wilhelm, W.W. No-tillage increases soil profile carbon and nitrogen under long-term rainfed cropping systems. Soil Tillage Res. 2011, 114, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Kolosova, E.M.; Sutormin, O.S.; Stepanova, L.V.; Shpedt, A.A.; Rimatskaya, N.V.; Sukovataya, I.E.; Kratasyuk, V.A. Bioluminescent enzyme inhibition-based assay for the prediction of toxicity of pollutants in urban soils. Environ. Technol. Innov. 2021, 24, 101842. [Google Scholar] [CrossRef]
- Kratasyuk, V.A.; Kolosova, E.M.; Sutormin, O.S.; Lonshakova-Mukina, V.I.; Baygin, M.M.; Rimatskaya, N.V.; Sukovataya, I.E.; Shpedt, A.A. Software for matching standard activity enzyme biosensors for soil pollution analysis. Sensors 2021, 21, 1017. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, E.M.; Sutormin, O.S.; Esimbekova, E.N.; Lonshakova-Mukina, V.I.; Kratasyuk, V.A. Set of enzymatic bioassays for assessment of soil contamination. Dokl. Biol. Sci. 2019, 489, 165. [Google Scholar] [CrossRef] [PubMed]
- Sutormin, O.S.; Kolosova, E.M.; Nemtseva, E.V.; Iskorneva, I.V.; Lisitsa, A.A.; Matvienko, V.S.; Esimbekova, E.N.; Kratasyuk, V.A. Enzymatic bioassay of soil: Sensitivity comparison of mono-, double-and triple-enzyme systems to soil toxicants. Tsitologiia 2018, 60, 826–829. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Nemtseva, E.V.; Bezrukikh, A.E.; Jukova, G.V.; Lisitsa, A.E.; Lonshakova-Mukina, V.I.; Rimatskaya, N.V.; Sutormin, O.S.; Kratasyuk, V.A. Bioluminescent enzyme inhibition-based assay to predict the potential toxicity of carbon nanomaterials. Toxicol. Vitr. 2017, 45, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Lukyanenko, K.A.; Denisov, I.A.; Sorokin, V.V.; Yakimov, A.S.; Esimbekova, E.N.; Belobrov, P.I. Handheld enzymatic luminescent biosensor for rapid detection of heavy metals in water samples. Chemosensors 2019, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Trubnikov, Y.N.; Shpedt, A.A. Assessment of Natural-Resource Potential and Extent of Anthropogenic Transformation of the Agricultural Territory of the Krasnoyarsk Region. In Ecosystems of the Central Asia: Research, Concervation and Sustainable Use; Federal State Budgetary Educational Institution of Higher Professional Education “Tuva State University”: Kyzyl, Russia, 2016; pp. 227–229. [Google Scholar]
- Trubnikov, Y.N.; Shpedt, A.A. Influence of Fertilizers on Agrochemical Properties and Productive Capability of Chernozem of Yenisei Siberia. In Soil Fertility and Assessment of Productivity in Agriculture; State Agrarian University of the Northern Trans-Urals: Tyumen, Russia, 2018; pp. 182–189. [Google Scholar]
- Shishov, L.L.; Tonkonogov, V.D.; Gerasimova, M.I.; Lebedeva, I.I. New classification system of Russian soils. Eurasian Soil Sci. E 2005, 38, S35–S43. [Google Scholar]
- GOST 26483–85. Soils. Preparations of Salt Extract and Determination of Its pH by CINAO Method. Available online: https://www.russiangost.com/p-50090-gost-26483-85.aspx (accessed on 25 April 2022).
- GOST 26213–91. Soils. Methods for Determination of Organic Matter. Available online: https://www.russiangost.com/p-52750-gost-26213-91.aspx (accessed on 25 April 2022).
- Tarasova, A.S.; Stom, D.I.; Kudryasheva, N.S. Effect of humic substances on toxicity of inorganic oxidizer bioluminescent monitoring. Environ. Toxicol. Chem. 2011, 30, 1013–1017. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Kislan, S.L.; Fedorova, E.S.; Kuznetsov, A.M.; Mogilnaya, O.A.; Stom, D.I.; Kudryasheva, N.S. Bioluminescence as a tool for studying detoxification processes in metal salt solutions involving humic substances. J. Photochem. Photobiol. B 2012, 117, 164–170. [Google Scholar] [CrossRef]
- Haque, M.A. Variation in salinity through the soil profile in south coastal region of Bangladesh. J. Bangladesh Acad. Sci. 2018, 42, 11–23. [Google Scholar] [CrossRef]
- Vogel, C.; Heister, K.; Buegger, F.; Tanuwidjaja, I.; Haug, S.; Schloter, M.; Kögel-Knabner, I. Clay mineral composition modifies decomposition and sequestration of organic carbon and nitrogen in fine soil fractions. Biol. Fertil. Soils 2015, 51, 427–442. [Google Scholar] [CrossRef]
- Pronk, G.J.; Heister, K.; Kögel-Knabner, I. Is turnover and development of organic matter controlled by mineral composition? Soil Biol. Biochem. 2013, 67, 235–244. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolosova, E.M.; Sutormin, O.S.; Shpedt, A.A.; Stepanova, L.V.; Kratasyuk, V.A. Bioluminescent-Inhibition-Based Biosensor for Full-Profile Soil Contamination Assessment. Biosensors 2022, 12, 353. https://doi.org/10.3390/bios12050353
Kolosova EM, Sutormin OS, Shpedt AA, Stepanova LV, Kratasyuk VA. Bioluminescent-Inhibition-Based Biosensor for Full-Profile Soil Contamination Assessment. Biosensors. 2022; 12(5):353. https://doi.org/10.3390/bios12050353
Chicago/Turabian StyleKolosova, Elizaveta M., Oleg S. Sutormin, Aleksandr A. Shpedt, Ludmila V. Stepanova, and Valentina A. Kratasyuk. 2022. "Bioluminescent-Inhibition-Based Biosensor for Full-Profile Soil Contamination Assessment" Biosensors 12, no. 5: 353. https://doi.org/10.3390/bios12050353
APA StyleKolosova, E. M., Sutormin, O. S., Shpedt, A. A., Stepanova, L. V., & Kratasyuk, V. A. (2022). Bioluminescent-Inhibition-Based Biosensor for Full-Profile Soil Contamination Assessment. Biosensors, 12(5), 353. https://doi.org/10.3390/bios12050353






