The Impact of Glucose Oxidase Immobilization on Dendritic Gold Nanostructures on the Performance of Glucose Biosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Pretreatment of the Working Electrode and Electrochemical Deposition of DGNs
2.4. Immobilization of GOx on a DGNs/GR Electrode
2.5. Electrochemical Measurement
2.6. Calculations
3. Results
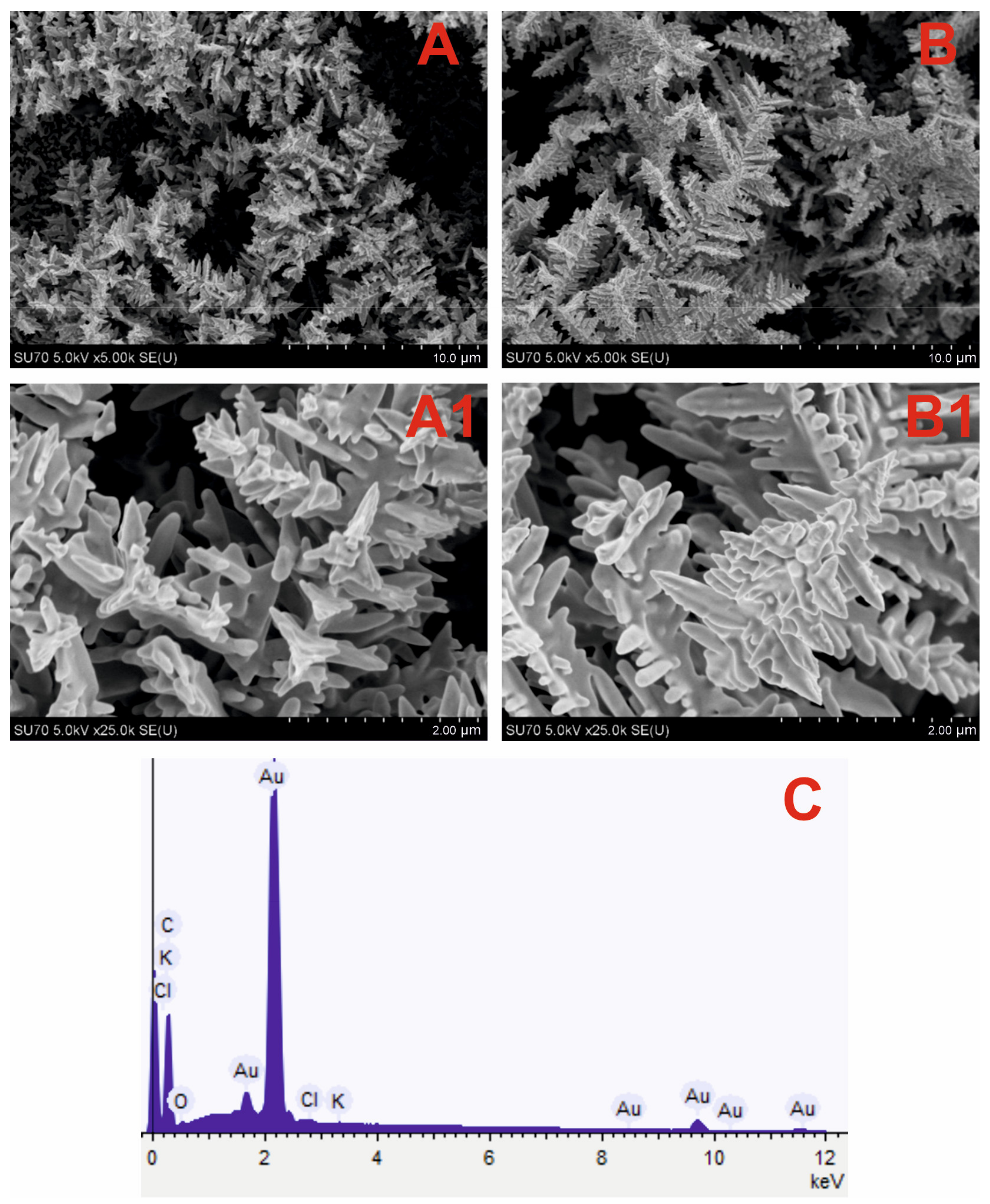
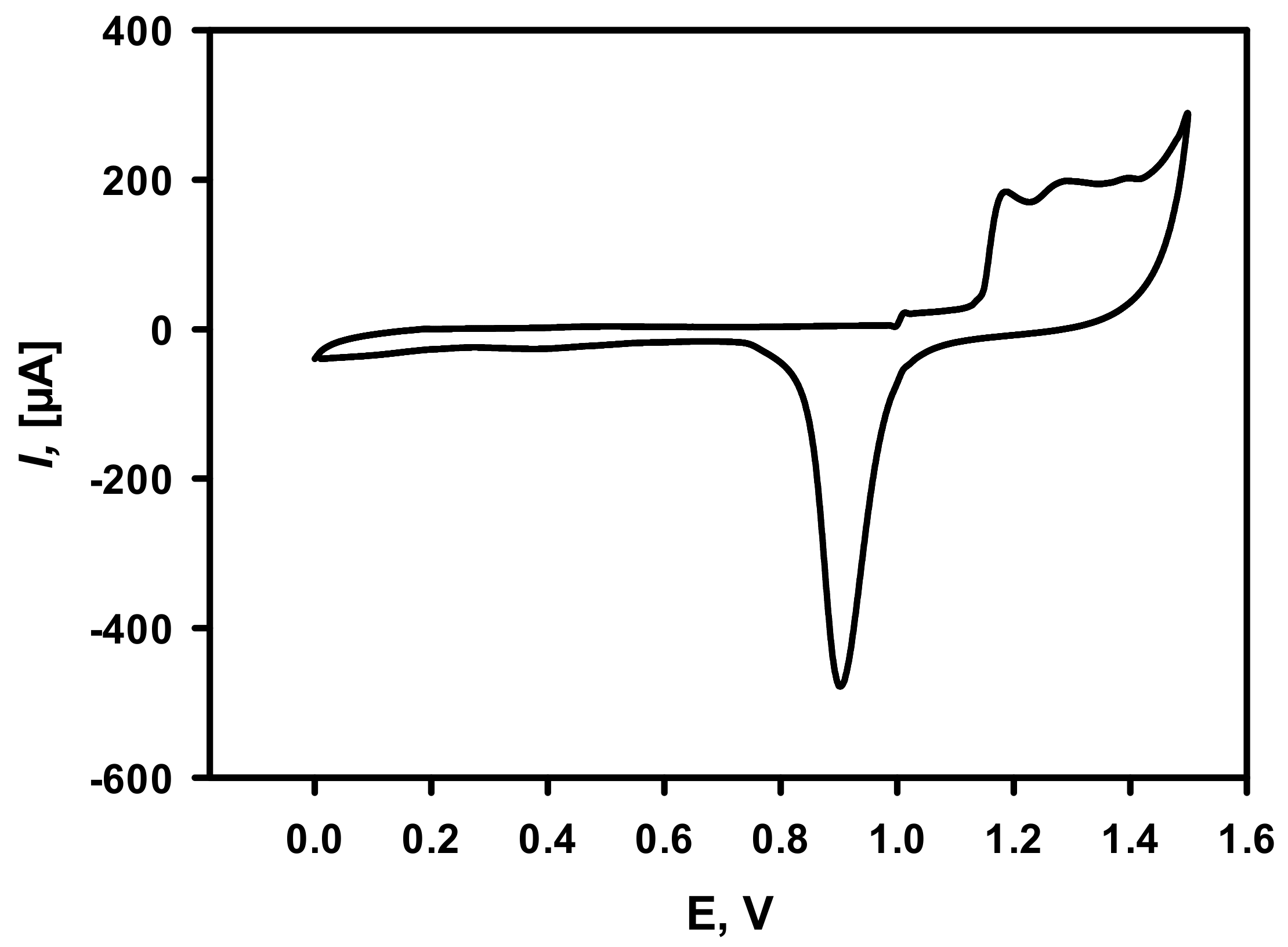
3.1. Optimization of Electrochemical Deposition of DGNs
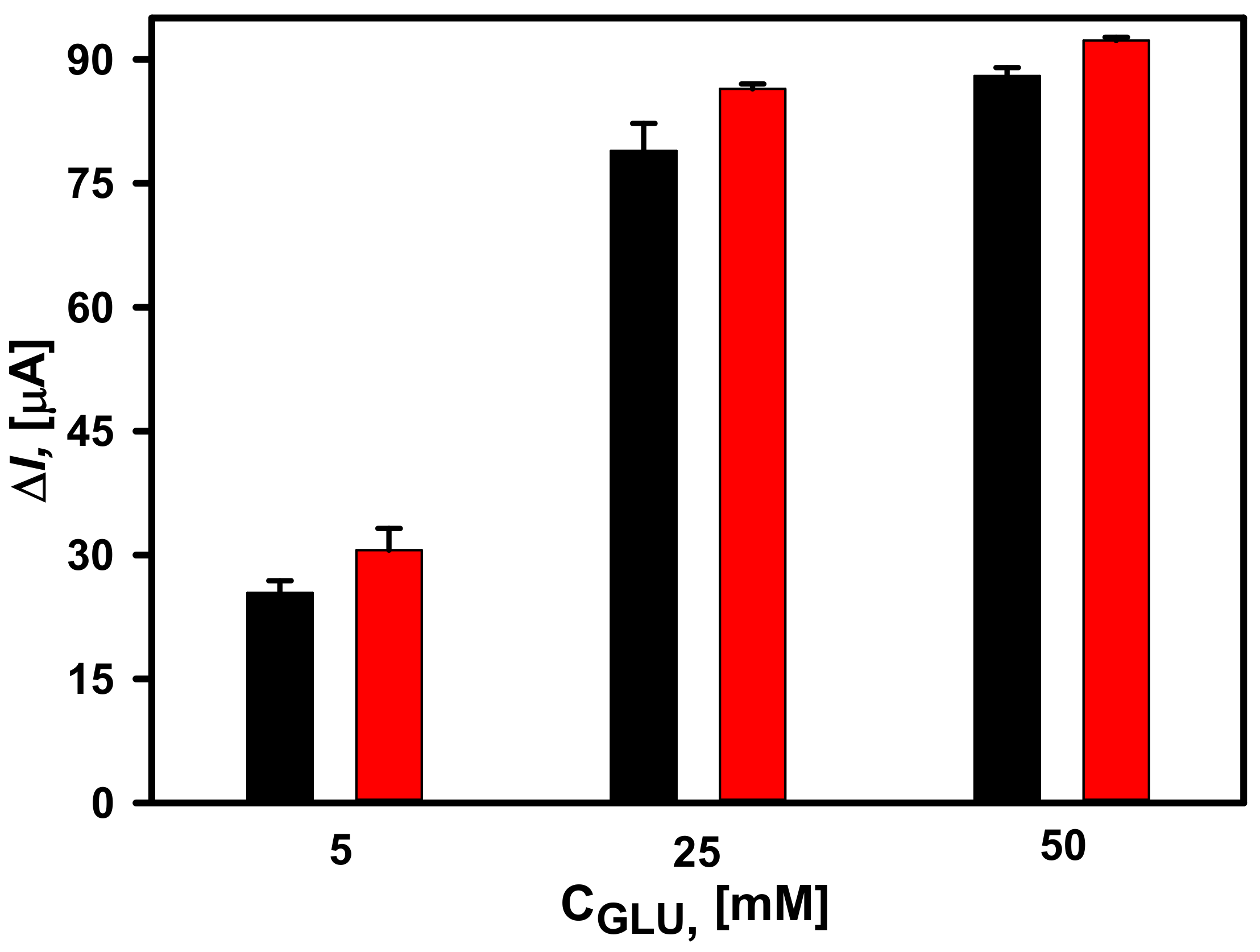
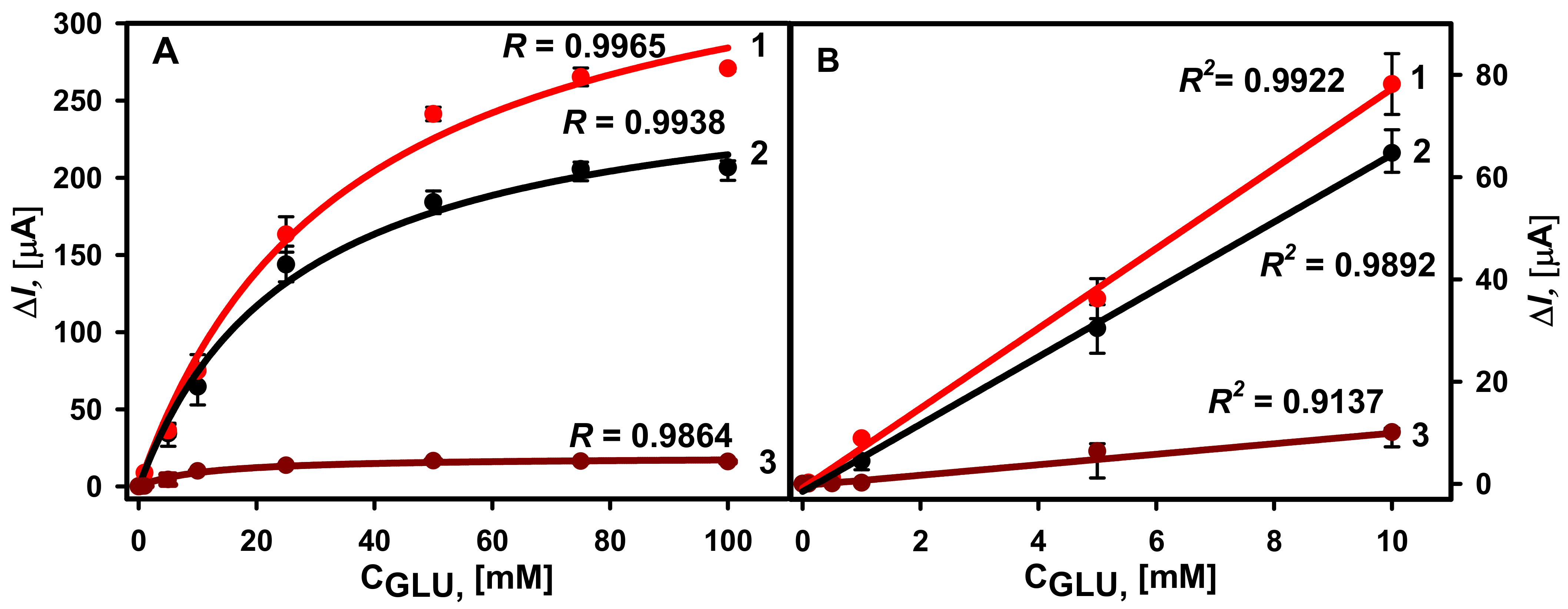
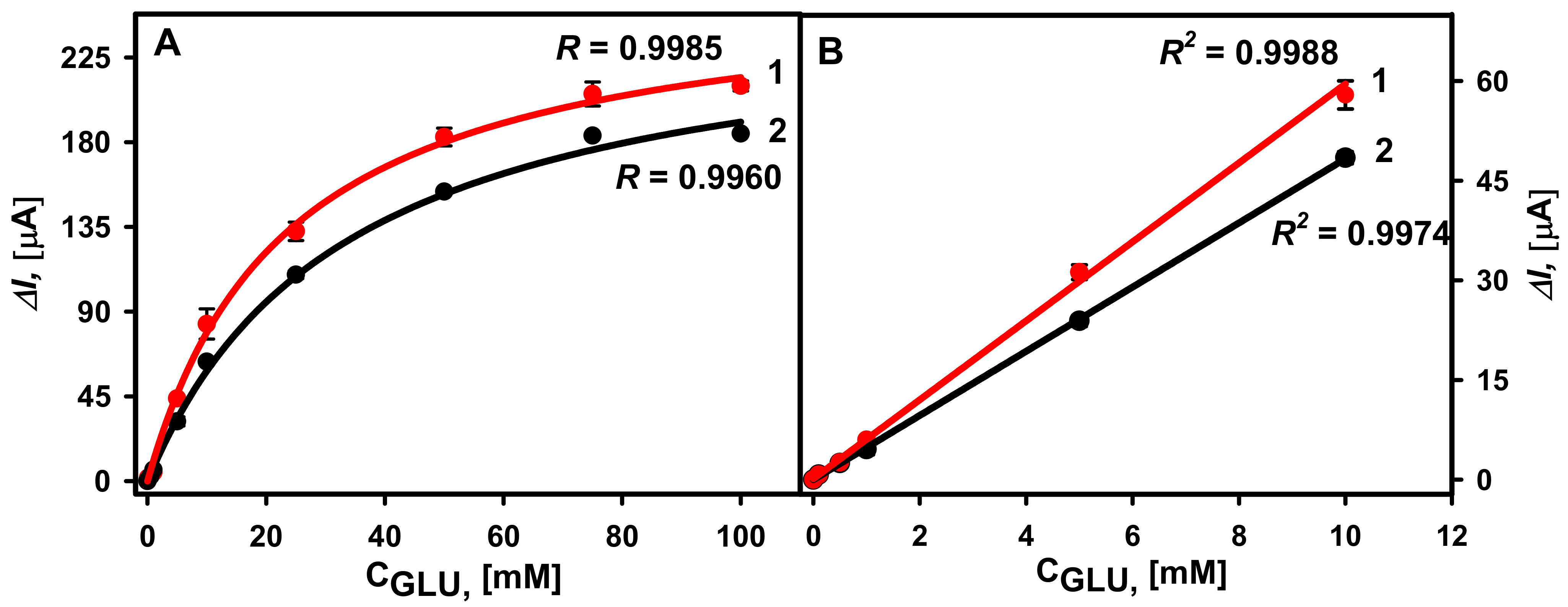
3.2. Comparison of Different GOx Immobilization Methods on the Performance of Glucose Biosensors
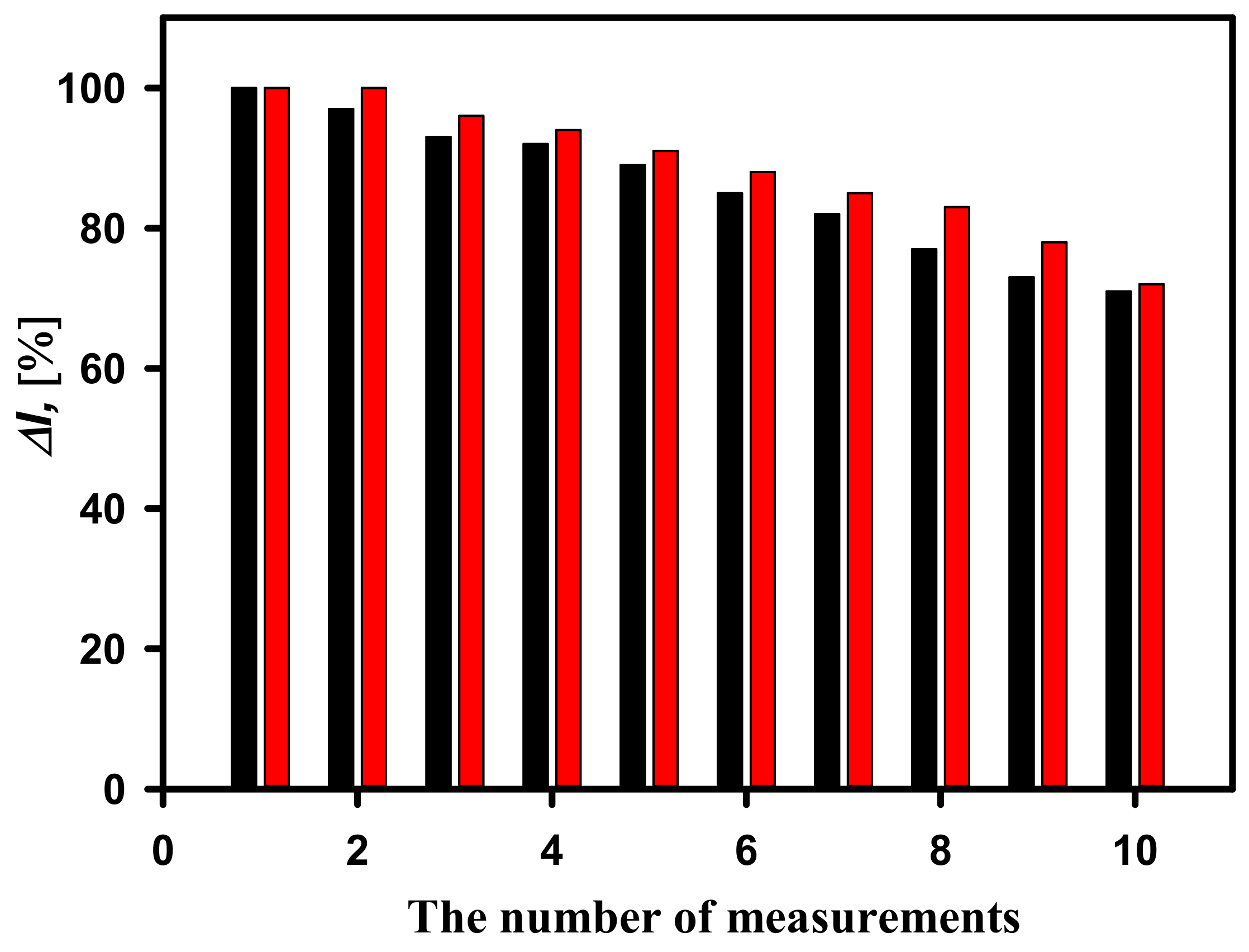
3.3. Study of Analytical Signal Repeatability
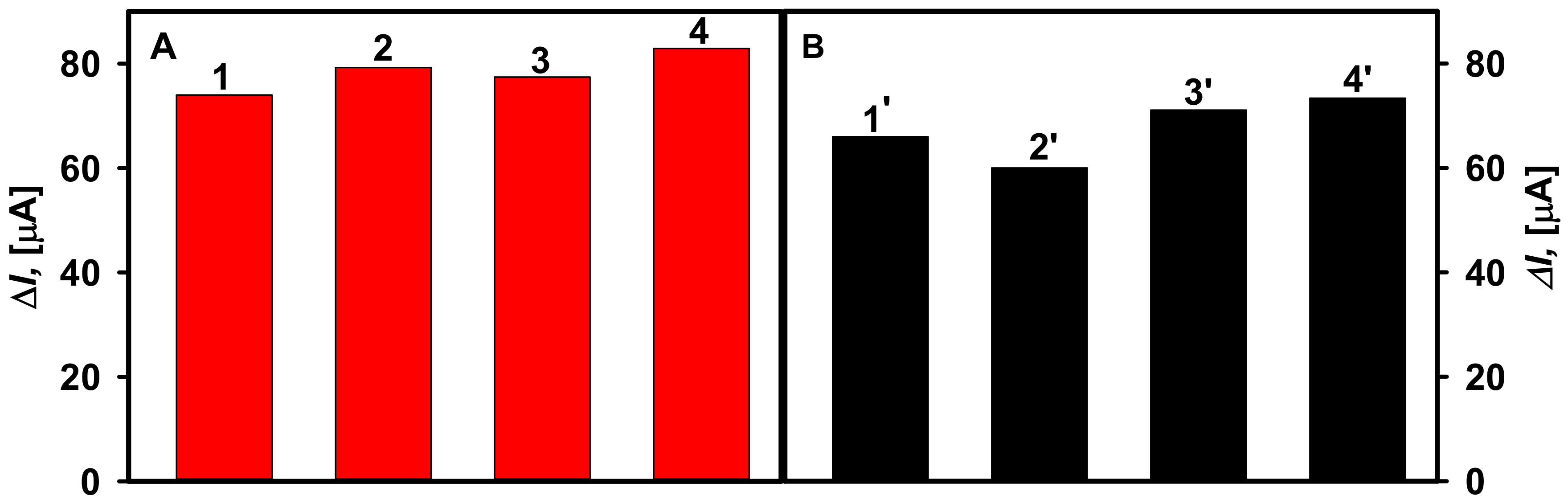
3.4. Detection of Glucose in Human Serum Sample
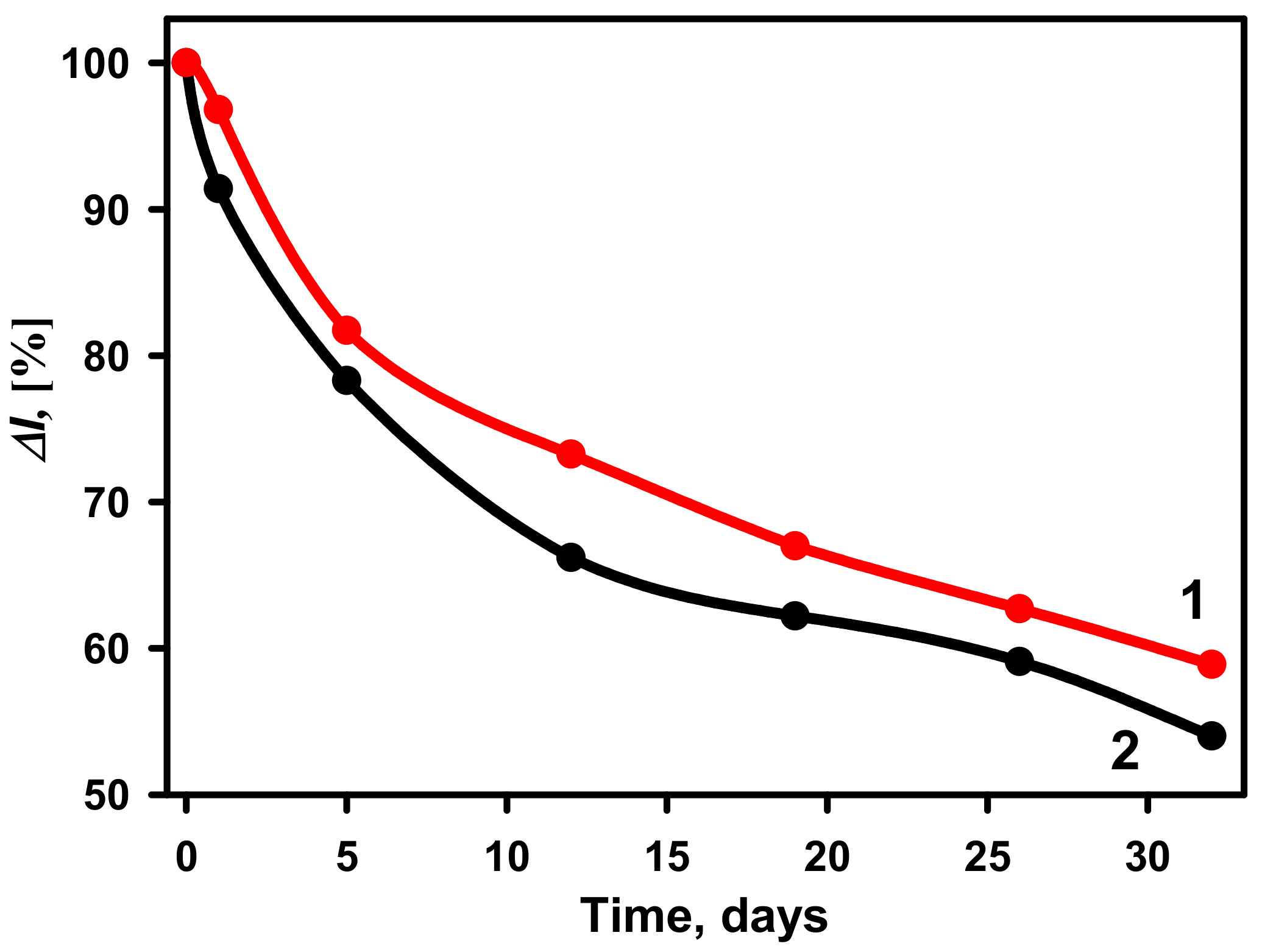
3.5. The Stability of the Developed Glucose Biosensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 31 March 2022).
- Mean Fasting Blood Glucose. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/2380 (accessed on 31 March 2022).
- Yu, Z.; Jiang, N.; Kazarian, S.G.; Tasoglu, S.; Yetisen, A.K. Optical sensors for continuous glucose monitoring. Prog. Biomed. Eng. 2021, 3, 22004. [Google Scholar] [CrossRef]
- Witkowska Nery, E.; Kundys, M.; Jeleń, P.S.; Jönsson-Niedziółka, M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016, 88, 11271–11282. [Google Scholar] [CrossRef] [PubMed]
- Donmez, S. A novel electrochemical glucose biosensor based on a poly (L-aspartic acid)-modified carbon-paste electrode. Prep. Biochem. Biotechnol. 2020, 50, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Hamade, F.; Bubniene, U.; Engblom, J.; Ramanavicius, A.; Ramanaviciene, A.; Ruzgas, T. In-vitro model for assessing glucose diffusion through skin. Biosens. Bioelectron. 2018, 11, 175–179. [Google Scholar] [CrossRef]
- German, N.; Ramanavicius, A.; Ramanaviciene, A. Electrochemical deposition of gold nanoparticles on graphite rod for glucose biosensing. Sens. Actuators B Chem. 2014, 203, 25–34. [Google Scholar] [CrossRef]
- House, J.L.; Anderson, E.M.; Ward, W.K. Immobilization Techniques to Avoid Enzyme Loss from Oxidase-Based Biosensors: A One-Year Study. J. Diabetes Sci. Technol. 2007, 1, 18–27. [Google Scholar] [CrossRef]
- Das, A.K.; Samdani, J.; Kim, H.Y.; Lee, J.H. Nicotinamide adenine dinucleotide assisted direct electrodeposition of gold nanodendrites and its electrochemical applications. Electrochim. Acta 2015, 158, 129–137. [Google Scholar] [CrossRef]
- Shu, H.; Cao, L.; Chang, G.; He, H.; Zhang, Y.; He, Y. Direct Electrodeposition of Gold Nanostructures onto Glassy Carbon Electrodes for Non-enzymatic Detection of Glucose. Electrochim. Acta 2014, 132, 524–532. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A. Glucose Biosensor Based on Dendritic Gold Nanostructures Electrodeposited on Graphite Electrode by Different Electrochemical Methods. Chemosensors 2021, 9, 188. [Google Scholar]
- German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A.; Semashko, T.; Mikhailova, R.; Ramanaviciene, A. The use of different glucose oxidases for the development of an amperometric reagentless glucose biosensor based on gold nanoparticles covered by polypyrrole. Electrochim. Acta 2015, 169, 326–333. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Simanaityte, R.; Ramanaviciene, A.; Glumbokaite, L.; Ramanavicius, A. Reagent-less amperometric glucose biosensor based on a graphite rod electrode layer-by-layer modified with 1,10-phenanthroline-5,6-dione and glucose oxidase. Talanta 2017, 171, 204–212. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Engel, A.B.; Cretin, M.; Tingry, S. Gold particles growth on carbon felt for efficient micropower generation in a hybrid biofuel cell. Electrochim. Acta 2016, 219, 121–129. [Google Scholar] [CrossRef]
- Seker, E.; Reed, M.L.; Begley, M.R. Nanoporous Gold: Fabrication, Characterization, and Applications. Materials 2009, 2, 2188–2215. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Sui, Y.; Xiao, G.; Yang, X.; Wei, Y.; Zou, B. Kinetically controlled synthesis of nanoporous Au and its enhanced electrocatalytic activity for glucose-based biofuel cells. Nanoscale 2017, 9, 2514–2520. [Google Scholar] [CrossRef]
- Huo, W.S.; Zeng, H.; Yang, Y.; Zhang, Y.H. Performance of glucose/O2 enzymatic fuel cell based on supporting electrodes over-coated by polymer-nanogold particle composite with entrapped enzymes. Chem. Phys. Lett. 2017, 671, 15–20. [Google Scholar] [CrossRef]
- Gholami, F.; Navaee, A.; Salimi, A.; Ahmadi, R.; Korani, A.; Hallaj, R. Direct Enzymatic Glucose/O2 Biofuel Cell based on Poly-Thiophene Carboxylic Acid alongside Gold Nanostructures Substrates Derived through Bipolar Electrochemistry. Sci. Rep. 2018, 8, 15103. [Google Scholar] [CrossRef]
- Jayapiriya, U.S.; Goel, S. Flexible and optimized carbon paste electrodes for direct electron transfer-based glucose biofuel cell fed by various physiological fluids. Appl. Nanosci. 2020, 10, 4315–4324. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Yu, X.; Liu, H.; Fu, Y.; Wang, Z.; Jiang, L.; Li, X. Polyelectrolyte Multilayer as Matrix for Electrochemical Deposition of Gold Clusters: Toward Super-Hydrophobic Surface. J. Am. Chem. Soc. 2004, 126, 3064–3065. [Google Scholar] [CrossRef]
- Yi, S.; Sun, L.; Lenaghan, S.C.; Wang, Y.; Chong, X.; Zhang, Z.; Zhang, M. One-step synthesis of dendritic gold nanoflowers with high surface-enhanced Raman scattering (SERS) properties. RSC Adv. 2013, 3, 10139–10144. [Google Scholar] [CrossRef]
- Huang, T.; Meng, F.; Qi, L. Controlled Synthesis of Dendritic Gold Nanostructures Assisted by Supramolecular Complexes of Surfactant with Cyclodextrin. Langmuir 2010, 26, 7582–7589. [Google Scholar] [CrossRef]
- Shanmugam, M.; Kim, K. Electrodeposited gold dendrites at reduced graphene oxide as an electrocatalyst for nitrite and glucose oxidation. J. Electroanal. Chem. 2016, 776, 82–92. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, N.; Zhang, K.; Wang, Z.; Hu, H.; Wang, X. Fabrication of dendrite-like Au nanostructures and their enhanced photoluminescence emission. Phys. Status Solidi 2007, 204, 3398–3404. [Google Scholar] [CrossRef]
- Gustafsson, H.; Küchler, A.; Holmberg, K.; Walde, P. Co-immobilization of enzymes with the help of a dendronized polymer and mesoporous silica nanoparticles. J. Mater. Chem. B 2015, 3, 6174–6184. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Glumbokaite, L.; Ramanaviciene, A.; Ramanavicius, A. Reagent-less amperometric glucose biosensor based on nanobiocomposite consisting of poly(1,10-phenanthroline-5,6-dione), poly(pyrrole-2-carboxylic acid), gold nanoparticles and glucose oxidase. Microchem. J. 2020, 154, 104665. [Google Scholar] [CrossRef]
- Gooding, J.J.; Erokhin, P.; Losic, D.; Yang, W.; Policarpio, V.; Liu, J.; Ho, F.M.; Situmorang, M.; Hibbert, D.B.; Shapter, J.G. Parameters Important in Fabricating Enzyme Electrodes Using Self-Assembled Monolayers of Alkanethiols. Anal. Sci. 2001, 17, 3–9. [Google Scholar] [CrossRef] [Green Version]
- German, N.; Ramanaviciene, A.; Voronovic, J.; Ramanavicius, A. Glucose biosensor based on graphite electrodes modified with glucose oxidase and colloidal gold nanoparticles. Microchim. Acta 2010, 168, 221–229. [Google Scholar] [CrossRef]
- Ameku, W.A.; de Araujo, W.R.; Rangel, C.J.; Ando, R.A.; Paixão, T.R.L.C. Gold Nanoparticle Paper-Based Dual-Detection Device for Forensics Applications. ACS Appl. Nano Mater. 2019, 2, 5460–5468. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI–PEDOT Film Deposited on Transparent Electrode. Polymers 2020, 12, 2778. [Google Scholar] [CrossRef]
- Ramanavicius, A.; German, N.; Ramanaviciene, A. Evaluation of electron transfer in electrochemical system based on immobilized gold nanoparticles and glucose oxidase. J. Electrochem. Soc. 2017, 164, G45–G49. [Google Scholar] [CrossRef]
- Nikolaev, K.; Ermakov, S.; Ermolenko, Y.; Averyaskina, E.; Offenhäusser, A.; Mourzina, Y. A novel bioelectrochemical interface based on in situ synthesis of gold nanostructures on electrode surfaces and surface activation by Meerwein’s salt. A bioelectrochemical sensor for glucose determination. Bioelectrochemistry 2015, 105, 34–43. [Google Scholar] [CrossRef]
- Sadak, O. One-pot scalable synthesis of rGO/AuNPs nanocomposite and its application in enzymatic glucose biosensor. Nanocomposites 2021, 7, 44–52. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, N.; Yu, H.; Niu, Y.; Sun, C. Covalent attachment of glucose oxidase to an Au electrode modified with gold nanoparticles for use as glucose biosensor. Bioelectrochemistry 2005, 67, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, H.; Wang, M.; Zhang, K.; Si, P. Examining the effects of self-assembled monolayers on nanoporous gold based amperometric glucose biosensors. Analyst 2014, 139, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, J.; Zhao, S.; Sun, Y.; Sun, C. Multilayered construction of glucose oxidase and gold nanoparticles on Au electrodes based on layer-by-layer covalent attachment. Electrochem. Commun. 2006, 8, 665–672. [Google Scholar] [CrossRef]
- German, N.; Ramanavicius, A.; Ramanaviciene, A. Amperometric Glucose Biosensor Based on Electrochemically Deposited Gold Nanoparticles Covered by Polypyrrole. Electroanalysis 2017, 29, 1267–1277. [Google Scholar] [CrossRef]

| GOx Immobilization Method | LOD/Sensitivity | LDR | Method/Mediator | Ref. |
|---|---|---|---|---|
| GOx adsorbed on the dendritic gold nanostructures modified graphite rod electrode and cross-linked with glutaraldehide vapour | 0.059 mM | 0.1–9.97 mM | Amperometry Phenazine methosulfate | [11] |
| GOx and 2,5-dihydroxybenzaldehyde cross-linked with glutaraldehyde and adsorbed on nanocomposite consisting of gold nanoparticles/reduced graphene oxide within gelatin deposited on a screen-printed electrode | 0.640 mM | 1–11 mM | Cyclic voltammetry 2,5-dihydroxybenzaldehyde | [33] |
| GOx adsorbed on gold nanoparticles (3.5 nm) and redox mediator modified graphite rod electrode and cross-linked with glutaraldehide vapour | 0.024 mM 52.1 μA mM−1 cm−2 | 0.1–10 mM | Amperometry 1,10-phenanthroline-5,6-dione | [12] |
| GOx adsorbed on gold nanoparticles electrochemically deposited on graphite rod electrode and cross-linked with glutaraldehide vapour | 0.083 mM 101.02 μA mM−1 cm−2 | 0.1–10 mM | Amperometry Phenazine methosulfate | [7] |
| GOx covalent attachment to gold nanoparticles (2.6 nm) monolayer modified gold electrode | 0.0082 mmol L−1 8.8 μA mM−1 cm−2 | 0.02–5.7 mM | Amperometry Ferrocenemethanol | [34] |
| GOx covalently immobilised on nanoporous gold modified with self-assembled monolayers (3,3′-dithiodipropionic acid/6-mercaptohexanoic acid/11-mercaptoundecanoic acid) via carbodiimide chemistry | 2.187 μA mM−1 cm−2 1.564 μA mM−1 cm−2 1.160 μA mM−1 cm−2 | 0.1–10 mM | Amperometry p-benzoquinone | [35] |
| Multilayers of GOx covalently immobilised on the gold electrode and gold nanoparticles (12 nm) obtained using cysteamine (6 layers) | 0.008 mM 5.72 μA mM−1 cm−2 | 0.01–13 mM | Amperometry Ferrocenemethanol | [36] |
| GOx covalently immobilized on 11-MUA SAM modified DGNs/GR electrode and cross-linked with GA vapour (GA-GOx-SAM/DGNs/GR) | 0.019 mM | 0.1 to 10 mM | Amperometry Phenazine methosulfate | This work |
| GOx adsorbed on the DGNs/GR electrode and cross-linked with GA vapour (GA-GOx/DGNs/GR) | 0.022 mM | 0.1 to 10 mM | Amperometry Phenazine methosulfate | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakalauskiene, L.; Popov, A.; Kausaite-Minkstimiene, A.; Ramanavicius, A.; Ramanaviciene, A. The Impact of Glucose Oxidase Immobilization on Dendritic Gold Nanostructures on the Performance of Glucose Biosensors. Biosensors 2022, 12, 320. https://doi.org/10.3390/bios12050320
Sakalauskiene L, Popov A, Kausaite-Minkstimiene A, Ramanavicius A, Ramanaviciene A. The Impact of Glucose Oxidase Immobilization on Dendritic Gold Nanostructures on the Performance of Glucose Biosensors. Biosensors. 2022; 12(5):320. https://doi.org/10.3390/bios12050320
Chicago/Turabian StyleSakalauskiene, Laura, Anton Popov, Asta Kausaite-Minkstimiene, Arunas Ramanavicius, and Almira Ramanaviciene. 2022. "The Impact of Glucose Oxidase Immobilization on Dendritic Gold Nanostructures on the Performance of Glucose Biosensors" Biosensors 12, no. 5: 320. https://doi.org/10.3390/bios12050320
APA StyleSakalauskiene, L., Popov, A., Kausaite-Minkstimiene, A., Ramanavicius, A., & Ramanaviciene, A. (2022). The Impact of Glucose Oxidase Immobilization on Dendritic Gold Nanostructures on the Performance of Glucose Biosensors. Biosensors, 12(5), 320. https://doi.org/10.3390/bios12050320









