SERS Determination of Trace Phosphate in Aquaculture Water Based on a Rhodamine 6G Molecular Probe Association Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Reagents
2.3. Synthesis of the AgNPs
2.4. Testing Procedure
2.5. Sample Preparation
3. Results and Discussion
3.1. Detection Principle
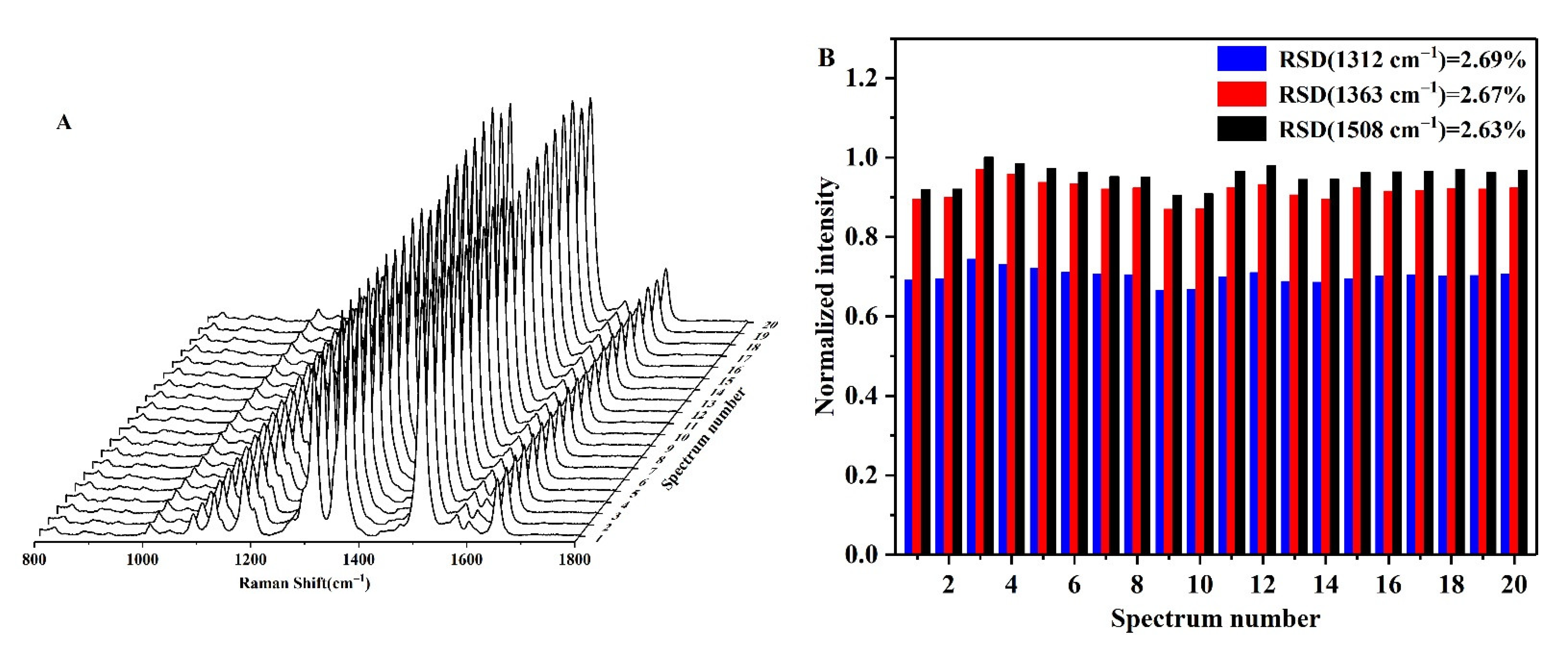
3.2. SERS Detection of Pi
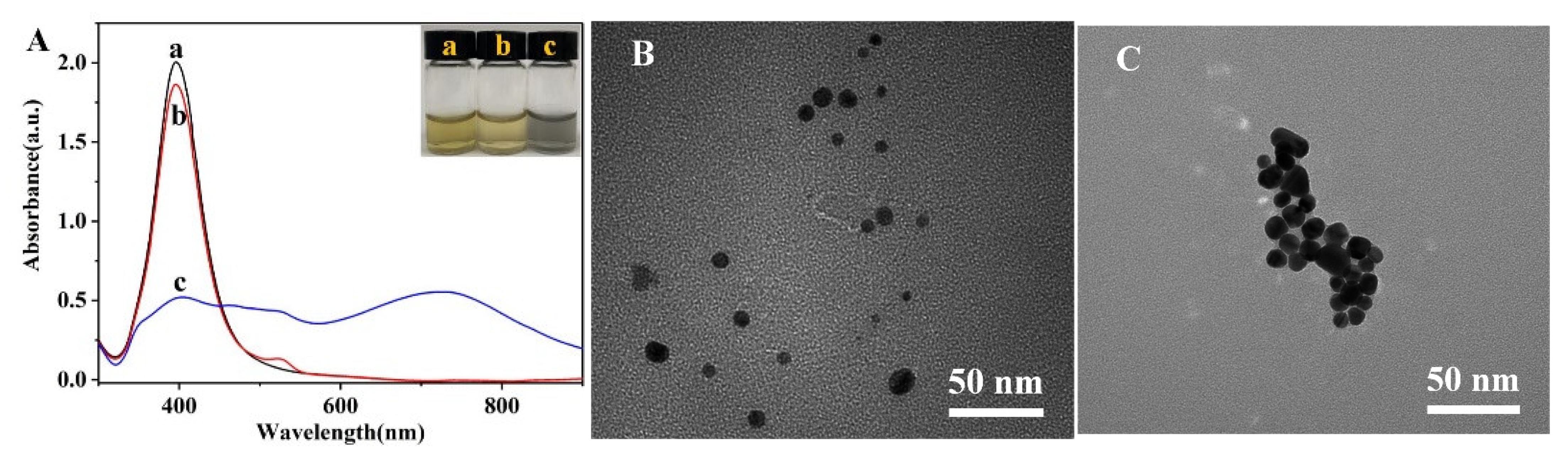
3.3. Characterization of the AgNPs-R6G System
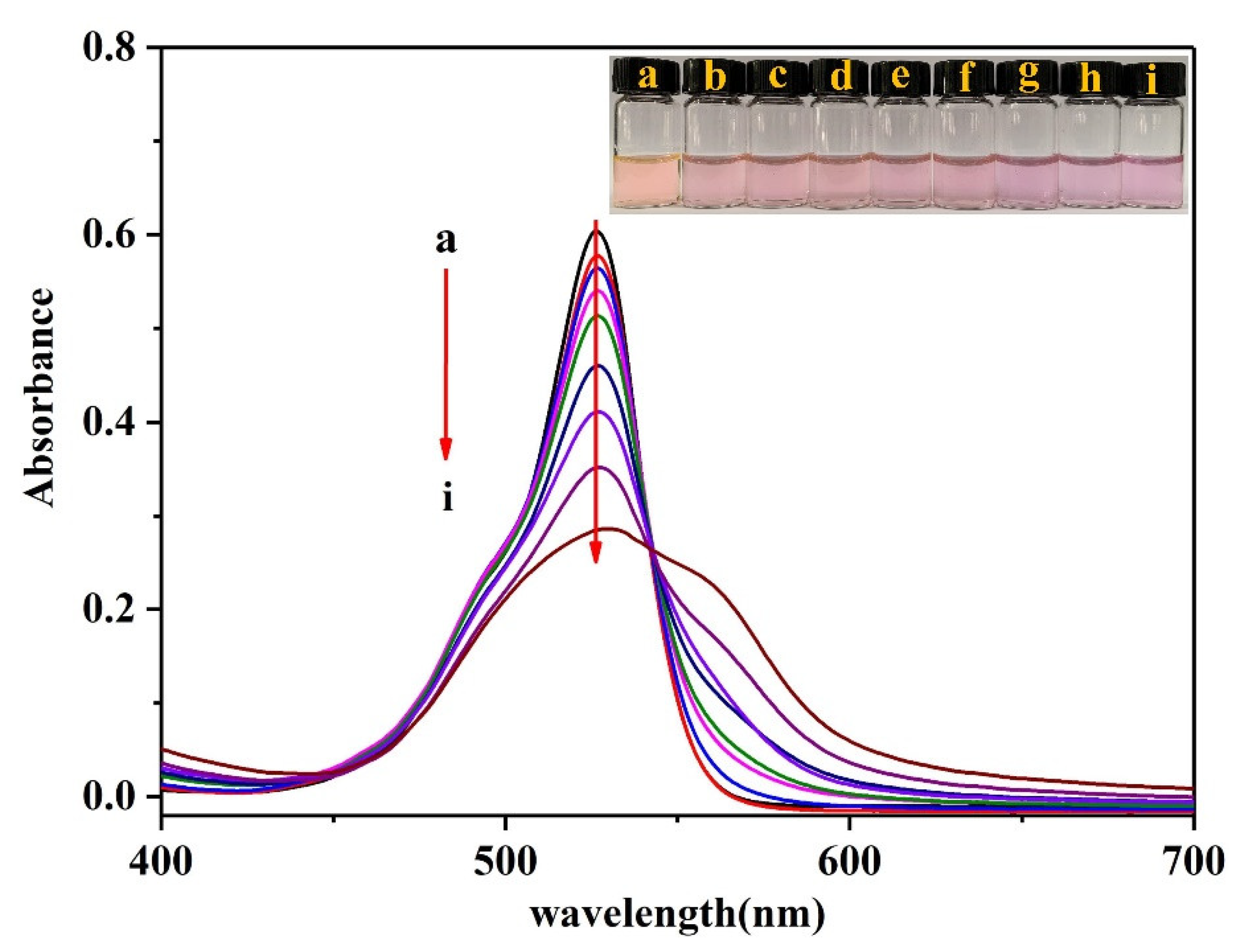
3.4. UV-Vis Characterization of R6G-PMo12O403−
3.5. Method Optimization
3.5.1. Effect of the R6G Concentration
3.5.2. Effect of the NaCl Concentration
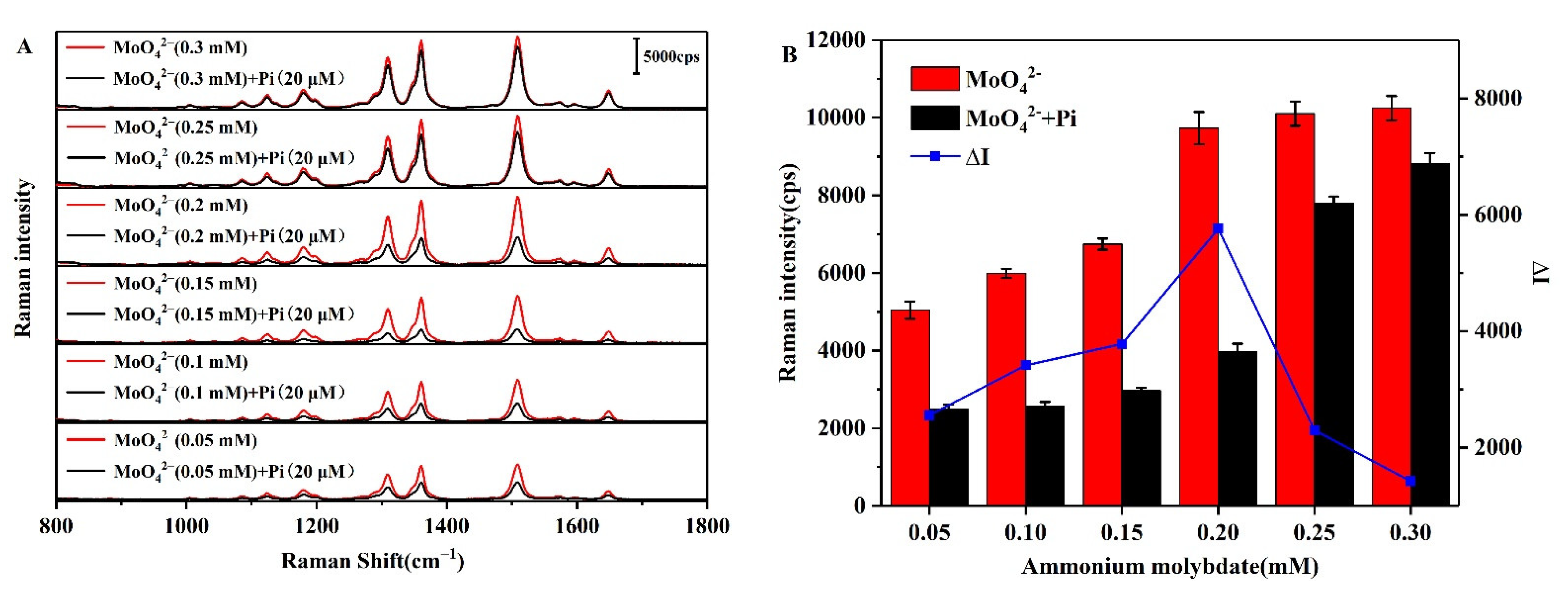
3.5.3. Effect of the Ammonium Molybdate Concentration
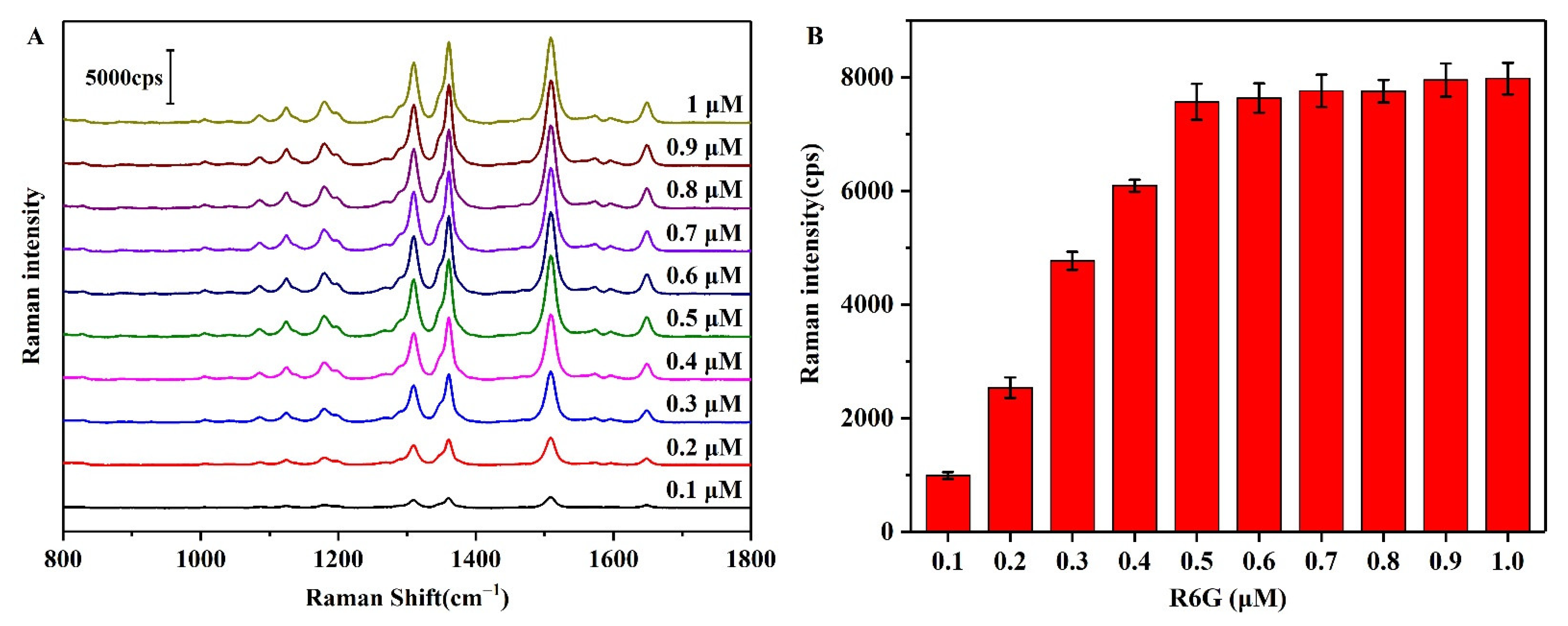
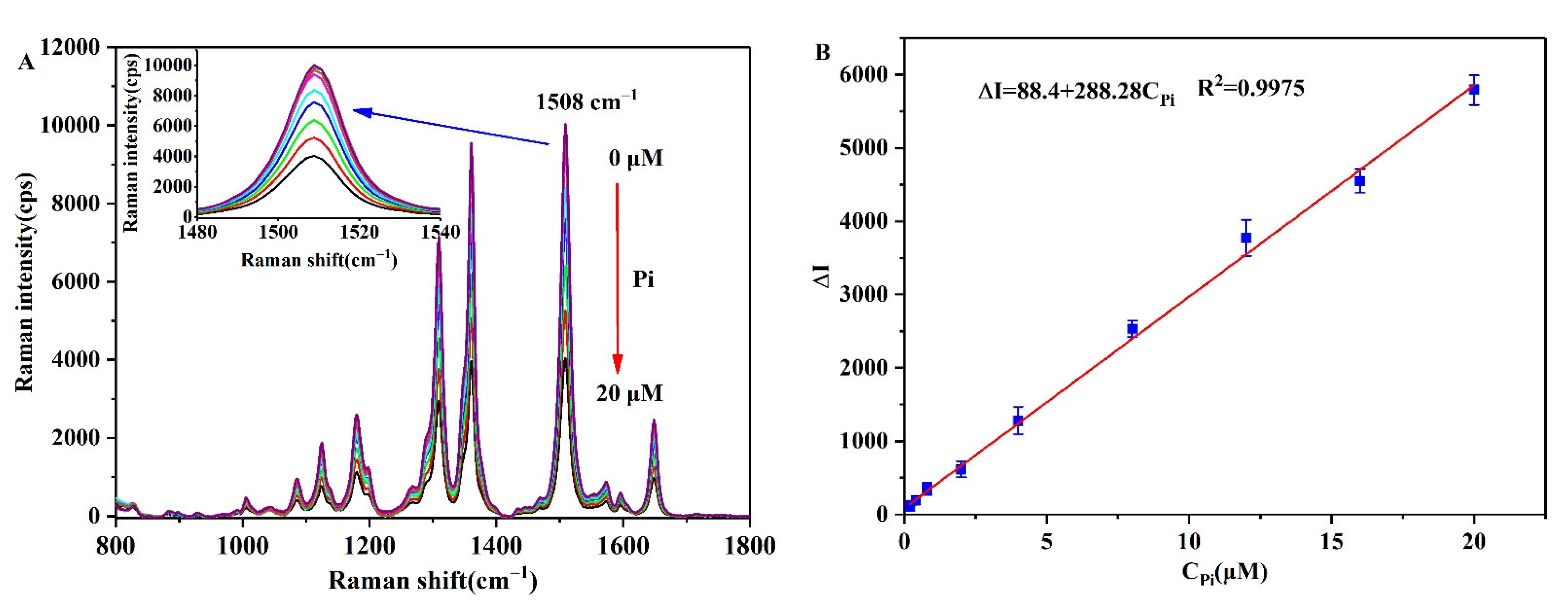
3.6. Analytical Performance of the Pi Detection Method
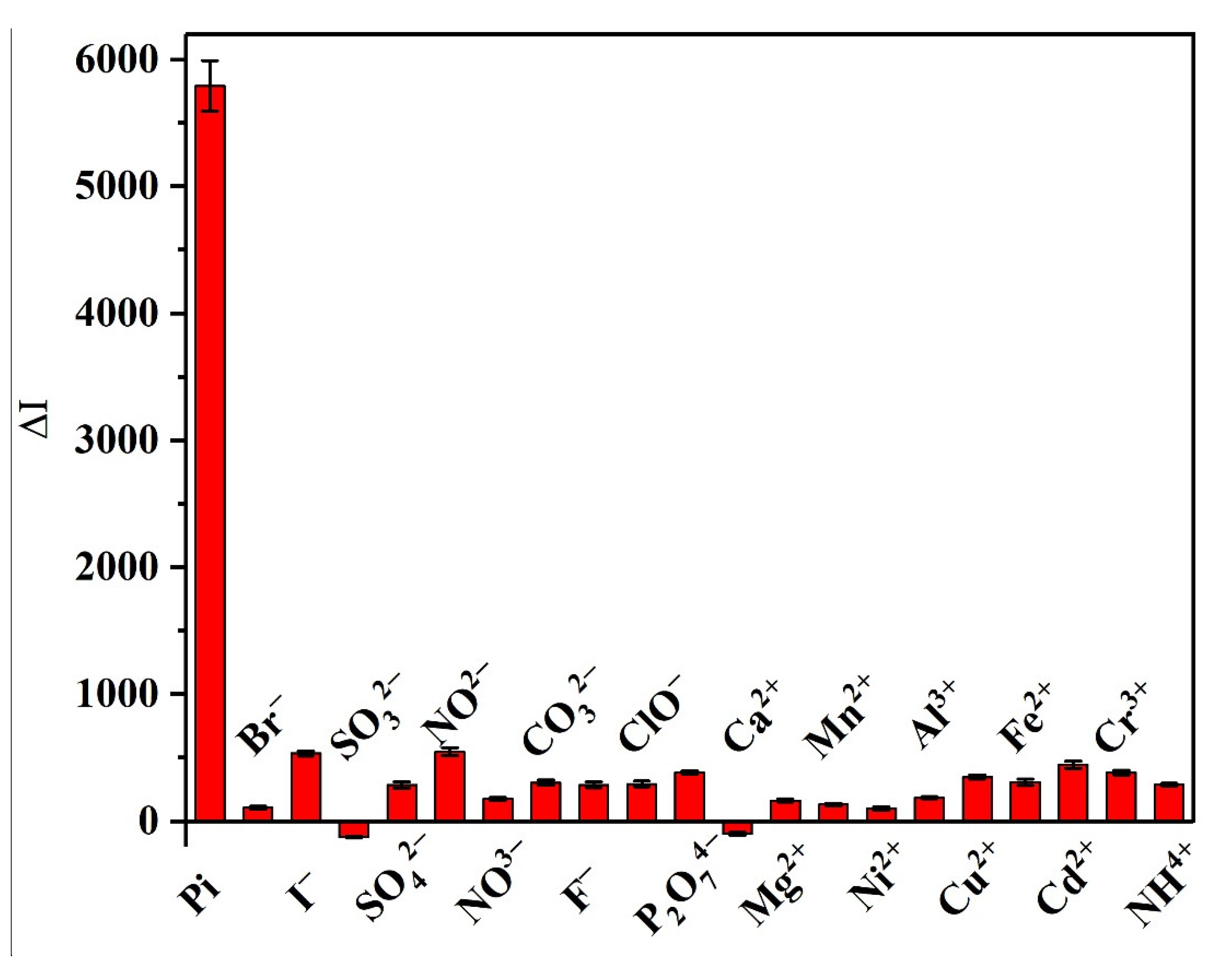
3.7. Selectivity of the Developed Method
3.8. Determination of Pi in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hudson, J.J.; Taylor, W.D.; Schindler, D.W. Phosphate concentrations in lakes. Nature 2000, 406, 54–56. [Google Scholar] [CrossRef]
- Tay, C.J.; Mohd, M.H.; Teh, S.Y.; Koh, H.L. Internal phosphorus recycling promotes rich and complex dynamics in an algae-phosphorus model: Implications for eutrophication management. J. Theor. Biol. 2022, 532, 110913. [Google Scholar] [CrossRef]
- Wu, B.; Wan, J.; Zhang, Y.; Pan, B.; Lo, I.M.C. Selective Phosphate Removal from Water and Wastewater using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol. 2020, 54, 50–66. [Google Scholar] [CrossRef]
- De Oliveira, D.A.V.; Botero, W.G.; Santos, J.C.C.; da Silva, R.M.; Pitombo, L.M.; do Carmo, J.B.; Rosa, L.M.T.; de Oliveira, L.C. Interaction Study Between Humin and Phosphate: Possible Environmental Remediation for Domestic Wastewater. Water Air Soil Pollut. 2017, 228, 265. [Google Scholar] [CrossRef]
- Parsons, S.A.; Smith, J.A. Phosphorus Removal and Recovery from Municipal Wastewaters. Elements 2008, 4, 109–112. [Google Scholar] [CrossRef]
- Gao, G.; Yang, S.; Xu, H.; Dzakpasu, M.; Jin, P.; Wang, X.C. Interception of sediment-liberated phosphate in a surface aquatic system for eutrophication control. Water Supply 2019, 20, 197–208. [Google Scholar] [CrossRef]
- Fink, G.; Alcamo, J.; Flörke, M.; Reder, K. Phosphorus Loadings to the World’s Largest Lakes: Sources and Trends. Global Biogeochem. Cycles 2018, 32, 617–634. [Google Scholar] [CrossRef]
- Ibnul, N.K.; Tripp, C.P. A solventless method for detecting trace level phosphate and arsenate in water using a transparent membrane and visible spectroscopy. Talanta 2021, 225, 122023. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Li, X.; Yang, P.; Yue, J.-Y.; Jiang, Y.; Tang, B. Linker-Eliminated Nano Metal–Organic Framework Fluorescent Probe for Highly Selective and Sensitive Phosphate Ratiometric Detection in Water and Body Fluids. Anal. Chem. 2020, 92, 3722–3727. [Google Scholar] [CrossRef]
- Yang, J.; Dai, Y.; Zhu, X.; Wang, Z.; Li, Y.; Zhuang, Q.; Shi, J.; Gu, J. Metal–organic frameworks with inherent recognition sites for selective phosphate sensing through their coordination-induced fluorescence enhancement effect. J. Mater. Chem. A 2015, 3, 7445–7452. [Google Scholar] [CrossRef]
- Qin, J.; Li, D.; Miao, Y.; Yan, G. Detection of phosphate based on phosphorescence of Mn doped ZnS quantum dots combined with cerium(iii). RSC Adv. 2017, 7, 46657–46664. [Google Scholar] [CrossRef] [Green Version]
- Kabir, M.F.; Rahman, M.T.; Gurung, A.; Qiao, Q. Electrochemical Phosphate Sensors Using Silver Nanowires Treated Screen Printed Electrodes. IEEE Sens. J. 2018, 18, 3480–3485. [Google Scholar] [CrossRef]
- Xu, F.; Wang, P.; Bian, S.; Wei, Y.; Kong, D.; Wang, H. A Co-Nanoparticles Modified Electrode for On-Site and Rapid Phosphate Detection in Hydroponic Solutions. Sensors 2021, 21, 299. [Google Scholar] [CrossRef]
- Heidari-Bafroui, H.; Charbaji, A.; Anagnostopoulos, C.; Faghri, M. A Colorimetric Dip Strip Assay for Detection of Low Concentrations of Phosphate in Seawater. Sensors 2021, 21, 3125. [Google Scholar] [CrossRef]
- Pinyorospathum, C.; Rattanarat, P.; Chaiyo, S.; Siangproh, W.; Chailapakul, O. Colorimetric sensor for determination of phosphate ions using anti-aggregation of 2-mercaptoethanesulfonate-modified silver nanoplates and europium ions. Sens. Actuators B Chem. 2019, 290, 226–232. [Google Scholar] [CrossRef]
- Nakatani, N.; Kozaki, D.; Mori, M.; Hasebe, K.; Nakagoshi, N.; Tanaka, K. Ion-exclusion/cation-exchange Chromatography with Dual Detection of the Conductivity and Spectrophotometry for the Simultaneous Determination of Common Inorganic Anionic Species and Cations in River and Wastewater. Anal. Sci. 2011, 27, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.-X.; Cai, Q.; Yang, Z. Determination of glyphosate and phosphate in water by ion chromatography—Inductively coupled plasma mass spectrometry detection. J. Chromatogr. A 2005, 1100, 160–167. [Google Scholar] [CrossRef]
- Cui, J.; Ogabiela, E.E.; Hui, J.; Wang, Y.; Zhang, Y.; Tong, L.; Zhang, J.; Adeloju, S.B.; Zhang, X.; Wu, Y. Electrochemical Biosensor based on Pt/Au Alloy Nanowire Arrays for Phosphate Detection. J. Electrochem. Soc. 2014, 162, B62–B67. [Google Scholar] [CrossRef]
- Basheer, S.; Samyn, D.; Hedström, M.; Thakur, M.S.; Persson, B.L.; Mattiasson, B. A membrane protein based biosensor: Use of a phosphate—H+ symporter membrane protein (Pho84) in the sensing of phosphate ions. Biosens. Bioelectron. 2011, 27, 58–63. [Google Scholar] [CrossRef]
- Mahmud, M.A.P.; Ejeian, F.; Azadi, S.; Myers, M.; Pejcic, B.; Abbassi, R.; Razmjou, A.; Asadnia, M. Recent progress in sensing nitrate, nitrite, phosphate, and ammonium in aquatic environment. Chemosphere 2020, 259, 127492. [Google Scholar] [CrossRef]
- Berchmans, S.; Issa, T.B.; Singh, P. Determination of inorganic phosphate by electroanalytical methods: A review. Anal. Chim. Acta 2012, 729, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.-Y.; Yi, J.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Panneerselvam, R.; Tian, Z.-Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Li, D.; Ma, Y.; Duan, H.; Deng, W.; Li, D. Griess reaction-based paper strip for colorimetric/fluorescent/SERS triple sensing of nitrite. Biosens. Bioelectron. 2018, 99, 389–398. [Google Scholar] [CrossRef]
- Ji, W.; Song, W.; Tanabe, I.; Wang, Y.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering for highly robust SERS sensing: The case of phosphate analysis. Chem. Commun. 2015, 51, 7641–7644. [Google Scholar] [CrossRef]
- Piotrowski, P.; Bukowska, J. 2-Mercaptoethanesulfonate (MES) anion-functionalized silver nanoparticles as an efficient SERS-based sensor of metal cations. Sens. Actuators B Chem. 2015, 221, 700–707. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Lai, Y.; Wu, C.; Sun, S.; He, Y.; Ma, H. Ultrasensitive and selective detection of copper (II) and mercury (II) ions by dye-coded silver nanoparticle-based SERS probes. Biosens. Bioelectron. 2013, 39, 82–87. [Google Scholar] [CrossRef]
- Murugan, E.; Santhoshkumar, S.; Govindaraju, S.; Palanichamy, M. Silver nanoparticles decorated g-C3N4: An efficient SERS substrate for monitoring catalytic reduction and selective Hg2+ions detection. Spectrochim. Acta Part A 2021, 246, 119036. [Google Scholar] [CrossRef]
- Yin, J.; Wu, T.; Song, J.; Zhang, Q.; Liu, S.; Xu, R.; Duan, H. SERS-Active Nanoparticles for Sensitive and Selective Detection of Cadmium Ion (Cd2+). Chem. Mater. 2011, 23, 4756–4764. [Google Scholar] [CrossRef]
- Lv, L.; He, L.; Jiang, S.; Chen, J.; Zhou, C.; Qu, J.; Lu, Y.; Hong, P.; Sun, S.; Li, C. In situ surface-enhanced Raman spectroscopy for detecting microplastics and nanoplastics in aquatic environments. Sci. Total Environ. 2020, 728, 138449. [Google Scholar] [CrossRef] [PubMed]
- Lê, Q.T.; Ly, N.H.; Kim, M.-K.; Lim, S.H.; Son, S.J.; Zoh, K.-D.; Joo, S.-W. Nanostructured Raman substrates for the sensitive detection of submicrometer-sized plastic pollutants in water. J. Hazard. Mater. 2021, 402, 123499. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gong, M.; Jiang, X.; Chen, Y.; Han, X.; Song, K.; Sun, X.; Zhang, Y.; Zhao, B. SERS investigation and detection of levofloxacin drug molecules on semiconductor TiO2: Charge transfer contribution. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 142–149. [Google Scholar] [CrossRef]
- Wang, W.; Sang, Q.; Yang, M.; Du, J.; Yang, L.; Jiang, X.; Han, X.; Zhao, B. Detection of several quinolone antibiotic residues in water based on Ag-TiO2 SERS strategy. Sci. Total Environ. 2020, 702, 134956. [Google Scholar] [CrossRef]
- Barveen, N.R.; Wang, T.-J.; Chang, Y.-H. Photochemical decoration of silver nanoparticles on silver vanadate nanorods as an efficient SERS probe for ultrasensitive detection of chloramphenicol residue in real samples. Chemosphere 2021, 275, 130115. [Google Scholar] [CrossRef]
- Luo, L.; Chen, Y.; Zhang, L.; Li, Y.; Li, H.; Zhang, H.; Tian, Y. SERS assay for pyrophosphate based on its competitive binding to Cu(II) ion on silver nanoparticles modified with cysteine and rhodamine 6G. Microchim. Acta 2017, 184, 595–601. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Wang, C.; Zhou, X.; Li, J.; Li, D. Highly sensitive and selective method for detection of trace amounts of nitrite in aquaculture water by SERRS coupled with diazo reaction. Sens. Actuators B Chem. 2019, 297, 126757. [Google Scholar] [CrossRef]
- Jia, S.; Li, D.; Fodjo, E.K.; Xu, H.; Deng, W.; Wu, Y.; Wang, Y. Simultaneous preconcentration and ultrasensitive on-site SERS detection of polycyclic aromatic hydrocarbons in seawater using hexanethiol-modified silver decorated graphene nanomaterials. Anal. Methods 2016, 8, 7587–7596. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Si, M.; Wu, R.; Zhang, P. The enhanced mechanism of Cl− to SERS in silver colloid. Spectrosc. Spectral Anal. 2001, 21, 343–346. (In Chinese) [Google Scholar] [CrossRef]
- Lin, X.; Hasi, W.-L.-J.; Lou, X.-T.; Lin, S.; Yang, F.; Jia, B.-S.; Cui, Y.; Ba, D.-X.; Lin, D.-Y.; Lu, Z.-W. Rapid and simple detection of sodium thiocyanate in milk using surface-enhanced Raman spectroscopy based on silver aggregates. J. Raman Spectrosc. 2014, 45, 162–167. [Google Scholar] [CrossRef]
- Michaels, A.M.; Jiang, J.; Brus, L. Ag Nanocrystal Junctions as the Site for Surface-Enhanced Raman Scattering of Single Rhodamine 6G Molecules. J. Phys. Chem. B 2000, 104, 11965–11971. [Google Scholar] [CrossRef] [Green Version]
- Kan, M.; Nasu, T.; Taga, M. Fluorophotometric Determination of Phosphate after Collection on a Membrane Filter as Ion Pair of Molybdophosphate with Rhodamine 6G. Anal. Sci. 1991, 7, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Li, H.; Mo, Y.; Huang, X.; Chen, L. Surface enhanced resonance Raman spectroscopy of rhodamine 6G adsorbed on silver electrode in lithium batteries. Chem. Phys. Lett. 2000, 330, 249–254. [Google Scholar] [CrossRef]
- Armas, L.E.G.; Menezes, J.W.; Huila, M.G.; Araki, K.; Toma, H.E. Gold Nanohole Arrays Fabricated by Interference Lithography Technique as SERS Probes for Chemical Species Such As Rhodamine 6G and 4,4′-Bipyridine. Plasmonics 2017, 12, 1015–1020. [Google Scholar] [CrossRef]
- Shim, S.; Stuart, C.M.; Mathies, R.A. Resonance Raman Cross-Sections and Vibronic Analysis of Rhodamine 6G from Broadband Stimulated Raman Spectroscopy. Chemphyschem 2008, 9, 697–699. [Google Scholar] [CrossRef]
- Liu, S.; Chen, G.; Prasad, P.N.; Swihart, M.T. Synthesis of Monodisperse Au, Ag, and Au–Ag Alloy Nanoparticles with Tunable Size and Surface Plasmon Resonance Frequency. Chem. Mater. 2011, 23, 4098–4101. [Google Scholar] [CrossRef]
- Sarangi, S.N.; Hussain, A.M.P.; Sahu, S.N. Strong UV absorption and emission from L-cysteine capped monodispersed gold nanoparticles. Appl. Phys. Lett. 2009, 95, 073109. [Google Scholar] [CrossRef]
- Pan, H.; Zhu, J.; Jiang, L. Synthesis, characterization and application of rhodamine-phosphomolybdic acid hybrids. J. Analyt. Sci. 2015, 31, 1–5. (In Chinese) [Google Scholar] [CrossRef]
- Liu, W.; Du, Z.; Qian, Y.; Li, F. A specific colorimetric probe for phosphate detection based on anti-aggregation of gold nanoparticles. Sens. Actuators B Chem. 2013, 176, 927–931. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, W.; Xu, H.; Luo, Y.; Guo, D.; Li, D.; Li, D. Negatively charged molybdate mediated nitrogen-doped graphene quantum dots as a fluorescence turn on probe for phosphate ion in aqueous media and living cells. Anal. Chim. Acta 2019, 1080, 196–205. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, M.; Yu, J.; Zhang, X.; Li, Z.; Li, H. Using the interfacial barrier effects of p–n junction on electrochemistry for detection of phosphate. Analyst 2020, 145, 3217–3221. [Google Scholar] [CrossRef]
- National Environmental Protection Standards of the People’s Republic of China: Water Quality-Determination of Orthophosphate and Total Phosphorus-Continuous Flow Analysis(CFA) and Ammonium Molybdate Spectrophotometry. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201311/t20131106_262958.shtml (accessed on 2 May 2022).




| Method | Sample | Linear Range | LOD | Reference |
|---|---|---|---|---|
| Fluorimetry | Drinking water | 0–10 μM | 54 nM | [10] |
| Phosphorescence | Environmental water | 8–320 mM | 2.71 mM | [12] |
| Chromatography | Reservoir water | 0–100 μg/L | 0.7 μg/L | [18] |
| Biosensor | Water | 248–1456 μM | 45 μM | [19] |
| Colorimetry | River water | 0.5–30 μM | 76 nM | [50] |
| Fluorimetry | River water | 7–30 μM | 50 nM | [51] |
| Electrochemistry | Natural water | 0–0.045 mg/L | 3.01 μg/L | [52] |
| SERS | Aquaculture water | 0.2–20 μM | 29.3 nM | This work |
| Sample | Added Pi (μM) | This Work | Spectrophotometry (μM) | ||
|---|---|---|---|---|---|
| Found (μM) | Recovery (%) | RSD (%) | |||
| Pond aquaculture water | 0 | 1.91 | 2.66 | 2.03 | |
| 5 | 6.63 | 94.4 | 5.06 | 7.13 | |
| 10 | 11.58 | 96.7 | 4.52 | 12.09 | |
| Yangtze River water | 0 | 2.14 | 5.03 | 2.32 | |
| 5 | 7.05 | 98.2 | 3.07 | 7.27 | |
| 10 | 12.26 | 101.2 | 2.62 | 12.62 | |
| Aquaponics water | 0 | 6.99 | 5.78 | 7.13 | |
| 5 | 12.1 | 102.2 | 1.77 | 12.02 | |
| 10 | 16.68 | 95.5 | 2.65 | 17.24 | |
| Tap water | 0 | 0.79 | 6.18 | 0.71 | |
| 5 | 6.15 | 107.2 | 3.55 | 5.93 | |
| 10 | 10.83 | 100.4 | 2.41 | 10.55 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Wang, X.; Zhao, G.; Shi, Y.; Thuy, N.T.D.; Yang, H. SERS Determination of Trace Phosphate in Aquaculture Water Based on a Rhodamine 6G Molecular Probe Association Reaction. Biosensors 2022, 12, 319. https://doi.org/10.3390/bios12050319
Jiang Y, Wang X, Zhao G, Shi Y, Thuy NTD, Yang H. SERS Determination of Trace Phosphate in Aquaculture Water Based on a Rhodamine 6G Molecular Probe Association Reaction. Biosensors. 2022; 12(5):319. https://doi.org/10.3390/bios12050319
Chicago/Turabian StyleJiang, Ye, Xiaochan Wang, Guo Zhao, Yinyan Shi, Nguyen Thi Dieu Thuy, and Haolin Yang. 2022. "SERS Determination of Trace Phosphate in Aquaculture Water Based on a Rhodamine 6G Molecular Probe Association Reaction" Biosensors 12, no. 5: 319. https://doi.org/10.3390/bios12050319
APA StyleJiang, Y., Wang, X., Zhao, G., Shi, Y., Thuy, N. T. D., & Yang, H. (2022). SERS Determination of Trace Phosphate in Aquaculture Water Based on a Rhodamine 6G Molecular Probe Association Reaction. Biosensors, 12(5), 319. https://doi.org/10.3390/bios12050319






